Abstract
The aim of this article is to correct a very general error in scientific articles, in textbooks and in the Internet that has become an accepted fact. In this literature, the term “vitamin E″ is used for several similar molecules (both tocopherols and tocotrienols) that have never been shown to have vitamin property, i.e. a protective effect against the human deficiency disease. In fact, the name “vitamin E″ should only be used to define molecules that prevent the human deficiency disease “Ataxia with Vitamin E Deficiency” (AVED). Only one such molecule is known, α-tocopherol. This error may confuse consumers as well as medical doctors, who prescribe vitamin E without realizing that the current use of the name includes molecules of unknown, if not unwanted functions.
Graphical abstract
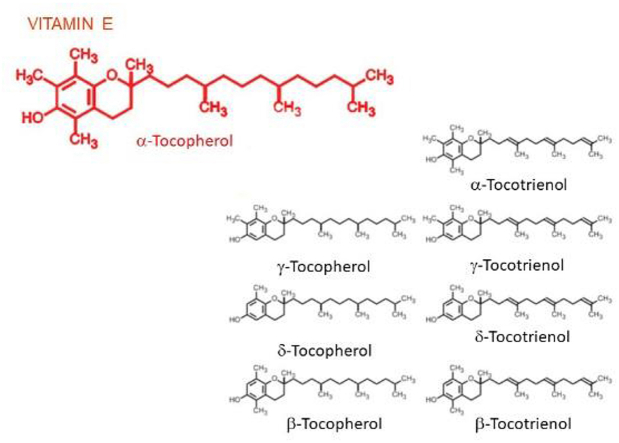
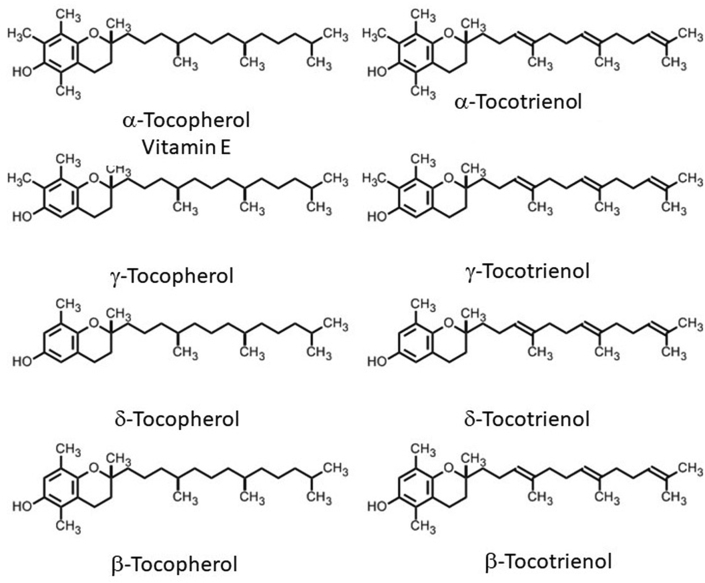
According to most scientific and popular publications (see for instance [[1], [2]] and https://lpi.oregonstate.edu/mic/vitamins/vitamin-E) “vitamin E includes eight fat-soluble isoforms: α-, β-, γ-, and δ-tocopherol and α-, β-, γ-, and δ-tocotrienol”. (Fig. 1).
Fig. 1.
The differences in the α, β, γ and δ forms are in the number and position of the methyl groups on the chromanol ring. Only the β- and γ- forms of tocopherols or tocotrienols can be called isomers, having the same formula but a different arrangement of atoms in the molecule. The difference between the tocopherols and the tocotrienols is in the presence of three double bonds in the side chain of the latter.
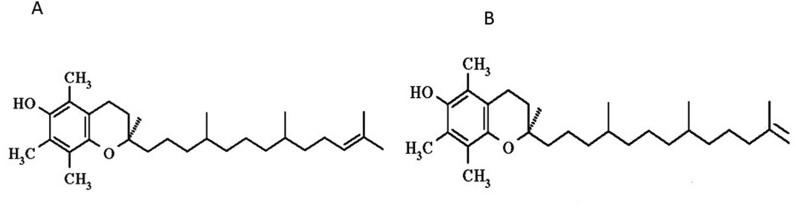
In addition, more recently, very similar molecules have been described, the tocomonoenols, with only one double bond in the sidechain (Fig. 2)
Fig. 2.
Matsumoto et al. [4] discovered α-tocomonoenol in palm oil, (A) and Yamamoto et al. [5] isolated an isomeric and chemically distinct α-tocomonoenol from the lipophilic fraction of salmon eggs having an unusual methylene unsaturation at the isoprenoid-chain terminus (B).
Do all those molecules, tocopherols, tocotrienols and tocomonoenols act in the human body in a way that they can be called vitamins?
Vitamins are organic substances essential in small quantities to normal metabolism, found in minute amounts in natural foodstuffs or sometimes produced synthetically: deficiencies of vitamins produce specific disorders which can be prevented by the administration of the missing vitamin. https://www.dictionary.com/browse/vitamin.
According to this definition, molecules such as essential fatty acids or essential amino acids cannot be called vitamins being needed not in minute amounts. Also trace metals, like zinc, copper, iron and molibdenum cannot be called vitamins, given their nature of inorganig compounds. In addition, vitamins are compounds whose deficiency results in a disease.
Are the molecular structures of vitamins specific?
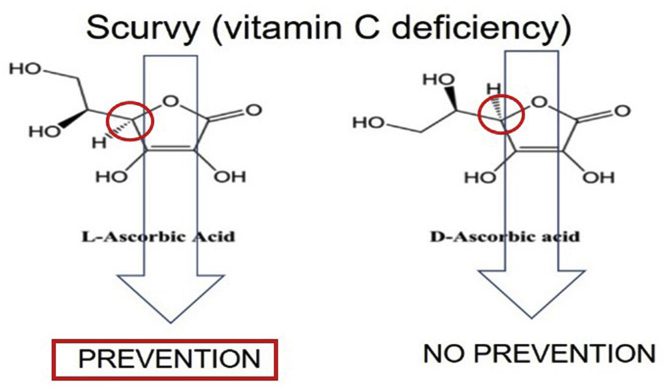
Scurvy is a disease caused by deficiency of vitamin C, historically famous because it affected sailors until the end of the 18th century, whose diets were lacking fresh fruits and vegetables. The disease can be prevented by l-ascorbic acid but not by the enantiomer d-ascorbic acid and only l-ascorbic acid can be called vitamin C (Fig. 3).
Fig. 3.
l-ascorbic acid and d-ascorbic acid. The circles indicate the only difference of the two molecules of a hydrogen atom in opposite stereo position. l-ascorbic acid prevents scurvy but d-ascorbic acid does not.
l-Ascorbic acid but not the enantiomer d-ascorbic acid prevents the deficiency disease . Only l-Ascorbic acid is called vitamin C.
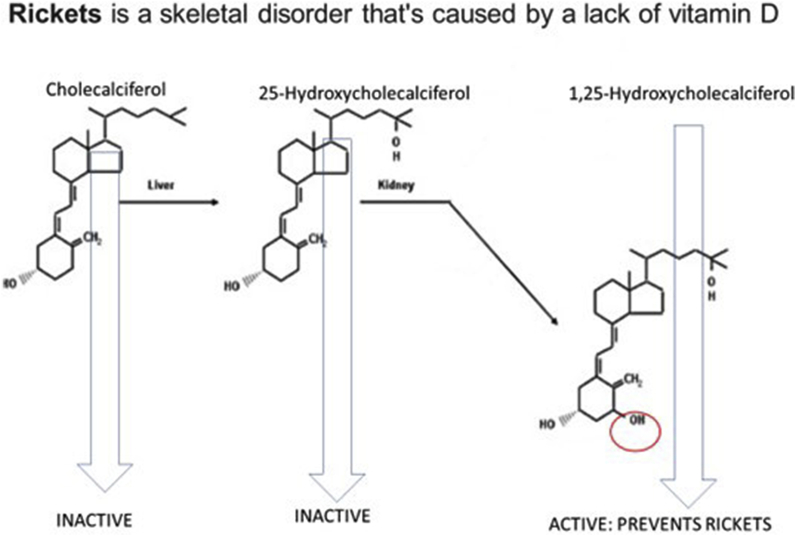
Another example is given by rickets prevention by vitamin D (Fig. 4),Are all tocopherols and tocotrienols vitamins?
Fig. 4.
Rickets is a skeletal disease caused by lack of vitamin D. Vitamin D is also structurally specific: 1,25-dihydroxycholecalciferol prevents the development of rickets but 25-hydroxycholecalciferol, just lacking one OH group (circled in the active molecule), is inactive. Consequently, only 1,25-dihydroxycholecalciferol is called Vitamin D [6].
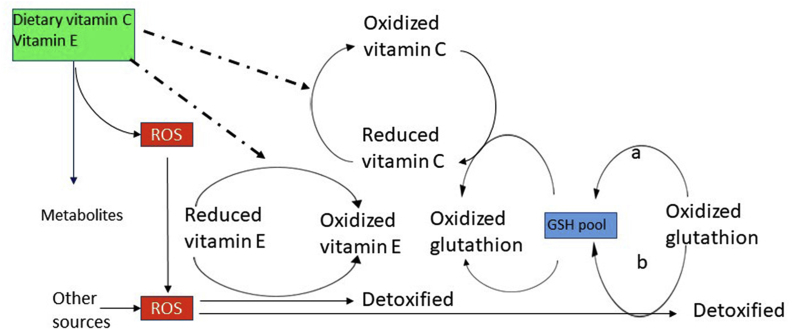
Tocopherols and tocotrienols act as free radical scavengers in membranes and lipoproteins[3] . They quench fatty acid peroxyl radicals by becoming tocopheroxyl radicals. Tocopheroxyl radicals potential damage would be avoided via reduction by an appropriate reducing agent (Fig. 5).
Fig. 5.
The recycling of vitamin E. The radical scavenging property of tocopherols [3] has been assumed to be at the basis of disease prevention, but this assumption was never proven. An additional claim of vitamin function for β-, γ-, and δ-tocopherol was based on their protection against fetus resorption in rats [6], but was incorrectly extended to the prevention of the human disease (AVED), the human neurological disease [7]. Based on a wrong assumption and an illogical extension of the animal effects to human disease, all tocopherols (wrongly defined frequently as “vitamin E isomers”) and tocotrienols, have been therefore called vitamin E.
Different tocopherols have gene regulatory functions, not related to their radical scavenging capacity.
In adddition to their radical scavenging action, non-antioxidant functions of tocopherols have been shown, both in vivo and in vitro [8,9]. In T cells from mouse spleen, α-tocopherol affects signal transduction, transcriptional regulation, apoptosis pathways and other genes associated with the regulation of cell cycle. The modulation of gene expression is dependent on the fine molecular structure of α-tocopherol: S and R isomers of the molecule side chain act differently and marker genes respond uniquely to natural vitamin E (RRR-α-tocopherol) and not to the SSS isomers, that have identical radical scavenging properties [10]. The two vitamers α and γ tocopherols differ only by a methyl group on the chromane ring and have essentially the same antioxidant properties certain genes uniquely respond to either α-tocopherol or γ tocopherol [11].
Different tocopherols and tocotrienols have largely different cellular effects.
β-Tocopherol has similar antioxidant properties as α-tocopherol but does not inhibit protein kinase C activity, cell proliferation and gene expression, and it is 10-fold less active than α-tocopherol in inhibiting thrombin-induced PKC activity. β-Tocopherol, has no effect on IL-1β release in monocytes, in endothelial cells, β-tocopherol, contrary to α-tocopherol has no effect on caspase-3 activity. β-Tocopherol and γ-tocopherol but not α-tocopherol strongly inhibit intracellular tyrosinase [12].
γ−Tocopherol, and its metabolite γ−CEHC, exhibit activities different from α-tocopherol such as natriuretic, anti-inflammatory, antitumoral, and more. α-Tocopherol does not exhibit anticancer properties, but it reduces anticancer actions of γ-tocopherol in vivo. δ-Tocopherol is responsible for a proinflammatory response promoted by reactive oxygen species, it prevents hormone-dependent breast cancer progression, colon carcinogenesis, lung tumorigenesis and prostate cancer cell growth. δ-Tocopherol reduces lipid accumulation, has antiangiogenic effects and elevates L-type calcium channel activity to increase neuronal differentiation [12]. In summary, the activities of β-, γ-, and δ-tocopherols do not correspond to their radical scavenging behavior: they reflect anti-inflammatory, antineoplastic, and natriuretic actions probably mediated through specific binding interactions and have no property suggesting a protective effect agains AVED.
In vitro studies with tocotrienols indicate effects against cancer (mostly due to suppression of angiogenesis), cardiovascular and neurodegenerative diseases (ability to suppress glutamate-induced activation of c-Src kinase) [2], It has been concluded that tocotrienols are more potent than tocopherols and that the different tocotrienols, which differ in their number of methyl groups and their position on the chroman ring, also differ in their biological activities. While various studies have indicated that α-tocotrienol is neuroprotective, δ- and γ-tocotrienol have been shown to exhibit the greatest anticancer effects. However, none of these molecules has been tested for an effect on AVED and only α-tocopherol has been shown to protect against AVED, the only human disease associated with vitamin E deficiency.
Since it is known that small structural changes can eliminate the vitamin effect of a molecule and since only α-tocopherol, and none of the other tocopherols, have been shown to protect against the human deficiency disease, only α-tocopherol can be called bona fide vitamin E. Why the other seven fat-soluble tocopherols and tocotrienols, that have never shown any protective effect against a human deficiency disease, should be called (and sold as) vitamin E? The recently described α-tocomonoenol should also be called “vitamin E”?
Conclusions
-
•
α-Tocopherol only has been tested and shown to prevent AVED, the vitamin E deficiency disease
-
•
Tocopherols and tocotrienols have different structures: the protection by α-tocopherol against AVED cannot be extrapolated to other similar molecules
-
•
Due to their different in vitro and in vivo molecular effects tocopherols and tocotrienols cannot be automatically exchanged with each other, even if they have similar radical scavenging properties
-
•
Only α-tocopherol can be called vitamin E. The possible future discovery of new preventive/curing functions against specific diseases of the different tocopherols and tocotrienols may prompt to a change in nomenclature, as it was done for the vitamin B family,
In a more general perspective, it is not a good policy that molecules with no vitamin effect are called and sold as vitamins, especially when their unknown properties may even endanger, rather than protect, human health. For the time being, medical doctors should be aware of the confusion: if they want to prescribe vitamin E it should be clear to them that all molecules called vitamin E, except for α-tocopherol, do not have the vitamin property implied in their name.
References
- 1.Wallert M., Bauer J., Kluge S., Schmolz L., Chen Y.C., Ziegler M., Searle A.K., Maxones A., Schubert M., Thurmer M., Pein H., Koeberle A., Werz O., Birringer M., Peter K., Lorkowski S. The vitamin E derivative garcinoic acid from Garcinia kola nut seeds attenuates the inflammatory response. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal B.B., Sundaram C., Prasad S., Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010;80:1613–1631. doi: 10.1016/j.bcp.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kagan V.E., Serbinova E.A., Forte T., Scita G., Packer L. Recycling of vitamin E in human low density lipoproteins. J. Lipid Res. 1992;33:385–397. [PubMed] [Google Scholar]
- 4.Matsumoto A., Takahashi S., Nakano K., Kijima S. Identification of a new vitamin E in a plant oil. J. Jpn. Oil Chem. Soc. 1995;44:593–597. [Google Scholar]
- 5.Yamamoto Y., Fujisawa A., Hara A., Dunlap W.C. An unusual vitamin E constituent (α-tocomonoenol) provides enhanced antioxidant protection in marine organisms adapted to cold-water environments. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13144–13148. doi: 10.1073/pnas.241024298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krinsky N.I., Beecher G.R., Burk R.F., Chan A.C., Erdman jJW., Robert A.J., Jialal I., Kolonel L.N., Marshall J.R., Taylor Mayne S., Prentice Rl, Schwarz K.B., Steinberg D., Traber M.G. 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington (DC) [Google Scholar]
- 7.Ouahchi K., Arita M., Kayden H., Hentati F., Ben Hamida M., Sokol R., Arai H., Inoue K., Mandel J.L., Koenig M. Ataxia with isolated vitamin E deficiency is caused by mutations in the α-tocopherol transfer protein. Nat. Genet. 1995;9:141–145. doi: 10.1038/ng0295-141. [DOI] [PubMed] [Google Scholar]
- 8.Azzi A. Molecular mechanism of α-tocopherol action. Free Radic. Biol. Med. 2007;43:16–21. doi: 10.1016/j.freeradbiomed.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Zingg J.M., Libinaki R., Lai C.Q., Meydani M., Gianello R., Ogru E., Azzi A. Modulation of gene expression by α-tocopherol and α-tocopheryl phosphate in THP-1 monocytes. Free Radic. Biol. Med. 2010;49:1989–2000. doi: 10.1016/j.freeradbiomed.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Han S.N., Pang E., Zingg J.M., Meydani S.N., Meydani M., Azzi A. Differential effects of natural and synthetic vitamin E on gene transcription in murine T lymphocytes. Arch. Biochem. Biophys. 2010;495:49–55. doi: 10.1016/j.abb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Zingg J.M., Han S.N., Pang E., Meydani M., Meydani S.N., Azzi A. In vivo regulation of gene transcription by α- and γ-tocopherol in murine T lymphocytes. Arch. Biochem. Biophys. 2013;538:111–119. doi: 10.1016/j.abb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Azzi A. Many tocopherols, one vitamin E. Mol. Asp. Med. 2018;61:92–103. doi: 10.1016/j.mam.2017.06.004. [DOI] [PubMed] [Google Scholar]