Abstract
Background
Clinical studies assessing the feasibility and accuracy of three stone scoring systems’s (SSSs: Guy’s stone score, CROES nomogram and S.T.O.N.E nephrolithometry scoring system) have reported contradictory outcomes. This systematic evaluation was performed to obtain comprehensive evidence with regard to the feasibility and accuracy of three SSSs.
Methods
A systematic search of Embase, Pubmed, Medline, and the Cochrane Library was conducted to identify studies that compared three SSSs up to Mar 2018. Patients were categorized according to stone free (SF) and no-stone free (NSF), Outcomes of interest included perioperative variables, stone-free rate (SFR), and complications.
Results
Ten studies estimating three SSSs were included for meta-analysis. The results showed that SF patients had a significantly lower proportion of male (OR = 1.48, P = 0.0007), lower stone burden (WMD = -504.28, P < 0.0001), fewer No of involved calyces (OR = -1.23, P = 0.0007) and lower proportion of staghorn stone (OR = 0.33, P < 0.0001). Moreover, SF patients had significantly lower score of Guy score (WMD = -0.64, P < 0.0001), but, S.T.O.N.E. score (WMD = -1.23, P < 0.0001) and a higher score of CROES nomogram (WMD = 29.48, P = 0.003). However, the comparison of area under curves (AUC) of predicting SFR indicated that there was no remarkable difference between three SSSs. Nonetheless, Guy score was the only stone scoring system that predicted complications after PCNL (WMD = -0.29, 95% CI: − 0.57 to − 0.02, P = 0.03).
Conclusions
Our meta-analysis indicated that the three SSSs were equally, feasible and accurate for predicting SFR after PCNL. However, Guy score was the only stone scoring system that predicted complications after PCNL.
Electronic supplementary material
The online version of this article (10.1186/s12894-019-0488-y) contains supplementary material, which is available to authorized users.
Keywords: Guy’s score, S.T.O.N.E. score, CROES nomogram, Stone free rate, Meta-analysis
Background
The recommended treatment option for renal calculi and staghorn calculi is percutaneous nephrolithotomy (PCNL) according to the guidelines of the European Association of Urology (EAU) [1]. PCNL has increasingly been used over the past few decades and may continue in the future [2, 3]. However, PCNL outcomes among the authors are different, because of the vast heterogeneity in the methods for clinical and academic characterization of nephrolithiasis besides the evaluation of surgical outcomes. So assessing the preoperative factors that affect SFR and complications is critical.
The Guy’s stone score, the Clinical Research Office of the Endourological Society(CROES) nomogram and the S.T.O.N.E.(stone size, tract length, obstruction, number of involved calices and essence) stone score are seen as predictors of stone-free status (SFS) and complications after PCNL [4–6]. The widespread use of a standardized stone scoring system is very precious for counseling patient, clinical decision, and assessment of outcomes, in addition to improving academic reporting [7]. However, no universally accepted stone scoring system for predicting SFR and complications after PCNL exists. Comparison of the SSSs in different clinical studies indicated some advantages as well as disadvantages of one nomogram to another for different variables. Hence, we performed a systematic review of the literature with a meta-analysis of the available published literature to compare the feasibility and accuracy of three SSSs in predicting PCNL outcomes concerning SFR and complications.
Methods
Study selection
According to the Cochrane Handbook recommendations, a systematic review of published literature was performed [8]. To identify all studies published up to Dec 31, 2018, which assessed the feasibility and accuracy of three SSSs. The following MESH search headings were used: “comparative studies”, “Guy”, “CROES”, “S.T.O.N.E”, “percutaneous nephrolithotomy”, “stone free rate”, and “complication”.
Inclusion and exclusion criteria
All studies included in this meta-analysis satisfied the following requirements: (a) compare the two or three SSSs, (b) report the outcomes of two or three SSSs, (c) document the surgery as PCNL, (d) document indications for PCNL with renal stones. Studies were excluded if: (a) the article did not meet the inclusion criteria, (b) no outcomes were mentioned or the parameters were impossible to analyze the three SSSs from the published findings.
Data extraction and outcomes of interest
Two of the authors (JKH and SF) extracted data from the included studies including: author identification, country, publication years, study design, age, and the number of patients. All disagreements about eligibility were resolved by consensus through author discussions. The outcomes, including SFR and, overall complications, were extracted to compare between three SSSs. Overall complications were graded based on the Clavien-Dindo system [9].
Study quality assessment
In accordance with the criteria of Centre for Evidence-Based Medicine in Oxford, we evaluated the level of evidence (LOE) of the included ten studies. Furthermore, Jaded Score was applied to evaluate the methodological quality of RCTs [10]. while the Newcastle-Ottawa Scale (NOS) assessed the methodological quality of non-RCTs observational studies [11]. Besides, JKH and ZJG evaluated the quality of the articles and discrepancies were rechecked and resolved by discussion.
Statistical analysis
All analyses were conducted by Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). Continuous and dichotomous variables were analyzed by weighted mean differences (WMDs) and odds ratios (ORs). All analysis results were reported with 95% CIs. I2 and X2 statistics were applied to evaluate the quantity of heterogeneity, and when I2>50%, the evidence was considered to have substantial heterogeneity, the random-effects (RE) model would be applied, otherwise, the fixed effects (FE) model was applied. Egger’s test and funnel plot evaluated the publication bias. Sensitivity analyses estimated the influence of studies with a high risk of bias on the overall effect.
Results
Characteristics of eligible studies
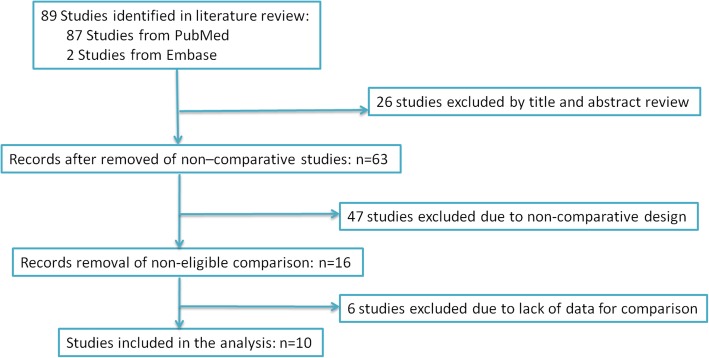
Ten studies [12–21] conformed to the inclusion criteria of this meta-analysis and were there included in the analysis of three SSSs (Fig. 1). The demographic and clinical characteristics of the included literature were shown in Table 1.
Fig. 1.
PRISMA diagram. The search strategy and number of studies identified for inclusion in this meta-analysis
Table 1.
Characteristics of included studies
| First author year | Country | Study interval | Design | LOE | No.of patients | Matching/comparablea |
|---|---|---|---|---|---|---|
| Bozkurt, 2015 [12] | Turekey | 2012–2015 | Retrospective | 3b | 437 | 1,2,3,4,5 |
| Choi, 2016 [13] | Korea | 2003–2014 | Retrospective | 3b | 217 | 1,2,3,4,5,6 |
| Choi, 2016 [14] | Korea | 2012–2015 | Retrospective | 3b | 141 | 1,2,3,4, 5,6 |
| Jaipuria, 2016 [15] | India | 2014–2015 | prospective | 3b | 606 | 1,3 |
| Kocaaslan, 2016 [16] | Turekey | 2010–2015 | Retrospective | 3b | 137 | 1,2,3,4, 5,6 |
| Labadie, 2015 [17] | USA | 2009–2012 | Retrospective | 3b | 246 | 1,2,3,4, 5,6 |
| Noureldin, 2015 [18] | Canada | 2009–2013 | Retrospective | 3b | 185 | 1,2,3,5 |
| Sfoungaristos, 2016 [19] | Israel | 2010–2015 | Retrospective | 3b | 73 | 1,3,4, 5,6 |
| Tailly, 2016 [20] | Canada | 2006–2013 | Retrospective | 3b | 586 | 1,2,3,4, 5,6 |
| Yarimoglu, 2016 [21] | Turekey | 2012–2015 | Retrospective | 3b | 262 | 1,2,3,4, 5 |
a Matching/comparable variable: 1 = age, 2 = BMI, 3 = gender, 4 = laterality(right/left), 5 = stone burden, 6 = stone density
Quality of the studies and level of evidence(Table 1)
In this meta-analysis, the NOS quality assessment method of the observational studies [11], and the US Preventive Services Task Force grading system were applied to evaluate the quality of all studies [10]. Included studies were all level 3b. Also, the demographic variables of the three SSSs were extracted from included articles (Table 1).
Description of included studies and preoperative characteristics of the patients (Table 2)
Table 2.
Overall analysis of demographic and clinical characteristics compared stone-free with not stone-free after PCNL
| Outcomes of interest | No. of studies | No. of patients SF/NSF | OR/WMD(95% CI) a | p-value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| Chi2 | df | I2 | p-value | |||||
| Age(year) | 7 | 1101/559 | 0.02[−1.48,1.52] a | 0.98 | 5.57 | 6 | 0% | 0.47 |
| BMI(kg/m2) | 6 | 1053/534 | −0.59[− 1.80,0.63] a | 0.34 | 21.87 | 5 | 77% | 0.0006 |
| Proportion/male | 6 | 914/484 | 1.48[1.18,1.86] | 0.0007 | 2.38 | 5 | 0% | 0.80 |
| Laterality(right/left) | 6 | 914/484 | 1.10[0.88,1.38] | 0.41 | 8.43 | 5 | 41% | 0.13 |
| Stone burden(mm2) | 6 | 914/484 | −504.28[−673.88,-334.67] a | < 0.0001 | 13.47 | 5 | 63% | 0.02 |
| No of involved calyces | 3 | 560/316 | −1.23[−1.94,-0.52] | 0.0007 | 9.15 | 2 | 78% | 0.01 |
| Staghorn | 6 | 914/484 | 0.33[0.26,0.43] | < 0.0001 | 6.58 | 5 | 24% | 0.25 |
| Stone density(HU) | 4 | 777/377 | −37.65[−81.95,6.65] a | 0.10 | 4.90 | 4 | 18% | 0.30 |
| Guy score | 5 | 777/377 | −0.64[−0.90,-0.37] a | < 0.0001 | 26.42 | 4 | 85% | < 0.0001 |
| CROES nomogram | 6 | 964/452 | 29.48[9.84,49.12] a | 0.003 | 57.50 | 5 | 91% | < 0.0001 |
| S.T.O.N.E. score | 6 | 964/452 | −1.23[−1.78,-0.67] a | < 0.0001 | 35.04 | 5 | 86% | < 0.0001 |
SF stone free, NSF not stone free, PCNL percutaneous nephrolithotomy, OR odds ratio, WMD weighted mean difference, CI confidence interval, BMI body mass index, a WMD
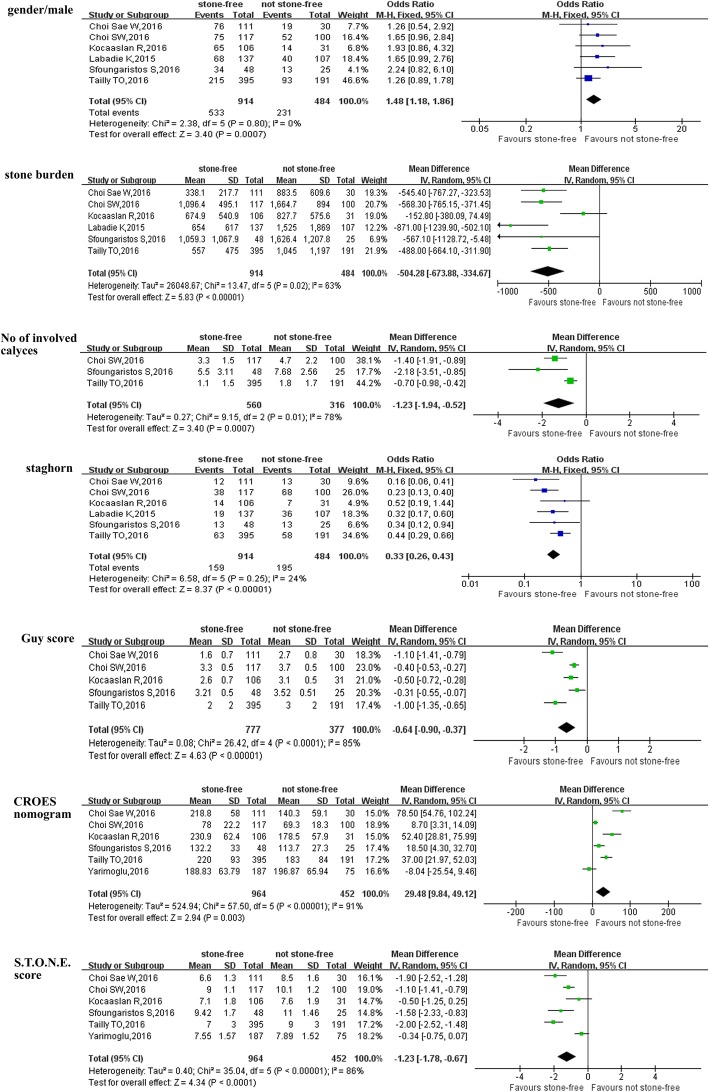
Patients were categorized as stone free(SF) and no-stone free(NSF), and the pooled data of included studies [13, 14, 16, 17, 19, 20] showed that stone free patients had significantly lower proportion of male(OR = 1.48, 95% CI: 1.18 to 1.86, P = 0.0007) (Fig. 2), lower stone burden (WMD = -504.28, 95% CI: − 673.88 to − 334.67, P < 0.0001) (Fig. 2), fewer No of involved calyces (OR = -1.23, 95% CI: − 1.94 to − 0.52, P = 0.0007) (Fig. 2) and lower proportion of staghorn stone (OR = 0.33, 95% CI: 0.26 to 0.43, P < 0.0001) (Fig. 2). And stone free patients had significantly lower score of Guy score (WMD = -0.64, 95% CI: − 0.90 to − 0.37, P < 0.0001) (Fig. 2), lower score of S.T.O.N.E. score (WMD = -1.23, 95% CI: − 1.78 to − 0.67, P < 0.0001) (Fig. 2) and higher score of CROES nomogram (WMD = 29.48, 95% CI: 9.84 to 49.12, P = 0.003) (Fig. 2) (Table 2). However, there were no remarkable difference in terms of age, BMI, laterality and stone density between SF and NSF patients (Table 2, Additional file 1: Figure S1).
Fig. 2.
Forest plot and meta-analysis of preoperative characteristics between the stone-free patients and not stone-free patients
Outcomes of perioperative variables (Table 3)
Table 3.
Overall analysis of perioperative outcomes comparing stone free with not stone free after PCNL
| Outcome of interest | No. of studies | No.of patients SF/NSF |
OR/WMD(95%CI) a | p-value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| Chi2 | df | I 2 | p-value | |||||
| Operative time, min | 6 | 706/368 | −19.85[−25.52,-14.18] | <0.0001 | 8.62 | 5 | 42% | 0.13 |
| No of tract | 3 | 618/322 | −0.03[−0.14,0.08] a | 0.62 | 4.76 | 2 | 58% | 0.09 |
| Mean tract length | 3 | 623/321 | 0.77[−2.06,3.61] | 0.59 | 1.92 | 2 | 0% | 0.38 |
| LOS, days | 6 | 706/368 | −0.53[−0.88,-0.17] | 0.003 | 5.00 | 5 | 0% | 0.42 |
| Transfusion rate | 2 | 228/130 | 0.22[0.08,0.55] a | 0.001 | 0.34 | 1 | 0% | 0.56 |
| Change in Hb level(g/mL) | 2 | 228/130 | −0.21[−0.55,0.13] | 0.22 | 0.01 | 1 | 0% | 0.92 |
NS stone free, NSF not stone free, LOS length of stay, PCNL percutaneous nephrolithotomy, OR odds ratio, WMD weighted mean difference, CI confidence interval, a OR
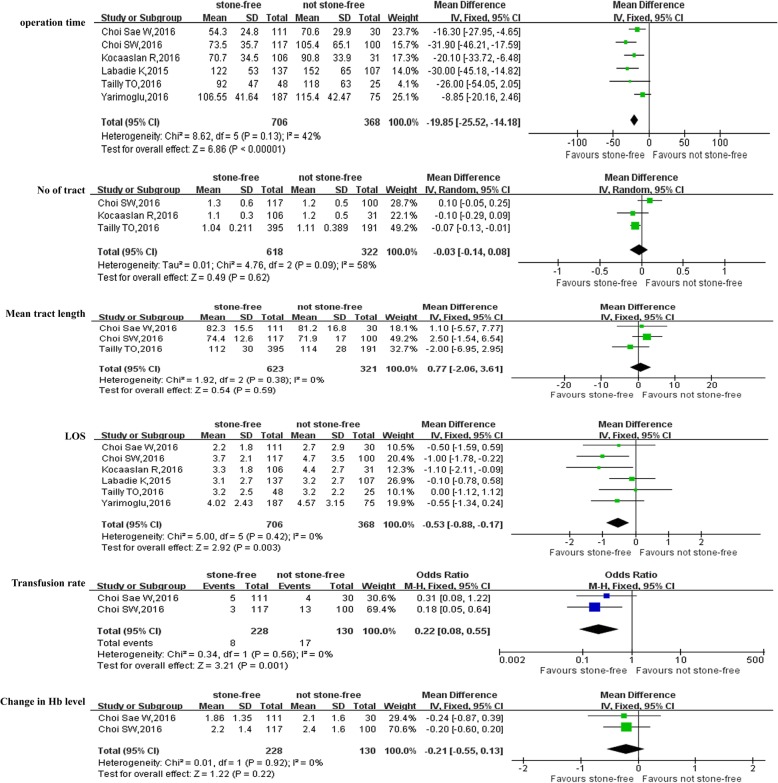
With respect to perioperative variables, pooled data of 6 studies [13, 14, 16, 17, 20, 21] involving 1074 participants demonstrated that SF patients were associated with shorter operative time than NSF patients (WMD:-19.85 min; 95% CI: − 25.52 to − 14.18; P<0.0001)(Fig. 3). SF patients were also associated with shorter length of hospital stay (LOS) (WMD: − 0.53 days; 95% CI: − 0.88 to − 0.17; P = 0.003) (Fig. 3) and lower transfusion rate (OR: 0.22; 95% CI: 0.08 to 0.55; P = 0.001) (Fig. 3) than NSF patients, respectively. However, there were no statistically difference between SF and NSF patients in term of No of tract (OR = -0.03; 95% CI: − 0.14 to 0.08; P = 0.62) (Fig. 3), mean tract length (WMD = 0.77; 95% CI: − 2.06 to 3.61; P = 0.59) (Fig. 3) and change in Hb level (WMD = − 0.21 g/mL; 95% CI: − 0.55 to 0.13; P = 0.22) (Fig. 3)(Table 3).
Fig. 3.
Forest plot and meta-analysis of outcomes of perioperative variables between stone-free patients and not stone-free patients
Outcomes of three scoring systems for predicting SFR after PCNL (Table 4)
Table 4.
Overall analysis of three stone-scoring systems for predicting SFR after PCNL
| Outcome of interest | No.of studies | No. of patients | MD (95%CI) | p-value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| Chi2 | df | I 2 | p-value | |||||
| CROES/Guy | ||||||||
| AUC | 7 | 1837/1837 | 0.09[− 0.12,0.29] | 0.41 | 14,350.0 | 6 | 100% | <0.0001 |
| STONE/Guy | ||||||||
| AUC | 8 | 2191/2191 | 0.00[−0.03,0.04] | 0.92 | 699.92 | 7 | 99% | <0.0001 |
| CROES/STONE | ||||||||
| AUC | 7 | 1662/1662 | −0.00[−0.05,0.04] | 0.94 | 491.21 | 6 | 99% | <0.0001 |
SFR stone free rate, PCNL percutaneous nephrolithotomy, AUC area under curve, CROES clinical research office of the endourological society scoring system, Guy Guy scoring system, S.T.O.N.E S.T.O.N.E scoring system, MD mean difference, CI confidence interval
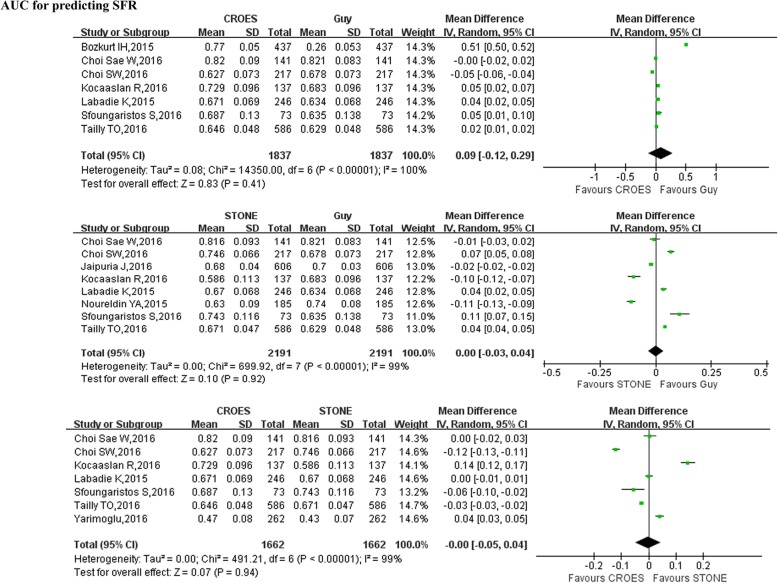
Pooled data of 7 studies [5, 12–14, 16, 17, 20] reported an accuracy of three SSSs in predicting post-PCNL SFR, the forest plot indicated that there was no statistical difference between Guy score and CROES nomogram with respect to area under curves(AUC) of prediction of SFR(WMD = 0.09; 95% CI:-0.12 to 0.29; P = 0.41) (Table 4, Fig. 4). There was also no significant difference between S.T.O.N.E. score and Guy score with respect to area under curves (AUC) of prediction of SFR (WMD = 0.00; 95% CI:-0.03 to 0.04; P = 0.92) (Table 4, Fig. 4) and no significant difference between CROES nomogram and S.T.O.N.E. score with respect to area under curves (AUC) of prediction of SFR (WMD = 0.00; 95% CI: − 0.05 to 0.04; P = 0.94) (Table 4, Fig. 4).
Fig. 4.
Forest plot and meta-analysis of outcomes of three scoring systems for predicting SFR after PCNL. SFR = stone free rate
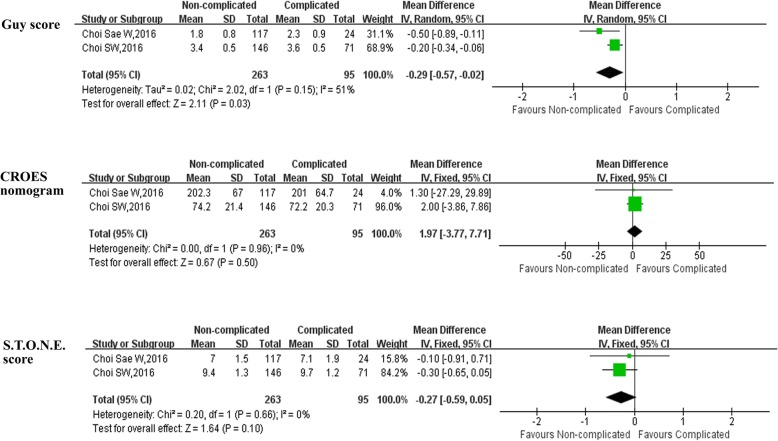
Outcomes of three scoring systems for predicting complications after PCNL (Table 5)
Table 5.
Overall analysis of three scoring systems for predicting complications after PCNL
| Outcome of interest | No. of studies | No.of patients Non-complicated/complicated |
OR/WMD(95%CI) a | p-value | Study heterogeneity | |||
|---|---|---|---|---|---|---|---|---|
| Chi2 | df | I 2 | p-value | |||||
| Guy score | 2 | 263/95 | −0.29[− 0.57,-0.02] | 0.03 | 2.02 | 1 | 51% | 0.15 |
| CROES nomogram | 2 | 263/95 | 1.97[−3.77,7.71] | 0.50 | 0.00 | 1 | 0% | 0.96 |
| S.T.O.N.E. score | 2 | 263/95 | −0.27[−0.59,0.05] | 0.10 | 0.20 | 1 | 0% | 0.66 |
PCNL percutaneous nephrolithotomy, CROES clinical research office of the endourological society scoring system, Guy Guy scoring system, S.T.O.N.E S.T.O.N.E scoring system, OR odds ratio, WMD weighted mean difference, CI confidence interval, a OR
Pooled data of reported that the three SSSs in predicting post-PCNL complications, the forest plot indicated that Guy score was the only stone scoring system for predicting post-PCNL complications (WMD = -0.29, 95% CI: − 0.57 to − 0.02, P = 0.03) (Table 5, Fig. 5). No association between the two other scoring systems (CROES nomogram and S.T.O.N.E. score) and post-PCNL complications (Table 5, Fig. 5).
Fig. 5.
Forest plot and meta-analysis of outcomes of three scoring systems for predicting complications after PCNL
Discussion
Widely applicable and straightforward tools will highly improve patient counseling, clinical decision making, assessment of operation outcomes and academic study after PCNL for renal stones [22, 23]. These can allow reliable and accurate comparisons of treatment safety and efficacy, and facilitate the meaningful comparison of clinical studies [24]. We considered that the commonest and validated SSSs (Guy score, CROES nomogram, and S.T.O.N.E. score) could be predictive of SFR and complications after PCNL.
Both similar and divergent variables between the three SSSs has to be known. The Guy stone score consists of four grades based on stone burden and patient anatomy [7, 25]. The CROES nomogram is highly generalizable based on global data and uniquely grades risk across a continuous scale rather than dividing stones of varying complexity into discrete groups [7, 25]. The S.T.O.N.E. stone score stratifies patients into low-, moderate-, and high-risk groups, and it is more useful for decision making [7, 25]. The three SSSs included different parameters, however, the stone location, stone count and staghorn calculi were pivotal variables in the three SSSs [7].
On the other hand, Guy stone score included renal anatomy but not stone burden, and this was a difference from CROES nomogram and S.T.O.N.E. score. The CROES nomogram included prior treatment as well as operation volume, and these variables had remarkable relationships to the SFR. However, CROES nomogram lacked imageology information on hydronephrosis and calyceal abnormalities. The S.T.O.N.E. score comprised stone size, tract length, obstruction, number of involved calices and essence, and it had greater feasibility and accuracy than any of the individual variables alone. In our meta-analysis, the results showed preoperative variables gender, stone burden, number of involved calyces and staghorn calculi were remarkably correlating with SFR after PCNL for kidney calculi.
However, there is still no widely accepted stone scoring system for the prediction of outcomes after PCNL, and contradictions between different authors exist concerning the prediction of outcomes by the SSSs. Some experts reported that three SSSs were efficacy and equally predictive of SFR by estimating and comparing the three SSSs in 246 patients after PCNL [17]. Tailly et al. reported that three SSSs have similar predictive accuracy of SFS by comparing the three SSSs in 586 patients after PCNL, but no association between three SSSs and complications [20]. Moreover, Bozkurt et al. reported that both the Guy and CROES nomogram had a remarkable relationship with SFS and complications [12]. Noureldin et al. also reported that the Guy score and S.T.O.N.E. score were equally associated with SFS, but there were no significant correlations with complications [18]. However, when other experts evaluated and compared three SSSs in predicting post-PCNL SFR and complications, the results showed that only S.T.O.N.E. score was a predictor of SFS after PCNL for renal stones [13]. Kocaaslan et al. reported that the CROES nomogram was well correlated with the success of PCNL in cases with anatomical abnormalities, and that the Guy score and S.T.O.N.E. score failed to predict the SFS and complications after PCNL [16]. Choi et al. compared the predictability and accuracy of the Guy score, CROES nomogram and S.T.O.N.E. score, the results showed that only Guy score could predict SFS and complications after PCNL [14]. Sfoungaristos et al. also compared three SSSs, and the results indicated that S.T.O.N.E. score was the only predictor of the SFR for post-PCNL, and the three SSSs were not associated with complications [19]. In our meta-analysis, the results indicated that the three SSSs were remarkably associated with SFS and equally predictive of SFR, but only Guy score was a predictor of complications.
However, some critical limitations exist in the three SSSs. Firstly, stone burden and density as the important parameters dide not reflect in Guy’s score. Moreover, it is also failed to describe procedure difficulties as well as clinical variability. Secondly, the CROES nomogram is also did not reflect stone density and lacked important variables affecting the outcomes, including imaging information on hydronephrosis and pelvicalyceal abnormalities [5, 26]. Moreover, the CROES nomogram was complex in the clinical applications [17]. Thirdly, the limitations of S.T.O.N.E. score were validated with a small cohort which may limit its widespread usage [4, 7].
Similarly, several limitations existed while analyzing and interpreting results in our meta-analysis. Firstly, to identify prognostic factors, we acknowledge that other variables, such as surgeon experience and advanced surgical instruments, may need further investigation. These factors in the future may need to be incorporated in multicenter and larger samples clinical applications. Secondly, there existed heterogeneities of studies, some studies in this meta-analysis had the risk of selection bias. Lastly, all patients were evaluated for SFR after PCNL by KUB but not by CT, which may have overstated the SFR. Therefore, a need to develop more accurate and practical SSS to assess the relationship between the SSS and SFR, complications.In conjunction, our meta-analysis thus provides some up to date conclusions for the advantages and disadvantages of three SSSs in predicting of SFS and complications.
Conclusions
The Guy score, CROES nomogram and S.T.O.N.E. score were equally accurate predictive of SFR in patients undergoing PCNL, but the Guy score is the only SSS for predicting complications.
Additional file
Figure S1. Forest plot and meta-analysis of demographic and clinical characteristics compared stone-free with not stone-free after PCNL. (TIF 3058 kb)
Acknowledgements
Not applicable.
Abbreviations
- AUC
Area under curves
- CROES
The Clinical Research Office of the Endourological Society nomogram
- EAU
European Association of Urology
- FE
Fixed effects
- LOE
Level of evidence
- NOS
The Newcastle-Ottawa Scale
- ORs
Odds ratios
- PCNL
Percutaneous nephrolithotomy
- RE
Random-effects
- SFR
Stone free rate
- SSS
Three stone scoring systems
- the S.T.O.N.E
stone size, tract length, obstruction, number of involved calices and essence
- WMDs
Weighted mean differences
Authors’ contributions
Manuscript writing and editing: JKH, SF. Project development: SF. Data collection: ZP, BY, SG, data management/analysis: LGH, ZJG, LCX. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by National Natural Science Cultivate Foundation of Guizhou Provincial People’s Hospital (Number: [2018]5764–01) and Doctoral Foundation of Guizhou Provincial People’s Hospital (GZSYBS[2018]02) and National Natural Science Foundation of China (Number: 81873608).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kehua Jiang, Email: tjjkh@sina.com.
Fa Sun, Phone: 86-851-85922979, Email: sfgmc@sina.com.
Jianguo Zhu, Email: 4158273@qq.com.
Guangheng Luo, Email: 271561636@qq.com.
Peng Zhang, Email: 146060658@qq.com.
Yong Ban, Email: 155186194@qq.com.
Gang Shan, Email: 996097330@qq.com.
Changxiang Liu, Email: 2196777089@qq.com.
References
- 1.Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69(3):475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 2.Ganpule AP, Vijayakumar M, Malpani A, Desai MR. Percutaneous nephrolithotomy (PCNL) a critical review. Int J Surg. 2016;36(Pt D):660–664. doi: 10.1016/j.ijsu.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Ghani KR, Andonian S, Bultitude M, Desai M, Giusti G, Okhunov Z, Preminger GM, de la Rosette J. Percutaneous Nephrolithotomy: update, trends, and future directions. Eur Urol. 2016;70(2):382–396. doi: 10.1016/j.eururo.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Okhunov Z, Friedlander JI, George AK, Duty BD, Moreira DM, Srinivasan AK, Hillelsohn J, Smith AD, Okeke Z. S.T.O.N.E. nephrolithometry: novel surgical classification system for kidney calculi. Urology. 2013;81(6):1154–1159. doi: 10.1016/j.urology.2012.10.083. [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Averch TD, Shahrour K, Opondo D, Daels FP, Labate G, Turna B, de la Rosette JJ, Group CPS A nephrolithometric nomogram to predict treatment success of percutaneous nephrolithotomy. J Urol. 2013;190(1):149–156. doi: 10.1016/j.juro.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 6.Thomas K, Smith NC, Hegarty N, Glass JM. The Guy's stone score--grading the complexity of percutaneous nephrolithotomy procedures. Urology. 2011;78(2):277–281. doi: 10.1016/j.urology.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Vernez SLOZ, Motamedinia P, Bird V, Okeke Z, Smith A. Nephrolithometric scoring systems to predict outcomes of percutaneous Nephrolithotomy. Rev Urol. 2016;18(1):15–27. [PMC free article] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark HD, Wells GA, Huet C, McAlister FA, Salmi LR, Fergusson D, Laupacis A. Assessing the quality of randomized trials: reliability of the Jadad scale. Control Clin Trials. 1999;20(5):448–452. doi: 10.1016/s0197-2456(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 11.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 12.Bozkurt IH, Aydogdu O, Yonguc T, Yarimoglu S, Sen V, Gunlusoy B, Degirmenci T. Comparison of guy and clinical research Office of the Endourological Society Nephrolithometry Scoring Systems for predicting stone-free status and complication rates after percutaneous Nephrolithotomy: a single center study with 437 cases. J Endourol. 2015;29(9):1006–1010. doi: 10.1089/end.2015.0199. [DOI] [PubMed] [Google Scholar]
- 13.Choi SW, Bae WJ, Ha US, Hong SH, Lee JY, Kim SW, Cho HJ. Prognostic impact of stone-scoring systems after percutaneous Nephrolithotomy for staghorn calculi: a single Center's experience over 10 years. J Endourol. 2016;30(9):975–981. doi: 10.1089/end.2016.0188. [DOI] [PubMed] [Google Scholar]
- 14.Choi Sae Woong, Bae Woong Jin, Ha U.-Syn, Hong Sung-Hoo, Lee Ji Youl, Kim Sae Woong, Cho Hyuk Jin. Prediction of stone-free status and complication rates after tubeless percutaneous nephrolithotomy: a comparative and retrospective study using three stone-scoring systems and preoperative parameters. World Journal of Urology. 2016;35(3):449–457. doi: 10.1007/s00345-016-1891-6. [DOI] [PubMed] [Google Scholar]
- 15.Jaipuria J, Suryavanshi M, Sen TK. Comparative testing of reliability and audit utility of ordinal objective calculus complexity scores. Can we make an informed choice yet? BJU Int. 2016;118(6):958–968. doi: 10.1111/bju.13597. [DOI] [PubMed] [Google Scholar]
- 16.Kocaaslan Ramazan, Tepeler Abdulkadir, Buldu Ibrahim, Tosun Muhammed, Utangac Mehmet Mazhar, Karakan Tolga, Ozyuvali Ekrem, Hatipoglu Namik Kemal, Unsal Ali, Sarica Kemal. Do the urolithiasis scoring systems predict the success of percutaneous nephrolithotomy in cases with anatomical abnormalities? Urolithiasis. 2016;45(3):305–310. doi: 10.1007/s00240-016-0903-8. [DOI] [PubMed] [Google Scholar]
- 17.Labadie K, Okhunov Z, Akhavein A, Moreira DM, Moreno-Palacios J, Del Junco M, Okeke Z, Bird V, Smith AD, Landman J. Evaluation and comparison of urolithiasis scoring systems used in percutaneous kidney stone surgery. J Urol. 2015;193(1):154–159. doi: 10.1016/j.juro.2014.07.104. [DOI] [PubMed] [Google Scholar]
- 18.Noureldin YA, Elkoushy MA, Andonian S. Which is better? Guy's versus S.T.O.N.E. nephrolithometry scoring systems in predicting stone-free status post-percutaneous nephrolithotomy. World J Urol. 2015;33(11):1821–1825. doi: 10.1007/s00345-015-1508-5. [DOI] [PubMed] [Google Scholar]
- 19.Sfoungaristos S, Gofrit ON, Pode D, Landau EH, Duvdevani M. Percutaneous nephrolithotomy for staghorn stones: which nomogram can better predict postoperative outcomes? World J Urol. 2016;34(8):1163–1168. doi: 10.1007/s00345-015-1743-9. [DOI] [PubMed] [Google Scholar]
- 20.Tailly TO. Okhunov Z, Nadeau BR, Huynh MJ, Labadie K, Akhavein A, Violette PD, Olvera-Posada D, Alenezi H, Amann J, et al. Multicenter external validation and comparison of stone scoring Systems in Predicting Outcomes after Percutaneous Nephrolithotomy. J Endourol. 2016;30(5):594–601. doi: 10.1089/end.2015.0700. [DOI] [PubMed] [Google Scholar]
- 21.Yarimoglu Serkan, Polat Salih, Bozkurt Ibrahim Halil, Yonguc Tarık, Aydogdu Ozgu, Aydın Erhan, Degirmenci Tansu. Comparison of S.T.O.N.E and CROES nephrolithometry scoring systems for predicting stone-free status and complication rates after percutaneous nephrolithotomy: a single center study with 262 cases. Urolithiasis. 2016;45(5):489–494. doi: 10.1007/s00240-016-0935-0. [DOI] [PubMed] [Google Scholar]
- 22.Allen DOBT, Tiptaft R, Glass J. Defining the learning curve for percutaneous nephrolithotomy. J Endourol. 2005;19(3):279–282. doi: 10.1089/end.2005.19.279. [DOI] [PubMed] [Google Scholar]
- 23.de la Rosette JJ, Laguna MP, Rassweiler JJ, Conort P. Training in percutaneous nephrolithotomy--a critical review. Eur Urol. 2008;54(5):994–1001. doi: 10.1016/j.eururo.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 24.Autorino RQG, Sio MD, Lima E, Quarto E, Damiano R, Oliviero R, Osorio L, Marcelo F, D'Armiento M. Fate of abstracts presented at the world congress of Endourology: are they followed by publication in peer-reviewed journals? J Endourol. 2006;20(12):996–1001. doi: 10.1089/end.2006.20.996. [DOI] [PubMed] [Google Scholar]
- 25.Withington J, Armitage J, Finch W, Wiseman O, Glass J, Burgess N. Assessment of stone complexity for PCNL: a systematic review of the literature, how best can we record stone complexity in PCNL? J Endourol. 2016;30(1):13–23. doi: 10.1089/end.2015.0278. [DOI] [PubMed] [Google Scholar]
- 26.Akhavein A, Henriksen C, Syed J, Bird VG. Prediction of single procedure success rate using S.T.O.N.E. Nephrolithometry surgical classification system with strict criteria for surgical outcome. Urology. 2015;85(1):69–73. doi: 10.1016/j.urology.2014.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot and meta-analysis of demographic and clinical characteristics compared stone-free with not stone-free after PCNL. (TIF 3058 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.