Abstract
The intrauterine environment provides a key interface between the mother and the developing fetus during pregnancy, and is a target for investigating mechanisms of fetal programming. Studies have demonstrated an association between prenatal stress and neurodevelopmental disorders. The role of the intrauterine environment in mediating this effect is still being elucidated. In this review, we discuss emerging preclinical and clinical evidence suggesting the existence of microbial communities in utero. We also outline possible mechanisms of bacterial translocation to the intrauterine environment and immune responses to the presence of microbes or microbial components. Lastly, we overview the effects of intrauterine inflammation on neurodevelopment. We hypothesize that maternal gestational stress leads to disruptions in the maternal oral, gut, and vaginal microbiome that may lead to the translocation of bacteria to the intrauterine environment, eliciting an inflammatory response and resulting in deficits in neurodevelopment.
Keywords: Intrauterine environment, prenatal stress, placenta, uterus, neurodevelopment, microbiome, inflammation
Developmental Origins
The intrauterine environment contains a milieu of hormones, metabolites, cytokines (see Glossary), and nutrients, and plays a critical role in the development of the fetus during pregnancy. The placenta acts as the primary interface between the maternal and the fetal environment by selectively transferring nutrients and oxygen to the fetus, removing waste, producing hormones, serving as a protective physical barrier, and harboring its own immune microenvironment [1-3]. Meanwhile, the uterus provides a site for the implantation of the embryo during pregnancy and contracts during labor and delivery [4]. For these reasons, the intrauterine environment provides an enticing target for investigating mechanisms by which changes in the mother can impact the development of the offspring.
Epidemiologist David Barker, demonstrated an association between maternal nutritional status and ischemic heart disease in the offspring in adulthood [5], building upon ideas first posed by James Neel [6]. From this landmark study, the Developmental Origins of Health and Disease Theory, or the idea of “fetal programming,” was established, suggesting that adverse experiences in utero permanently alter organ development and physiology and influence health outcomes later in life. Since then, various fields have investigated links between metabolic and psychosocial stress in the mother during pregnancy and a wide array of conditions and diseases in the offspring, including asthma, diabetes, and obesity [7,8]. Furthermore, in both preclinical and clinical studies, prenatal stress has been linked to preterm birth [9], neurodevelopmental disorders [10,11], and mood disorders [12]. In this context, we use “stress” to refer to the body’s physiological response to demand, as originally defined by Hans Selye [13], and “stressors” to refer to experiences that induce these physiological changes. Stressors commonly include experiences such as socioeconomic difficulties or exposures to natural disasters in humans, and restraint exposure to cold, food deprivation, or social isolation in rodent models.
Although a relationship has been established between prenatal stress and neurodevelopmental disorders, the mechanisms underlying this association have yet to be fully elucidated. A role for the intrauterine environment in this context seems plausible, given that it consists of a complex mixture of hormones, cytokines, and metabolites, and that each of these elements could contribute to fetal programming, both individually and through interactions among these components.
This review will primarily explore intrauterine microbiota and inflammation as potential mechanisms by which prenatal stress can impact neurodevelopment. We acknowledge that these are not the sole factors that may mediate the programming effects of stress, and we refer the readers to prior reviews of other potential mechanisms, including for instance changes in serotonin signaling [14,15], excess glucocorticoids [16-18], and epigenetic regulation [19,20].
The Intrauterine Environment
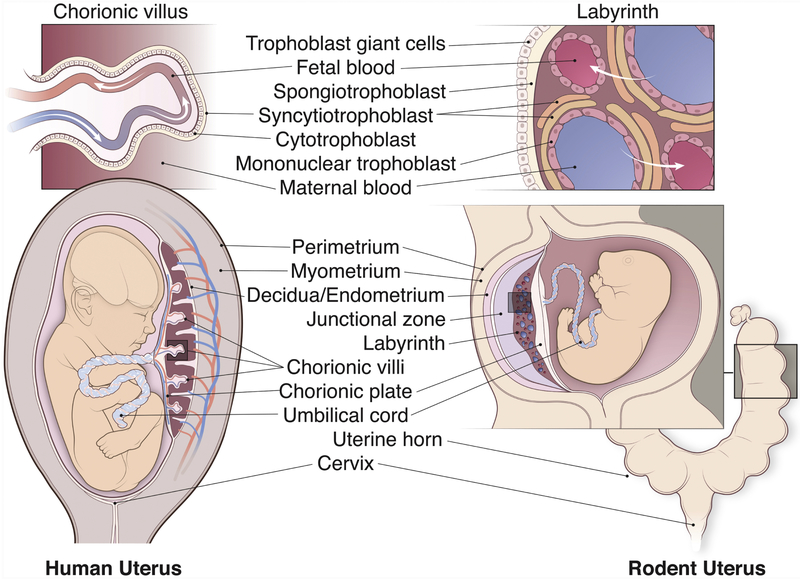
The key components of the pregnant intrauterine environment include the uterus, placenta, fetal membranes, and umbilical cord. Discussions in this review will focus primarily on humans and rodents, because most of the current literature on the links between maternal gestational stress and offspring neurodevelopment is based on these models. We first briefly present and compare the relevant components of the placenta and the uterus in these two species (Figure 1].
Figure 1: Comparative Anatomy of the Human and Rodent Intrauterine Environment.
A cross section of the human (left) and the rodent (right) pregnant uterus. The human uterus is composed of three layers of tissue: the perimetrium, the myometrium, and the endometrium. The fetus sits within the amniotic sac. Fetal blood flows through the umbilical cord to interface with the maternal circulation in the placenta. The placenta consists of 1) decidua, containing uterine cells, spiral arteries and veins, immune cells, and extravillous trophoblast cells; 2) chorionic villi, the functional unit of the placenta for nutrient, oxygen, and waste exchange; 3)chorionic plate, made of fetal trophoblast cells, extracellular matrix, and fetal vessels. The chorionic villi (see top panel) contain a layer of multinucleated syncytiotrophoblast cells and a layer of cytotrophoblast cells separating the maternal and the fetal blood. The rodent uterus consists of perimetrium, the myometrium, and the endometrium. The rodent uterus has two uterine horns (see schematics on the bottom right) that may contain multiple embryos. Within the uterus, each fetus sits in its own amniotic sac and is connected to its own placenta via an umbilical cord. The rodent placenta consists of 1) decidua, containing uterine cells, maternal vasculature, immune cells, and invading trophoblast cells; 2) junctional zone, which includes spongiogtrophoblast cells, trophoblastic glycogen cells, and trophoblastic giant cells; 3) labyrinth, analogous to the chorionic villi in the human placenta; and 4) chorionic plate, made up of fetal trophoblast cells, extracellular matrix, and fetal vessels. The labyrinth (top panel) is composed of two layers of syncytiotrophoblast and one mononuclear trophoblast layer that separate the maternal and fetal circulation. Image by Anthony S. Baker, CMI, Reproduced with the permission of The Ohio State University.
The Placenta
The placenta is an elaborately complex and heterogenous organ [21-23]. The human and the rodent placenta share structural features and functions; however, they diverge in morphogenesis, nomenclature, and specific cell types within the organ. For this reason, translation of findings from the rodent placenta to the human one should be conducted with caution. With that, the rodent model provides a valuable tool, for instance when aiming to assess causality between maternal gestational stress and the development of the fetus.
Both the human and the rodent placenta contain distinct layers of maternal and fetal tissue. The maternal component is primarily derived from uterine cells and maternal vasculature, whereas the fetal aspect includes trophoblast cells, fetal vasculature, and mesenchyme [24].
In humans, the outer layer, called the decidua, is composed of uterine cells derived from the endometrium, spiral arteries and veins [1], and immune cells [25] (see Inflammatory Response). The decidua also contains fetally-derived extravillous trophoblasts, which differentiate from trophoblast progenitor cells and invade the uterine stroma [3,24]. Beneath the decidua is a layer of chorionic villi, which comprise the functional unit of the placenta for the exchange of nutrients, waste, and oxygen [2]. The villi are made up of fetally-derived multinucleated syncytiotrophoblasts, cytotrophoblasts, a mesenchymal core, and fetal capillaries, and are bathed in maternal blood [2,3,24,26]. Single-cell RNA sequencing of human placentas has further revealed distinct subsets of cytotrophoblast and extravillous trophoblast cells based on gene expression [21,27]. The decidua and the villi directly adjacent to the decidua, in combination, are called the basal plate of the placenta [26]. Finally, the innermost layer of the human placenta is the chorionic plate, which consists of trophoblast cells, extracellular matrix, and fetal vessels [24]. In rodents, the decidua and mesometrial triangle together are comparable to the human decidua, containing uterine cells, maternal vasculature, immune cells, and fetal trophoblast cells that invade the uterine lining [3,28]. Different from the human placenta, in rodents, a layer of spongiotrophoblast cells, trophoblastic glycogen cells, and trophoblastic giant cells lie beneath the decidual surface, forming the junctional zone [3]. The junctional zone and the decidua together correspond to the basal plate of the human placenta [24]. The labyrinth is the region of nutrient, waste, and oxygen exchange in the rodent placenta, analogous to the chorionic villi in the human placenta. The labyrinth is composed of two layers of syncytiotrophoblast and a mononuclear trophoblast layer that separate the maternal and the fetal blood [2,3,29]. Unlike the branching villi of the human placenta, this layer forms a maze-like pattern [24,29]. Finally, the chorionic plate is the innermost layer of the rodent placenta, closely resembling that of the human placenta.
In addition to the heterogenous cellular composition of the placenta, single cell transcriptomic studies have revealed a myriad of ligand and receptor pairings facilitating communication between the maternal and fetal interface [22,23]. Due to the inherent complexity of this transient organ, future single-cell transcriptomic analyses can facilitate a better understaning of how maternal exposures may affect fetal development.
The Uterus
Anatomically, the human uterus differs in structure from the rodent uterus. Whereas the human uterus consists of a single body, the rodent uterus contains two uterine horns that extend from the main uterine body. Microscopically, human and rodent uteri are more similar; both consist of three layers of tissue: the perimetrium is the thin outer layer of epithelial cells; the myometrium is the middle layer of the uterus, consisting of smooth muscle cells with contractile functions; and the endometrium is the innermost layer of the uterus that responds to hormonal changes during the menstrual cycle and interfaces with the fetus during gestation [4].
Although rodents are commonly used as animal models, the bovine uterus has also been used in many experiments, particularly in those investigating intrauterine microbiota [30,31]. With two uterine horns extending from the body, the bovine uterus more closely resembles the rodent uterus. Microscopically, the bovine uterus is similar to both the rodent and the human uterus, also comprising three distinct tissue layers.
Intrauterine Microbiota
The existence of an intrauterine microbiome in a healthy pregnancy is a controversial and highly debated topic. Historically, the intrauterine environment was thought to be sterile. However, a number of recent studies using next-generation sequencing techniques have demonstrated the presence of commensal bacteria in the healthy placenta and uterus in both humans and animal models [32-36]. In contrast other studies argue that the bacteria identified using these techniques result from reagent contamination [37-39]. Even the studies that do argue for the presence microbes in the intrauterine environment acknowledge the low bacterial load [32,33,35,36,40], which contributes to the heated debate. Next, we briefly review these contradictory lines of evidence that seem to support and refute the existence of commensal intrauterine microbiota, and then explore emerging findings pointing at possible changes in the intrauterine microbiota associated with prenatal stress.
Placental Microbiota
Through the use of 16S-based metagenomic and whole genome shotgun sequencing, a unique microbiome has been identified in the human placenta [32-35]. However, the species that were found in different studies are inconsistent. While several showed that the placental microbiome closely resembles the oral microbiome, specifically identifying Escherichia coli, Prevotella tannarae, and non-pathogenic Neisseria species as the most abundant [32,33], others demonstrated that the bacterial communities in the placenta were more similar to those in the vagina [34]. In these studies, the placental microbiome significantly differed from DNA extraction and PCR blanks [32-34]. Furthermore, the resemblance between the vaginal and placental microbiome remained after controlling for delivery method, suggesting that the finding was not due to contamination of the placenta from passage through the vaginal canal [34]. Separately, Enterobacteriaceae have also been identified as a dominant family in the healthy human placenta [41]. The variability in these findings could be explained by the different sections of placental tissue collected in each experiment suggesting that the layers of the placenta could harbor unique microbial communities. The studies that found similarities between the placental and oral microbiome used chorionic villi samples [32], whereas vaginal microbiota were present in samples from the entire placenta [34] and Enterobacteriaceae were found in decidual samples [41]. Evidence also shows that the fetal membrane and the basal plate of the human placenta differ in terms of bacterial diversity and abundance [25]. Additionally, intracellular bacteria have been identified in the basal plate, regardless of clinical or histologic diagnosis of chorioamnionitis [42]. Extravillous trophoblasts, in particular, have been identified as the target of infection through the use of an in vitro culture of human placental explants [43].
Notably, several groups have cultured viable microbes in human placenta, amniotic fluid, and umbilical cord blood samples, including Escherichia coli and Enterococcus faecalis [40,41,44]. However, the viable microbes also included Propionibacterium acnes and Staphylococcus epidermidis, which are both essential components of the skin flora and could potentially result from contamination through contact with the skin [40].
Despite these compelling findings, there is also evidence suggesting that the bacteria found in human placental samples does not differ from contamination controls [37,39,45]. In one experiment, DNA was purified from the basal plate and fetal aspect of human placental tissue and total 16S rRNA gene copies were quantified in the tissue, showing that the placenta contained a low bacterial load comparable to that observed in the negative contamination controls. Additionally, the bacterial lineages in the placenta determined by sequencing were not readily distinguishable from those in the air swab, sterile swab, and extraction blank contamination controls [37]. This work was later replicated by the same group and extended to apply to placentas from spontaneous preterm and term births. Using 16S rRNA sequencing and shotgun metagenomic sequencing, the placental samples were found to closely resemble the contamination controls, and no differences were found based on timing or mode of delivery [39]. A second group also failed to define a consistent placental microbiome using 16S rRNA sequencing on human basal plate samples [45]. However, in contrast to the first study, Mycoplasma and Ureaplasma were identified in spontaneous preterm birth, but not the term birth, samples [45]. Furthermore, several reviews and commentaries assessing the evidence supporting and refuting the existence of a placental microbiome ultimately concluded that microbiota are not present under non-pathologic conditions [46,47]. Indeed, a commonly cited argument is that the ability to generate germ-free mice by delivering fetuses via cesarean section and raising the offspring in sterile environments refutes the idea that the placenta contains microbes [46]. With such controversy regarding the existence of a placental microbiome, it is imperative that stringent environmental and reagent contamination controls are used.
To date, the only study (to our knowledge) that has investigated the effect of maternal gestational stress on placental microbes was completed in a rodent model, showing that restraint stress in pregnant mice tended to alter the microbial community structure in the placenta [36]. Furthermore, the changes in microbial composition with stress were associated with increased anxiety-like behavior and decreased brain-derived neurotrophic factor in the amygdala of adult female offspring [36]. Additional research is warranted to examine the direct effect of alterations in placental microbial communities on the development of the fetal brain. Further work is also required to extend these findings to the human placenta following prenatal stress.
Uterine Microbiota
Similar to the placental microbiome, the presence of microbiota in the healthy uterus has sparked much discourse and debate. Using 16S rRNA sequencing, the dominant taxa identified in human non-pregnant endometrial samples were in the Bacteroidetes phylum, which is commonly found in the gut microbiome [48]. This finding was further supported by evidence of Bacteroidetes in virgin and pregnant bovine uteri [30,31]. Firmicutes and Proteobacteria were also abundant in the bovine uteri [30]. By contrast, another study of the human non-pregnant uterus found that Lactobacillus iners, Prevotella spp., and Lactobacillus crispatus were the dominant species, which are also present in the vaginal microbiome [49].
To date, no work has been completed on changes in the uterine microbiome under conditions of prenatal stress. However, due to the intimate interface between the uterus and the fetus, the uterine microbiome could provide an important avenue for future investigation.
Possible Sources and Sequelae of Intrauterine Microbiota
The close resemblance between the microbial communities found in the intrauterine environment (according to some of the studies arguing for their presence) and those found in the oral cavity, the gut, and the vagina raises a question about the origin of the intrauterine microbiota. Two primary mechanisms of transmission have been hypothesized: direct ascension from the vaginal canal or hematogenous spread from distal sites such as the oral cavity and the gut [50].
In support of the hypothesis that microbiota ascend from the vaginal canal through the cervix to seed the intrauterine environment, human and rodent uteri have been shown to uptake radioactively labeled particles and bioluminescent bacteria from the vagina [51,52]. Additionally, vaginal microbiota have been implicated in shaping the gut microbiome of the offspring [53,54], presumably through seeding during delivery. The bacterial communities in the neonatal murine gut have been shown to most closely resemble the composition of the vaginal microbiome of the mother [53]. Indeed, maternal stress during pregnancy in a rodent model has been shown to reduce the abundance of Lactobacillus species in the maternal vaginal microbiome and in the neonatal offspring gut [55]. The neonates from this study also had dysregulated metabolic profiles in the developing brain [55]. Furthermore, transplantation of vaginal microbiota from stressed dams to naïve pups enhanced the stress response in male offspring, implicating the vaginal microbiome in fetal programming [54]. Although ascension of stress-altered vaginal microbiota into the intrauterine environment presents a possible hypothesis for mediating the neurodevelopmental effects of prenatal stress, vertical transmission of microbes directly from the vagina to the neonatal gut is an equally if not more plausible and compelling mechanism.
Alternatively, evidence from a bovine model suggests that the blood and the uterus contain similar pathogens, supporting the idea that these pathogens may be transmitted hematogenously [31]. To directly investigate hematogenous transmission, additional studies have been conducted using experimentally inoculated rodents, allowing investigators to trace bacterium to the intrauterine environment. Injections of human salivary and subgingival plaque samples into the tail veins of pregnant mice resulted in the presence of bacterial DNA in the placenta that closely resembled the communities identified in the salivary and plaque samples [56]. Orally inoculating pregnant mice with bacteria, specifically Enterococcus spp., also led to the translocation of bacteria into the amniotic fluid, placenta, and fetus [44,57]. Finally, bacterial DNA has been identified in peripheral blood mononuclear cells that resembles the bacterial DNA found in breast milk, suggesting that bacteria may be transported to distal sites via peripheral blood mononuclear cells [58].
The significance of the hematogenous transmission of bacteria lies in the potential for microbes in the oral cavity or the gut to enter the bloodstream and translocate to the intrauterine environment following prenatal stress. There is accumulating evidence that stress leads to alterations in the microbial communities in the gut [36,59,60]. Furthermore, disruptions of the gut microbiome have been shown to increase permeability of the gut epithelial barrier, allowing microbes or microbial components to escape from the lumen of the gastrointestinal tract and enter the bloodstream [61,62]. Once in circulation, these microbes are capable of translocating to other organs, including the spleen [63], suggesting that the bacteria or bacterial DNA may be able to seed the intrauterine environment as well.
Although it is unclear if placental microbiota can directly influence neurodevelopment the presence of microbiota can elicit an inflammatory response within the intrauterine environment, which can lead to adverse effects on the development of the fetal brain (see Inflammatory Response). Furthermore, even if viable bacteria are not present in the placenta, toll-like receptors can respond to nucleic acids from microbes (Reviewed in [64]), and are therefore capable of unleashing an immune response. Studies of intrauterine infections in animal models and in humans have both demonstrated that the presence of microbes or microbial components can lead to the recruitment of immune cells to the placenta and the release of pro-inflammatory cytokines [65-67]. Specifically, the presence of anaerobic Streptococcus spp. and Mycoplasma spp. in the human placenta have been associated with differential CpG methylation of immune-related genes [68]. Additionally, bacterial cell wall peptidoglycan has been shown to be capable of passing into the murine placenta and into the fetal brain, inducing neuroproliferation, and resulting in deficits in spatial learning and working memory in the offspring [69].
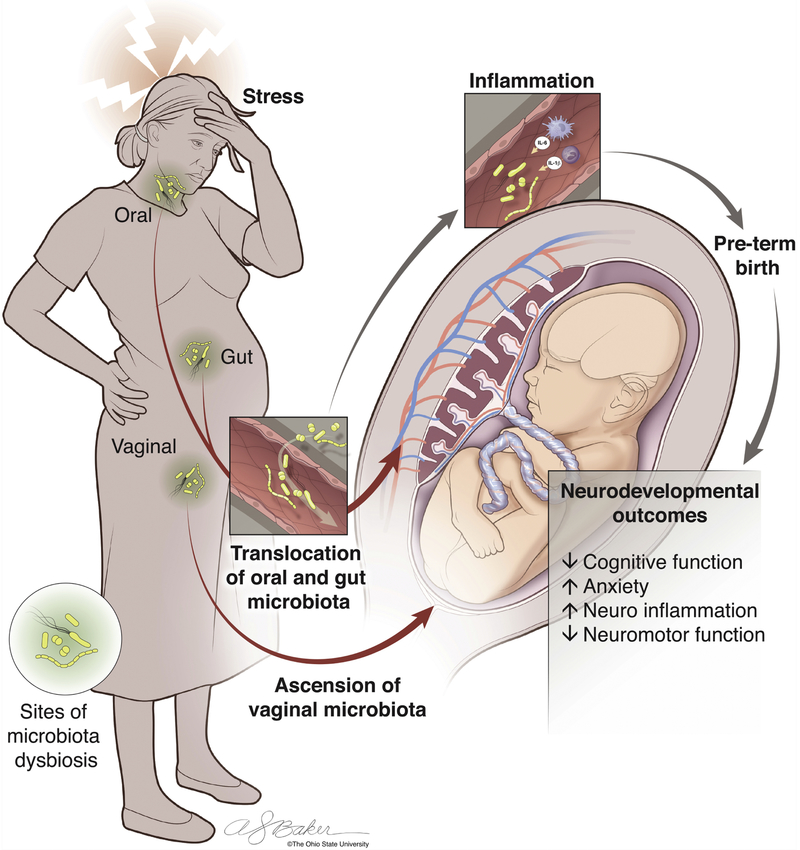
Given the associations between maternal gestational stress and alterations in maternal gastrointestinal and vaginal microbial communities, the potential mechanisms of bacterial translocation, and the immune responses elicited by the presence of microbiota in the intrauterine environment, we hypothesize that prenatal stress leads to changes in the gut, oral, and vaginal microbiome that allow for the translocation of bacteria to the intrauterine environment either hematogenously or through direct ascension, resulting in a maternal inflammatory response that may impact neurodevelopment by way of the placenta (Figure 2, Key Figure]. To further support this hypothesis, we will explore the inflammatory sequelae of prenatal stress, and the effects of intrauterine inflammation on neurodevelopment.
Figure 2, Key Figure: The Effect of Maternal Gestational Stress on Translocation of Microbiota, Inflammation, and Neurodevelopment.
Maternal stress during pregnancy has been shown to lead to behavioral and neurodevelopmental deficits in the offspring. Here, we propose a mechanism that may mediate these effects. Exposure to prenatal stress can lead to alterations in the bacterial communities within the gut, oral cavity, or vaginal canal of the mother. Microbiota can translocate to the intrauterine environment either hematogenously from the gut or oral cavity or through direct ascension from the vaginal canal. The presence of microbes or microbial components in the uterus or placenta can elicit a inflammatory response in utero. Intrauterine inflammation is associated with adverse obstetrical outcomes such as preterm birth, which has been linked to neurodevelopmental deficits. However, intrauterine inflammation has also been shown to result in neurodevelopmental deficits independently from preterm birth. Thus, we hypothesize that intrauterine microbiota and inflammation make up a key link between prenatal stress and adverse neurodevelopmental outcomes. Image by Anthony S. Baker, CMI, Reproduced with the permission of The Ohio State University.
Inflammatory Response
Stress leads to an increase in inflammation both peripherally and in the central nervous system (reviewed in [70]). Additionally, mothers who have experienced stress, depression, or anxiety during pregnancy have increased inflammatory markers in their plasma [71,72]. Furthermore, there is growing evidence that inflammation during pregnancy can lead to the emergence of behavioral and cognitive changes in the offspring [73,74]. As the interface between the mother and the fetus, the intrauterine environment provides a semi-permeable bridge by which inflammatory molecules in the mother’s circulation could influence the neurodevelopment of the fetus.
Intrauterine Immune Components
Immune cells are present within the decidua and the chorionic villi of the placenta, as well as within the non-pregnant endometrium [75]. During the first trimester of pregnancy, approximately 30-40% of the stromal cells within the decidua are leukocytes[75]. Uterine natural killer (uNK) cells (also called decidual NK cells) comprise 70% of the leukocytes within the decidua and modulate the invasion of extravillous trophoblast cells into the decidua early in gestation [22]. Single cell transcriptomic analysis of the placenta has revealed three distinct populations of uNK cells that tightly regulate the immune microenvironment of the placenta [22]. Additionally, uNK cells produce various cytokines, including TNF-α, IL-1β, and IFN-γ, and have moderate cytotoxic activity [75]. The second major population of immune cells within the placenta are specialized fetal macrophages called Hofbaeur cells that reside in the placental villi [75]. These macrophages typically display an alternatively activated phenotype, producing anti-inflammatory mediators such as IL-10 and TGF-β [76], but can also be involved in phagocytosing cellular debris [75].
Effect of Maternal Gestational Stress on Inflammation
The strong association between stress and inflammation has led to an increased interest in examining inflammatory markers in the intrauterine environment both in rodents and in humans. In rodents, studies have shown that maternal stress during pregnancy is associated with an increase in pro-inflammatory cytokines such as IL-1β and IL-6 in the placenta [36,77,78]. Cold stress in pregnant rats resulted in an increase in IL-1β in the labyrinth [78]. In this study, placental samples per dam were of mixed sex, and accordingly conclusions about sex-specific effects were precluded. Restraint stress in a mouse model was shown to increase IL-1β in female placentas [36], while chronic variable stress led to an increase in IL-1β and IL-6 only in male placentas [77]. These seemingly contradictory sex-specific findings could be explained by the differences in timing of the prenatal stress used in the two studies: the former employed a mid-to-late gestation stress model, while the latter utilized an early gestational model. This emphasizes the importance of timing of prenatal stress in influencing placental function.
Evidence from human studies further supports the link between maternal gestational stress and placental inflammation. For instance, through the use of transcriptional profiling, one study found that socioeconomic disadvantage was associated with the upregulation of genes involved in immune activation and the downregulation of genes involved in fetal immune tolerance in the chorionic villi [79]. Furthermore, these transcriptional changes associated with socioeconomic disadvantage were ameliorated with stress reduction and enhanced social support [79]. High levels of social stress and moderate to severe symptoms of depression were also shown to be associated with an increase in proinflammatory factors, necrotic villi, and polymorphonuclear cell infiltration in the decidua [80].
Effect of Intrauterine Inflammation on Neurodevelopment
Intrauterine inflammation has been associated with alterations in fetal neurodevelopment and offspring behavior in adulthood [81-83]. Notably, intrauterine inflammation is also linked to preterm birth [84,85], which may mediate the neurodevelopmental effects [86], though intrauterine inflammation insufficient to induce preterm birth can still result in neuronal injury [83].
Elevated levels of IL-1β in placentas was associated with enhanced anxiety-like behavior in female murine offspring from prenatally stressed dams [36]. In male offspring, by contrast, increased IL-1β and IL-6 in the placentas as a result of prenatal stress was associated with hyperactivity [77]. Furthermore, administration of an anti-inflammatory agent ameliorated the hyperactive phenotype [77].
Although several studies have examined the role of intrauterine inflammation on neurodevelopment in the context of prenatal stress, most current data exploring this relationship comes from rodent maternal immune activation models. In these models, lipopolysaccharide (LPS) or polyinosinic-polycytidylic acid (poly(I:C)) are used to induce an inflammatory response in the dams during pregnancy(Reviewed in [87]). Briefly, injections of LPS have been shown to increase pro-inflammatory cytokine levels in the fetal brain and compromise blood-brain barrier integrity [88]. Furthermore, intrauterine injections of LPS were associated with an increase in microglial density and activation, as well as an increase in excitatory synaptic strength in the hippocampus of mice exposed to LPS in utero [89]. Additionally, injections of poly(I:C) have been shown to increase levels of IL-6 and IL-17a in the intrauterine environment, leading to abnormal cortical development in the fetal brain and deficits in social behavior in the offspring [90]. Furthermore, the cortical and behavioral findings were recapitulated with injections of IL-17a into the fetal brain [90]. Of note, these findings were shown to be dependent on the presence of segmented filamentous bacteria in the maternal gut microbiome [91].
Further experiments implicate IL-1, IL-6, CD4+, and CD8+ T cells in the mechanism underlying adverse fetal brain development following intrauterine inflammation [92-94]. Exposure to IL-1β in mice resulted in infiltration of CD4+ and CD8+ T cells to the placenta and led to cortical thinning [95], whereas administration of an IL-1 receptor antagonist exposed to IL-1β ameliorated the cortical microvascular degeneration [93]. Intraperitoneal injections of IL-6 recapitulated the increase in density of multivacuolated microglia, delay in migration of GABAergic progenitors, and anxiety-like behavioral phenotypes observed with prenatal stress in mice [94]. In addition, the administration of anti-IL-6 prior to prenatal stress rescued the microglial and GABAergic changes, although the behavioral phenotype persisted [94].
Concluding Remarks and Future Perspectives
There is accumulating evidence that the intrauterine environment plays an important role in mediating the effects of maternal stress during pregnancy on offspring neurodevelopment. Ongoing research aims to elucidate the mechanisms underlying these influences. Several studies have provided evidence suggesting the existence of a placental and uterine microbiome during a healthy pregnancy, though others have argued that the microbial communities found in the placenta could not be distinguished from reagent contamination controls. Of note, emerging evidence suggests that even if the healthy intrauterine microbiome does not exist, bacteria or bacterial components may be able to translocate to the intrauterine environment hematogenously, from the gut or the oral cavity, or directly through ascension from the vaginal canal. Furthermore, the presence of microbes or microbial components in the intrauterine environment can elicit an immune response with deleterious neurodevelopmental consequences. Based on the findings in the current literature, we hypothesize that intrauterine microbiota and the resulting inflammatory response could be key players in sculpting the fetal brain following maternal gestational stress and warrant additional investigation to further elucidate mechanistic elements and localize findings within the placenta and uterus. According to the proposed framework, one could envision that targeting the intrauterine microbiota and inflammatory response, for instance through the use of probiotics and prebiotics or anti-inflammatory agents, could provide ways to ameliorate the detrimental neurodevelopmental effects of maternal stress during pregnancy.
Highlights.
The existence of an intrauterine microbiome in a healthy pregnancy is highly controversial. While several recent experiments have identified bacterial communities within the placenta and uterus, others indicate that such findings could be due to contamination.
There is emerging evidence that the microbiota within the intrauterine environment may originate from the oral cavity, gut, or vagina.
Maternal gestational stress has been associated with inflammation in utero.
Intrauterine inflammation has been linked to detrimental neurodevelopmental outcomes, both structurally and functionally.
Acknowledgements
The authors would like to thank Reghan Borer and Adrienne Antonson, PhD for providing insightful and helpful edits to the manuscript and Anthony Baker for his graphical design expertise in designing the figures. Work in the Gur lab is supported by K08MH112892, KL2TR001068, and start-up funds from the Ohio State University to T.L.G.
Glossary
- Cytokines:
proteins released by cells in the immune system, endothelial and epithelial cells, and stromal cells that play an integral role in immune signaling. Cytokines can either be pro-inflammatory or anti-inflammatory, i.e., leading to an increase or decrease in the activation and recruitment of immune cells, respectively.
- Lipopolysaccharide (LPS):
a component of the cell wall of gram-negative bacteria that is a potent activator of the innate immune system. LPS is commonly administrated to elicit an immune response in animal models.
- Microbiome:
the genetic material of the commensal microorganisms, including bacteria, viruses, fungi, and archaea, found on the skin or on mucosal surfaces, such as in the gut, the oral cavity, or the vagina.
- Polyinosinic-Polycytidylic Acid (Poly(I:C)):
a synthetic double-stranded RNA polymer that is used to simulate a viral infection. Poly(I:C) is commonly administered to elicit an immune response in animal models.
- Trophoblast Cells:
the first cell type that differentiates from the fertilized egg. These cells are the precursor cells for the fetal components of the placenta, and mediate implantation of the embryo in the uterus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox B et al. (2009) Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol 5, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson ED and Cross JC (2005) Development of structures and transport functions in the mouse placenta. Physiol. 20, 180–193 [DOI] [PubMed] [Google Scholar]
- 3.Malassiné A et al. (2003) A comparison of placental development and endocrine functions between the human and mouse model. Hum. Reprod. Update 9, 531–539 [DOI] [PubMed] [Google Scholar]
- 4.Sosa-Stanley JN and Bhimji SS Anatomy, Pelvis, Uterus.. (2017), StatPearls Publishing; [PubMed] [Google Scholar]
- 5.Barker DJP and Osmond C (1986) Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet 84, 475–478 [DOI] [PubMed] [Google Scholar]
- 6.Neel JV (1962) Diabetes Mellitus: A “Thrifty” Genotype Rendered Detrimental by “Progress”? Am J Hum Genet 14, 353–362 [PMC free article] [PubMed] [Google Scholar]
- 7.Flanigan C et al. (2018) Prenatal maternal psychosocial stress and offspring’s asthma and allergic disease: A systematic review and meta-analysis. Clin Exp Allergy 48, 403–414 [DOI] [PubMed] [Google Scholar]
- 8.Entringer S (2013) Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutr Metab Care 16, 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro GD et al. (2013) Psychosocial stress in pregnancy and preterm birth: associations and mechanisms. J Perinat Med 41,631–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bale TL et al. (2010) Early life programming and neurodevelopmental disorders. Biol Psychiatry 68, 314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Bergh BRH et al. (2017) Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev DOI: 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell KJ et al. (2014) The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev. Psychopathol. 26,393–403 [DOI] [PubMed] [Google Scholar]
- 13.Selye H (1956) The Stress of Life, McGraw-Hill [Google Scholar]
- 14.St-Pierre J et al. (2016) Effects of prenatal maternal stress on serotonin and fetal development. Placenta 48 Suppl 1, S66–S71 [DOI] [PubMed] [Google Scholar]
- 15.Velasquez JC et al. (2013) Placental serotonin: implications for the developmental effects of SSRIs and maternal depression. Front. Cell. Neurosci. 7,1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds RM (2013) Glucocorticoid excess and the developmental origins of disease: Two decades of testing the hypothesis - 2012 Curt Richter Award Winner. Psychoneuroendocrinology 38,1–11 [DOI] [PubMed] [Google Scholar]
- 17.Painter RC et al. (2012) Long-term effects of prenatal stress and glucocorticoid exposure. Birth Defects Res C Embryo Today 96,315–324 [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell K et al. (2009) Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev. Neurosci. 31,285–292 [DOI] [PubMed] [Google Scholar]
- 19.Nugent BM and Bale TL (2016) The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front Neuroendocr. 39, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bronson SL and Bale TL (2016) The Placenta as a Mediator of Stress Effects on Neurodevelopmental Reprogramming. Neuropsychopharmacology 41, 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang JCH et al. (2017) Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics. Proc. Natl. Acad. Sci. 114, E7786–E7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vento-Tormo R et al. (2018) Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 563, 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavličev M et al. (2017) Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface. Genome Res 27, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgiades P et al. (2002) Comparative developmental anatomy of the murine and human definitive placentae. Placenta 23, 3–19 [DOI] [PubMed] [Google Scholar]
- 25.Parnell LA et al. (2017) Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci. Rep. 7,1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Enders A and Blankenship T (1999) Comparative placental structure. Adv Drug Deliv Rev. 38, 3–15 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y et al. (2018) Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res 28, 819–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faas MM and de Vos P (2017) Uterine NK cells and macrophages in pregnancy. Placenta 56,44–52 [DOI] [PubMed] [Google Scholar]
- 29.Simmons DG and Cross JC (2005) Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol 284,12–24 [DOI] [PubMed] [Google Scholar]
- 30.Moore SG et al. (2017) Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci 100,4953–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeon SJ et al. (2017) Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 5, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aagaard K et al. (2015) The Placenta Harbors a Unique Microbiome. Sci. Transl. Med 6, 229–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Arango LF et al. (2017) Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci. Rep 7,1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doyle RM et al. (2017) Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS One 12, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu L et al. (2018) Bacterial communities in the womb during healthy pregnancy. Front. Microbiol 9,1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gur TL et al. (2017) Prenatal stress affects placental cytokines and neurotrophins, commensal microbes, and anxiety-like behavior in adult female offspring. Brain. Behav. Immun 64, 50–58 [DOI] [PubMed] [Google Scholar]
- 37.Lauder AP et al. (2016) Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome 4, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Goffau MC et al. (2018) Recognizing the reagent microbiome. Nat Microbiol 3, 851–853 [DOI] [PubMed] [Google Scholar]
- 39.Leiby JS et al. (2018) Lack of detection of a human placenta microbiome in samples from preterm and term deliveries. Microbiome 6,196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collado MC et al. (2016) Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci. Rep 6,1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu L et al. (2018) Bacterial Communities in the Womb During Healthy Pregnancy. Front Microbiol 9, 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stout MJ et al. (2013) Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obs. Gynecol 208, 226.e1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao B and Mysorekar IU (2014) Intracellular bacteria in placental basal plate localize to extravillous trophoblasts. Placenta 35,139–142 [DOI] [PubMed] [Google Scholar]
- 44.Jiménez E et al. (2005) Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol 51,270–274 [DOI] [PubMed] [Google Scholar]
- 45.Leon LJ et al. (2018) Enrichment of Clinically Relevant Organisms in Spontaneous Preterm-Delivered Placentas and Reagent Contamination across All Clinical Groups in a Large Pregnancy Cohort in the United Kingdom. 84,1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Muñoz ME et al. (2017) A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 5,1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hornef M and Penders J (2017) Does a prenatal bacterial microbiota exist? Mucosal Immunol 10,598–601 [DOI] [PubMed] [Google Scholar]
- 48.Verstraelen H et al. (2016) Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 4, e1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell CM et al. (2015) Colonization of the upper genital tract by vaginal bacterial species in nonpregnant women. Am J Obs. Gynecol 212, 611.e1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker JM et al. (2018) Uterine microbiota: Residents, tourists, or invaders? Front. Immunol 9, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zervomanolakis I et al. (2007) Physiology of upward transport in the human female genital tract. Ann N Y Acad Sci 1101,1–20 [DOI] [PubMed] [Google Scholar]
- 52.Suff N et al. (2018) Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am J Pathol 188,2164–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jašarević E et al. (2017) Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jašarević E et al. (2018) The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci 21,1061–1071 [DOI] [PubMed] [Google Scholar]
- 55.Jašarević E et al. (2015) Alterations in the Vaginal Microbiome by Maternal Stress are Associated with Metabolic Reprogramming of the Offspring Gut and Brain. Endocrinology 156, 3265–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fardini Y et al. (2010) Transmission of diverse oral bacteria to murine placenta: Evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun 78,1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan Q et al. (2013) Survival, distribution, and translocation of Enterococcus faecalis and implications for pregnant mice. FEMS Microbiol Lett 349,32–39 [DOI] [PubMed] [Google Scholar]
- 58.Perez PF et al. (2007) Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119, e724–32 [DOI] [PubMed] [Google Scholar]
- 59.Bailey MT et al. (2011) Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain. Behav. Immun 25, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galley JD and Bailey MT (2014) Impact of stressor exposure on the interplay between commensal microbiota and host inflammation. Gut Microbes 5, 390–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiecolt-Glaser JK et al. (2018) Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 98, 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maes M et al. (2008) The gut-brain barrier in major depression- translocation of LPS from gram negative enterobacteria plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett 29,117–124 [PubMed] [Google Scholar]
- 63.Lafuse WP et al. (2013) Exposure to a Social Stressor Induces Translocation of Commensal Lactobacilli to the Spleen and Priming of the Innate Immune System William. Phys. Rev. B - Condens. Matter Mater. Phys 87, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasaki A and Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol 16, 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spinillo A et al. (2014) The role of the placenta in feto-neonatal infections. Early Hum Dev 90 Suppl 1, S7–9 [DOI] [PubMed] [Google Scholar]
- 66.Redline RW (2012) Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med 17,20–25 [DOI] [PubMed] [Google Scholar]
- 67.Turner ML et al. (2012) Immunity and inflammation in the uterus. Reprod Domest Anim 47 Suppl 4, 402–409 [DOI] [PubMed] [Google Scholar]
- 68.Tomlinson MS et al. (2017) Microorganisms in the human placenta are associated with altered CpG methylation of immune and inflammation-related genes. PLoS One 12, e0188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Humann J et al. (2016) Bacterial Peptidoglycan Transverses the Placenta to Induce Fetal Neuroproliferation and Aberrant Postnatal Behavior. Cell Host Microbe 19, 388–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slavich GM and Irwin MR (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140,774–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coussons-Read ME et al. (2007) Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun 21, 343–350 [DOI] [PubMed] [Google Scholar]
- 72.Ashdown H et al. (2006) The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol. Psychiatry 11,47–55 [DOI] [PubMed] [Google Scholar]
- 73.Bennet L et al. (2018) Chronic inflammation and impaired development of the preterm brain. J Reprod Immunol 125, 45–55 [DOI] [PubMed] [Google Scholar]
- 74.Rasmussen JM et al. (2018) Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage DOI: 10.1016/j.neuroimage.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bulmer JN et al. (2009) Immune cells in the placental bed. Int. J. Dev. Biol 101, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 76.Johnson EL and Chakraborty R (2012) Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 9, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bronson SL and Bale TL (2014) Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155,2635–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lian S et al. (2017) Impact of prenatal cold stress on placental physiology, inflammatory response, and apoptosis in rats. Oncotarget 8,115304–115314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller GE et al. (2017) Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain. Behav. Immun 64, 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marinescu IP et al. (2014) Prenatal depression and stress - Risk factors for placental pathology and spontaneous abortion. Rom. J. Morphol. Embryol 55,1155–1160 [PubMed] [Google Scholar]
- 81.Hsiao EY and Patterson PH (2012) Placental regulation of maternal-fetal interactions and brain development. Dev. Neurobiol 72,1317–1326 [DOI] [PubMed] [Google Scholar]
- 82.Paton MCB et al. (2017) Perinatal brain injury as a consequence of preterm birth and intrauterine inflammation: Designing targeted stem cell therapies. Front. Neurosci 11,1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elovitz MA et al. (2011) Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int. J. Dev. Neurosci 29, 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Elovitz MA and Mrinalini C (2004) Animal models of preterm birth. Trends Endocrinol. Metab. 15,479–487 [DOI] [PubMed] [Google Scholar]
- 85.Kemp MW (2014) Preterm birth, intrauterine infection, and fetal inflammation. Front Immunol 5, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodyl NA et al. (2017) Child neurodevelopmental outcomes following preterm and term birth: What can the placenta tell us? Placenta 57, 79–86 [DOI] [PubMed] [Google Scholar]
- 87.Solek CM et al. (2018) Maternal immune activation in neurodevelopmental disorders. Dev Dyn 247,588–619 [DOI] [PubMed] [Google Scholar]
- 88.Simões LR et al. (2018) Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats. J Psychiatr Res 100, 71–83 [DOI] [PubMed] [Google Scholar]
- 89.Kelley MH et al. (2017) Functional changes in hippocampal synaptic signaling in offspring survivors of a mouse model of intrauterine inflammation. J. Neuroinflammation 14,1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi GB et al. (2016) The maternal ROR γ t / IL-17a pathway promotes autism-like phenotypes in offspring. Science (80-.). 6, 933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim S et al. (2017) Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549, 528–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lei J et al. (2018) Maternal CD8+T-cell depletion alleviates intrauterine inflammation-induced perinatal brain injury. Am. J. Reprod. Immunol 79, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nadeau-Vallée M et al. (2017) Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J. Immunol 198, 2047–2062 [DOI] [PubMed] [Google Scholar]
- 94.Gumusoglu SB et al. (2017) The role of IL-6 in neurodevelopment after prenatal stress. Brain. Behav. Immun 65,274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Novak CM et al. (2018) Increased placental T cell trafficking results in adverse neurobehavioral outcomes in offspring exposed to sub -chronic maternal inflammation. Brain Behav Immun DOI: 10.1016/j.bbi.2018.09.025 [DOI] [PubMed] [Google Scholar]