Key Points
Question
Is epilepsy a progressive neurodegenerative disease?
Findings
In this case-control study of 331 adults with 678 magnetic resonance imaging scans, widespread progressive cortical thinning distinct from normal aging was detected in 146 of 190 individuals with epilepsy (76.8%). Cortical thinning was most pronounced in individuals with epilepsy older than 55 years and during the first 5 years after the onset of seizures.
Meaning
People with focal epilepsy may develop progressive cortical atrophy that can affect widespread cortical areas.
Abstract
Importance
It is controversial whether epilepsy is a static or progressive disease. Evidence of progressive gray matter loss in epilepsy would support early diagnosis, rapid treatment, and early referral for surgical interventions.
Objective
To demonstrate progressive cortical thinning in patients with focal epilepsy distinct from cortical thinning associated with normal aging.
Design, Setting, and Participants
A case-control neuroimaging study was conducted from August 3, 2004, to January 26, 2016, among 190 patients with focal epilepsy at a tertiary epilepsy referral center (epilepsy data) and 3 independent comparison cohorts matched for age and sex (healthy volunteer data; n = 141).
Exposures
Two or more high-resolution T1-weighted magnetic resonance imaging scans at least 6 months apart (mean [SD] interval, 2.5 [1.6] years).
Main Outcomes and Measures
Global and vertexwise rate of progressive cortical thinning.
Results
A total of 190 people with focal epilepsy (99 women and 91 men; mean [SD] age, 36 [11] years; 396 magnetic resonance imaging scans) were compared with 141 healthy volunteers (76 women and 65 men; mean [SD] age, 35 [17] years; 282 magnetic resonance imaging scans). Widespread highly significant progressive cortical thinning exceeding normal aging effects, mainly involving the bilateral temporal lobes, medial parietal and occipital cortices, pericentral gyri, and opercula, was seen in 146 individuals with epilepsy (76.8%; 95% CI, 58%-95%). The mean (SD) annualized rate of global cortical thinning in patients with epilepsy was twice the rate of age-associated thinning observed in healthy volunteers (0.024 [0.061] vs 0.011 [0.029] mm/y; P = .01). Progression was most pronounced in adults older than 55 years and during the first 5 years after the onset of seizures. Areas of accelerated cortical thinning were detected in patients with early onset of epilepsy and in patients with hippocampal sclerosis. Accelerated thinning was not associated with seizure frequency, history of generalized seizures, or antiepileptic drug load and did not differ between patients with or without ongoing seizures. Progressive atrophy in temporal (n = 101) and frontal (n = 28) lobe epilepsy was most pronounced ipsilaterally to the epileptic focus but also affected a widespread area extending beyond the focus and commonly affected the contralateral hemisphere. For patients with temporal lobe epilepsy, accelerated cortical thinning was observed within areas structurally connected with the ipsilateral hippocampus.
Conclusions and Relevance
Widespread progressive cortical thinning exceeding that seen with normal aging may occur in patients with focal epilepsy. These findings appear to highlight the need to develop epilepsy disease-modifying treatments to disrupt or slow ongoing atrophy. Longitudinal cortical thickness measurements may have the potential to serve as biomarkers for such studies.
This case-control study examines whether progressive cortical thinning in patients with focal epilepsy is distinct from cortical thinning associated with normal aging.
Introduction
For more than 100 years it, has been hypothesized that “seizures beget seizures,”1 but it remains controversial whether epilepsy is a static or progressive disease.2,3,4 Knowledge about whether epilepsy in humans is associated with ongoing neuronal damage has important practical implications for rapid diagnosis and early treatment. Evidence of progressive neurodegeneration in drug-resistant epilepsy would support early surgical interventions, with an incentive to reduce the mean delay of 18 to 23 years between the onset of seizures and referral for surgery5 and further stimulate the search for disease-modifying therapies.
Measurements of cortical thickness with structural magnetic resonance imaging (MRI) scans are a quantitative, reproducible, and biologically valid biomarker of neurodegeneration.6,7 Neuroimaging studies have demonstrated widespread patterns of neocortical atrophy in people with epilepsy.8,9 The predominantly cross-sectional design of these studies did not allow determination of whether gray matter loss was static or progressive.10 Some cross-sectional studies correlated morphologic changes with duration of epilepsy, but this approach is inherently confounded by the effects of aging because disease duration is highly correlated with age.11
Longitudinal structural neuroimaging provides an objective and statistically powerful12 framework to assess disease progression. Few whole-brain longitudinal studies have been performed in epilepsy.11,13,14,15,16,17,18 Several of these studies were restricted to small cohorts and did not directly compare patients with epilepsy with healthy controls, thus failing to differentiate possible disease progression in epilepsy from normal aging.10 A previous population-based study from our center detected new neocortical volume loss using visual analysis of subtracted images during a 3.5-year period in 54% of patients with chronic epilepsy, 39% of newly diagnosed patients, and 24% of healthy controls.17 The observed changes were widespread, could be remote from the putative epileptic focus, and were not associated with frequency of seizures. Overall cerebral and cerebellar volume losses were similar in the control and patient groups,16,18 concluding that more sensitive methods are required to quantitatively assess progression of epilepsy. Two recent meta-analyses identified an urgent need for large longitudinal neuroimaging studies in epilepsy.9,10
We evaluated changes in cortical thickness over time in a large neuroimaging data set of people with focal epilepsy and matched healthy controls, with the aim to separate out the effects of epilepsy-associated progression from normal aging.
Methods
Participants
We analyzed anonymized data from August 3, 2004, to January 26, 2016, of people with focal epilepsy from a cohort of consecutive patients undergoing follow-up at the National Hospital for Neurology and Neurosurgery, London, United Kingdom. We identified individuals who had at least 2 high-resolution T1-weighted MRI scans performed on the same scanner more than 6 months apart (mean [SD] interscan interval, 3.0 [1.8] years). We excluded individuals with brain lesions other than hippocampal sclerosis, those with insufficient MRI scan quality (ie, patient movement or technical artifacts), and those missing clinical data. The MRI acquisition protocol is described in eAppendix 1 in the Supplement. Presumed lateralization and localization of epilepsy was based on the clinical workup by experienced neurologists specializing in epilepsy, which included clinical, electrophysiological, and neuroimaging data and, where available (108 of 190 [56.8% of the sample]), video electroencephalography telemetry or ambulatory long-term electroencephalography monitoring. Ambiguous or unclear lateralization or localization was classified as undetermined. The study was classified by the National Hospital for Neurology and Neurosurgery Institutional Review Board as a service evaluation involving further anonymized analysis of previously acquired data that did not require individual participant consent. The institutional review board approved the project as a service evaluation.
Several online repositories include publicly available anonymized MRI scan data of healthy volunteers, but most of these cohorts are cross-sectional and involve young (<20 years) or old (>70 years) individuals.19 We selected 3 longitudinal data sets (eAppendix 2 and eTable 1 in the Supplement) with data on healthy volunteers aged between 20 and 70 years, each having 2 high-resolution T1-weighted scans more than 6 months apart (mean [SD] interscan interval, 1.7 [0.7] years).20,21,22 We matched the healthy volunteers with the epilepsy data set for age and sex.
MRI Preprocessing
Cortical thickness was estimated using the fully automated,23 validated,23,24,25 and reliable23,24 Computational Anatomy Toolbox (CAT12) (eAppendix 3 in the Supplement) with an inverse-consistent longitudinal surface registration approach. Cortical thickness maps were smoothed with a 15-mm surface-based kernel. All data were quality controlled according to procedures implemented in CAT12 and scans with misalignment, misregistration, or inaccurate thickness estimation were excluded. Image quality ratings were estimated by scaling image noise, inhomogeneities, and resolution to a single score within the CAT12 quality assurance framework.
Statistical Analysis
Categorical variables are displayed as numbers and percentages and were analyzed with the Fisher exact test. Continuous variables are displayed as mean (SD) and were analyzed with 2-sample t tests. Calculations were done in SPSS, version 24.0 (IBM Corp).
Vertexwise cortical thickness measurements were analyzed with SurfStat within Matlab (http://www.math.mcgill.ca/keith/surfstat). We fitted linear mixed-effects models, a flexible framework for longitudinal analysis of multiple repeated measurements per participant with irregular measurement intervals. To test for differences between groups (eg, patients vs controls) or the influence of clinical variables (eg, seizure frequency) on change of cortical thickness over time, we tested for a main effect of an interaction between the variable of interest (ie, group allocation or clinical variable) and age at MRI scan. All models were corrected for a random effect of participant and fixed effects of age at scan, sex, and group. With this approach, we were able to test for within-participant thickness changes over time while correcting for baseline demographic differences and for different interscan intervals. We report findings considered significant at P < .05 corrected for multiple comparisons using random field theory for nonisotropic images on a cluster level.26
Annualized cortical thinning was determined by subtracting vertexwise thickness values of aligned baseline and follow-up MRI scan pairs and dividing by the interscan interval. Mean annualized thinning was calculated in age subgroups27 with approximately 20-year intervals (18 to <35, 35 to <55, and 55 to <75 years) and subgroups of short (<5 years) and long (≥5 years) duration of epilepsy.
We fitted a machine-learning model (Bayesian logistic ridge regression)28 to separate (classify) epilepsy cases from healthy controls based on their annualized cortical thinning. We trained the model using the top 100 principal components describing the vertexwise data (1000 posterior and 1000 burnin samples), adjusted for age and sex, and tested it using a 10-fold cross-validation procedure. We used t-distributed stochastic neighbor embedding to visualize high-dimensional differences (100 dimensions, perplexity 30) between progressive thinning in patients and controls in 2-dimensional space.29
We performed structural connectivity analyses using 10 diffusion-weighted data sets from healthy volunteers included in BCBtoolkit,30 measuring the regional proportion of voxels connected with the hippocampus. The detailed methods are given in eAppendix 4 and eFigures 1 and 2 in the Supplement.
Results
We included 190 people with focal epilepsy with 396 MRI scans and 141 healthy volunteers with 282 MRI scans. Patients and controls were comparable for mean (SD) age (36 [11] vs 35 [17] years; P = .56), sex (99 women [52.1%] vs 76 women [53.9%]; P = .75), and mean (SD) image quality rating scores (2.04 [0.14] vs 2.05 [0.17]; P = .66). Demographic characteristics of the patients with epilepsy and the controls are displayed in the Table.
Table. Demographic and Clinical Characteristics.
| Characteristic | Patients With Epilepsy (n = 190) | Healthy Volunteers (n = 141) | P Value |
|---|---|---|---|
| Sex, No. (%) | |||
| Male | 91 (47.9) | 65 (46.1) | .75 |
| Female | 99 (52.1) | 76 (53.9) | |
| Age, mean (SD), y | |||
| At baseline MRI scan | 36 (11) | 35 (17) | .56 |
| At seizure onset | 16 (12) | NA | |
| Duration of epilepsy at baseline MRI scan, mean (SD), y | 22 (13) | NA | |
| Lateralization of epilepsy, No. (%) | |||
| Left | 72 (37.9) | NA | NA |
| Right | 68 (35.8) | NA | NA |
| Bilateral | 21 (11.1) | NA | NA |
| Undetermined | 29 (15.3) | NA | NA |
| Localization of epilepsy, No. (%) | |||
| Temporal | 101 (53.2) | NA | NA |
| Frontal | 28 (14.7) | NA | NA |
| Parietal | 5 (2.6) | NA | NA |
| Multilobar | 7 (3.7) | NA | NA |
| Undetermined | 49 (25.8) | NA | NA |
| Hippocampal sclerosis, No. (%) | 38 (20.0) | NA | NA |
| Seizures | |||
| Seizure frequency/mo, mean (SD) | 29 (145) | NA | NA |
| No seizures, No. (%) | 14 (7.4) | NA | NA |
| Less than once a month, No. (%) | 29 (15.3) | NA | NA |
| Once a month to once a week, No. (%) | 62 (32.6) | NA | NA |
| Once a week to daily, No. (%) | 63 (33.2) | NA | NA |
| Daily seizures, No. (%) | 22 (11.6) | NA | NA |
| Generalized tonic-clonic seizures, No. (%) | 92 (48.2) | NA | NA |
| No. of AEDs, mean (SD) | 2 (1) | NA | NA |
Abbreviations: AED, antiepileptic drug; MRI, magnetic resonance imaging; NA, not applicable.
Annualized Cortical Thinning
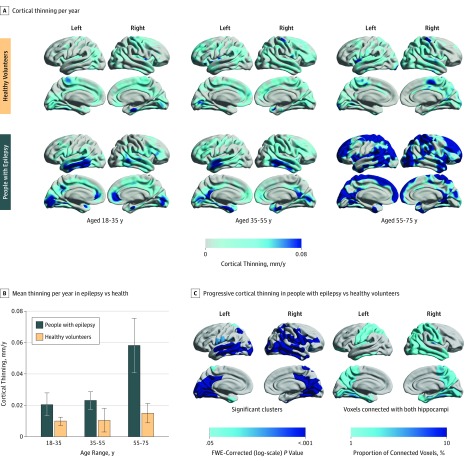
People with epilepsy had a higher mean (SD) overall yearly rate of global cortical thinning compared with healthy volunteers (0.024 [0.061] vs 0.011 [0.029] mm/y; P = .01; Figure 1A). The mean (SD) rate of yearly cortical thinning increased in people with epilepsy older than 55 years (aged 18 to <35 years: n = 83, 0.021 [0.066] mm/y; P = .07 compared with the oldest group; aged 35 to <55 years: n = 95, 0.023 [0.055] mm/y; P = .04 compared with the oldest group; aged 55 to <75 years: n = 12, 0.058 [0.060] mm/y; Figure 1B). There were no differences between age groups in seizure frequency, number of antiepileptic drugs (AEDs) taken, or history of secondarily generalized seizures. Clinical characteristics of patients with epilepsy aged 55 years or older are displayed in eAppendix 5 and eTable 2 in the Supplement. For aging-associated thinning in healthy volunteers, see eAppendix 6 in the Supplement.
Figure 1. Progressive Epilepsy-Associated Cortical Thinning Compared With Thinning Associated With Normal Aging.
A, Annualized rate of cortical thinning in healthy volunteers (top) and people with epilepsy (bottom), stratified to 3 age groups (18 to <35, 35 to <55, and 55 to <75 years). B, Comparison of annualized cortical thinning rates between people with epilepsy and controls (vertical lines indicate SEM). C, Vertexwise statistical comparison of progressive cortical thinning in people with epilepsy vs healthy volunteers using linear mixed-effects models. Hemispheric surface templates are displaying a map of significant clusters after random field theory correction for multiple comparisons (left). Structural connectivity with left and right hippocampi in 10 healthy volunteers is presented as the regional proportion of connected voxels (right). FWE indicates familywise error.
Progressive Cortical Thinning Associated With Epilepsy
When comparing progressive cortical thinning in epilepsy with aging-associated thinning in healthy volunteers, we found widespread areas of greater progressive atrophy developing in those with focal epilepsy (right-sided cluster involving 63 884 vertices; P < .001; largest left-sided cluster involving 37 189 vertices, P < .001; Figure 1C). Areas that were affected bilaterally included the lateral and posterior temporal lobes, posterior cingulate gyri, occipital lobes, pericentral gyri, and the opercula. The ventrolateral prefrontal cortex and the inferior parietal lobule were affected more in the right hemisphere than the left hemisphere.
To analyze whether the spatial patterns of progressive thinning reflect areas of connectivity to the epileptic focus, we performed structural connectivity measurements based on data from 10 healthy volunteers (eAppendix 4 and eFigures 1 and 2 in the Supplement). We found that the distribution of progressive thinning in all patients with epilepsy was similar to regions connected to both hippocampi (Figure 1C).
The results were largely unchanged after adjustment for number of AEDs taken between the 2 MRI scans (eAppendix 7 and eFigure 3 in the Supplement). We performed a sensitivity analysis excluding data from a healthy control cohort involving Asian volunteers22 (n = 69) and the results remained similar (eAppendix 8 and eFigure 4 in the Supplement). Post hoc analyses also showed that the multisite character of the healthy volunteer cohort and the Parkinson Progression Marker Initiative study20 in particular did not increase the variability of cortical thickness measurements compared with patients with epilepsy and thus cannot explain our findings (eAppendix 9 and eFigure 5 in the Supplement). There were no areas of greater cortical thinning in controls than in people with epilepsy.
To detect the frequency of progressive changes in individual participants, we fitted a machine-learning model (pseudo-R2 = 0.55; C statistic, 0.74; 95% CI, 0.52-0.97). The model separated annualized cortical thinning in epilepsy from normal aging in 242 of 331 participants (73.1%; 95% CI, 58%-88%), with a sensitivity of 77% (95% CI, 58%-95%) and specificity of 69% (95% CI, 35%-100%). A dimensionality-reduction plot (eFigure 6 in the Supplement) shows 2 separable but overlapping clusters between people with epilepsy and healthy volunteers.
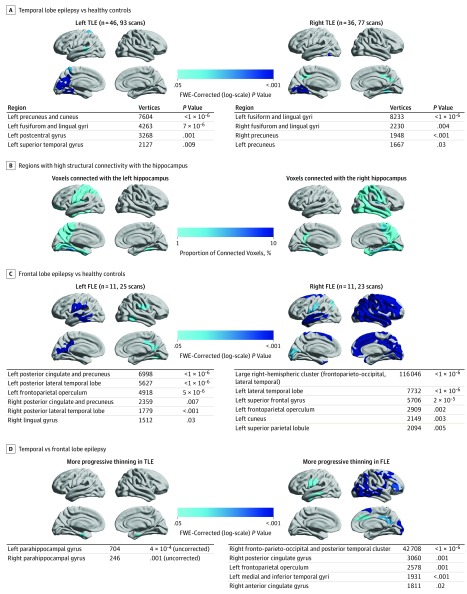
Localization and Lateralization of Epilepsy
To assess the association between epilepsy localization and lateralization and the spatial patterns of progressive atrophy, we compared cortical thinning in patients with left and right temporal lobe epilepsy (TLE), patients with left and right frontal lobe epilepsy (FLE), and healthy volunteers (Figure 2). Those with left TLE (n = 46; 93 MRI scans; Figure 2A) showed progressive cortical thinning in the left precuneus, cuneus, fusiform, lingual, postcentral, and superior temporal gyri. Those with right TLE (n = 36; 77 MRI scans; Figure 2A) showed progressive atrophy in bilateral fusiform and lingual gyri and bilateral precunei. The distribution of accelerated cortical thinning in patients with left and right TLE was similar to areas structurally connected with the ipsilateral hippocampus (Figure 2B). Comparing the effects of TLE lateralization (eFigure 7A in the Supplement), patients with left TLE showed more progressive thinning in the left postcentral gyrus than did those with right TLE.
Figure 2. Localization and Lateralization of Epilepsy and Their Association With Progressive Cortical Thinning.
A, Comparison of left and right temporal lobe epilepsy vs healthy controls. B, Structural connectivity with the left and right hippocampi in 10 healthy volunteers is presented as the regional proportion of connected voxels. C, Comparison of left and right frontal lobe epilepsy vs healthy controls. D, Comparison of temporal vs frontal lobe epilepsy. Significant clusters (P < .05; random field theory corrected) are displayed on hemispheric surface templates and in overview tables. FLE indicates frontal lobe epilepsy; FWE, familywise error; and TLE, temporal lobe epilepsy.
Patients with left FLE (n = 11; 25 MRI scans; Figure 2C) showed progressive cortical thinning in bilateral posterior cingulate gyri and precunei, bilateral posterior lateral temporal lobes, and bilateral frontoparietal opercula, whereas the effects were more pronounced in the left hemisphere. Those with right FLE (n = 11; 23 MRI scans; Figure 2C) showed progressive atrophy in a large cluster affecting most of the right hemisphere but not involving the right medial and ventral temporal lobe. Patients with right FLE also showed progressive atrophy in the left hemisphere, including the lateral temporal lobe, superior frontal gyrus, frontoparietal operculum, cuneus, and superior parietal lobule. Cortical thinning was more progressive in those with right than left FLE, particularly in several right parieto-temporal and right frontal areas (eFigure 7B in the Supplement).
Comparing the patterns of progressive atrophy between patients with a temporal vs frontal epileptic focus (Figure 2D), those with FLE showed more progressive thinning in a large right fronto-parieto-occipital and posterolateral temporal cluster and in the right cingulate gyrus, left operculum, and left lateral temporal lobe. Patients with TLE showed more progressive thinning in both parahippocampal gyri, but these small clusters did not reach the threshold after random field theory correction.
Clinical Characteristics and Progressive Cortical Thinning
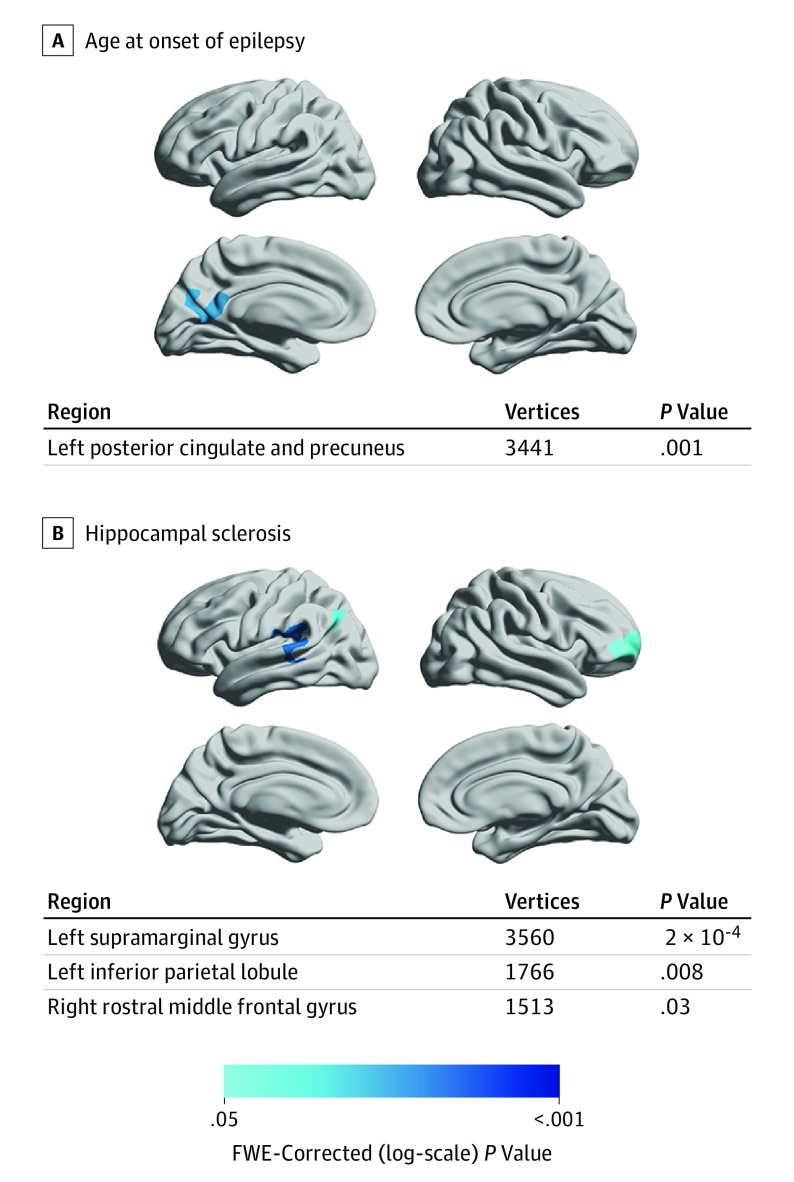
We analyzed the association of clinical characteristics in people with epilepsy with the rate and spatial extent of progressive cortical thinning (Figure 3). An earlier age at onset of epilepsy correlated with accelerated thinning in the left posterior cingulate gyrus and left precuneus (Figure 3A). In those with epilepsy and hippocampal sclerosis, areas of accelerated cortical thinning were detected in the left supramarginal gyrus, left inferior parietal lobule, and right middle frontal gyrus (Figure 3B). There was no association of the rate of cortical thinning with seizure frequency, history of secondarily generalized seizures, or the number of AEDs taken between scans. There was no difference in the rate of atrophy between patients with epilepsy with (n = 176) vs without (n = 14) ongoing seizures.
Figure 3. Clinical Characteristics in People With Epilepsy and Their Association With Progressive Cortical Thinning.
A, Association of progressive cortical thinning in people with epilepsy (n = 190) with younger age at onset of epilepsy. B, Association of progressive cortical thinning in people with epilepsy with presence of hippocampal sclerosis. Significant clusters (P < .05; random field theory corrected) are displayed on hemispheric surface templates and in overview tables. No areas of progressive thinning associated with seizure frequency, history of secondary generalized seizures, and number of antiepileptic drugs taken were detected, and there were no differences between patients with vs without ongoing seizures. FWE indicates familywise error.
Duration of Epilepsy and Cortical Thinning
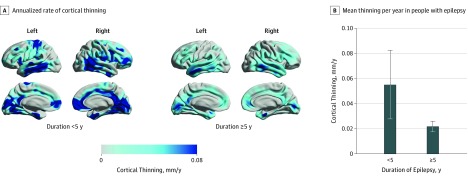
People with a short (<5 years) duration of epilepsy had a higher yearly mean (SD) rate of cortical thinning (n = 14; 0.055 [0.102] mm/y; Figure 4) compared with those with an onset of epilepsy 5 years or more ago (n = 176; 0.022 [0.056] mm/y; P = .049). There were no between-group differences in seizure frequency, number of AEDs, or history of secondary generalized seizures. For analyses of an interaction between short epilepsy duration and older age, see eAppendix 10 and eTable 3 in the Supplement.
Figure 4. Duration of Epilepsy and Its Association With Progressive Cortical Thinning.
A, Annualized rate of cortical thinning in people with epilepsy with disease duration of less than 5 years or 5 years or more after the onset of first seizure. B, Comparison of annualized cortical thinning rates in people with epilepsy with short and long disease duration (vertical lines indicate SEM).
Discussion
We used a large longitudinal neuroimaging data set to demonstrate that morphologic abnormalities in focal epilepsy are dynamic rather than static. We showed that progressive cerebral atrophy may occur in epilepsy that is widespread and exceeds gradual atrophy associated with normal aging.
A total of 76.8% of people with epilepsy in our cohort showed progressive cortical thinning that was distinct from that seen with normal aging, suggesting that epilepsy-associated progressive atrophy is a common phenomenon in patients with epilepsy undergoing follow-up in a tertiary epilepsy center. Our annualized cortical thinning estimates are comparable with those in previous reports that documented thinning of 0.02 to 0.05 mm/y in people with epilepsy11,14 and between 0.001 and 0.008 mm/y in healthy adults.10,31,32 The most likely cause of cortical thinning is neuronal loss, suggesting that these measurements are a surrogate marker for neurodegeneration.6
In our study, the yearly rate of cortical thinning in epilepsy was double that of normal aging in people younger than 55 years and almost 4-fold in those older than 55 years (Figure 1A). This significant increase of epilepsy-associated gray matter loss in older adults suggests a higher vulnerability of the aging brain to cortical damage caused by epilepsy.33,34 Alternatively, it could reflect a concomitant preclinical neurodegenerative condition in these cases, whereas none of the patients in our study had confirmed dementia at time of scanning. It is unlikely that the increased rate of atrophy in older individuals would be explained by a higher severity of epilepsy because there were no differences in seizure frequency or number of AEDs between age groups.
Progressive morphologic changes in epilepsy were most pronounced in the first 5 years after the onset of seizures (Figure 4A), with a doubling of the progression rate within the first 5 years after epilepsy onset compared with the later disease course. This finding supports the need for early diagnosis, rapid treatment, and reduction of delays of surgical referral5 in people with epilepsy.
Areas of accelerated cortical thinning were detected in patients who had hippocampal sclerosis (Figure 3B) and also in those with earlier age at onset of seizures (Figure 3A), in accordance with findings of previous cross-sectional studies.9,35 The rate of cortical thinning was not associated with the number of AEDs (Figure 3F), suggesting that the outcomes observed in our study are independent of AED load. A higher AED load is usually associated with more severe epilepsy, but certain drugs might also have neuroprotective effects.36 We did not differentiate between AED types, and future research will need to determine whether specific AEDs have a differential influence on progressive morphologic changes in epilepsy.
One longitudinal study15 and several cross-sectional studies10 found more severe atrophy in people with more frequent seizures. However, a higher frequency of seizures also points to a phenotype of more severe epilepsy that could have confounded these results. Seizures are notoriously difficult to count and recorded seizures underestimate the numbers of ictal events that actually occur.37 In our study, the rate of cortical thinning was not associated with seizure frequency or history of secondarily generalized seizures, and it did not differ between those who were and were not reported to be seizure free. In line with this observation, 2 previous longitudinal studies found progressive atrophy even in people with epilepsy who were seizure free.13,17 A potential explanation could be that progressive morphologic changes in epilepsy are not a direct consequence of seizures but are a seizure-independent phenomenon. This possibility is in accordance with the observation that medical treatment of seizures does not have a long-term disease-modifying effect.38 We speculate that seizures could be but one manifestation of a widespread progressive pathologic process affecting neuronal networks in epilepsy that continues even in the absence of detectable seizures. This possibility highlights the need to identify truly disease-modifying treatments for epilepsy in humans.
We found distinct patterns of progressive atrophy in all studied epilepsy subgroups (left and right TLE and FLE). Progressive thinning was most pronounced ipsilaterally to the epileptic focus but also affected widespread areas extending beyond the focus and frequently involved the contralateral hemisphere (Figure 2). The observed patterns are largely similar to atrophy detected in cross-sectional studies8,9 and they suggest that epilepsy is associated with networks beyond what is traditionally considered the epileptic focus. The spatial distribution of progressive thinning in all patients with epilepsy and in subgroups with left and right TLE was similar to the brain areas structurally connected with the hippocampus (Figure 1C and Figure 2B; eAppendix 4 and eFigures 1 and 2 in the Supplement). This spatial correlation suggests that regions that are closely interconnected with the epileptic focus are more likely to be affected by progressive atrophy. This finding needs to be further evaluated in studies combining longitudinal anatomical imaging with structural and functional connectivity measurements. In line with previous cross-sectional data,9,39,40 progressive atrophy was more pronounced in left TLE than right TLE and in right FLE compared with left FLE. The extent of progressive atrophy was syndrome specific, with FLE affecting a more widespread cortical area, whereas progressive atrophy in TLE was most pronounced in the medial temporal lobes (Figure 2D).
Strengths and Limitations
Strengths of our study are the inclusion of a large, diverse, and clinically representative tertiary center cohort of people with focal epilepsy and the use of a robust and statistically powerful12 longitudinal neuroimaging pipeline. A limitation is that data from patients with epilepsy and healthy volunteers were acquired on different 3-T MRI scanners. The statistical analyses focused on within-individual changes and all individuals were rescanned on the same equipment, potentially reducing the effect of between-cohort differences. Moreover, there were no between-group differences in image quality (combination of noise, inhomogeneities, and resolution), and a sensitivity analysis excluding a cohort of Asian volunteers produced largely unchanged results (eAppendix 8 and eFigure 4 in the Supplement). Post hoc analyses (eAppendix 9 and eFigure 5 in the Supplement) showed that our results cannot be explained by a reduced sensitivity to detect cortical thinning in healthy volunteers compared with patients with epilepsy. Future studies will, nevertheless, need to address this issue by acquiring longitudinal data from patients with epilepsy and healthy controls using the same MRI scanner.
A limitation inherent to most epilepsy studies is the possible influence of AED intake in patients compared with controls. It is unlikely, however, that our results could be explained by differences in medication only because a sensitivity analysis adjusting for AED intake produced largely similar results (eAppendix 7 and eFigure 3 in the Supplement), and we did not find any association of AED load with progressive atrophy. The subgroups of patients with epilepsy who were not taking AEDs (n = 14) and those with short disease duration (n = 14) were small and these findings will need to be replicated in larger cohorts specifically targeting these subgroups.
We acknowledge that there will have been a referral bias in the patients included in this study, with patients with more complicated cases being referred to our services and undergoing multiple MRI scans for clinical reasons, mainly for refractory seizures or concerns about cognitive impairment. Our findings, thus, apply to individuals receiving follow-up in a tertiary epilepsy center and cannot be readily generalized to the overall population. Previous population-based studies16,17,18 included less severely affected individuals, which may explain the lower magnitude and frequency of progressive changes observed in those studies. Although the patient and control groups were matched for age and sex, we did not have data on controls’ educational attainment or intelligence and we cannot be certain whether this factor may have contributed to our findings.
Conclusions
We provide evidence for widespread progressive neocortical atrophy in almost three-quarters of our cohort of patients with epilepsy, but it remains unknown how to prevent these morphologic changes. Our findings appear to highlight the need for longitudinal studies to develop disease-modifying treatments for epilepsy. Anticonvulsants that suppress seizures alone might not have an effect on progressive cerebral atrophy because it was not affected by seizure frequency. Future longitudinal studies should thus determine whether medical treatment, either with certain AEDs or alternative approaches (eg, anti-inflammatory therapies), or successful epilepsy surgery prevent or slow accelerated cortical thinning. Longitudinal measurements of cortical thickness could provide a practical surrogate marker for such studies. It appears that future research should also address whether progressive changes in cortical morphologic characteristics correlate with deficits on serial cognitive testing41 or spreading of the irritative zone on electroencephalographic recordings.42 If confirmed, such results would provide a rationale to include longitudinal imaging in the clinical care of patients with epilepsy and to inform them about these potential risks.
eAppendix 1. MRI Acquisition Protocol in Epilepsy Cohort
eAppendix 2. Description of Healthy Volunteer Cohorts
eAppendix 3. Computational Anatomy Toolbox
eAppendix 4. Connectivity Analyses
eAppendix 5. Clinical Characteristics in Patients Aged 55 Years or Older
eAppendix 6. Aging-Related Thinning in Healthy Volunteers
eAppendix 7. Adjustment for Number of Antiepileptic Drugs (AEDs) Taken
eAppendix 8. Sensitivity Analysis Excluding SLIM Cohort
eAppendix 9. Sensitivity and Post-Hoc Analyses of the PPMI Cohort
eAppendix 10. Interaction of Age and Duration Effects
eTable 1. Healthy Volunteer Demographic by Cohort
eTable 2. Clinical Characteristics of Epilepsy Patients Aged 55 Years or Older
eTable 3. Interaction of Age >55 Years and Duration of Epilepsy <5 Years
eFigure 1. Comparison of Regions Connected to the Hippocampus and Findings of Progressive Cortical Thinning
eFigure 2. Comparison of Regions Connected to the Left and Right Hippocampi and Findings of Progressive Cortical Thinning in Left and Right Temporal Lobe Epilepsy (TLE)
eFigure 3. Progressive Cortical Thinning in People With Epilepsy vs Healthy Volunteers Corrected for AED Load
eFigure 4. Progressive Cortical Thinning in People With Epilepsy vs Healthy Volunteers Excluding the SLIM Cohort
eFigure 5. Annualised Cortical Thinning Rates in the PPMI Cohort, Other Healthy Volunteers (HV), and in People With Epilepsy
eFigure 6. Dimensionality-Reduction Plot
eFigure 7. Comparison of Left and Right Temporal Lobe Epilepsy and of Left and Right Frontal Lobe Epilepsy
References
- 1.Gowers WR. Epilepsy and Other Chronic Convulsive Disorders. London, UK: J. & A. Churchill; 1881. [Google Scholar]
- 2.Rossini L, Garbelli R, Gnatkovsky V, et al. Seizure activity per se does not induce tissue damage markers in human neocortical focal epilepsy. Ann Neurol. 2017;82(3):331-341. doi: 10.1002/ana.25005 [DOI] [PubMed] [Google Scholar]
- 3.Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain? Curr Opin Neurol. 2003;16(2):189-195. doi: 10.1097/00019052-200304000-00012 [DOI] [PubMed] [Google Scholar]
- 4.Cole AJ. Is epilepsy a progressive disease? the neurobiological consequences of epilepsy. Epilepsia. 2000;41(suppl 2):S13-S22. doi: 10.1111/j.1528-1157.2000.tb01520.x [DOI] [PubMed] [Google Scholar]
- 5.Haneef Z, Stern J, Dewar S, Engel J Jr. Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology. 2010;75(8):699-704. doi: 10.1212/WNL.0b013e3181eee457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497-510. doi: 10.1093/cercor/bhn113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonilha L, Keller SS. Quantitative MRI in refractory temporal lobe epilepsy: relationship with surgical outcomes. Quant Imaging Med Surg. 2015;5(2):204-224. doi: 10.3978/j.issn.2223-4292.2015.01.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan CD, Altmann A, Botía JA, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain. 2018;141(2):391-408. doi: 10.1093/brain/awx341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caciagli L, Bernasconi A, Wiebe S, Koepp MJ, Bernasconi N, Bernhardt BC. A meta-analysis on progressive atrophy in intractable temporal lobe epilepsy: time is brain? Neurology. 2017;89(5):506-516. doi: 10.1212/WNL.0000000000004176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72(20):1747-1754. doi: 10.1212/01.wnl.0000345969.57574.f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steen RG, Hamer RM, Lieberman JA. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. AJNR Am J Neuroradiol. 2007;28(6):1119-1125. doi: 10.3174/ajnr.A0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvim MKM, Coan AC, Campos BM, et al. Progression of gray matter atrophy in seizure-free patients with temporal lobe epilepsy. Epilepsia. 2016;57(4):621-629. doi: 10.1111/epi.13334 [DOI] [PubMed] [Google Scholar]
- 14.Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology. 2010;74(22):1776-1784. doi: 10.1212/WNL.0b013e3181e0f80a [DOI] [PubMed] [Google Scholar]
- 15.Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009;73(11):834-842. doi: 10.1212/WNL.0b013e3181b783dd [DOI] [PubMed] [Google Scholar]
- 16.Liu RSN, Lemieux L, Bell GS, et al. Cerebral damage in epilepsy: a population-based longitudinal quantitative MRI study. Epilepsia. 2005;46(9):1482-1494. doi: 10.1111/j.1528-1167.2005.51603.x [DOI] [PubMed] [Google Scholar]
- 17.Liu RSN, Lemieux L, Bell GS, et al. Progressive neocortical damage in epilepsy. Ann Neurol. 2003;53(3):312-324. doi: 10.1002/ana.10463 [DOI] [PubMed] [Google Scholar]
- 18.Liu RSN, Lemieux L, Bell GS, et al. The structural consequences of newly diagnosed seizures. Ann Neurol. 2002;52(5):573-580. doi: 10.1002/ana.10338 [DOI] [PubMed] [Google Scholar]
- 19.Potvin O, Dieumegarde L, Duchesne S; Alzheimer’s Disease Neuroimaging Initiative . Normative morphometric data for cerebral cortical areas over the lifetime of the adult human brain. Neuroimage. 2017;156:315-339. doi: 10.1016/j.neuroimage.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 20.Parkinson Progression Marker Initiative The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol. 2011;95(4):629-635. doi: 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kogan A, Alpert K, Ambite JL, Marcus DS, Wang L. Northwestern University schizophrenia data sharing for SchizConnect: a longitudinal dataset for large-scale integration. Neuroimage. 2016;124(pt B):1196-1201. doi: 10.1016/j.neuroimage.2015.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Wei D, Chen Q, et al. Longitudinal test-retest neuroimaging data from healthy young adults in southwest China. Sci Data. 2017;4:170017. doi: 10.1038/sdata.2017.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahnke R, Yotter RA, Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336-348. doi: 10.1016/j.neuroimage.2012.09.050 [DOI] [PubMed] [Google Scholar]
- 24.Seiger R, Ganger S, Kranz GS, Hahn A, Lanzenberger R. Cortical thickness estimations of FreeSurfer and the CAT12 toolbox in patients with Alzheimer’s disease and healthy controls. J Neuroimaging. 2018;28(5):515-523. doi: 10.1111/jon.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Righart R, Schmidt P, Dahnke R, et al. Volume versus surface-based cortical thickness measurements: a comparative study with healthy controls and multiple sclerosis patients. PLoS One. 2017;12(7):e0179590. doi: 10.1371/journal.pone.0179590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8(2-3):98-101. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu RSN, Lemieux L, Bell GS, et al. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20(1):22-33. doi: 10.1016/S1053-8119(03)00219-2 [DOI] [PubMed] [Google Scholar]
- 28.Makalic E, Schmidt DF High-dimensional Bayesian regularised regression with the BayesReg package. https://arxiv.org/abs/1611.06649. Updated December 20, 2016. Accessed October 10, 2018.
- 29.van der Maaten L, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. 2008;9:2579-2605. [Google Scholar]
- 30.Foulon C, Cerliani L, Kinkingnéhun S, et al. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. Gigascience. 2018;7(3):1-17. doi: 10.1093/gigascience/giy004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage. 2010;52(4):1215-1223. doi: 10.1016/j.neuroimage.2010.04.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemaitre H, Goldman AL, Sambataro F, et al. Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging. 2012;33(3):617.e1-617.e9. doi: 10.1016/j.neurobiolaging.2010.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douaud G, Groves AR, Tamnes CK, et al. A common brain network links development, aging, and vulnerability to disease. Proc Natl Acad Sci U S A. 2014;111(49):17648-17653. doi: 10.1073/pnas.1410378111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu T, Pan Y, Kao S-Y, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883-891. doi: 10.1038/nature02661 [DOI] [PubMed] [Google Scholar]
- 35.Mueller SG, Laxer KD, Cashdollar N, Buckley S, Paul C, Weiner MW. Voxel-based optimized morphometry (VBM) of gray and white matter in temporal lobe epilepsy (TLE) with and without mesial temporal sclerosis. Epilepsia. 2006;47(5):900-907. doi: 10.1111/j.1528-1167.2006.00512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willmore LJ. Antiepileptic drugs and neuroprotection: current status and future roles. Epilepsy Behav. 2005;7(suppl 3):S25-S28. doi: 10.1016/j.yebeh.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 37.Cook MJ, O’Brien TJ, Berkovic SF, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: a first-in-man study. Lancet Neurol. 2013;12(6):563-571. doi: 10.1016/S1474-4422(13)70075-9 [DOI] [PubMed] [Google Scholar]
- 38.Marson A, Jacoby A, Johnson A, Kim L, Gamble C, Chadwick D; Medical Research Council MESS Study Group . Immediate versus deferred antiepileptic drug treatment for early epilepsy and single seizures: a randomised controlled trial. Lancet. 2005;365(9476):2007-2013. doi: 10.1016/S0140-6736(05)66694-9 [DOI] [PubMed] [Google Scholar]
- 39.Centeno M, Vollmar C, Stretton J, et al. Structural changes in the temporal lobe and piriform cortex in frontal lobe epilepsy. Epilepsy Res. 2014;108(5):978-981. doi: 10.1016/j.eplepsyres.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Widjaja E, Mahmoodabadi SZ, Snead OC III, Almehdar A, Smith M-L. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52(9):1685-1691. doi: 10.1111/j.1528-1167.2011.03085.x [DOI] [PubMed] [Google Scholar]
- 41.Thompson PJ, Duncan JS. Cognitive decline in severe intractable epilepsy. Epilepsia. 2005;46(11):1780-1787. doi: 10.1111/j.1528-1167.2005.00279.x [DOI] [PubMed] [Google Scholar]
- 42.Gollwitzer S, Scott CA, Farrell F, et al. The long-term course of temporal lobe epilepsy: from unilateral to bilateral interictal epileptiform discharges in repeated video-EEG monitorings. Epilepsy Behav. 2017;68:17-21. doi: 10.1016/j.yebeh.2016.12.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. MRI Acquisition Protocol in Epilepsy Cohort
eAppendix 2. Description of Healthy Volunteer Cohorts
eAppendix 3. Computational Anatomy Toolbox
eAppendix 4. Connectivity Analyses
eAppendix 5. Clinical Characteristics in Patients Aged 55 Years or Older
eAppendix 6. Aging-Related Thinning in Healthy Volunteers
eAppendix 7. Adjustment for Number of Antiepileptic Drugs (AEDs) Taken
eAppendix 8. Sensitivity Analysis Excluding SLIM Cohort
eAppendix 9. Sensitivity and Post-Hoc Analyses of the PPMI Cohort
eAppendix 10. Interaction of Age and Duration Effects
eTable 1. Healthy Volunteer Demographic by Cohort
eTable 2. Clinical Characteristics of Epilepsy Patients Aged 55 Years or Older
eTable 3. Interaction of Age >55 Years and Duration of Epilepsy <5 Years
eFigure 1. Comparison of Regions Connected to the Hippocampus and Findings of Progressive Cortical Thinning
eFigure 2. Comparison of Regions Connected to the Left and Right Hippocampi and Findings of Progressive Cortical Thinning in Left and Right Temporal Lobe Epilepsy (TLE)
eFigure 3. Progressive Cortical Thinning in People With Epilepsy vs Healthy Volunteers Corrected for AED Load
eFigure 4. Progressive Cortical Thinning in People With Epilepsy vs Healthy Volunteers Excluding the SLIM Cohort
eFigure 5. Annualised Cortical Thinning Rates in the PPMI Cohort, Other Healthy Volunteers (HV), and in People With Epilepsy
eFigure 6. Dimensionality-Reduction Plot
eFigure 7. Comparison of Left and Right Temporal Lobe Epilepsy and of Left and Right Frontal Lobe Epilepsy