Abstract
Endogenous IL-15 deficiency promotes lung fibrosis; therefore, we examined the effect of induced IL-15 in restricting the progression of lung fibrosis. Our objective in this work was to establish a novel therapeutic molecule for pulmonary fibrosis. Western blot, qPCR, and ELISA were performed on the lung tissues of IL-15–deficient mice, and recombinant IL-15 (rIL-15)–treated CC10–IL-13 and CC10–TGF-α mice, and allergen-challenged CC10–IL-15 mice were examined to establish the antifibrotic effect of IL-15 in lung fibrosis. We show that endogenous IL-15 deficiency induces baseline profibrotic cytokine and collagen accumulation in the lung, and pharmacological delivery of rIL-15 downregulates Aspergillus antigen–induced lung collagen, the profibrotic cytokines IL-13 and TGF-β1, and α-SMA+ and FSP1+ cells in mice. To confirm that overexpression of IL-15 diminishes pulmonary fibrosis, we generated CC10-rtTA-tetO7–IL-15 transgenic mice and challenged them with Aspergillus antigen. Aspergillus antigen–challenged, doxycycline (DOX)-treated CC10–IL-15 transgenic mice exhibited decreased collagen accumulation, profibrotic cytokine (IL-13 and TGF-β1) expression, and α-SMA+ and FSP1+ cells compared with IL-15–overexpressing mice not treated with DOX. Additionally, to establish that the antifibrotic effect of IL-15 is not limited to allergen-induced fibrosis, we showed that rIL-15 or IL-15 agonist treatment restricted pulmonary fibrosis even in CC10–IL-13 and CC10–TGF-α mice. Mechanistically, we show that T-helper cell type 17 suppressor IL-15–responsive RORγ+ T regulatory cells are induced in DOX-treated, allergen-challenged IL-15–overexpressing mice, which may be a novel pathway for restricting progression of pulmonary fibrosis. Taken together, our data establishes antifibrotic activity of IL-15 that might be a novel therapeutic molecule to combat the development of pulmonary fibrosis.
Keywords: allergen, fibrosis, IL-13, lung, TGF-β
Clinical Relevance
IL-15 gene deficiency promotes pulmonary fibrosis. Overexpression of IL-15 protects against allergen- or cytokine-induced pulmonary fibrosis. We report for the first time the significance of T-helper cell type 17 suppressor RORγ+ T regulatory cells in IL-15–mediated protection of pulmonary fibrosis.
In the past decade, an increase in immune-based diseases has been noted in both developed and undeveloped countries (1, 2). Several reports have indicated that T-helper cell type 2 (Th2) and Th3 cells, and their derived cytokines, play a role in promoting allergic diseases, including various pulmonary complications and fibrosis (1, 2). Earlier reports indicated that bronchial or interstitial fibrosis is the result of the immune cells and structural cells induced cytokines and growth factors, including transforming growth factor (TGF)-α/β and IL-13. Notably, the roles of both TGF-α/β and IL-13 have been implicated in the accumulation and propagation of fibroblasts and collagen, and the progression of pulmonary fibrosis (3, 4). Human and mouse lung fibroblasts have an exceptional characteristics that produce several mediators, including inhibitors of metalloproteinases (5). It is well established that the TGF family of molecules is a key driving force in fibroblast-to-myofibroblast differentiation. Allergen- or cytokine-induced lung fibrosis is defined by the overgrowth, hardening, and/or scarring of various tissues, and is attributed to excess deposition of extracellular matrix components, including collagen. The key cellular mediator of fibrosis is the myofibroblast, when activated serves as the primary collagen-producing cell. Lung fibrosis involves complex mechanisms, and clinical management using antiinflammatory therapy alone has limited effectiveness. Therefore, it is critical to explore new therapeutic strategies to restrict the progression of lung airway and interstitial fibrosis. Currently, several antiinflammatory medications are used to treat airway inflammation in patients, but these medications have no major effect on protecting against the progression of allergen- or cytokine-induced lung fibrosis (6). IL-15 is critical for the generation and maintenance of several innate naive and memory T-cell subsets, including natural killer cells (7–9). IL-15 also has a significant role in antiviral immunity and protecting against asthma and airway obstruction (10, 11). We previously reported a critical role of IL-15–responsive T regulatory (Treg) cells in protecting against airway inflammation (11). Furthermore, a report implicated IL-15 deficiency in exacerbating asthma in pediatric population (9). Interestingly, even induced IL-15 was observed after steroid treatment in human asthma (10). Therefore, in this study, we sought to establish the significance of IL-15 in restricting pulmonary fibrosis.
Our current study shows that IL-15 synchronizes pulmonary fibrosis induced by allergens or cytokines. IL-15 is an innate cytokine that is induced in several allergic and nonallergic diseases. For example, IL-15’s role in inhibiting eosinophil apoptosis involves activating the autocrine production of macrophage colony–stimulating factor (12). Herein, we demonstrate for the first time that IL-15 has antifibrotic properties that protect mice from developing lung fibrosis by downregulating proinflammatory and profibrotic cytokines. The current studies provide strong evidence that IL-15 restricts the development of fibrosis in allergen- and IL-13–induced airways, as well as the TGF-α–induced interstitial type of pulmonary fibrosis.
Methods
Mice
BALB/c mice were obtained from Taconic Farms, Inc., and housed under specific-pathogen-free conditions. Doxycycline (Dox)-inducible CC10–IL-13 bitransgenic mice were previously used at the Cincinnati Children’s Hospital and Medical Center (CCHMC) and brought to Tulane from Dr. Marc Rothernberg’s laboratory. CC10–TGF-α mice were obtained from Dr. William D. Hardie and used at the CCHMC. IL-15 gene-deficient mice were obtained from the laboratory of De Freed Finkelman at CCHMC. All of the mice were kept in a barrier faculty and used according to the protocols and guidelines of the Tulane University School of Medicine. The details and generation of CC10–IL-15 transgenic mice were previously reported (11).
Allergen-induced Experimental Asthma
A mouse model of allergic lung inflammation was established using methods described previously (11). In brief, mice were lightly anesthetized with isoflurane and then given 100 μg in 50 μl Aspergillus fumigatus extract (GEER Laboratories) or 50 μl normal saline alone via intranasal route using a micropipette. After instillation, the mice were held upright for a few minutes. Aspergillus extract or saline was administered three times a week for 3 weeks. All of the mice were killed 20–24 hours after the last intranasal challenge.
Fibroblast Analysis by Immunostaining
Rabbit anti-FSP1 antibody was used to immunostain tissue sections overnight at 4°C, followed by secondary biotinylated goat anti-rabbit horseradish peroxidase–conjugated antibody (Santa Cruz Biotechnology) for 1 hour at room temperature. The tissue sections were further developed with a nickel diaminobenzidine–cobalt chloride solution and counterstained with Nuclear Fast Red.
Cytokine Estimation in Tissue and Lung Lavage Fluid
Lung lavage fluid was centrifuged at 250 × g for 5 minutes at 4°C. Lung tissue was collected in protein buffer (Invitrogen) and homogenized. Cytokines were measured using the R&D ELISA duo set kits (R&D Systems, Inc.) for specific cytokines according to the protocol provided by the manufacturer.
Masson’s Trichrome Blue Collagen Staining
Tissue sections obtained from the different groups of mice were fixed with 4% formaldehyde and embedded in paraffin. Then, 5-μm sections were cut and placed on positively charged glass slides. Tissue sections were used to detect collagen using Masson’s trichrome staining kit (Poly Scientific R&D Corp.) according to the protocol provided by the manufacturer.
Western Blotting for Expression Analysis
Protein samples obtained from the different treatment groups were homogenized in lysis buffer, and 40 μg of protein was separated on 8–15% SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were then incubated with blocking buffer and treated with primary antibodies for collagen I, TGF-β, α-SMA, and GAPDH at 4°C overnight, followed by three to four washes, and incubated with horseradish peroxidase–conjugated secondary antibodies obtained from Santa Cruz Biotechnology. Blots were then developed using an enhanced chemiluminescence system provided by Thermo Scientific.
RT-PCR Analysis
RNA was isolated from the lung by the TRIZOL method (11). In brief, RNA samples (500 ng) were subjected to reverse transcription analysis using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s instructions. Collagen-I, collagen-III, α-SMA, TGF-β1 and GAPDH were quantified by real-time PCR using the CFX connect Real-Time System (Bio-Rad) and SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). Results were normalized with GAPDH amplified from the same cDNA mix and expressed as relative expression compared with controls. cDNA was amplified using the primers: mCollagen-I, F- 5′ TGTTCAGCTTTGTGGACCTC-3′; R-5′-GGTTTCCACGT CTCACCATT-3′; mCollagen-III, F-5′-CAGGATCTGTCCTTTG CGAT-3′; R-5-CCCACTCCAGACTTGACATC-3′; mSMA, F-5′-CCTGGAGAAGAGCTACGA A C-3′; R-5- CCCCTGACAGGACGTTGTTA-3′; mTGF-β1, F-5′-GCAACAA TTCCTGGCGTTAC-3′; R-5- GCTGAATCGAAA GCCCTGTA-3′; mGAPDH, F-5′-ACCCAG AAGACTGTGGATGG-3′; R-5-CACATTGGGGGTAGGAACAC-3.
Flow-Cytometric Analysis of RORγ+CD4+CD25+Foxp3+ Treg Cells
Isolated mediastinal lymph node cells of DOX- and no-DOX–treated CC10–IL-15 asthmatic mice were stained for cell-surface markers with anti-CD45-FITC, anti-CD4-PerCP, anti-RORγ-PE, and anti-CD25-PE-Cy7, followed by intracellular Foxp3 staining (eBioscience) using an anti-Foxp3-APC antibody. The samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences) (11).
Statistical Analysis
Statistical analysis was performed with the use of GraphPad Prism software (version 5). A nonparametric t test or one-way ANOVA followed by a Newman-Keuls post hoc test was used as indicated. A P value of <0.05 was considered statistically significant.
Results
Endogenous IL-15 Deficiency Results in TGF-β1 Induction and Collagen Accumulation in the Murine Lung
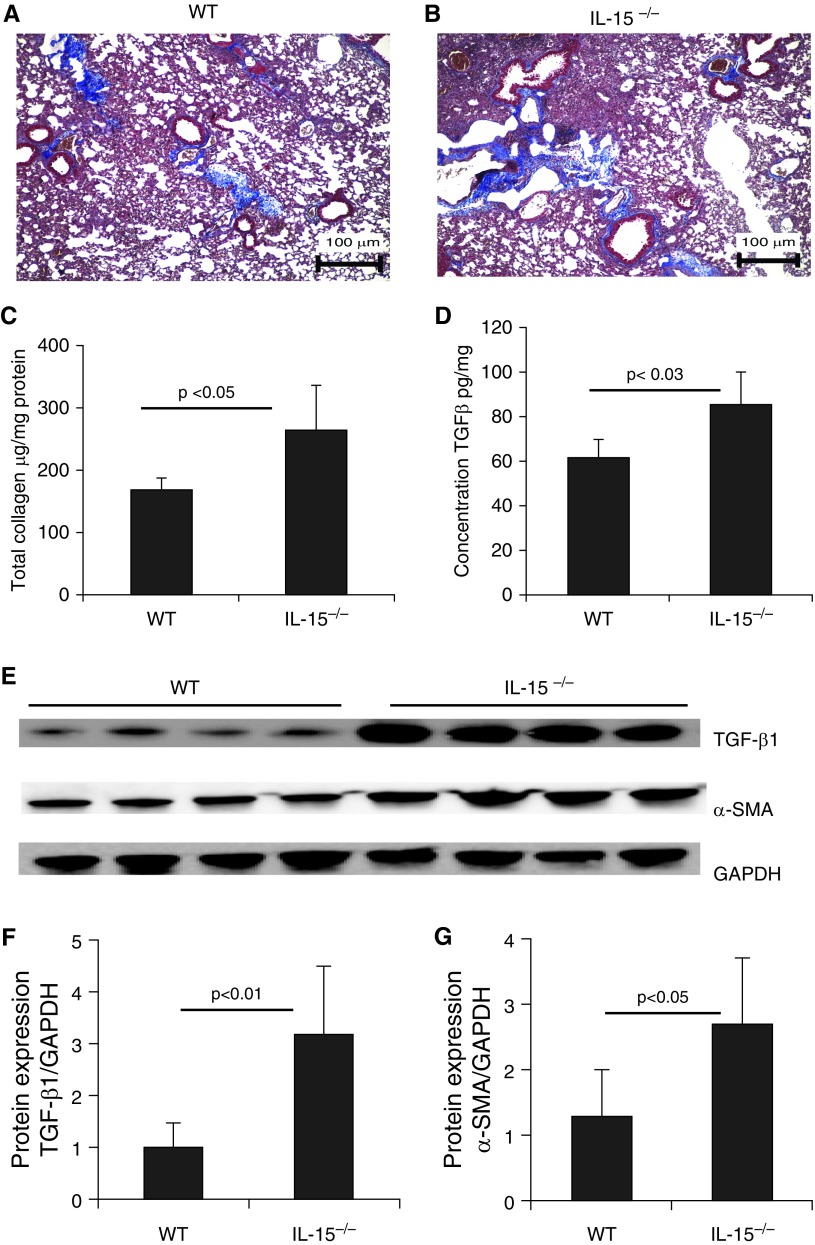
A previous study examined the relationship between serum IL-15 levels and lung fibrosis in systemic sclerosis, and the results suggested that IL-15 may possess antifibrotic properties (13). Therefore, we examined and compared baseline TGF-β1 levels and collagen accumulation in endogenous IL-15–deficient mice and wild-type (WT) mice. Our analysis showed that baseline collagen accumulation was increased in the lung tissue of endogenous IL-15–deficient mice compared with the WT mice (Figures 1A and 1B). We also observed increased levels of total collagen and TGF-β1 in the lungs of endogenous IL-15–deficient mice compared with the WT mice (Figures 1C and 1D). Furthermore, a Western blot analysis validated the induction of the profibrotic cytokines TGF-β1 and α-SMA in endogenous IL-15–deficient mice compared with WT mice (Figure 1E). GAPDH-normalized densitometry of TGF-β1 and α-SMA analyses confirmed that lL-15 regulated both of the examined profibrotic cytokines (Figures 1F and 1G), which encouraged us to examine its role as a novel molecule in restricting the progression of lung fibrosis.
Figure 1.
Analysis of baseline tissue remodeling in endogenous IL-15 gene–deficient (IL-15−/−) mice. (A and B) Baseline collagen deposition was examined using Masson’s trichrome staining of lung tissue sections from 20-weeks-old naive wild-type (WT) and (C and D) IL-15−/− mice. (C) Total lung collagen was assessed using Sircol reagent, and (D) transforming growth factor β1 (TGF-β1) levels were assessed by ELISA. (E–G) Western blot analysis showed enhanced expression of TGF-β1 and α-SMA in IL-15−/− mice (E), which was confirmed by performing GAPDH-normalized densitometry (F and G). Data are expressed as mean ± SD, n = 8 mice/group in each group, except for the Western blot analysis. Scale bars: 100 μm. SMA = smooth muscle actin.
Pharmacological Delivery of Recombinant IL-15 Improves Aspergillus fumigatus Extract–induced Bronchial Fibrosis in Mice
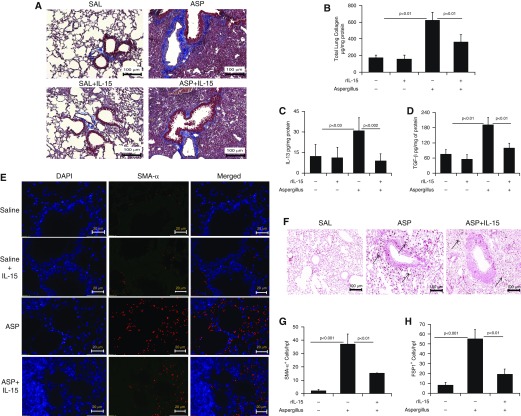
Recently, we reported the importance of IL-15 in Aspergillus extract–induced lung airway disorder. Airway obstruction is well associated with bronchial fibrosis in chronic obstructive pulmonary disease (14, 15). Therefore, we investigated the role of IL-15 in the development of Aspergillus-induced bronchial fibrosis using an experimental mouse model of asthma. First, we delivered intranasal Aspergillus antigen according to a previously established protocol to induce experimental asthma in mice (11). Second, we determined the role of IL-15 pharmacologically delivered into the lungs of allergen-challenged mice. We observed that intranasal Aspergillus challenge significantly increased collagen accumulation compared with saline control in the mouse lungs. Third, we evaluated whether recombinant IL-15 (rIL-15) delivery affected perivascular and peribronchial collagen accumulation in the allergen-challenged mice. We found that rIL-15 downregulated accumulation of lung collagen in IL-15–pretreated, Aspergillus-challenged mice compared with saline-treated, Aspergillus-challenged mice (Figure 2A). Total collagen in saline-, Aspergillus-, and rIL-15–treated Aspergillus-challenged mice was analyzed using Sircol reagent, which validated the Masson’s trichrome histological collagen analysis (Figure 2B). Fourth, we showed downregulation of Aspergillus extract–induced levels of IL-13, TGF-β1, collagen I, and collagen III transcripts (Figure E1 in the data supplement), and protein levels of IL-13 and TGF-β1 (Figures 2C–2D), including α-SMA+ and FSP1+ cells (fibroblasts and myofibroblasts) (Figures 2E–2H) after rIL-15 treatment compared with saline-treated, Aspergillus-challenged mice. Mechanistically, the reduction of FSP1+ and SMA+ cells indicates that IL-15 may be a novel molecule to restrict fibroblast proliferation and TGF-β1–induced transformation of fibroblasts into myofibroblasts.
Figure 2.
Consequences of rIL-15 pretreatment in Aspergillus-induced bronchial fibrosis in mice. (A) Representative light-microscopy photomicrographs of lung sections with Masson’s trichrome staining show perivascular and peribronchiolar collagen accumulation after 3 weeks of saline or Aspergillus challenge in mice treated with and without rIL-15. (B) Total collagen expression in saline- and rIL-15–treated, Aspergillus-challenged mice. ELISA results for profibrotic IL-13 and TGF-β1 expression in saline- and rIL-15–treated, Aspergillus-challenged mice are shown. (C and D) Immunofluorescence staining revealed α-SMA+ cells in Aspergillus- and rIL-15–treated, Aspergillus-challenged mice. (E) Very few eosinophils are seen in the saline-treated (for 3 wk) mice. (F) Arrows indicated FSP1+ cell expression in Aspergillus and rIL-15–treated Aspergillus-challenged mice. Quantitation of α-SMA+ and FSP1+ cells is shown in saline-, (G and H) Aspergillus-, and rIL-15–treated Aspergillus-challenged mice. Data are expressed as mean ± SD, n = 12 mice/group. Scale bars: 100 μm and 20 μm. ASP = Aspergillus; FSP1 = fibroblast-specific protein 1; rIL-15 = recombinant IL-15; SAL = saline.
DOX-Inducible, IL-15–Overexpressing Mice Are Protected from Allergen-induced Bronchial Fibrosis
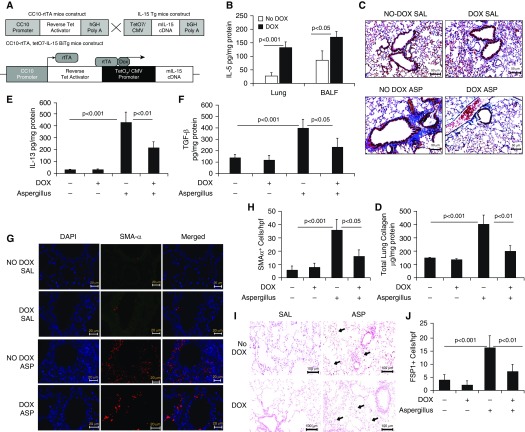
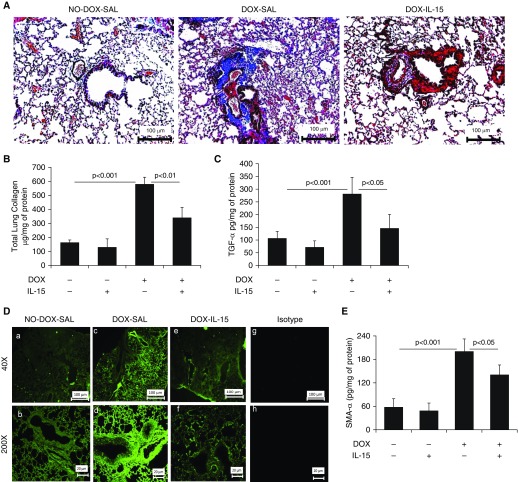
Next, to show that overexpression of IL-15 protects against Aspergillus extract–induced bronchial fibrosis in mice, DOX-inducible CC10–IL15 mice were generated as described recently (11) and the construct shown (Figure 3A). After 3 weeks of DOX exposure, CC10–IL-15 mice showed highly induced IL-15 protein levels in lung homogenates and BAL fluid (Figure 3B) compared with the no-DOX mice. Furthermore, we induced experimental asthma in DOX- and no-DOX–treated CC10–IL-15 mice to establish that IL-15 overexpression indeed protects against allergen-induced bronchial fibrosis. Histological analysis showed some baseline airway collagen in both the no-DOX and DOX-treated saline-challenged mice (Figure 3C); however, the no-DOX mice challenged with Aspergillus antigen showed a greater accumulation of perivascular and peribronchiolar collagen than the Aspergillus-challenged, DOX-treated mice (Figure 3C). A quantitative Sircol reagent assay further confirmed significantly reduced total lung collagen in Aspergillus-induced, DOX-treated mice compared with no-DOX mice (Figure 3D). Significantly decreased collagen I, TGF-β1, and α-SMA transcript levels (Figures E2A–E2C), and IL-13 and TGF-β1 protein levels were observed in Aspergillus-induced, DOX-treated mice compared with no-DOX mice (Figures 3E–3F). Mechanistically, we showed that Aspergillus-challenged, DOX-exposed IL-15–overexpressing mice exhibited reduced peribronchial and perivascular recruitment of α-SMA+ lung myofibroblasts (Figures 3G and 3H) and FSP1+ lung fibroblasts (Figures 3I and 3J) compared with the no-DOX, allergen-challenged mice. DOX-exposed BALB/c mice did not develop any lung remodeling characteristics and showed normal baseline collagen accumulation in lung tissue sections (data not shown).
Figure 3.
Generation of doxycycline (DOX)-regulated IL-15 transgenic mice, and analysis of tissue collagen and profibrotic cytokines in saline- and Aspergillus-exposed CC10–IL-15 bitransgenic mice. (A) Construct of (tetO) 7 CMV-IL15 transgenic mice and rtTA-CC10 transgenic mice to generate rtTA-CC10–IL-15 bitransgenic mice. (B) Induction of IL-15 levels in the lung and BAL fluid (BALF) after 3 weeks of DOX exposure to rtTA-CC10–IL-15 bitransgenic mice. (C) A representative photomicrograph of Masson’s trichrome-stained saline- and Aspergillus-challenged DOX- and no-DOX–exposed CC10–IL-15 bitransgenic mice. (D) Total lung collagen, and the profibrotic cytokines (IL-13 and TGF-β1) in saline and Aspergillus-challenged no-DOX and DOX exposed IL-15 transgenic mice are shown (E and F). Immunofluorescence staining revealed α-SMA+ cells in saline- and Aspergillus-challenged no-DOX– and DOX-exposed IL-15 transgenic mice. (G) Very few α-SMA+ cells are seen in the saline-treated (for 3 wk) mice. (H) Quantitation of α-SMA+ cells in saline- and Aspergillus-challenged IL-15 transgenic mice. (I) Arrows indicated FSP1+ cells detected in Aspergillus-challenged no-Dox– and Dox-exposed IL-15 transgenic mice. (J) Quantitation of FSP1+ cells in saline- and Aspergillus-challenged Dox- and no-Dox–exposed transgenic mice. Data are expressed as mean ± SD, n = 12 mice/group. Scale bars: 20 μm, 50 μm, and 100 μm. bGH = bovine growth hormone; CC10 = CC10-Clara cell 10 kD protein; CMV = cytomegalovirus (promoter); hGH = human growth hormone; rtTA = express a tet repressor-VP16 transcriptional activator fusion gene; TetO7 = tetracycline; Tg = transgenic.
IL-15 Treatment Attenuates IL-13 Overexpression–induced Lung Fibrosis
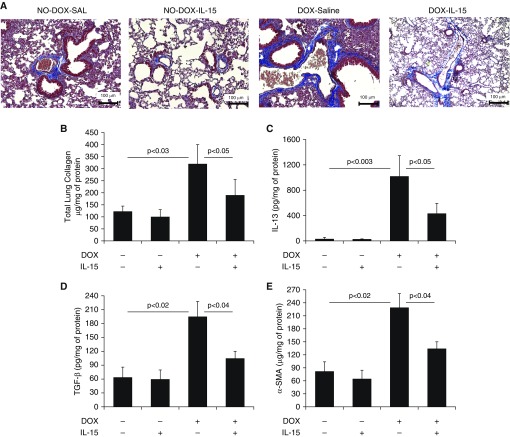
Overexpression of IL-13 was previously reported to induce a TGF-β1–associated rapidly progressing form of bronchial and interstitial lung fibrosis (16, 17). Accordingly, we examined the profibrotic characteristics of IL-15 in the IL-13–induced mouse model of pulmonary fibrosis by delivering intratracheal rIL-15 directly to the lungs in DOX-inducible CC10–IL-13 bitransgenic mice. Within 3 weeks, the DOX-treated CC10–IL-13 bitransgenic mice developed significantly greater lung collagen accumulation compared with the no-DOX CC10–IL-13 bitransgenic mice (Figure 4A). Pharmacological delivery of rIL-15 significantly downregulated collagen accumulation in the lungs of DOX-treated CC10–IL-13 transgenic mice, to a level comparable to that observed in the saline-only DOX-treated CC10–IL-13 mice (Figure 4A). A quantitative Sircol reagent assay of total lung collagen confirmed that rIL-15 treatment significantly reduced the lung collagen in DOX-fed mice (Figure 4B). Lung IL-13 levels were higher in the DOX-fed transgenic mice than in the no-DOX mice. Of note, transgene-induced levels of IL-13 were significantly reduced by rIL-15 delivery to the CC10–IL-13 mice (Figure 4C). Furthermore, a significant increase of collagen I, collagen III, TGF-β1 and α-SMA transcripts (Figures E3A–E3D) and in the protein levels of TGF-β1, α-SMA in the lungs of DOX-fed CC10–IL-13 mice compared with no-DOX mice, which were reduced after rIL-15 treatment in DOX-exposed CC10–IL-13 mice (Figures 4D and 4E).
Figure 4.
Pharmacological delivery of IL-15 significantly reduces IL-13–induced collagen accumulation and profibrotic cytokines in the lungs of CC10–IL-13 transgenic mice. (A) Light-microscopy photomicrographs of Masson’s trichome–stained lung sections from saline- or IL-15–treated, no-DOX– or DOX-exposed CC10–IL-13 bitransgenic mice. (B–E) Total lung collagen (B) and the profibrotic cytokines IL-13 (C), TGF-β1 (D), and α-SMA (E) in saline- and IL-15–treated no-DOX– and DOX-exposed IL-13 bitransgenic mice are shown. Data are expressed as mean ± SD, n = 12 mice/group. Scale bars: 100 μm.
TGF-α–induced Pulmonary Fibrosis Is Diminished by rIL-15 Treatment
TGF-α–overexpressing mice develop pulmonary fibrosis that mimics human idiopathic pulmonary fibrosis (IPF) (18–20). Accordingly, we sought to determine whether IL-15 would reduce pulmonary fibrosis in TGF-α–overexpressing mice. To that end, we delivered 10 μg rIL-15 via the intratracheal route, two times a week for 3 weeks, to CC10–TGF-α bitransgenic mice that were fed DOX for 3 weeks. Control no-DOX– and DOX-fed mice were treated only with intratracheal saline. Saline-treated CC10–TGF-α bitransgenic mice that were fed DOX for 3 weeks showed significantly greater lung collagen accumulation compared with the baseline collagen in saline-treated, no-DOX-fed CC10–TGF-α bitransgenic mice. All of the murine lungs were examined histologically for collagen accumulation (Figure 5A). Pharmacological delivery of rIL-15 significantly diminished collagen accumulation in the lungs of DOX-fed CC10–TGF-α transgenic mice compared with the saline-treated, DOX-fed CC10–TGF-α bitransgenic mice (Figure 5A). Similarly, rIL-15–treated CC10–TGF-α bitransgenic mice showed significantly reduced total lung collagen compared with saline-treated, DOX-exposed mice examined by Sircol reagent assay (Figure 5B). The levels of TGF-α in the lungs of transgenic mice fed DOX for 3 weeks were highly induced compared with those in the no-DOX mice. It is interesting to note that even transgene-induced TGF-α levels were significantly reduced in response to rIL-15 delivery to the CC10–TGF-α transgenic mice (Figure 5C). We further confirmed this TGF-α downregulation by performing an immunofluorescence analysis in the lungs of rIL-15– and DOX-treated CC10–TGF-α bitransgenic mice, which showed reduced expression of TGF-α compared with saline-treated CC10–TGF-α bitransgenic mice (Figure 5D). α-SMA a marker for myofibroblasts, were also significantly reduced in rIL-15– and DOX-treated CC10–TGF-α mice compared with saline- and DOX-treated CC10–TGF-α mice (Figure 5E).
Figure 5.
Pharmacological delivery of IL-15 significantly reduces TGF-α–induced collagen accumulation and profibrotic cytokines in the lungs of CC10–TGF-α transgenic mice. (A) Light-microscopy photomicrographs of Masson’s trichome–stained lung sections of saline- or IL-15–treated, no-DOX– or DOX-exposed CC10–TGF-α bitransgenic mice are shown. (B–D) Total lung collagen (B) and the profibrotic cytokine TGF-β1 (C), α-SMA (D), and the expression of lung TGF-α (E) in saline- or IL-15–treated, no-DOX– or DOX-exposed TGF-α bitransgenic mice are shown. Data are expressed as mean ± SD, n = 4 mice/group. Scale bars: 100 μm and 20 μm.
Induced RORγ+ Treg Cells in Allergen-challenged, DOX-Fed CC10–IL-15 Bitransgenic Mice
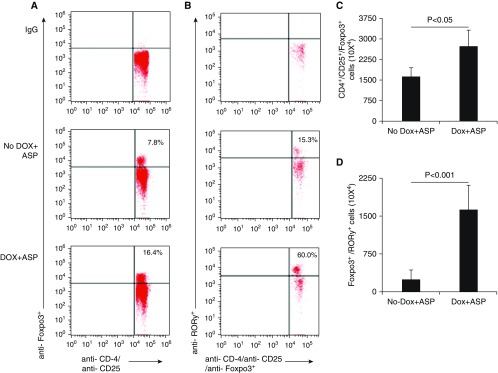
Antiinflammatory Th17 cell suppressor RORγ+Foxp3+-expressing Treg cells have been reported in healthy individuals as well as in several inflammatory diseases, including ulcerative colitis (21), periodontitis (22), and arthritis (21). However, the role of these Treg cells is not understood with respect to pulmonary inflammation and fibrosis. We previously reported IL-15–induced Treg cells in an asthma mouse model, but did not examine the specific population of Treg cells in the lungs of these mice. Here, we sought to determine whether these Treg cells are RORγ+Foxp3+ Treg subsets. Therefore, we examined mediastinal lymph nodes of Aspergillus extract–challenged DOX- and no-DOX-fed CC10–IL-15 mice for RORγ+Foxp3+ Treg cells. We examined isolated mediastinal lymph node single-cell suspensions by performing flow-cytometry analyses for the expression of RORγ in Foxp3+ Treg cells of Aspergillus extract–challenged, 3-week DOX- and no-DOX–fed mice. The mediastinal lymph node cells were incubated with a combination of anti-CD4, anti-CD25, and anti-RORγ for 45 min on ice, followed by intracellular anti-Foxp3 staining according to the manufacturer’s protocol (eBioscience). Our analysis showed that a population of CD4+CD25+Foxp3+ Treg cells was induced in the mediastinal lymph nodes of DOX-fed, Aspergillus extract–challenged CC10–IL-15 mice compared with no-DOX-fed, Aspergillus extract–challenged CC10–IL-15 mice (Figure 6A). Notably, the DOX-fed, Aspergillus extract–challenged CC10–IL-15 mice displayed significantly higher numbers of RORγ+CD4+CD25+Foxp3+–specific Treg cells than the no-DOX-fed, Aspergillus-challenged mice (Figure 6B). The absolute numbers of CD4+CD25+Foxp3+ Treg cells and RORγ+CD4+CD25+Foxp3+ Treg cells in DOX- and no-DOX-fed Aspergillus extract–challenged CC10–IL-15 mice are shown in Figures 6C and 6D. The data indicate that the RORγ+CD4+CD25+Foxp3+-specific Treg cell lineage may be critical for protecting against allergen- or proinflammatory cytokine-induced pulmonary fibrosis in mice.
Figure 6.
Analysis of T regulatory (Treg) cells in DOX-regulated CC10–IL-15 transgenic mice after Aspergillus challenge. (A and B) Treg cells in the mediastinal lymph nodes of Aspergillus-challenged DOX- and non-DOX–exposed CC10–IL-15 overexpressing mice were examined by flow cytometry using anti-CD45-FITC, anti-CD4-PerCP, anti-RORγ-PE, anti-CD25-PE-Cy7, and anti-Foxp3-APC along with matched IgG antibodies. (C) The average percentage of CD4+CD25+Foxp3+ and (D) RORγ+CD4+CD25+Foxp3+ Treg cells, and their absolute number of cells from three independent experiments in mediastinal lymph nodes of mice. Data are expressed as mean ± SD.
The IL-15 Agonist ALT-803 May Be a Therapeutic Drug to Restrict Pulmonary Fibrosis in Humans
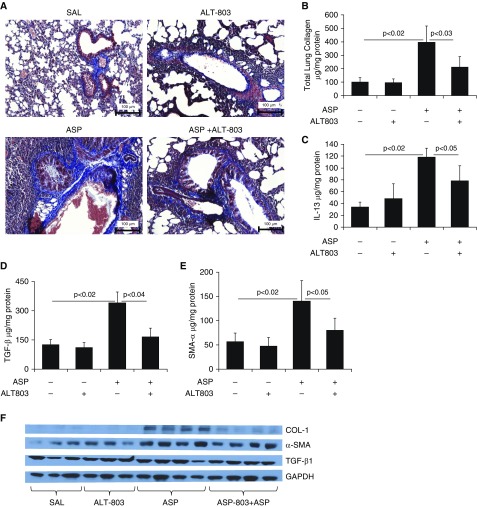
Finally, to show that our findings may be clinically relevant for human studies, we tested the human IL-15 agonist ALT-803 (Altor BioScience Corp.) as a novel therapeutic agent in our allergen-induced bronchial fibrosis model. We administered 5 μg of human ALT-803 on alternate days for 3 weeks to an Aspergillus extract–challenged established asthma mouse model. Our data show that ALT-803 pretreatment in Aspergillus extract–challenged mice protected the lungs from the induction of perivascular and peribronchial collagen accumulation compared with saline-treated, Aspergillus extract–challenged mice (Figure 7A). We further confirmed this by examining the total collagen in the lungs using the Sircol reagent method (Figure 7B). Significantly reduced mRNA levels of collagen I, collagen III, TGF-β1, and α-SMA (Figures E4A–E4D), and protein levels of IL-13, TGF-β1, and α-SMA were observed in ALT-803–treated, Aspergillus extract–challenged mice compared with saline-treated, Aspergillus extract–challenged mice (Figures 7C–7E) by RT-PCR, ELISA, or Western blot analyses for collagen I, TGF-β1, and α-SMA (Figure 7F). GAPDH-normalized densitometry of collagen I, TGF-β1, and α-SMA confirmed that ALT-803 treatment significantly downregulated collagen and profibrotic cytokines (Figure 7G).
Figure 7.
Reduction in lung collagen and the levels of profibrotic cytokines in response to pretreatment with the human IL-15 agonist ALT-803 in the lungs of Aspergillus-challenged mice. The potential of a human IL-15 agonist as a therapeutic agent was examined using an allergen-induced lung fibrosis model. (A) A highly significant reduction in collagen accumulation is shown in response to ALT-803 treatment (regimen of 5 μg on alternate days for 3 wk) in Aspergillus-challenged mice compared with saline-treated, Aspergillus-challenged mice. (B–E) Total lung collagen (B) and the profibrotic cytokines IL-13 (C), TGF-β1 (D), and α-SMA (E) in saline- and ALT-803–treated saline or Aspergillus-challenged mice are shown. (F and G) Western blot analysis showed that ALT-803 treatment downregulated Aspergillus-induced levels of profibrotic cytokines (collagen I, TGF-β1, and α-SMA) in mice (F), and this was further confirmed by GAPDH-normalized densitometry of collagen I, TGF-β1, and α-SMA (G). Data are expressed as mean ± SD, n = 12 mice/group in each group, except for the Western blot analysis. Scale bars: 100 μm.
Discussion
Fibroblasts are the initial source of matrix proteins that are deposited in tissue during the development of fibrosis (23). Matrix protein deposition is reported in almost all inflammatory diseases, but affects lung function more severely (24, 25). Lung inflammation is induced by the accumulation of several types of inflammatory cells, and the induction of proinflammatory and profibrotic cytokines and chemokines. Asthma is an allergen-induced lung inflammation related to tissue remodeling, fibrosis, and airway functional abnormalities. Herein, we report that IL-15 gene deficiency in mice promotes proinflammatory and profibrotic cytokines, and results in excess lung collagen deposition. Additionally, we show the significance of IL-15 in regulating proinflammatory and profibrotic cytokines, namely, IL-13, TGF-β1, and α-SMA, in an allergen-mediated experimental model of asthma. IL-15 is an innate cytokine with antiviral properties, and several experimental and clinical reports have linked IL-15 downregulation to pulmonary dysfunction (25). We used multiple strategies and murine models to test the hypothesis that IL-15 is critical for the induction and protection of allergen- or cytokine-induced pulmonary fibrosis. Accordingly, to establish that IL-15 overexpression indeed protects against pulmonary fibrosis, we used allergen-induced and profibrotic cytokine-induced mouse models of fibrosis that mimic the human disease. Our experimental data showed that both pharmacological lung delivery of rIL-15 and overexpression of IL-15 by transgene insertion in mice downregulated the levels of lung IL-13, TGF-β1, and α-SMA in the murine models. These cytokines have been proven to play a critical role in promoting pulmonary fibrosis.
IL-13 is a profibrotic cytokine that extensively promotes tissue inflammation, remodeling, and fibrosis in several inflammatory diseases (26, 27), and promotes functional abnormalities in the lungs during asthma (28). IL-13 is elevated in several types of lung inflammation and in patients with asthma (29). It is critical to demonstrate that IL-13 is required to induce TGF-β1 signaling that promotes tissue fibrosis (28). Therefore, inhibiting IL-13 may provide a way to inhibit the development of TGF-β1–mediated allergen- or cytokine-induced tissue fibrosis. In addition, IL-13–mediated inflammatory responses demonstrated the induction of several inflammatory cells and related cytokines and chemokines. Therefore, we employed several strategies to examine whether IL-15 regulation is important for the improvement of allergen- or cytokine-induced pulmonary fibrosis in murine models. In the current study, we showed that endogenous IL-15 deficiency induced proinflammatory and profibrotic cytokines, and accumulation of lung collagen. It has been shown that IL-15 is involved in antiviral immunity, and several experimental and clinical reports have indicated that IL-15 regulates pulmonary dysfunction (25). Furthermore, to determine whether IL-15 protects the progression of pulmonary fibrosis, we used different cytokine-induced, gene-manipulated murine models of lung fibrosis that represent fibrotic lung diseases in patients. Our analysis showed that pharmacological transgene insertion of IL-15 into the lung downregulated the profibrotic cytokines IL-13, TGF-β, and α-SMA after allergen exposure to mice that are prone to develop pulmonary fibrosis (30, 31). It is well established that TGF-α/β is a major cytokine that mediates epithelial, mesenchymal, and immune cell transformation to induce tissue remodeling and fibrosis (32, 33).
Our earlier reports suggested that IL-15 interacts with lung macrophages and may regulate TGF-β1 production during chronic inflammation. We show that IL-15 protects against airway remodeling, using allergen- and various cytokine-induced mouse models of lung inflammation (34). Our data demonstrate that IL-15 significantly downregulates IL-13 production and inhibits TGF-β1 expression and subsequent fibrosis. Importantly, IL-13 is required for the growth and development of fibroblasts (35). Our data clearly show that IL-15 significantly limits lung fibrosis by restricting the induction of IL-13 and TGF-β1, and reduces the accumulation of FSP1+ and SMA+ cells. This is in accordance with our data demonstrating that administration of rIL-15 via the intratracheal route reduces fibrosis in CC10–TGF-α transgenic mice. Fibroblasts and myofibroblasts are key mediators in altering the lung immune system; however, the underlying mechanisms during this process are largely unknown (36–38). IL-13 and TGF-β1 downregulated fibroblast metaloprotease-1 expression, which is also involved in the clearance of fibrosis (39, 40). Our data also indicate that IL-15 overexpression by pharmacological delivery of rIL-15 or by transgene insertion reduces the proliferation and transformation of fibroblasts into myofibroblasts.
IL-15 is a pleiotropic molecule that may exert its effects to reduce the excessive development of tissue remodeling and fibrosis by more than one mechanism (41). Recently, we reported that IL-15 induces IFN-γ– and IL-10–producing regulatory CD4+CD25+Foxp3+ T cells (11), which is critical for improving lung inflammation and functional abnormalities. Herein, we mechanistically show that the IL-15–mediated protection in lung fibrosis may related to the induction of high numbers of Th17 suppressor RORγ+CD4+CD25+Foxp3+ Treg cells. For the first time, we report that DOX-regulated CC10–IL-15 increases the numbers of RORγ+CD4+CD25+Foxp3+ Treg cells that produce the antiinflammatory cytokine IL-10 in the lungs of mice with asthma. Our observation is supported by previous reports that IL-10–producing Treg cells protect Th2 cells and cytokine-mediated lung inflammation (42). Therefore, the increase in the number of RORγ+CD4+CD25+Foxp3+ Treg cells by IL-15 overexpression may regulate the mechanism that improves allergen- or cytokine-mediated pulmonary inflammation and fibrosis. Reduced levels of IL-10 and Treg cells (CD4+CD25+Foxp3+) have been observed in the blood and lungs of individuals with IPF (43). Additionally, loss of FoxP3 has been implicated in the depletion or deregulation of T-cell responses that play a role in exacerbating fibrosis in the mammalian lung (44, 45). These reports provide additional mechanistic support for our finding that IL-15 protects against lung fibrogenesis. We provide clinically relevant supportive data demonstrating the effectiveness of an IL-15 agonist (ALT-803) in diminishing allergen-induced IL-13–mediated airway fibrosis. These investigations highlight the importance of IL-15 signaling and provide a strong rationale for developing an IL-15 treatment strategy for human lung fibrosis. IL-15 agonists are being investigated in several clinical trials for their antitumor effects in humans. ALT-803 is an IL-15 superagonist and was previously reported to have stronger in vivo biologic activity than rIL-15.
This study has several main findings. First, we show that endogenous IL-15–deficient mice develop induced accumulation of lung collagen and profibrotic cytokines. Second, we provide evidence that rIL-15 treatment improves Aspergillus extract–induced IL-13 and TGF-β1–mediated fibrosis. Third, we show that CC10–IL-15 transgenic mouse lungs are protected from allergen-induced lung fibrosis via downregulating profibrotic cytokines. Furthermore, we demonstrate that IL-13– and TGF-α–induced interstitial lung fibrosis is abrogated by pharmacological delivery of rIL-15. A previous work reported reduced levels of IL-10 and Treg cells in the blood and lungs of patients with IPF (43), and our data show that IL-15 induced Th17 suppressor RORγ+CD4+CD25+Foxp3+ Treg cells. These RORγ+CD4+CD25+Foxp3+ Treg cells may be mechanistic pathways in promoting IL-15 overexpression–mediated improvement in pulmonary fibrosis. Taken together, these current and earlier findings reveal a detailed mechanism that is operational in IL-15–mediated protection from lung fibrosis, and provide a strong basis for using IL-15 agonists as a treatment strategy for patients suffering from lung fibrosis.
Conclusion
We report that IL-15 protects against airway remodeling and interstitial fibrosis in allergen challenge– and cytokine-induced models. IL-15 significantly reduces IL-13 and TGF-β1 profibrotic cytokine expression and significantly inhibits the accumulation of fibroblasts and their transformation into myofibroblasts. We contend that IL-15 is an antifibrotic molecule and should be considered in future treatment strategies to restrict the development of progressive lung fibrosis.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Drs. William D. Hardie, M.D., and Freed Finkelman, M.D. (CCHMC) for providing TGF-α transgenic and IL-15 gene-deficient mice, respectively.
Footnotes
Supported by National Institutes of Health grant R01 AI080581 (A.M.) and the Bridge Funding Support program. A.M. holds an Endowed Schlieder Chair, supported by the Edward G. Schlieder Educational Foundation.
Author Contributions: S.U.V. and R.N.: performed most experiments related to allergen- and cytokine-induced lung fibrosis in mouse models. M.M. and A.K.V.: performed most of the PCR, ELISA, and statistical data analyses, and helped prepare the figures. H.K.K.: mouse maintenance and genotyping. J.A.L.: critical review of the manuscript; A.M.: study concept and design, analysis interpretation, study supervision, and funding.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0254OC on January 31, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogan SP, Matthaei KI, Young JM, Koskinen A, Young IG, Foster PS. A novel T cell-regulated mechanism modulating allergen-induced airways hyperreactivity in BALB/c mice independently of IL-4 and IL-5. J Immunol. 1998;161:1501–1509. [PubMed] [Google Scholar]
- 2.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 3.Wilson MS, Wynn TA. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185:660–669. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quesnel C, Nardelli L, Piednoir P, Leçon V, Marchal-Somme J, Lasocki S, et al. Alveolar fibroblasts in acute lung injury: biological behaviour and clinical relevance. Eur Respir J. 2010;35:1312–1321. doi: 10.1183/09031936.00074709. [DOI] [PubMed] [Google Scholar]
- 6.Royce SG, Cheng V, Samuel CS, Tang ML. The regulation of fibrosis in airway remodeling in asthma. Mol Cell Endocrinol. 2012;351:167–175. doi: 10.1016/j.mce.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 8.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy MK, Park LS. Characterization of interleukin-15 (IL-15) and the IL-15 receptor complex. J Clin Immunol. 1996;16:134–143. doi: 10.1007/BF01540911. [DOI] [PubMed] [Google Scholar]
- 10.Komai-Koma M, McKay A, Thomson L, McSharry C, Chalmers GW, Liew FY, et al. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin Exp Allergy. 2001;31:1441–1448. doi: 10.1046/j.1365-2222.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 11.Venkateshaiah SU, Zhu X, Rajavelu P, Niranjan R, Manohar M, Verma AK, et al. Regulatory effects of interleukin (IL)-15 on allergen-induced airway obstruction. J Allergy Clin Immunol. 2018;141:906–917. doi: 10.1016/j.jaci.2017.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoontrakoon R, Chu HW, Gardai SJ, Wenzel SE, McDonald P, Fadok VA, et al. Interleukin-15 inhibits spontaneous apoptosis in human eosinophils via autocrine production of granulocyte macrophage-colony stimulating factor and nuclear factor-κB activation. Am J Respir Cell Mol Biol. 2002;26:404–412. doi: 10.1165/ajrcmb.26.4.4517. [DOI] [PubMed] [Google Scholar]
- 13.Wuttge DM, Wildt M, Scheja A, Westergren-Thorsson G. Interleukin-15 attenuates transforming growth factor-β1-induced myofibroblast differentiation in human fetal lung fibroblasts. Eur Cytokine Netw. 2010;21:165–176. doi: 10.1684/ecn.2010.0202. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RJ, Djukanovic R, Tashkin DP, Millar AB, du Bois RM, Orr PA. The role of small airways in lung disease. Respir Med. 2002;96:67–80. doi: 10.1053/rmed.2001.1216. [DOI] [PubMed] [Google Scholar]
- 15.Niewoehner DE, Cosio MG. Chronic obstructive lung disease: the role of airway disease, with special emphasis on the pathology of small airways. Monogr Pathol. 1978;19:160–179. [PubMed] [Google Scholar]
- 16.Blanchard C, Mishra A, Saito-Akei H, Monk P, Anderson I, Rothenberg ME. Inhibition of human interleukin-13-induced respiratory and oesophageal inflammation by anti-human-interleukin-13 antibody (CAT-354) Clin Exp Allergy. 2005;35:1096–1103. doi: 10.1111/j.1365-2222.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 17.Passalacqua G, Mincarini M, Colombo D, Troisi G, Ferrari M, Bagnasco D, et al. IL-13 and idiopathic pulmonary fibrosis: possible links and new therapeutic strategies. Pulm Pharmacol Ther. 2017;45:95–100. doi: 10.1016/j.pupt.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Baughman RP, Lower EE, Miller MA, Bejarano PA, Heffelfinger SC. Overexpression of transforming growth factor-alpha and epidermal growth factor-receptor in idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:57–61. [PubMed] [Google Scholar]
- 19.Hardie WD, Bruno MD, Huelsman KM, Iwamoto HS, Carrigan PE, Leikauf GD, et al. Postnatal lung function and morphology in transgenic mice expressing transforming growth factor-alpha. Am J Pathol. 1997;151:1075–1083. [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie WD, Kerlakian CB, Bruno MD, Huelsman KM, Wert SE, Glasser SW, et al. Reversal of lung lesions in transgenic transforming growth factor alpha mice by expression of mutant epidermal growth factor receptor. Am J Respir Cell Mol Biol. 1996;15:499–508. doi: 10.1165/ajrcmb.15.4.8879184. [DOI] [PubMed] [Google Scholar]
- 21.Kluger MA, Meyer MC, Nosko A, Goerke B, Luig M, Wegscheid C, et al. RORγt(+)Foxp3(+) cells are an independent bifunctional regulatory T cell lineage and mediate crescentic GN. J Am Soc Nephrol. 2016;27:454–465. doi: 10.1681/ASN.2014090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okui T, Aoki Y, Ito H, Honda T, Yamazaki K. The presence of IL-17+/FOXP3+ double-positive cells in periodontitis. J Dent Res. 2012;91:574–579. doi: 10.1177/0022034512446341. [DOI] [PubMed] [Google Scholar]
- 23.Drakopanagiotakis F, Xifteri A, Polychronopoulos V, Bouros D. Apoptosis in lung injury and fibrosis. Eur Respir J. 2008;32:1631–1638. doi: 10.1183/09031936.00176807. [DOI] [PubMed] [Google Scholar]
- 24.Collardeau-Frachon S, Hervieu V, Scoazec JY. [Eosinophilic esophagitis: an “emerging disease”] Ann Pathol. 2007;27:417–425. doi: 10.1016/S0242-6498(07)71413-X. [DOI] [PubMed] [Google Scholar]
- 25.Choe JY, Jung HJ, Park KY, Kum YS, Song GG, Hyun DS, et al. Anti-fibrotic effect of thalidomide through inhibiting TGF-beta-induced ERK1/2 pathways in bleomycin-induced lung fibrosis in mice. Inflamm Res. 2010;59:177–188. doi: 10.1007/s00011-009-0084-9. [DOI] [PubMed] [Google Scholar]
- 26.Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1175–G1187. doi: 10.1152/ajpgi.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L, Li ZH, Hou XM, Yu RJ. [Evaluation of interleukin-13 in the serum and bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis] Zhonghua Jie He He Hu Xi Za Zhi. 2003;26:686–688. [PubMed] [Google Scholar]
- 30.Howell JE, McAnulty RJ. TGF-beta: its role in asthma and therapeutic potential. Curr Drug Targets. 2006;7:547–565. doi: 10.2174/138945006776818692. [DOI] [PubMed] [Google Scholar]
- 31.Leppäranta O, Sens C, Salmenkivi K, Kinnula VL, Keski-Oja J, Myllärniemi M, et al. Regulation of TGF-β storage and activation in the human idiopathic pulmonary fibrosis lung. Cell Tissue Res. 2012;348:491–503. doi: 10.1007/s00441-012-1385-9. [DOI] [PubMed] [Google Scholar]
- 32.Halwani R, Al-Muhsen S, Al-Jahdali H, Hamid Q. Role of transforming growth factor-β in airway remodeling in asthma. Am J Respir Cell Mol Biol. 2011;44:127–133. doi: 10.1165/rcmb.2010-0027TR. [DOI] [PubMed] [Google Scholar]
- 33.Martin MM, Buckenberger JA, Jiang J, Malana GE, Knoell DL, Feldman DS, Elton Elton TS.TGF-beta1 stimulates human AT1 receptor expression in lung fibroblasts by cross talk between the Smad, p38 MAPK, JNK, and PI3K signaling pathways Am J Physiol Lung Cell Mol Physiol 2007293L790–L799.[Retraction in Am J Physiol Lung Cell Mol Physiol 302:2012] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Zhu X, Wang M, Mavi P, Rayapudi M, Pandey AK, Kaul A, et al. Interleukin-15 expression is increased in human eosinophilic esophagitis and mediates pathogenesis in mice. Gastroenterology. 2010;139:182–193.e7. doi: 10.1053/j.gastro.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram JL, Rice AB, Geisenhoffer K, Madtes DK, Bonner JC. IL-13 and IL-1beta promote lung fibroblast growth through coordinated up-regulation of PDGF-AA and PDGF-Ralpha. FASEB J. 2004;18:1132–1134. doi: 10.1096/fj.03-1492fje. [DOI] [PubMed] [Google Scholar]
- 36.Stumm CL, Wettlaufer SH, Jancar S, Peters-Golden M. Airway remodeling in murine asthma correlates with a defect in PGE2 synthesis by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2011;301:L636–L644. doi: 10.1152/ajplung.00158.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integr Biol. 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 38.Bonner JC. Mesenchymal cell survival in airway and interstitial pulmonary fibrosis. Fibrogenesis Tissue Repair. 2010;3:15. doi: 10.1186/1755-1536-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Hu H, Huynh ML, Kotaru C, Balzar S, Trudeau JB, et al. Mechanisms of tissue inhibitor of metalloproteinase 1 augmentation by IL-13 on TGF-beta 1-stimulated primary human fibroblasts. J Allergy Clin Immunol. 2007;119:1388–1397. doi: 10.1016/j.jaci.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 40.Borowski A, Kuepper M, Horn U, Knüpfer U, Zissel G, Höhne K, et al. Interleukin-13 acts as an apoptotic effector on lung epithelial cells and induces pro-fibrotic gene expression in lung fibroblasts. Clin Exp Allergy. 2008;38:619–628. doi: 10.1111/j.1365-2222.2008.02944.x. [DOI] [PubMed] [Google Scholar]
- 41.Argilés JM, López-Soriano FJ, Busquets S. Therapeutic potential of interleukin-15: a myokine involved in muscle wasting and adiposity. Drug Discov Today. 2009;14:208–213. doi: 10.1016/j.drudis.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:1121–1130. doi: 10.1164/rccm.200812-1936OC. [DOI] [PubMed] [Google Scholar]
- 44.Birjandi SZ, Palchevskiy V, Xue YY, Nunez S, Kern R, Weigt SS, et al. CD4(+)CD25(hi)Foxp3(+) cells exacerbate bleomycin-induced pulmonary fibrosis. Am J Pathol. 2016;186:2008–2020. doi: 10.1016/j.ajpath.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu F, Liu J, Weng D, Chen Y, Song L, He Q, et al. CD4+CD25+Foxp3+ regulatory T cells depletion may attenuate the development of silica-induced lung fibrosis in mice. PLoS One. 2010;5:e15404. doi: 10.1371/journal.pone.0015404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.