Key Points
Question
What is the gastric cancer penetrance of CDH1 pathogenic variants in families not ascertained based on strict clinical hereditary diffuse gastric cancer criteria?
Findings
In this review of 75 families found to have pathogenic variants in CDH1, overall cumulative incidence of gastric cancer was estimated to be 42% for men and 33% for women with pathogenic variants in CDH1; previous estimates have shown a 40% to 70% lifetime risk for gastric cancer in men and 56% to 83% lifetime risk in women.
Meaning
Previously published CDH1 penetrance estimates may be inflated owing to ascertainment bias and likely overestimate cancer risks for most individuals with a CDH1 pathogenic variant.
This review of data from 75 families with a CDH1 pathologic variant compares estimates of the lifetime risk of gastric cancer in the families found to have a CDH1 pathologic variant through clinical ascertainment with those ascertained through multigene panel testing.
Abstract
Importance
CDH1 pathogenic variants have been estimated to confer a 40% to 70% and 56% to 83% lifetime risk for gastric cancer in men and women, respectively. These are likely to be overestimates owing to ascertainment of families with multiple cases of gastric cancer. To our knowledge, there are no penetrance estimates for CDH1 without this ascertainment bias.
Objective
To estimate CDH1 penetrance in a patient cohort not exclusively ascertained based on strict hereditary diffuse gastric cancer (HDGC) criteria.
Design, Setting, and Participants
Retrospective review of 75 families found to have pathogenic variants in CDH1 through clinical ascertainment and multigene panel testing at a large commercial diagnostic laboratory from August 5, 2013, to June 30, 2018. CDH1 pathogenic variants were identified in 238 individuals from 75 families. Pedigrees from those families included cancer status for 1679 relatives. Penetrance estimates are based on 41 families for which completed pedigrees were available.
Main Outcomes and Measures
Gastric cancer standardized incidence ratio estimates relative to Surveillance, Epidemiology, and End Results (SEER) Program incidence for pathogenic CDH1 variants from families ascertained without regard to HDGC criteria.
Results
Among the 238 individuals with a CDH1 pathogenic variant, mean (SD) age was 49.3 (18.1) years and 63.4% were female. Ethnicity was reported for 67 of 75 (89%) families; of these 67 families, 51 (76%) reported European ancestry, whereas Asian, African, Latino, and 2 or more ancestries were reported for 4 families (6%) each. Standardized incidence ratios for gastric and breast cancer were significantly elevated above SEER incidence. Extrapolated cumulative incidence of gastric cancer at age 80 years was 42% (95% CI, 30%-56%) for men and 33% (95% CI, 21%-43%) for women with pathogenic variants in CDH1, whereas cumulative incidence of female breast cancer was estimated at 55% (95% CI, 39%-68%). International Gastric Cancer Linkage Consortium criteria were met in 25 of the 75 (33%) families; however, dispensing with the requirement of confirmation of HDGC histologic subtype, 43 (57%) would meet criteria.
Conclusions and Relevance
The cumulative incidence of gastric cancer for individuals with pathogenic variants in CDH1 is significantly lower than previously described. Because prophylactic gastrectomy can have bearing upon both physical and psychological health, further discussion is warranted to assess whether this surgical recommendation is appropriate for all individuals with pathogenic variants in CDH1.
Introduction
The CDH1 gene (OMIM:192090) encodes the protein E-cadherin, a cell-to-cell adhesion molecule.1 Hereditary diffuse gastric cancer (HDGC) syndrome is caused by germline pathogenic variants in the CDH1 gene and is characterized by an increased risk for diffuse gastric cancer (DGC) and lobular breast cancer (LBC).2 Clinical testing criteria and medical management recommendations for HDGC were first established and published in 1999 by the International Gastric Cancer Linkage Consortium (IGCLC), a multidisciplinary collaboration of international clinical geneticists, gastroenterologists, surgeons, oncologists, pathologists, and molecular biologists.3 Revisions to the initial criteria were subsequently published in 2010 and 2015 (Table 1).2,4 Recent evaluation of the revised 2015 IGCLC clinical testing criteria in a laboratory-based CDH1 pathogenic variant cohort found that 65% of all individuals with pathogenic variants did not meet criteria.5 In addition, it has been suggested that CDH1 pathogenic variants may be present in women presenting with lobular breast cancer and no family history of gastric cancer.6,7 International Gastric Cancer Linkage Consortium criteria were developed to define a clinical syndrome and facilitate identification of risk genes rather than to determine risk in all individuals with a genetically defined syndrome. These observations suggest that criteria for clinical HDGC and genetic CDH1 syndromes may need to be separated.
Table 1. Evolution of the International Gastric Cancer Linkage Consortium Clinical Testing and Management Guidelines.
| Criteria or Management | van der Post et al,2 2015 | Fitzgerald et al,4 2010 | Caldas et al,3 1999 |
|---|---|---|---|
| Clinical testing criteria |
|
|
|
| Gastric cancer management |
|
|
|
| Breast cancer management |
|
|
|
Abbreviations: DGC, diffuse gastric cancer; GC, gastric cancer; LBC, lobular breast cancer; MRI, magnetic resonance imaging.
Including first-degree and second-degree relatives.
Current IGCLC medical management recommendations advise that individuals with a CDH1 pathogenic variant have a prophylactic total gastrectomy in early adulthood (20-30 years) regardless of endoscopic findings.2 These guidelines also stress the importance of well-informed decision-making that considers psychological, physiological, and metabolic health. Although the presence of incidental signet ring cell carcinoma foci and early-stage diffuse gastric cancer has been well documented in asymptomatic carriers, it has also been suggested that endoscopic surveillance can help rule out the presence of invasive disease and guide patients on the timing of prophylactic gastrectomy.8,9
Several publications have estimated that CDH1 pathogenic variants confer a 40% to 70% lifetime risk for gastric cancer in men, a 56% to 83% lifetime risk for gastric cancer in women, and a 39% to 52% lifetime risk for breast cancer in women.10,11,12,13 However, families used to calculate these penetrance estimates were ascertained based on IGCLC HDGC testing criteria (eTable 1 in the Supplement). Available penetrance figures are likely overestimates for individuals with CDH1 pathogenic variants from families not meeting these strict clinical criteria, as illustrated by results from results from multigene panel testing.2,5,14 Risk assessment, genetic counseling, and current medical management recommendations regarding HDGC for individuals with CDH1 pathogenic variants are often based on penetrance estimates subject to ascertainment bias owing to the common misconception that these estimates apply to all individuals with CDH1 pathogenic variants simply because there are not better estimates in any other context. Here we aimed to estimate CDH1 penetrance in a patient cohort not exclusively ascertained based on strict HDGC genetic testing criteria.
Methods
Study Sample
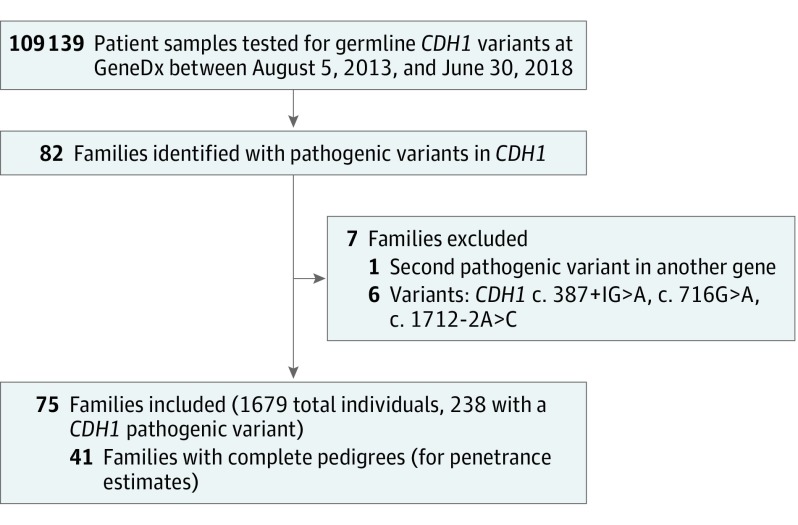
We retrospectively queried 109 139 samples, including panel tests and targeted variant tests, where CDH1 was evaluated that were submitted to GeneDx between August 5, 2013, and June 30, 2018. All individuals found to have a single germline pathogenic or likely pathogenic variant (collectively referred to as pathogenic variant) in CDH1 were included, but individuals with a second pathogenic variant in another gene (excluding single, heterozygous pathogenic variants in MUTYH) or individuals with 1 of 3 CDH1 variants, c.387 + 1G>A, c.715G>A, and c.1712-2A>C, were excluded due to concerns of complex splicing and reduced penetrance (Figure 1). A list of the pathogenic variants identified can be found in eTable 2 in the Supplement. Patient demographic features, clinical data, and family histories were abstracted from test requisition forms and accompanying clinical notes and pedigrees provided at the time of testing order. Pathology reports were not requested to confirm diagnoses but were reviewed when provided. Breast cancer was defined as any invasive breast neoplasm; ductal carcinoma in situ and lobular carcinoma in situ were not counted as breast cancer for the purposes of this study. Pedigrees were evaluated to determine whether each family met the 2015 revised IGCLC HDGC testing criteria for subset analysis.2 This study was conducted in accordance with all guidelines set forth by the Western Institutional Review Board, Puyallup, Washington (WIRB 20162523). Informed consent for genetic testing was obtained from all individuals undergoing testing, and Western Institutional Review Board waived authorization for use of deidentified aggregate data. Institutions who opted out of this type of data use were excluded.
Figure 1. Cohort Assembly.
Technical methods for DNA extraction, next-generation sequencing, and analysis have been previously described.15 For a known familial variant identifiable by sequencing, the targeted gene region was amplified by polymerase chain reaction, conventional dideoxy DNA sequencing was performed, and bidirectional sequence was evaluated for the specific variant. For a known familial deletion or duplication at the exon or gene level, exon-level array CGH was performed and analyzed using gene- specific filtering. Variants were described according to the Human Genome Variation Society nomenclature guidelines and classified following the 2008 and 2015 guidelines from the American College of Medical Genetics and Genomics/Association for Molecular Pathology standards and guidelines for variant classification.16
Statistical Analysis
The Surveillance, Epidemiology, and End Results (SEER) Program is a publicly available source of cancer incidence statistics for the United States with detailed online methods documentation.17 When estimating elevated risk of cancer, it is necessary to choose a baseline population risk. Although study-specific baselines would be ideal, the SEER data are widely accepted and used as an estimate for general cancer incidence in the Unites States. We estimated cumulative incidence in our cohort by first estimating standardized incidence ratio (SIR) relative to SEER and then multiplying these by the SEER cumulative incidence at the desired age. Standardized incidence ratio was calculated using only families with complete pedigrees, excluding those families for which information on only affected family members was provided, whether in pedigree or text format. The Poisson binomial distribution was used to model the number of cancer cases in each family given the age and sex of each individual who carried the variant using SEER incidence values.17 Data from individuals with unknown genotypes were weighted according to the probability that they carried the variant. Standardized incidence ratio was estimated as the ratio of the weighted observed number of affected individuals to the weighted expected number of affected individuals. Specifically, it was estimated as the ratio of the weighted observed number of affected individuals to the weighted mean of the corresponding Poisson binomial distribution. Although weighting enriched the sample for carriers, it is possible for noncarriers to be included in the data set. We adjusted for this by assuming that the estimated SIR is a mixture of the true SIR and SEER SIR, which is 1, with the proportion of each determined by the percent of carriers in the data set. We used the following equation: Estimated SIR = (% Carriers) × (True SIR) + (1–% Carriers) × (SEER SIR).
Substituting SEER SIR with 1 and solving for the True SIR gives the desired estimate. We calculated 95% CIs for the SIR by bootstrapping the data 10 000 times. To compare these values to previous estimates, we translated the SIR, and its corresponding CIs, into cumulative incidence at age 80 years, and CIs, by multiplying the former by the published SEER cumulative incidence at 80 years.
To illustrate the association of selection for families meeting HDGC criteria with cumulative incidence, data were divided into subsets based on the number of gastric cancers reported in each family, 3 or more or 2 or fewer. Two of 3 previous publications used the presence of 3 or more gastric cancers as their primary ascertainment criteria and a third used various criteria that resulted in a sample with a mean of 4 and a median of 3 gastric cancers per family.11,12,13
Results
Cohort Characteristics
Among the 238 individuals with a CDH1 pathogenic variant, 151 were female (63.4%), 70 were male (29.4%), and 17 individuals had an unknown sex (7.1%); mean (SD) age was 49.3 (18.1) years. Ethnicity was reported for 67 of 75 (89%) families. Of these 67 families, 51 (76%) reported European ancestry whereas Asian, African, Latino, and 2 or more ancestries were reported by 4 families (6%) each.
Pedigrees from 75 families included cancer status for 1679 individuals, of whom 238 individuals were identified as CDH1 pathogenic variant carriers (75 probands and 163 family members). Complete pedigrees were available for 41 of the 75 (55%) families. Fifty-two unique CDH1 pathogenic variants were identified: 31 (60%) frameshift or nonsense variants, 11 (21%) canonical splice variants, 6 (12%) large exonic deletions or duplications, 3 (6%) cryptic splice variants, and 1 (2%) small deletion crossing the exonic/intronic boundary (eTable 2 in the Supplement). Variants were distributed throughout the CDH1 gene and observed in all 16 exons and functional domains (eFigure in the Supplement).
Tests Ordered
Fifty-one of 75 probands (68%) were referred for testing with a multigene panel containing 20 to 46 genes, 9 (12%) had a smaller multigene panel consisting of 3 to 19 genes, and 11 (15%) had CDH1 sequencing, deletion, and duplication analysis only. The type of initial testing in the family was unknown for the remaining 4 (5%); in all of these cases, targeted variant testing for a family member was completed at GeneDx, but details about the proband’s testing were not provided.
Cancer History
Among probands and relatives, 238 were confirmed or obligate pathogenic variant carriers (Table 2). Among the carriers, 53 (22%) were reported to have a history of gastric cancer with the mean (SD) age at diagnosis of 46.7 (15.6) years. Thirty-one of the 53 (59%) reported diffuse histologic subtype, whereas 2 (4%) reported gastric adenocarcinoma and 20 (38%) did not specify histologic subtype. Of the 151 female carriers of CDH1 pathogenic variant, 47 (31%) reported a history of breast cancer, with the mean (SD) age at diagnosis of 48.2 (10.9) years. Histologic subtype was not provided in 22 (47%) of the breast cancer cases but indicated as lobular in 17 (36%) and ductal in 9 (19%). Fifteen of the 75 families (20%) reported breast cancer but not gastric cancer. Colon cancer was observed in 3 of 238 (1%) CDH1 pathogenic variant carriers, with 1 reporting signet ring cell features.
Table 2. Cancer History for Individuals With a CDH1 Pathogenic Variant.
| Clinical Characteristic | No. (%) | ||
|---|---|---|---|
| All (N = 238) | Probands (n = 75) | Family Members (n = 163) | |
| Cancer | |||
| Gastric | 53 (22.3) | 32 (42.7) | 21 (12.9) |
| Age at diagnosis, mean (SD) y | 46.7 (15.5) | 45.9 (16.2) | 48.1 (14.5) |
| Diffuse | 31 (58.5) | 23 (71.9) | 8 (38.1) |
| Adenocarcinoma | 2 (3.8) | 2 (6.3) | 0 (0) |
| Not otherwise specified | 20 (37.7) | 7 (21.9) | 13 (61.9) |
| Breast (n = 151 women) | 47 (31.1) | 28 (47.5) | 19 (20.7) |
| Age at earliest diagnosis, mean (SD) y | 48.2 (10.9) | 47.3 (10.2) | 49.6 (11.9) |
| Lobulara | 17 (36.2) | 10 (35.7) | 7 (36.8) |
| Ductala | 9 (19.1) | 8 (28.6) | 1 (5.3) |
| Not otherwise specified | 22 (46.8) | 10 (35.7) | 12 (63.2) |
| Colon | 3 (1.3) | 1 (1.3) | 2 (1.2) |
| Age at diagnosis, mean (SD) y | 80.5 (0.7) | 81.0 (NA) | 80.0 (NA) |
| Signet ring cell features | 1 (33.3) | 0 (0) | 1 (50.0) |
| Adenocarcinoma | 1 (33.3) | 1 (100) | 0 (0) |
| Not otherwise specified | 1 (33.3) | 0 (0) | 1 (50.0) |
Abbreviation: NA, not applicable.
One woman reported both invasive ductal and invasive lobular breast cancer and is counted in both categories.
Clinical Criteria
The following 2015 IGCLC revised testing criteria were met in 25 of the 75 families (33%): (1) two cases of gastric cancer regardless of age with at least 1 confirmed to be diffuse gastric cancer (12 families); (2) one case of diffuse gastric cancer diagnosed at younger than 40 years (6 families); (3) personal or family history of diffuse gastric cancer and lobular breast cancer with at least 1 diagnosed at younger than 50 years (1 family); and (4) more than 1 criterion (6 families).
When dispensing with the requirement of histologic confirmation of DGC, the proportion meeting criteria increased to 57% (43 families). In addition, 1 of 75 families (1%) met the following criterion for consideration of testing: Bilateral lobular breast cancer or family history of 2 or more cases of lobular breast cancer diagnosed at younger than 50 years.
SIRs and Cumulative Incidence
The SIR was significantly elevated relative to SEER for gastric cancer in men (SIR, 57.3; 95% CI, 30.4-89.7) and women (SIR, 82.4; 95% CI, 53.6.4-117.1). The SIR was also elevated for breast cancer in women (SIR, 6.5; 95% CI, 4.0-8.8); there was no male breast cancer observed in this cohort. The SIR for colorectal cancer was not significantly different than 1 for women (SIR, 1.1; 95% CI, 0-4.1) or men (SIR, 1.6; 95% CI, 0.2-4.1).
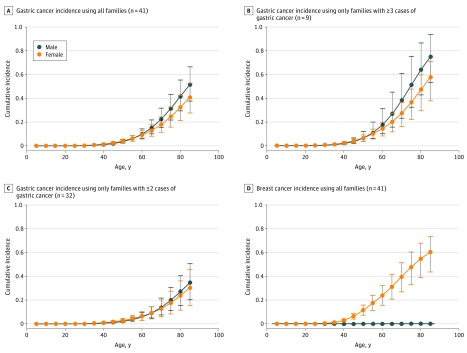
For comparison purposes, we converted the estimated SIR to cumulative incidence by multiplying the SIR, and the associated CI, by the SEER cumulative incidence (eTables 3, 4, and 5 in the Supplement). Considering only the 41 families with complete pedigrees, the cumulative incidence of gastric cancer by age 80 years was estimated to be 42% (95% CI, 30%-56%) for men and 33% (95% CI, 21%-43%) for women (Table 3; Figure 2). Stratifying by number of reported cases of gastric cancers per family, the estimated cumulative incidence of gastric cancer was 64% (95% CI, 43%-87%) for men and 47% (95% CI, 29%-60%) for women in families reporting 3 or more gastric cancers and 27% (95% CI, 15%-41%) for men and 24% (95% CI, 12%-36%) for women reporting 2 or fewer gastric cancers. Cumulative incidence of female breast cancer was estimated to be 55% (95% CI, 39%-68%). Colorectal cancer was estimated at 7% (95% CI, 0%-17%) for men and 4% (95% CI, 0%-11%) for women (Table 3), which was not notably different from the 4.2% lifetime colorectal cancer risk in the general population (SEER).
Table 3. Cumulative Incidence of Gastric Cancer in 41 Familiesa.
| Cancer Type | Study Cohort, % (95% CI) | |
|---|---|---|
| Male | Female | |
| Gastricb | 42 (30-56) | 33 (21-43) |
| >3 | 64 (43-87) | 47 (29-60) |
| <2 | 27 (15-41) | 24 (12-36) |
| Breast | 0 (0-0) | 55 (39-68) |
| Colorectal | 7 (0-17) | 4 (0-11) |
Families with complete pedigrees.
Divided into subgroups by total number of gastric cancers reported in the family.
Figure 2. Estimated Cumulative Incidence of Gastric and Breast Cancer in Individuals With CDH1 Pathogenic Variants.
Graphs show cumulative incidence of (A) gastric cancer in all 41 families with complete pedigrees; (B) gastric cancer in 9 families with 3 or more cases of gastric cancer; (C) gastric cancer in 32 families with 2 or fewer cases of gastric cancer; and (D) breast cancer in 41 families with complete pedigrees. Error bars indicate 95% CIs for cumulative incidence.
Discussion
Herein we report CDH1 penetrance estimates in a cohort of patients not ascertained exclusively based on IGCLC clinical testing criteria. We observed that previously published gastric cancer penetrance estimates are inflated, likely owing to study ascertainment. Although the penetrance estimates in this study are not completely representative of unselected CDH1 pathogenic variant carriers, they are likely a better representation of the penetrance in families with less-severe cancer histories, allowing for more accurate risk assessment.
These data show that the overall lifetime risk of gastric cancer conferred by a CDH1 pathogenic variant is lower than previously thought: 42% in men and 33% in women.10,11,12,13 When stratifying by the number of gastric cancers reported in each family, estimates for families with 3 or more individuals with gastric cancer (64% for men; 47% for women) were similar to past estimates, but were higher than estimates in families with 2 or fewer individuals with gastric cancer (27% for men; 24% for women). Our study was not designed to determine whether these differences are owing to environmental or other genetic modifiers, as previously hypothesized,11,13 or are simply owing to data on subsets.
The estimated CDH1 female breast cancer penetrance of 55% in the present study is similar to previously published estimates.11,12,13 The concordance of estimates reflects concordant ascertainment, because past studies did not use breast cancer as an inclusion or exclusion criterion. Of the 25 female participants with a CDH1 pathogenic variant who reported a personal history of breast cancer and specified histologic subtype, 64% reported invasive lobular carcinoma (ILC), 32% reported invasive ductal carcinoma (IDC), and 4% reported both ILC and IDC. In 2017, Lowstuter et al5 showed that when E-cadherin immunohistochemical staining was retrospectively completed on 3 IDC tumors from women with germline CDH1 pathogenic variants, two-thirds were confirmed as ductal, whereas one-third was reclassified as lobular based on lack of E-cadherin expression. Thus, it is unknown whether the IDCs reported in our cohort are incidental, histologically misclassified, or whether IDC could possibly be part of the CDH1 tumor spectrum.
Colon cancer has been speculated to be a CDH1-related cancer based on case reports in the literature.18,19,20 Our results showed no increased risk over that of the general population, further supporting the 2015 IGCLC’s statement regarding insufficient evidence to recommend increased colorectal cancer screening based on the presence of a CDH1 pathogenic variant only.2
Recent evaluations of the 2015 IGCLC genetic testing criteria have found that a significant proportion of individuals with CDH1 pathogenic variants do not meet these established clinical testing criteria.5,7 Assessment of the 2015 IGCLC HDGC testing criteria in this cohort showed that 33% of families met these criteria. However, without the gastric histology requirement, 57% of families met criteria. Whereas histologic confirmation is often available for probands with gastric cancer (provided for 78% in this cohort), it is often not available for family members (provided for 38% in this cohort), which limits the application of this criterion, especially when the proband is unaffected and referred based on family history.
In addition, current HDGC genetic testing criteria do not account for families presenting with breast cancer only despite evidence of families with CDH1 pathogenic variants and no gastric cancer in the literature.6 One family in this cohort of 75 (1%) would be considered for testing based on bilateral LBC or 2 or more cases of LBC in the family, yet 20% of families presented with breast cancer but not gastric cancer. The clinical picture and phenotypic variability that exists among families with CDH1 pathogenic variants will continue to evolve as clinical genetic testing grows.
Limitations
This study has several limitations. Cumulative incidence estimates are based on SIR relative to SEER incidence with the assumption that incidence is proportional; this assumption may not be true. Available clinical information was limited by clinician submission. In addition, this cohort consisted of all individuals who were considered candidates for genetic testing by their medical professional based on either a personal or family history of cancer and therefore represents a higher-risk population. Thus, the estimates presented may overestimate cancer risk for individuals with CDH1 pathogenic variants because individuals with no personal or family history of cancer are less likely to undergo testing. In addition, to our knowledge, tumor tissue samples were not reviewed and immunohistochemical studies for E-cadherin were not performed. Finally, clinical follow-up was not always available for the individuals identified to carry a CDH1 pathogenic variant in this study.
This study indicates gastric cancer risk in individuals with CDH1 pathogenic variants identified by multigene panel testing who do not meet established clinical testing criteria is significantly lower than CDH1 pathogenic variant risk estimates generated by studies with more biased ascertainment strategies. Because prophylactic gastrectomy has the potential to have a significant bearing upon both physical and psychological health, it is our hope that the updated gastric cancer penetrance estimates provided in this study will encourage new gastric cancer screening research to better assess whether prophylactic gastrectomy is appropriate for all individuals with a CDH1 pathogenic variant. In addition, because IDC was reported in an appreciable number of women with a CDH1 pathogenic variant, both in this and other cohorts, additional studies are warranted to assess whether IDC is in the CDH1 cancer spectrum.
Conclusions
The cumulative incidence of gastric cancer identified in this cohort is significantly lower than previously reported in cohorts ascertained for multiple gastric cancers. In addition, these data support previous assertions that the 2015 IGCLC clinical testing criteria are too restrictive and should be adapted.5,7 The penetrance estimates provided in this study will allow genetics professionals to present more accurate cancer risk estimates to patients, allowing for more informed medical management decisions.
eTable 1. Previously Published Penetrance Estimates
eTable 2. Unique Germline CDH1 Pathogenic Variants Identified
eTable 3. Gastric Cancer Observed by Age in Men From 41 Pedigrees With expected Cancers by Age Based on SEER Data and Estimated Cumulative Risk Presented for Comparison
eTable 4. Gastric Cancers Observed by Age in Women From 41 Pedigrees With Expected Cancers by Age Based on SEER Data and Estimated Cumulative Risk Presented for Comparison
eTable 5. Breast Cancer Observed by Age in Women From 41 Pedigrees With Expected Cancers by Age Based on SEER Data and Estimated Cumulative Risk Presented for Comparison
eFigure. Germline Pathogenic Variants Identified
References
- 1.Gayther SA, Gorringe KL, Ramus SJ, et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res. 1998;58(18):4086-4089. [PubMed] [Google Scholar]
- 2.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52(6):361-374. doi: 10.1136/jmedgenet-2015-103094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management [published correction appears in J Med Genet. 2011;48(3):216]. J Med Genet. 1999;36(12):873-880. [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald RC, Hardwick R, Huntsman D, et al. ; International Gastric Cancer Linkage Consortium . Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47(7):436-444. doi: 10.1136/jmg.2009.074237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowstuter K, Espenschied CR, Sturgeon D, et al. Unexpected CDH1 mutations identified on multigene panels pose clinical management challenges [published online March 29, 2017]. JCO Precis Oncol. doi: 10.1200/PO.16.00021 [DOI] [PubMed] [Google Scholar]
- 6.Corso G, Intra M, Trentin C, Veronesi P, Galimberti V. CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer. 2016;15(2):215-219. doi: 10.1007/s10689-016-9869-5 [DOI] [PubMed] [Google Scholar]
- 7.Corso G, Figueiredo J, La Vecchia C, et al. Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J Med Genet. 2018;55(7):431-441. doi: 10.1136/jmedgenet-2018-105337 [DOI] [PubMed] [Google Scholar]
- 8.Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc. 2018;87(2):408-418. doi: 10.1016/j.gie.2017.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moslim MA, Heald B, Tu C, Burke CA, Walsh RM. Early genetic counseling and detection of CDH1 mutation in asymptomatic carriers improves survival in hereditary diffuse gastric cancer. Surgery. 2018;164(4):754-759. doi: 10.1016/j.surg.2018.05.059 [DOI] [PubMed] [Google Scholar]
- 10.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392(6674):402-405. doi: 10.1038/32918 [DOI] [PubMed] [Google Scholar]
- 11.Pharoah PD, Guilford P, Caldas C; International Gastric Cancer Linkage Consortium . Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology. 2001;121(6):1348-1353. doi: 10.1053/gast.2001.29611 [DOI] [PubMed] [Google Scholar]
- 12.Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA. 2007;297(21):2360-2372. doi: 10.1001/jama.297.21.2360 [DOI] [PubMed] [Google Scholar]
- 13.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23-32. doi: 10.1001/jamaoncol.2014.168 [DOI] [PubMed] [Google Scholar]
- 14.Huynh JM, Laukaitis CM. Panel testing reveals nonsense and missense CDH1 mutations in families without hereditary diffuse gastric cancer. Mol Genet Genomic Med. 2016;4(2):232-236. doi: 10.1002/mgg3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts ME, Jackson SA, Susswein LR, et al. MSH6 and PMS2 germ-line pathogenic variants implicated in Lynch syndrome are associated with breast cancer. Genet Med. 2018;20(10):1167-1174. doi: 10.1038/gim.2017.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 18 Regs Research Data, Nov 2015 Sub (2000-2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission. https://seer.cancer.gov/data-software/documentation/seerstat/nov2016/. Accessed May 23, 2019.
- 18.Richards FM, McKee SA, Rajpar MH, et al. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet. 1999;8(4):607-610. doi: 10.1093/hmg/8.4.607 [DOI] [PubMed] [Google Scholar]
- 19.Oliveira C, Bordin MC, Grehan N, et al. Screening E-cadherin in gastric cancer families reveals germline mutations only in hereditary diffuse gastric cancer kindred. Hum Mutat. 2002;19(5):510-517. doi: 10.1002/humu.10068 [DOI] [PubMed] [Google Scholar]
- 20.Brooks-Wilson AR, Kaurah P, Suriano G, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet. 2004;41(7):508-517. doi: 10.1136/jmg.2004.018275 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Previously Published Penetrance Estimates
eTable 2. Unique Germline CDH1 Pathogenic Variants Identified
eTable 3. Gastric Cancer Observed by Age in Men From 41 Pedigrees With expected Cancers by Age Based on SEER Data and Estimated Cumulative Risk Presented for Comparison
eTable 4. Gastric Cancers Observed by Age in Women From 41 Pedigrees With Expected Cancers by Age Based on SEER Data and Estimated Cumulative Risk Presented for Comparison
eTable 5. Breast Cancer Observed by Age in Women From 41 Pedigrees With Expected Cancers by Age Based on SEER Data and Estimated Cumulative Risk Presented for Comparison
eFigure. Germline Pathogenic Variants Identified