This cohort study uses data from the Southern Community Cohort Study to evaluate whether race-specific adjustment of pack-year criteria in the US Preventive Services Task Force (USPSTF) lung cancer screening guidelines is associated with more equitable screening for African American smokers at high risk for lung cancer.
Key Points
Question
Can US Preventive Services Task Force (USPSTF) lung cancer screening guidelines be modified in order that screening eligibility and clinical validity are equitable for African American individuals?
Findings
In this cohort study of 48 364 adult smokers who were observed for up to 12 years, significantly fewer African American smokers with lung cancer met the USPSTF screening guidelines compared with white smokers. Large racial disparities in sensitivity and specificity were observed.
Meaning
The findings suggest that current lung cancer screening guidelines may be too conservative for African American smokers and that race-specific smoking pack-year eligibility should be considered to make screening operationally equitable.
Abstract
Importance
The United States Preventive Services Task Force (USPSTF) recommends low-dose computed tomography screening for lung cancer. However, USPSTF screening guidelines were derived from a study population including only 4% African American smokers, and racial differences in smoking patterns were not considered.
Objective
To evaluate the diagnostic accuracy of USPSTF lung cancer screening eligibility criteria in a predominantly African American and low-income cohort.
Design, Setting, and Participants
The Southern Community Cohort Study prospectively enrolled adults visiting community health centers across 12 southern US states from March 25, 2002, through September 24, 2009, and followed up for cancer incidence through December 31, 2014. Participants included African American and white current and former smokers aged 40 through 79 years. Statistical analysis was performed from May 11, 2016, to December 6, 2018.
Exposures
Self-reported race, age, and smoking history. Cumulative exposure smoking histories encompassed most recent follow-up questionnaires.
Main Outcomes and Measures
Incident lung cancer cases assessed for eligibility for lung cancer screening using USPSTF criteria.
Results
Among 48 364 ever smokers, 32 463 (67%) were African American and 15 901 (33%) were white, with 1269 incident lung cancers identified. Among all 48 364 Southern Community Cohort Study participants, 5654 of 32 463 African American smokers (17%) were eligible for USPSTF screening compared with 4992 of 15 901 white smokers (31%) (P < .001). Among persons diagnosed with lung cancer, a significantly lower percentage of African American smokers (255 of 791; 32%) was eligible for screening compared with white smokers (270 of 478; 56%) (P < .001). The lower percentage of eligible lung cancer cases in African American smokers was primarily associated with fewer smoking pack-years among African American vs white smokers (median pack-years: 25.8 [interquartile range, 16.9-42.0] vs 48.0 [interquartile range, 30.2-70.5]; P < .001). Racial disparity was observed in the sensitivity and specificity of USPSTF guidelines between African American and white smokers for all ages. Lowering the smoking pack-year eligibility criteria to a minimum 20-pack-year history was associated with an increased percentage of screening eligibility of African American smokers and with equitable performance of sensitivity and specificity compared with white smokers across all ages (for a 55-year-old current African American smoker, sensitivity increased from 32.2% to 49.0% vs 56.5% for a 55-year-old white current smoker; specificity decreased from 83.0% to 71.6% vs 69.4%; P < .001).
Conclusions and Relevance
Current USPSTF lung cancer screening guidelines may be too conservative for African American smokers. The findings suggest that race-specific adjustment of pack-year criteria in lung cancer screening guidelines would result in more equitable screening for African American smokers at high risk for lung cancer.
Introduction
The National Lung Screening Trial (NLST) showed that screening for lung cancer with annual low-dose computed tomography allows for early detection and reduces lung cancer mortality by 20%.1 As a result, the US Preventive Services Task Force (USPSTF) set forth screening guidelines based on age (55-80 years) and smoking history, targeting smokers with a 30-pack-year history who either currently smoke or quit within the previous 15 years. These guidelines are based on the NLST and microsimulation models2; however, they do not consider important racial differences in smoking patterns between racial groups. Despite targeted recruitment methods to increase enrollment of underrepresented populations, the NLST included only 4% African American smokers.3,4
African American smokers tend to smoke fewer cigarettes per day compared with white smokers and have a lower overall smoking pack-year history.5,6,7,8,9,10 Newly published data from the National Cancer Institute Tobacco Control Monograph shows that a larger percentage of adult racial minority populations are light smokers (usually defined as fewer than 10 cigarettes per day) compared with white adults.11 Despite smoking fewer cigarettes per day and having a lower pack-year history, African American smokers have a higher risk of lung cancer compared with white smokers, particularly among men.12,13,14 For the same quantity of cigarettes smoked per day, African American smokers remain at higher risk of lung cancer compared with white smokers, even after accounting for individual-level socioeconomic status and other risk factors.12,14 African American smokers also have fewer successful smoking cessation attempts compared with white smokers15,16; thus, a greater percentage of African American adults are current smokers compared with white adults. Furthermore, the mean age at lung cancer diagnosis occurs significantly earlier among African American smokers compared with white smokers.17 Together, these differences in smoking patterns and lung cancer risk suggest that the USPSTF lung cancer screening guidelines are not optimal for African American adults and could be improved. To our knowledge, there have been no previous studies assessing the lung cancer screening guidelines in prospective observational cohorts with large numbers of African American smokers and incident lung cancer diagnoses. In this study, we aimed to evaluate current USPSTF lung cancer screening guidelines in the largest US study of African American smokers, the Southern Community Cohort Study (SCCS).
Methods
Study Design and Participants
The SCCS is an ongoing prospective observational cohort study established to examine health disparities among a predominantly low-income and African American population. Details of the SCCS are provided elsewhere.18,19 In brief, nearly 85 000 adults were enrolled into the SCCS from March 25, 2002, to September 24, 2009, and were followed up for cancer incidence through December 31, 2014, mainly at community health clinics across a 12-state area of the southeastern United States (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia, and West Virginia). In this study, 85.4% of the cohort was recruited from community health centers, and 14.5% of the cohort was recruited by mailings to an age-, sex-, and race-stratified random sample of the general population. Participants were eligible to participate in the SCCS if they were English speaking, between the ages of 40 and 79 years, and not receiving treatment for cancer (except for nonmelanoma skin cancer) within the previous 12 months. The SCCS was approved by institutional review boards at Vanderbilt University, Nashville, Tennessee, and Meharry Medical College, Nashville, Tennessee. Written, informed consent was obtained from all study participants.
Incident Lung Cancer Identification, Self-reported Race, and Smoking Characteristics
Incident lung cancer cases (International Classification of Disease for Oncology, Third Edition diagnosis codes C340-C349) were identified through end of follow-up on December 31, 2014, via linkage with state cancer registries operating in the 12-state catchment area and/or the National Death Index mortality records. Baseline epidemiologic data were collected during in-person, computer-assisted personal interviews conducted by trained interviewers at community health clinics or from mailed questionnaires. Demographic characteristics and exposure histories were ascertained, including self-identified race, household income, educational level, tobacco smoking, body mass index, and family history of lung cancer and chronic obstructive pulmonary disease. Two follow-up surveys were conducted to ascertain smoking patterns. Smoking status was determined for current and former smokers with nonmissing smoking status at the last follow-up or at lung cancer diagnosis. For current and former smokers missing follow-up smoking status, we assumed their smoking status remained the same during the missing follow-up periods and pack-years increased for current smokers. Smoking pack-years at diagnosis were updated. For lung cancer cases, pack-years were estimated by dividing the follow-up into time windows, such that those who completed both follow-up surveys could have 3 time windows maximum, depending on when the lung cancer diagnosis occurred. Pack-years were estimated within each time window using smoking information from the start and end time points based on dates of the follow-up survey. If a participant started or stopped smoking within that time window, midpoint ages were used to estimate the calculated pack-years. Depending on when the diagnosis occurred in the time window, any pack-year variables before the diagnoses were summed to estimate the total pack-years at the time of lung cancer diagnosis. Smoking information for individuals without a diagnosis of lung cancer was updated in a similar manner through the end of follow-up. Age at diagnosis was used for persons with lung cancer, and age at end of follow-up was used for participants without a lung cancer diagnosis.
Statistical Analysis
Descriptive statistics were used to evaluate differences between African American and white smokers. The Pearson χ2 test was used to assess categorical variables, and the Wilcoxon rank sum test was used to assess continuous variables. We computed eligibility of SCCS smokers for lung cancer screening according to USPSTF guidelines. Among individuals diagnosed with lung cancer but ineligible for lung cancer screening according to USPSTF guidelines, we assessed characteristics leading to screening ineligibility between African American and white smokers. We estimated sensitivity and specificity for African American and white smokers across the smoking pack-year and age distribution, and McNemar’s test was used to compare paired proportions. We also assessed sex-specific smoking pack-years with eligibility. Also, 95% CIs were used for interval estimates. Reporting of significance testing P values follows the recent guidance from the American Statistical Association.20 The sample size almost guaranteed that even clinically negligible differences would appear statistically significant. Thus, emphasis was placed on observed effect sizes when the precision was high. Data were analyzed using R, version 3.5.1 (R Core Team).
Results
Baseline Characteristics and Lung Cancer Screening Eligibility
Among 84 522 SCCS participants followed up from March 25, 2002, through December 31, 2014, with linkage to cancer registries in the 12-state area, we identified a total of 32 463 African American and 15 901 white ever smokers (Table 1). We excluded never smokers (n = 29 758), those with missing smoking information (n = 4191), and those with self-reported unknown race (n = 188) or race other than African American or white (n = 2021) (eFigure 1 in the Supplement). Overall, 24 743 of 48 364 participants (51%) were female; the median age at cohort entry was 50 years (interquartile range [IQR], 45-57 years), and 15 489 participants (32%) had less than a high school education. A greater percentage of African American smokers (20 346 of 32 463 [63%]) vs white smokers (8533 of 15 901 [54%]) were current smokers. African American smokers, however, had significantly lower smoking pack-years at baseline compared with white smokers (median pack-years: 17.5 [IQR, 8.4-30.8] vs 32.0 [IQR, 15.0-50.0]) (Table 1). African American persons smoked fewer cigarettes per day at baseline compared with white persons (median cigarettes per day: 10.0 [IQR, 5.0-20.0] vs 20.0 [IQR, 10.0-30.0]). African American former smokers had a shorter median quit time duration compared with white former smokers (17.0 [IQR, 8.0-29.0] years vs 22.0 [IQR, 11.0-35.0] years). Overall, women had significantly lower median smoking pack-years at study baseline compared with men (15.5 [IQR, 6.8-30] pack-years vs 18.5 [IQR, 9.0-34.0] pack-years), and African American women had lower median pack-years at baseline compared with white women (12.5 [IQR, 5.6-23.0] pack-years vs 24.8 [IQR, 11.3-39.9] pack-years). Among the SCCS smokers, 5654 of 32 463 African American smokers (17.4%) vs 4992 of 15 901 white smokers (31.4%) were eligible for lung cancer screening using USPSTF criteria. All of the above demographic comparisons were statistically significant because of the sample size (P < .001).
Table 1. Descriptive Characteristics of Ever Smokers Participating in the Southern Community Cohort Study, 2002-2014a.
| Characteristic | Race | ||
|---|---|---|---|
| White (n = 15 901) | African American (n = 32 463) | Total (N = 48 364) | |
| Incident lung cancer | 478 (3) | 791 (2) | 1269 (3) |
| Female | 9058 (57) | 15 685 (48) | 24 743 (51) |
| Age at enrollment, median (IQR), y | 52.0 (46.0-59.0) | 49.0 (45.0-56.0) | 50.0 (45.0-57.0) |
| Age at lung cancer diagnosis, median (IQR), y | 64.0 (57.0-69.8) | 59.0 (55.0-67.0) | 61.0 (55.0-68.0) |
| Smoking statusb | |||
| Current | 8533 (54) | 20 346 (63) | 28 879 (60) |
| Former | 7368 (46) | 12 117 (37) | 19 485 (40) |
| Pack-years, median (IQR)b | 32.0 (15.0-50.0) | 17.5 (8.4-30.8) | 20.3 (9.5-38.0) |
| Smoking quit timeb,c | 22.0 (11.0-35.0) | 17.0 (8.0-29.0) | 19.0 (9.0-32.0) |
| Cigarettes per day at baseline, median (IQR) | 20.0 (10.0-30.0) | 10.0 (5.0-20.0) | 10.0 (7.0-20.0) |
| Highest educational level | |||
| Below high school | 4287 (27) | 11 202 (35) | 15 489 (32) |
| High school | 5263 (33) | 11 207 (35) | 16 470 (34) |
| Above high school | 6344 (40) | 10 040 (31) | 16 384 (34) |
| Income, US$ | |||
| <15 000 | 8249 (53) | 20 706 (64) | 28 955 (61) |
| 15 000-25 000 | 2980 (19) | 6846 (21) | 9826 (21) |
| >25 000 | 4483 (29) | 4582 (14) | 9065 (19) |
| Body mass index, median (IQR)d | 28.1 (24.2-33.0) | 27.9 (23.9-32.9) | 27.9 (24.0-32.9) |
| Self-reported COPD | 2751 (17) | 2599 (8) | 5350 (11) |
| Family history of lung cancer | 2301 (16) | 2546 (9) | 4847 (11) |
| USPSTF eligible | 4992 (31) | 5654 (17) | 10 646 (22) |
| AALCS eligiblee | 9388 (29) | ||
Abbreviations: AALCS, African American Lung Cancer Screening; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; USPSTF, United States Preventive Services Task Force.
Data are presented as number (percentage) of individuals unless otherwise indicated.
At the time of diagnosis for persons with incident lung cancer or in 2014 for controls.
Among former smokers only (n = 16 501).
Calculated as weight in kilograms divided by height in meters squared.
AALCS eligibility criteria: age 55 to 80 years and 20 pack-years or more of smoking.
Characteristics of Incident Lung Cancer Cases
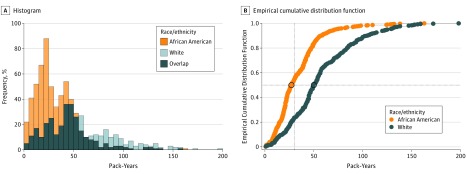
African American smokers diagnosed with lung cancer had significantly lower median smoking pack-years compared with white smokers (25.8 [IQR, 16.9-42.0] pack-years vs 48.0 [IQR, 30.2-70.5] pack-years) (Figure 1A and B), with respective median differences of 22.6 [IQR, 14.4-37.0] pack-years vs 45.0 [IQR, 28.0-59.9] pack-years for women and 28.5 [IQR, 18.1-45.0] pack-years vs 53.0 [IQR, 35.6-84.0] pack-years for men. African American smokers also tended to be diagnosed with lung cancer at an earlier age compared with white smokers (median age: 59.0 [IQR, 55.0-67.0] years vs 64.0 [IQR, 57.0-69.8] years). Among lung cancer cases, significantly fewer African American smokers (255 of 791; 32.2%) were eligible for screening based on USPSTF criteria compared with white smokers (270 of 478; 56.5%). All comparisons above were statistically significant because of the sample size (P < .001).
Figure 1. Distribution and Median Smoking Pack-Years at Diagnosis by Race for Incident Lung Cancer Cases.
Characteristics of Participants With Incident Lung Cancer Ineligible for Lung Cancer Screening
A total of 1269 incident lung cancers (2.6%) occurred among 48 364 smokers during the follow-up period from 2002 through 2014. Among individuals diagnosed with lung cancer, 536 of 791 African American smokers (67.8%) diagnosed with lung cancer were ineligible for screening compared with 208 of 478 white smokers (43.5%). Smoking and age characteristics of smokers with lung cancer who were ineligible for screening are provided in Table 2. A significantly greater percentage of African American smokers (358 of 791; 45.3%) vs white smokers (77 of 478; 16.1%) did not meet the minimum 30-pack-year requirement for screening. African American smokers (192 of 791; 24.3%) were also more likely than white smokers to be diagnosed with lung cancer at an earlier age compared with the USPSTF-required minimum age of 55 years (91 of 478; 19.0%) (P = .03) and thus were ineligible for screening. Conversely, a greater percentage of white former smokers diagnosed with lung cancer (43 of 478; 9.0%) were ineligible for screening because of quit times greater than 15 years compared with African American smokers (47 of 791; 5.9%). Among ineligible lung cancer cases, not meeting the 30-pack-year requirement was the primary reason for ineligibility for African American smokers; 358 of 536 ineligible African American smokers with lung cancer (66.8%) did not meet the requirement compared with 77 of 208 ineligible white smokers (37.0%). Among lung cancer cases ineligible for screening, 192 of 536 African American (35.8%) and 91 of 208 white smokers (43.8%) did not meet the minimum age criteria (P = .046), whereas a significantly greater percentage of ineligible white smokers with lung cancer (43 of 208; 20.7%) had quit times longer than 15 years compared with African American smokers (47 of 536; 8.8%). The above comparisons were significant at the P < .001 level unless otherwise noted.
Table 2. Reasons for USPSTF Lung Cancer Screening Ineligibility for SCCS Smokers With Lung Cancer.
| Characteristica | SCCS Smokers, No. (%) | P Value | ||
|---|---|---|---|---|
| White | African American | Total | ||
| All cancer cases | ||||
| No. | 478 | 791 | 1269 | NA |
| Age <55 y | 91 (19) | 192 (24) | 283 (22) | .03 |
| <30 Pack-years | 77 (16) | 358 (45) | 435 (34) | <.001 |
| Smoking cessation >15 y | 43 (9) | 47 (6) | 90 (7) | .04 |
| Ineligible lung cancer cases | ||||
| No. | 208 | 536 | 744 | NA |
| Age <55 y | 91 (44) | 192 (36) | 283 (38) | .046 |
| <30 Pack-years | 77 (37) | 358 (67) | 435 (58) | <.001 |
| Smoking cessation >15 y | 43 (21) | 47 (9) | 90 (12) | <.001 |
Abbreviations: NA, not applicable; SCCS, Southern Community Cohort Study; USPSTF, United States Preventive Services Task Force.
Categories are not mutually exclusive.
Current USPSTF Guidelines and Effectiveness of a Proposed Revision
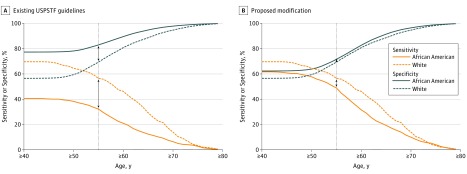
Revising the current USPSTF lung cancer screening guidelines by reducing the smoking pack-year eligibility requirement from 30 to 20 pack-years for African American smokers would increase the number of eligible African American smokers for screening from 17.4% (5654 of 32 463) to 28.5% (9388 of 32 963), making it similar to that of white smokers (Table 1). Lowering the smoking pack-year requirement for lung cancer screening eligibility would increase the eligibility for African American smokers diagnosed with lung cancer from 32.2% (255 of 791) to 48.9% (387 of 791), similar to white smokers (56.5%; 270 of 478). Reducing the minimum age criterion for screening to 50 years for African American smokers in addition to lowering the smoking pack-year requirement would further increase the eligibility of African American persons diagnosed with lung cancer to 57.8% (457 of 791), even more similar to white smokers. Revising the lung cancer screening eligibility guidelines would improve the sensitivity of lung cancer screening for African American smokers and narrow the gap between this group and white smokers. For a 55-year-old current smoker who smoked 30 pack-years or more, the sensitivity of USPSTF lung cancer screening eligibility criteria was 32.2% (95% CI, 29.0%-35.6%) among African American smokers and 56.5% (95% CI, 51.9%-61.0%) among white smokers (Figure 2A). Similarly, the specificity of the test was 83.0% (95% CI, 82.5%-83.4%) for an eligible African American smoker and 69.4% (95% CI, 68.6%-70.1%) for a white smoker (Figure 2A). Revising the criteria to a minimum 20-pack-year smoking history for a 55-year-old African American current smoker would improve the sensitivity of the screening test to 49.0% (95% CI, 45.4%-52.5%; McNemar P < .001) yet decrease the specificity to 71.6% (95% CI, 71.1%-72.1%; McNemar P < .001) (Figure 2B). As individuals age, the gap in sensitivity and specificity between white and African American smokers would narrow. Stratification by sex revealed similar patterns in sensitivity and specificity, although females had higher specificity and lower sensitivity compared with males (eFigure 2 in the Supplement).
Figure 2. Sensitivity and Specificity by Age and Smoking Pack-Years for Existing United States Preventive Services Task Force (USPSTF) Eligibility Guidelines and Their Proposed Modification.
Existing USPSTF eligibility guidelines are defined as 30 pack-years or more for all races; proposed modification is defined as 30 pack-years or more for white persons and 20 pack-years or more for African American persons.
We also observed an inverse association between age at diagnosis and stage of disease among both African American smokers (median age: stage I, 61.0 years [IQR, 56.0-68.0 years]; stage II, 61.0 [IQR, 55.2-69.8]; stage III, 59.5 [IQR, 55.0-67.8]; and stage IV, 59.0 [IQR, 54.0-66.0]) (P = .05) and white smokers (median age: stage I, 66.0 years [IQR, 57.0-71.0 years]; stage II, 64.0 [IQR, 58.0-70.0]; stage III, 64.0 [IQR, 57.0-69.0]; and stage IV, 63.0 [IQR, 57.0-69.0]) (P = .22), because earlier age at diagnosis occurred with increased stage of disease. These data provide additional evidence that reducing the minimum screening age to 50 years may allow greater detection of disease at preclinical early stage for African American individuals, assuming a 3- to 6-year progression of disease from stage 1A to IV.21
Discussion
We examined smokers for lung cancer screening eligibility established by the USPSTF guidelines. These guidelines use a combination of age and smoking history to determine eligibility and are used for reimbursement of health care costs associated with administration of low-dose computed tomography scans. We found that a larger percentage of African American smokers diagnosed with lung cancer compared with white smokers would not have been eligible for lung screening with low-dose computed tomography per USPSTF guidelines, mainly because of having smoking histories below the 30-pack-year eligibility cutoff. A simple modification of USPSTF guidelines to 20 pack-years for African American smokers would increase the percentage of eligible African American smokers to be nearly similar with that of eligible white smokers. Modifying the minimum age to 50 years for African American smokers would increase the percentage eligible for screening and also allow detection of earlier-stage disease that would otherwise be missed with the present age minimum of 55 years.
The USPSTF eligibility criteria present a challenge for effective implementation of lung screening for African American smokers because these individuals have a higher risk of lung cancer with fewer reported smoking pack-years.10,12 Within the SCCS cohort, we observed higher absolute rates of lung cancer among African American smokers compared with white smokers, with age-adjusted mortality rates of 39 per 100 000 person-years of follow-up among never-smoking African American individuals and 10 per 100 000 person-years of follow-up among never-smoking white individuals and increasing rates with increasing tobacco consumption regardless of race and type of tobacco product.22 Our results were consistent with previous studies and support the differential eligibility and exclusion of African American smokers from screening opportunities based on different risk profiles between these racial populations.10,15,23 Ryan15 found that a greater proportion of white smokers would be captured with current screening guidelines compared with African American smokers. Pinsky and Kramer23 analyzed National Health Interview Survey and Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial data and found that the percentage of screening-eligible minority groups and women smokers greatly increased by reducing the selection criteria to include 20- to 29-pack-year smokers. A recent evaluation of a minority group–based lung cancer screening program found that the characteristics did not align with the NLST characteristics.24 Our results and the findings from other researchers emphasize the need for reevaluation of screening guidelines to ensure equity in screening.
Racial differences in tobacco smoking are rooted in psychosocial and genetic determinants, among other factors such as tobacco control policies.25,26 While cultural norms and attitudes align with smoking initiation and behavior,25 genetic factors are also associated with smoking behavior.27 Genes such as CHRNA5 and CYP2A6 contribute to established racial differences in nicotine metabolism, smoking intensity, and underlying lung cancer risk.28,29,30,31,32,33,34,35 Consideration of factors, such as genetics, that are associated with racial differences in smoking behaviors may be useful to incorporate into lung cancer screening programs, particularly those paired with smoking cessation programs.36
Our results support revision of the USPSTF lung cancer screening guidelines to reduce the required number of smoking pack-years for African American smokers if simple eligibility criteria are maintained. Potential revisions to the minimum age criteria of 50 years could also be considered for African American smokers.37 A general consensus is emerging that risk-based models outperform and are a better approach for screening compared with USPSTF criteria based on age and smoking alone.38,39,40 Ultimately, a cost-effective and beneficial approach must be implemented to balance the risks and harms of false-positive screening results, radiation from computed tomography imaging, and overdiagnosis.40,41 Current lung cancer screening guidelines appear to bias screening toward white persons, excluding high-risk African American individuals and potentially exacerbating disparities in lung cancer outcomes.10,24 The effectiveness of screening will thus be inconsistent across races and may result in missed opportunities to screen African American persons at high risk of lung cancer if current eligibility guidelines remain in place. Existing USPSTF lung cancer screening guidelines do not appear to be optimized for African American smokers and may result in a widening of racial disparities in late-stage diagnosis, potentially leading to higher mortality and worse outcomes among African American persons with lung cancer.24,42
Strengths and Limitations
To our knowledge, this was the largest study to evaluate lung cancer screening guidelines among African American smokers. This study was strengthened by its prospective follow-up, identification of incident lung cancers, and detailed self-reported smoking information assessed at baseline and in follow-up surveys. Of importance, this study includes a large number of participants with low educational levels recruited from federally qualified health centers across the southern United States, thus portraying a low socioeconomic population and a geographic region with a high burden of smokers at risk for lung cancer.43,44
This study has several limitations. First, smoking information was self-reported, and thus there is potential for misclassification of smoking intensity and duration needed for the calculation of pack-years. Second, we did not have information about actual lung screening use, although it is believed to be low in underserved populations such as the SCCS cohort. Third, we were missing smoking information and race for 5566 participants (eTable in the Supplement). Of note, age for persons with cancer was defined at diagnosis, whereas age for controls was defined at the end of the follow-up period. Although consistent with the study design, this may yield a disparity in age distributions between persons with cancer and controls. However, with the analysis conditioned on case status (ie, with comparisons of sensitivity and specificity), this disparity in age distributions between cases and controls would not be associated with observed racial imbalances.
Conclusions
The findings suggest that current USPSTF lung cancer screening guidelines may be too conservative for African American smokers and that race-specific adjustment of pack-year criteria in lung cancer screening guidelines would result in more equitable screening for African American smokers at high risk for lung cancer.
eFigure 1. Flowchart of Southern Community Cohort Study Participants Included and Excluded From the Present Study
eFigure 2. Sensitivity and Specificity for Males and Females by Age and Smoking Pack-Years for Existing (A) and Proposed Modification (B) to USPSTF Screening Guidelines
eTable. Characteristics of Individuals Excluded From the Analysis Due to Missing Race or Smoking Information.
References
- 1.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deKoning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening programs for high-risk populations [published online July 30, 2013]. Modeling report. US Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Document/modeling-report/lung-cancer-screening. Accessed March 7, 2019.
- 3.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team . Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102(23):1771-1779. doi: 10.1093/jnci/djq434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial differences in outcomes within the National Lung Screening Trial: implications for widespread implementation. Am J Respir Crit Care Med. 2015;192(2):200-208. doi: 10.1164/rccm.201502-0259OC [DOI] [PubMed] [Google Scholar]
- 5.Ho MK, Faseru B, Choi WS, et al. . Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3426-3434. doi: 10.1158/1055-9965.EPI-09-0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho MK, Mwenifumbo JC, Al Koudsi N, et al. . Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635-643. doi: 10.1038/clpt.2009.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross KC, Dempsey DA, St Helen G, Delucchi K, Benowitz NL. The influence of puff characteristics, nicotine dependence, and rate of nicotine metabolism on daily nicotine exposure in African American smokers. Cancer Epidemiol Biomarkers Prev. 2016;25(6):936-943. doi: 10.1158/1055-9965.EPI-15-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699-706. doi: 10.2105/AJPH.2010.191668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3(8):461-469. doi: 10.1016/S1470-2045(02)00815-X [DOI] [PubMed] [Google Scholar]
- 10.Fiscella K, Winters P, Farah S, Sanders M, Mohile SG. Do lung cancer eligibility criteria align with risk among blacks and Hispanics? PLoS One. 2015;10(11):e0143789. doi: 10.1371/journal.pone.0143789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. National Cancer Institute A Socioecological Approach to Addressing Tobacco-Related Health Disparities. National Cancer Institute Tobacco Control Monograph 22. NIH Publication No. 17-CA-8035A. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017. [Google Scholar]
- 12.Haiman CA, Stram DO, Wilkens LR, et al. . Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med. 2006;354(4):333-342. doi: 10.1056/NEJMoa033250 [DOI] [PubMed] [Google Scholar]
- 13.Howlader N, Noone AM, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. [Google Scholar]
- 14.Pinsky PF. Racial and ethnic differences in lung cancer incidence: how much is explained by differences in smoking patterns? (United States). Cancer Causes Control. 2006;17(8):1017-1024. doi: 10.1007/s10552-006-0038-2 [DOI] [PubMed] [Google Scholar]
- 15.Ryan BM. Differential eligibility of African American and European American lung cancer cases using LDCT screening guidelines. BMJ Open Respir Res. 2016;3(1):e000166. doi: 10.1136/bmjresp-2016-000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457-1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 17.Robbins HA, Engels EA, Pfeiffer RM, Shiels MS. Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst. 2015;107(3):dju489. doi: 10.1093/jnci/dju489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signorello LB, Hargreaves MK, Blot WJ. The Southern Community Cohort Study: investigating health disparities. J Health Care Poor Underserved. 2010;21(1)(suppl):26-37. doi: 10.1353/hpu.0.0245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signorello LB, Hargreaves MK, Steinwandel MD, et al. . Southern Community Cohort Study: establishing a cohort to investigate health disparities. J Natl Med Assoc. 2005;97(7):972-979. [PMC free article] [PubMed] [Google Scholar]
- 20.Wasserstein RL, Lazar NA. The asa’s statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129-133. doi: 10.1080/00031305.2016.1154108 [DOI] [Google Scholar]
- 21.Ten Haaf K, van Rosmalen J, de Koning HJ. Lung cancer detectability by test, histology, stage, and gender: estimates from the NLST and the PLCO trials. Cancer Epidemiol Biomarkers Prev. 2015;24(1):154-161. doi: 10.1158/1055-9965.EPI-14-0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro HM, Tarone RE, Wang TJ, Blot WJ. Menthol and nonmenthol cigarette smoking: all-cause deaths, cardiovascular disease deaths, and other causes of death among blacks and whites. Circulation. 2016;133(19):1861-1866. doi: 10.1161/CIRCULATIONAHA.115.020536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinsky PF, Kramer BS. Lung cancer risk and demographic characteristics of current 20-29 pack-year smokers: implications for screening. J Natl Cancer Inst. 2015;107(11):djv226. doi: 10.1093/jnci/djv226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasquinelli MM, Kovitz KL, Koshy M, et al. . Outcomes from a minority-based lung cancer screening program vs the National Lung Screening Trial. JAMA Oncol. 2018;4(9):1291-1293. doi: 10.1001/jamaoncol.2018.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Tobacco use among U.S. racial/ethnic minority groups–African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary. MMWR Recomm Rep. 1998;47(RR-18):v-xv, 1-16. [PubMed] [Google Scholar]
- 26.Wang TW, Asman K, Gentzke AS, et al. . Tobacco product use among adults—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225-1232. doi: 10.15585/mmwr.mm6744a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amos CI, Spitz MR, Cinciripini P. Chipping away at the genetics of smoking behavior. Nat Genet. 2010;42(5):366-368. doi: 10.1038/ng0510-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez-Stable EJ, Herrera B, Jacob P III, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152-156. doi: 10.1001/jama.280.2.152 [DOI] [PubMed] [Google Scholar]
- 29.Tanner JA, Chenoweth MJ, Tyndale RF. Pharmacogenetics of nicotine and associated smoking behaviors. Curr Top Behav Neurosci. 2015;23:37-86. doi: 10.1007/978-3-319-13665-3_3 [DOI] [PubMed] [Google Scholar]
- 30.Bossé Y, Amos CI. A decade of GWAS results in lung cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(4):363-379. doi: 10.1158/1055-9965.EPI-16-0794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji X, Bossé Y, Landi MT, et al. . Identification of susceptibility pathways for the role of chromosome 15q25.1 in modifying lung cancer risk. Nat Commun. 2018;9(1):3221. doi: 10.1038/s41467-018-05074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanetti KA, Wang Z, Aldrich M, et al. . Genome-wide association study confirms lung cancer susceptibility loci on chromosomes 5p15 and 15q25 in an African-American population. Lung Cancer. 2016;98:33-42. doi: 10.1016/j.lungcan.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKay JD, Hung RJ, Han Y, et al. ; SpiroMeta Consortium . Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet. 2017;49(7):1126-1132. doi: 10.1038/ng.3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wassenaar CA, Ye Y, Cai Q, et al. . CYP2A6 reduced activity gene variants confer reduction in lung cancer risk in African American smokers—findings from two independent populations. Carcinogenesis. 2015;36(1):99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103(17):1342-1346. doi: 10.1093/jnci/djr237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bierut LJ, Tyndale RF. Preparing the way: exploiting genomic medicine to stop smoking. Trends Mol Med. 2018;24(2):187-196. doi: 10.1016/j.molmed.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Annangi S, Nutalapati S, Foreman MG, Pillai R, Flenaugh EL. Potential racial disparities using current lung cancer screening guidelines. J Racial Ethn Health Disparities. 2018;6(1):22-26. [DOI] [PubMed] [Google Scholar]
- 38.Kaaks R, Hüsing A, Fortner RT. Selecting high-risk individuals for lung cancer screening; the use of risk prediction models vs simplified eligibility criteria. Ann Transl Med. 2017;5(20):406. doi: 10.21037/atm.2017.07.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ten Haaf K, Jeon J, Tammemägi MC, et al. . Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14(4):e1002277. doi: 10.1371/journal.pmed.1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung LC, Katki HA, Chaturvedi AK, Jemal A, Berg CD. Preventing lung cancer mortality by computed tomography screening: the effect of risk-based versus US Preventive Services Task Force eligibility criteria, 2005-2015. Ann Intern Med. 2018;168(3):229-232. doi: 10.7326/M17-2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19(3):154-156. doi: 10.1258/jms.2012.012010 [DOI] [PubMed] [Google Scholar]
- 42.Criss SD, Sheehan DF, Palazzo L, Kong CY. Population impact of lung cancer screening in the United States: projections from a microsimulation model. PLoS Med. 2018;15(2):e1002506. doi: 10.1371/journal.pmed.1002506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. 2015;69(2):168-176. doi: 10.1136/jech-2014-204417 [DOI] [PubMed] [Google Scholar]
- 44.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of Southern Community Cohort Study Participants Included and Excluded From the Present Study
eFigure 2. Sensitivity and Specificity for Males and Females by Age and Smoking Pack-Years for Existing (A) and Proposed Modification (B) to USPSTF Screening Guidelines
eTable. Characteristics of Individuals Excluded From the Analysis Due to Missing Race or Smoking Information.