Abstract
Research to understand the contribution of macrophages to nonresolving airway inflammation in cystic fibrosis (CF) and other chronic suppurative airways diseases has been hindered by a lack of methods for isolating and studying these cells. With the development of technologies that can characterize small numbers of cells or individual cells, there is an even greater need for methodologies to isolate rare cells in heterogeneous specimens. Here, we describe a method that overcomes the technical obstacles imposed by sputum debris and apoptotic cells, and allows isolation of pure populations of macrophages from CF sputum. In addition to enhancing our ability to study human CF airway macrophages, this protocol can be adapted to study cells in sputum from other chronic suppurative lung diseases (e.g., chronic obstructive pulmonary disease) and used for isolation of individual cells for single cell analyses.
Keywords: macrophages, cystic fibrosis, sputum, single-cell analyses
Clinical Relevance
The ability to isolate rare cells from heterogeneous specimens that are easily collected by noninvasive methods, combined with newly developed techniques to characterize single cells, has the potential to significantly advance our understanding of the pathophysiology of chronic lung diseases. The method described here allows isolation of pure populations of macrophages from cystic fibrosis sputum and will permit studies to elucidate the role of macrophages in modulating inflammation in the cystic fibrosis airway. This protocol can also be adapted to study cells in sputum from patients with other chronic suppurative lung diseases, and to isolate individual cells for single-cell analyses.
Cystic fibrosis (CF) is characterized by chronic airway infections and nonresolving airway inflammation, and most affected individuals experience respiratory failure by their fourth decade (1). CF therapies, therefore, aim to decrease symptoms and maintain lung function by suppressing bacterial growth and reducing inflammation (2). However, the use of currently available antiinflammatory medications is limited by adverse side effects (2), and novel therapeutics are needed to dampen CF airway inflammation.
Macrophages are key regulators of inflammation (3, 4), and an imbalance in the ratio of pro- and antiinflammatory macrophages is believed to contribute to pathology in many chronic diseases (5, 6), including CF (7). Macrophages are present in CF sputum (8–10), and macrophage activation states have been shown to correlate with disease severity in chronic airways diseases such as CF, chronic obstructive pulmonary disease, and asthma (9, 11, 12). Furthermore, monocytes are recruited to airways from the circulation during CF disease flare-ups (10, 13), suggesting a role for monocyte-derived macrophages in airway inflammation. However, how different macrophages phenotypes contribute to CF disease remains unclear. Some studies suggest that suppressing inflammatory macrophages would improve health (14, 15), whereas others suggest that increasing inflammatory responses could eliminate infections (16, 17).
Animal models of CF have thus far not replicated chronic airway infections with variable airway inflammation, a hallmark of CF disease (18). Macrophages recovered from human CF airways provide a unique opportunity to study the role of macrophages in modulating chronic CF airway inflammation. Individuals with CF spontaneously expectorate sputum, providing a noninvasive method for sampling the CF airway environment (19, 20). Despite the ease of acquiring specimens, isolating individual immune cell populations from CF sputum has proven difficult because the copious debris in sputum (21) interferes with cell isolation methods. Characterization of macrophages in CF sputum has been mostly limited to flow cytometric descriptions of cell surface markers (8–10). Several recent studies of CF airway cell inflammatory states described analyses of total sputum cells, but not specific populations of leukocytes (22, 23).
With the continued development and more widespread availability of technologies to perform single-cell analysis (24), methods to isolate individual airway cells are needed to apply these new technologies to the study of chronic airway inflammation. Here, we describe a protocol for reliably isolating CF sputum macrophages for further study. Based on established protocols for solubilizing CF sputum (25), our protocol leverages flow cytometric strategies to mitigate challenges imposed by sputum debris and apoptotic cells, which nonspecifically bind antibodies and hinder conventional cell isolation methods.
Methods
Subjects and Specimen Collection
Specimens were obtained from patients in the University of Washington (UW) Adult Cystic Fibrosis Clinic with their informed consent, and the study was approved by the UW Human Subjects Division. Spontaneously expectorated sputum specimens were collected during clinic visits and kept on ice (0.5–4 h) until processing. Alternately, the subjects collected sputum at home, packaged the specimens in the appropriate biohazard packaging, and shipped them on ice overnight to the UW.
Sputum Solubilization and Filtration
Sputum specimens were weighed and mixed with 1 vol (assuming 1 g = 1 ml) of 0.1% DTT (Fisher Scientific) in 10 mM sodium acetate. The sputum was mechanically solubilized in DTT by pipetting. The specimens were incubated on ice for 30 minutes with intermittent vortex agitation to complete dissolution of the sputum. The reaction was stopped by adding RPMI 1640 media with L-glutamine (Fisher Scientific). RPMI was used instead of PBS to provide the leukocytes with nutrients and minimize cell death. Solubilized sputum was then pipetted through sterile gauze and poured through a 70 μM cell strainer. Strained, solubilized sputum was centrifuged at 300 × g. Pellets were washed twice in RPMI and resuspended for counting. Aliquots of cell suspensions were mixed with Trypan Blue, and live and dead cells were enumerated by visualization on a Neubauer hemocytometer (the method is depicted in Figure E1 in the data supplement).
Histologic Examination of Sputum Leukocytes
Cells were applied to microscope slides by cytospin, followed by staining with Romanowsky stain (Diff-Quick) to differentiate populations. Slides were evaluated by light microscopy, and digital images were obtained using a Nikon 90i microscope and Nikon Elements BR software.
Leukocyte Labeling for FACS
Cells were resuspended in fluorescence activated cell sorting (FACS) buffer (PBS+ 10% heat-inactivated fetal calf serum) at a concentration of up to 1 × 107/ml and incubated on ice with Fc receptor (FcR) blocking reagent (Miltenyi Biotec) per the manufacturer’s protocol for 15 minutes. Cells were labeled with Calcein AM (eBioscience/ThermoFisher), anti-CD15 (Clone VIMC6; Miltenyi Biotec), and anti-CD14 (Clone MϕP9; BD Pharmingen, or Clone 61D3; eBiosciences/ThermoFisher) per the manufacturer’s protocol. See Methods in the data supplement for additional details.
Cell Sorting
Samples were acquired on a FACS Aria II (BD Biosciences), with a flow rate of 1.0 and a 100-μM nozzle to minimize shear stress. Cells were sorted into cold RPMI. FACS data were exported as Flow Cytometry Standard (FCS) files and analyzed using FlowJo software (version 10.4.2).
Additional methods are described in detail in the data supplement.
Results
Sputum Cell Yield and Viability
CF sputum contains cells, proteins, immune complexes, bacteria, debris, and extracellular DNA that create a gel-like consistency (19, 21, 26). We applied the CF Foundation’s standard operating procedure for solubilizing CF sputum (25) and quantitated the number of leukocytes in each sample. Specimens collected from patients were sometimes measured in volume (range 1–60 ml), but often had more of a gel-like consistency and were weighed (range 0.6–10 g). Most specimens contained more than 1 × 106 leukocytes per milliliter or milligram of sputum (Table 1). Trypan Blue exclusion revealed that 90–100% of the sputum leukocytes were viable.
Table 1.
Quantification of Sputum Cells by Light Microscopy
| Subject | Number of Specimens | Average Leukocytes per Milliliter or Milligram | Median Leukocytes per Milliliter or Milligram | SEM | Range of Leukocytes per Milliliter or Milligram |
|---|---|---|---|---|---|
| 3 | 15 | 3.1 × 106 | 2.6 × 106 | 6.8 × 105 | 3.0 × 105 to 8 × 106 |
| 18 | 8 | 6.4 × 106 | 6.5 × 106 | 1.0 × 106 | 2.6 × 106 to 1.0 × 107 |
| 10 | 1 | 2.5 × 106 | — | — | — |
| 22 | 1 | 8.2 × 106 | — | — | — |
| 38 | 1 | 4.7 × 106 | — | — | — |
| 40 | 1 | 7.4 × 106 | — | — | — |
Modifications Made to Published Sputum-Processing Protocols Eliminate Debris
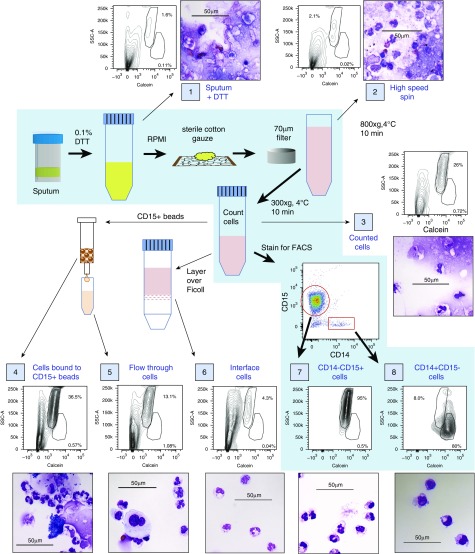
Sputum specimens solubilized according to published protocols contained significant quantities of squamous cells and debris that obscured identification and quantitation of leukocytes (Figures 1 and E2; sample 1). To eliminate additional debris while maximizing recovery of macrophages, we made three protocol modifications: 1) solubilized sputum was passaged through sterile gauze to eliminate large debris, 2) a 70 μM filter was used instead of a 40 μM filter to prevent filter retention of larger or activated macrophages, and 3) cells were pelleted using slow-speed centrifugation (300 × g) to eliminate lightweight debris (Figure E1). These steps substantially reduced debris in solubilized sputum specimens (Figures 1, E2, and E3; samples 1, 2, and 3).
Figure 1.
Efficacy of different methods for isolating leukocytes from spontaneously expectorated cystic fibrosis (CF) sputum evaluated by flow cytometry and histologic examination. Steps in the protocol described in this article are depicted on the blue background, with thick black arrows between each step. Samples of a single specimen were evaluated at steps throughout the protocol (each sample is identified by a number in a blue box) to determine the presence of debris and the purity of the leukocyte populations by both flow cytometry and histologic examination. Thin black arrows indicate samples produced either by initial steps in the protocol or by other protocols that are commonly used to isolate leukocyte populations but are ineffective for isolating sputum leukocytes. Side scatter-area (SSC-A) versus calcein (a viability dye that fluoresces only when taken up by intact, viable cells) can be used to separate live cells and debris, and can distinguish neutrophils from macrophages more effectively than forward scatter-area (FSC-A). Larger images of histology for each sample can be found in Figure E2. Scale bars: 50 μm.
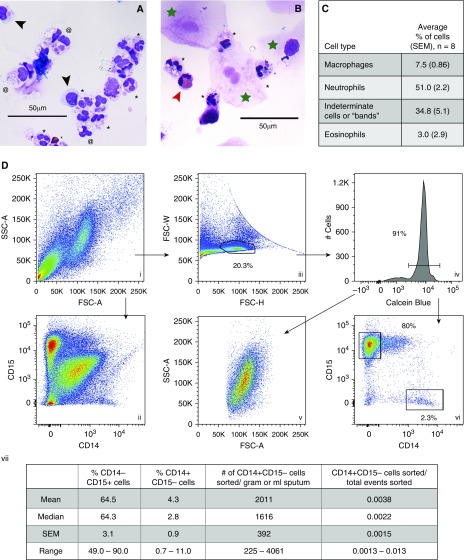
Consistent with previous reports (19), light microscopy revealed that CF sputum leukocytes were mostly neutrophils and macrophages, with occasional eosinophils observed in some patients (Figure 2). Many macrophages and neutrophils were large and highly vacuolated, but some macrophages appeared morphologically similar to blood monocytes. Specimens also contained cells that had pink cytoplasm containing granules (like neutrophils), but appeared to have a single-lobed nucleus; these cells were later determined by flow cytometry to be neutrophils (data not shown), most likely representing immature neutrophils or “bands” (27). In addition to leukocytes, the sputum contained squamous cells (larger than leukocytes, with a “fried egg” appearance) and debris (Figures 2 and E2).
Figure 2.
CF sputum contains macrophages and neutrophils that can be sorted as pure populations by FACS. (A and B) Morphologic identification of sputum leukocytes. Sputum specimens were solubilized with DTT, strained, filtered, and centrifuged before counting (as in Figure E1), and ∼30,000 leukocytes were applied to a microscope slide via cytospin and stained with Romanowsky stain. Two separate specimens are shown. Macrophages (black arrowheads) have purple-blue cytoplasm lacking granules, and a single, eccentric nucleus. Neutrophils (black asterisks) have pink cytoplasm containing granules, and a multilobular nucleus. Eosinophils (red arrowhead) are binucleate with red cytoplasmic granules. Indeterminate cells (at symbols) have a single nucleus, and pink and/or granular cytoplasm; these were determined later to be neutrophils by flow cytometry. Squamous cells are large, with a “fried egg” appearance (green star). (C) Quantitation of cell differentials from several sputum specimens as determined by light microscopy (n = 8, collected from three subjects). (D) Flow cytometric parameters for sorting macrophages and neutrophils from solubilized sputum specimens. (i, ii, and iii) Total events plotted for the indicated parameters. (iii) Gating strategy for single cells. (iv) Plot of single cells, gating on calcein+ cells. (v) Live single cells (identified in iv) plotted for FSC-A versus SSC-A (compare with i). (vi) Live single cells plotted for CD14 versus CD15 (compare with ii). (vii) Percentage of different cell subsets in sputum specimens from different subjects, as determined by flow cytometry, as well as the average numbers of macrophages per gram or milliliter of sputum that can be sorted using this method. n = 17 sputum specimens from 10 different subjects; repeat specimens (from subjects 3 and 18) were obtained on multiple days over several months. >50,000 neutrophils were sorted from virtually all sputum specimens, including those < 1.0 gram in weight. FSC-H = forward scatter-height; FSC-W = forward scatter-width. Scale bars: 50 μm.
Pure Populations of Neutrophils and Macrophages Can Be FACS Sorted from CF Sputum
We tested the feasibility of using cell-specific antibodies and FACS to identify and purify sputum macrophages. Prior studies documented forward and side scatter properties of induced sputum specimens from both healthy donors and subjects with other airways diseases (12, 28). We found similar forward and side scatter properties for specimens from subjects with CF, although the CF sputum specimens contained a particularly high quantity of debris (low forward scatter-area [FSC-A], low side scatter-area [SSC-A] events) (Figure 2D, i). Discrete leukocyte populations were difficult to distinguish when total acquired events (including debris and aggregates) were plotted for CD15 (a neutrophil marker) and CD14 (a macrophage marker) expression (Figure 2D, ii). Using forward scatter-height (FSC-H) versus forward scatter-width (FSC-W), we identified single cells within total acquired events (see Figures E5, E6, and E10), thus eliminating aggregates and doublets (Figure 2D, iii), and identified live cells using the fluorescent viability dye calcein (Figure 2D, iv). When only single viable cells were plotted for CD14 and CD15 expression, distinct populations of cells became apparent (Figure 2D, vi; see Figure E4 for controls).
Although CD15 and CD14 have previously been described as surface markers for neutrophils and macrophages, respectively (29, 30), lung tissue and CF disease–specific factors could alter cell surface proteins/markers (4, 31, 32). To confirm that expression of CD15 and CD14 can distinguish neutrophils and macrophages in CF sputum, we sorted CD15+/CD14− cells and CD15−/CD14+ cells from solubilized CF sputum, and examined their morphology using light microscopy (Figures 1 and E2; samples 7 and 8). CD15+/CD14− cells displayed the morphological characteristics of neutrophils, and CD15−/CD14+ cells displayed a morphology consistent with macrophages or monocytes (described in Figure 2).
We sorted sputum specimens from more than 15 subjects with CF. The amount of debris, squamous cells, leukocyte aggregates, and viable single leukocytes varied among subjects (Figure E6). Most events in the “single cell” gate were viable, or calcein+ (81% ± 3.7%), whereas single cells accounted for ∼50% of calcein+ events (±6.4%). Viable single cells in CF sputum were found to be 49–90% neutrophils and 1–11% macrophages (Figure 2D, vii), with debris and apoptotic neutrophils (see below) comprising the remainder of events. Variability in the number of viable cells and the abundance of macrophages and neutrophils was also present in sputum specimens obtained over time from a single subject (Table E1 and Figure E7). Differences in the composition of specimens obtained from the same individual over time may reflect variability inherent in spontaneously expectorated sputum specimens, or differences in the disease state of the subject’s airway (see Discussion below).
Sputum Contains Apoptotic Neutrophils, but Most Macrophages Are Viable
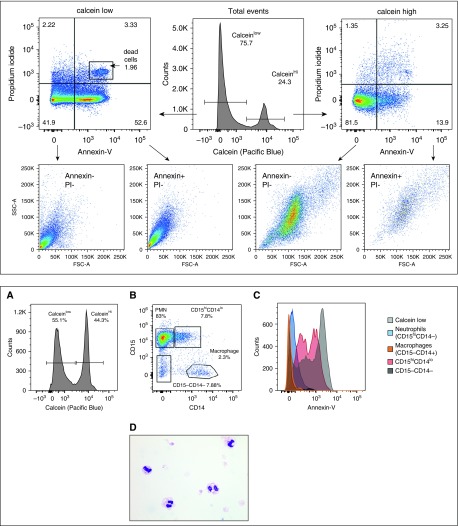
Previous studies have demonstrated that 20–70% of CF sputum neutrophils are undergoing either apoptosis or necrosis, with variability relating to differences in bacterial species causing chronic infection in patients’ airways (33, 34). Using calcein, propidium iodide (PI), and annexin-V, we determined the proportion of apoptotic (annexin+/PI−) and necrotic (annexin+/PI+) cells in our sputum samples (controls in Figure E8). Calcein+ events in sputum (n = 3) were mostly live, nonapoptotic cells (85.5% ± 2.1%), with ∼10% apoptotic cells (10.5% ± 2.9%) (Figure 3, top panel). In contrast, calcein− events contained annexin−/PI− debris (43.6% ± 4.2% of events), annexin+/PI+ necrotic cells (7.7% ± 2.7%), and 44.7% ± 5.0% annexin+/PI− events. Calcein−/annexin+/PI− events could represent late-stage apoptotic cells in which inactive enzymes can no longer cleave the ester bond in calcein that leads to fluorescence; alternately, annexin+/PI− events could be cell membranes (with exposed phosphatidylserine) that are not associated with DNA. Consistent with either possibility, calcein−/annexin+/PI− events cluster in the bottom left quadrant, where debris is found, when plotted for FSC-A versus SSC-A.
Figure 3.
Flow cytometric analysis of specimens stained with propidium iodide (PI) and annexin-V reveals apoptotic neutrophils in CF sputum. PI and annexin-V staining was used to identify apoptotic cell populations in CF sputum cells. PI binds to DNA in cells in which membrane integrity has been lost (dead cells), and annexin-V binds to accessible phosphatidylserine in apoptotic or dead cells. Thus, annexin+/PI− cells are apoptotic, and annexin+/PI+ cells are necrotic. Annexin−/PI− events are either debris (calcein−) or viable cells (calcein+). Top and bottom panels display data from different sputum specimens. Top: Total events were separated into calceinhi (live) and calceinlow (dead/debris) populations, and then plotted for annexin-V versus PI. All PI− cells were then plotted for FSC-A versus SSC-A. As expected, the majority of live cells (calceinhi) were comprised of nonapoptotic cells. The calceinlow events contained a majority of apoptotic and dead cells. Bottom: (A) In a different sample, total events were plotted to determine calcein positivity. (B) Viable cells (calceinhi) were then plotted for CD14 and CD15 expression, and four populations were defined (polymorphonuclear cells [PMN] or CD15hiCD14−, CD15hiCD14lo, CD15−CD14−, and macrophages or CD15−CD14+). (C) Annexin-V binding to the four populations delineated in B, plus the calceinlow cells in A; CD15−/CD14− cells in the black histogram are behind the macrophage histogram depicted in orange. (D) Cytospins of sorted apoptotic cells (calcein+/annexin+/PI−) reveal that these cells are neutrophils.
To evaluate which leukocytes were apoptotic, calceinhi cells in sputum specimens were plotted for CD15 and CD14 expression, and subpopulations were assessed for annexin staining (Figure 3, bottom panel, A and B). In addition to CD15+/CD14− cells (neutrophils) and CD15−/CD14+ cells (macrophages), many specimens also contained a CD15+/CD14lo population. Macrophages were annexin− (Figure 3, bottom panel, C), suggesting that very few or no macrophages were apoptotic. Neutrophils were also annexin−; however, CD15+/CD14lo cells were annexin+, raising the possibility that these cells could be neutrophils (high CD15 expression) undergoing apoptosis (annexin+) that were nonspecifically binding antibodies (e.g., anti-CD14). Three findings supported this conclusion: 1) all calcein+/annexin+/PI− cells stained CD15hi/CD14lo, but no cells expressed CD14 at levels (measured by mean fluorescence index [MFI]) seen on viable macrophages (Figure E9); 2) sorted calcein+/annexin+/PI− sputum cells had histologic features consistent with neutrophils (Figure 3, bottom panel, D); and 3) evaluation of CD15+/CD14lo events detected in total, ungated sputum revealed the presence of aggregates and calcein− events (Figure E10) in addition to apoptotic cells (Figure 3).
Discussion
Macrophages are postulated to be key regulatory cells in many chronic lung diseases; however, isolating human airway macrophages for study has proven difficult due to the high debris content in suppurative sputum. We describe a method for isolating macrophages from spontaneously expectorated CF sputum specimens. By minimizing debris and applying flow cytometric parameters, viable cells can be sorted as either individual cells or enriched leukocyte populations.
Our data suggest why many commonly used cell isolation techniques have been unsuccessful when applied to suppurative sputum specimens: debris, apoptotic cells, and necrotic cells nonspecifically bind antibodies (Figures 3, E9, and E10), which hinders cell isolation in several ways. First, sorted cell populations become contaminated with debris and dead cells, whether sorting occurs by FACS or magnetic bead–associated cell sorting (Figures 1 and E2, panels 4 and 5). Second, nonspecific antibody binding obscures distinct cell populations detected by FACS (see Figure 2D, ii vs. vi). Calcein-AM dye, a key component of this protocol, fluoresces only when taken up by viable cells, allowing discrimination of specific antibody binding to viable cells. Finally, nonspecific antibody binding by debris can sequester the antibody away from target leukocytes. For example, most kits that use magnetic bead–conjugated antibodies to purify cells do not contain an overwhelming excess of antibody-conjugated beads. When these kits are used to purify cells from CF sputum, many target cells fail to bind the antibody, resulting in cells that should be retained on the column washing off into the flow-through (Figures 1 and E2, panels 4 and 5, and E11).
Although this protocol provides technical advances, it has several limitations. First, as described here, this method purifies only CD14+ macrophages from CF sputum. CD14 is variably expressed on different macrophage populations, and is specifically low in abundance on alveolar macrophages (35, 36). Thus, anti-CD14 antibody is unlikely to recover alveolar macrophages present in sputum specimens. However, CF sputum likely does not contain many alveolar macrophages. CF disease primarily affects smaller airways, where most cystic fibrosis transmembrane receptor (CFTR) is expressed in healthy lungs (37), and the alveoli usually become involved only in individuals with advanced lung disease (38). Moreover, recent studies have demonstrated that macrophages in spontaneously expectorated CF sputum are “small macrophages,” and are believed to be infiltrating monocyte-derived macrophages rather than alveolar macrophages (10, 12, 13). The CD14+ macrophages described in our study are small and demonstrate forward and side scatter properties similar to those of cells previously described as “monocytes” (28). When single viable cells in our sputum specimens are replotted for forward versus side scatter (Figure 2D, v), the plot resembles published descriptions of induced sputum cells from healthy individuals (28), minus the alveolar macrophage population. FACS antibodies other than anti-CD14 could be used to distinguish CF airway macrophages; however, many markers commonly used to identify quiescent macrophages and monocytes are expressed on activated neutrophils (39), which are abundant in CF lungs. We tested several combinations of antibodies, and using light-microscopic examination of sorted cells for confirmation, we found that antibodies to CD14 and CD15 reliably distinguished macrophages from neutrophils.
Second, although the populations of cells isolated by this method are highly enriched, the number of macrophages recovered is always about 10-fold less than the number of cells predicted by calculating the percentage of total sputum leukocytes that are CD14+/CD15− viable single cells (Figure 2D, vii, and Table E2). This discrepancy may be due to macrophages being contained in aggregates. It may also reflect the fragility of sputum cells: microscopic examination revealed that sputum macrophages are often highly vacuolated, and sheer forces of flow cytometry may impact cell integrity. Despite this limitation, one can sort a sufficient number of cells (>10,000) to perform many cellular analyses, including proteomics, quantitative PCR analysis of gene expression, and whole-transcriptome analysis.
Application of this method to characterize CF sputum macrophages has the potential to answer numerous questions regarding the role of macrophages in CF airway inflammation, such as how macrophage populations change after initiation of new medications, whether macrophage phenotypes vary among people colonized with different airway pathogens, and whether pulmonary exacerbations are associated with increased proinflammatory macrophages. Damage and inflammation in CF lungs are heterogeneous (40, 41), and how different regions drain to produce sputum likely varies among individuals and over time within one individual (42). Thus, the cells in each sputum specimen represent a snapshot in time, and possibly also a region of the lung. Studies to understand sorted sputum macrophages will require prospective, longitudinal specimen collection, with multiple specimens collected during each different disease state or treatment condition. We successfully collected sputum specimens at regular intervals, and at times of increased symptoms/pulmonary exacerbations from several patients. In these pilot studies, we observed the absolute and relative abundance of sputum macrophages fluctuate over time, and these changes could reflect symptomatic changes in airway inflammation (Figure E12).
Isolation and characterization of sputum macrophages may also reveal new biomarkers of CF airway inflammation. Studies by our group and others suggest that CF sputum contains different populations of macrophages (8–10), which may vary in abundance with different treatments, infections, or disease states. Macrophage populations with different surface marker expression can be sorted using this method and characterized by gene or protein expression to determine their inflammatory potential. Changes in the relative abundance of different macrophage populations may aid in the diagnosis of pulmonary exacerbations, predict treatment failure or success, or herald the arrival of new airway pathogens. Our cell isolation method can also be adapted to characterize sputum macrophages and other cell types in other chronic lung diseases, such as non-CF bronchiectasis, chronic bronchitis, asthma, and pulmonary fibrosis. The ability to isolate cells from specimens that are easily collected by noninvasive methods, combined with newly developed techniques to characterize single cells, has the potential to significantly advance our understanding of the pathophysiology of chronic lung diseases.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the study participants for contributing specimens for this research. They also thank Michele Black, director of the Cell Analysis Facility (Department of Immunology, UW), and Donna Prunkard, manager of the Pathology Flow Cytometry Core Facility at the UW, for their assistance with flow cytometry and cell sorting. They thank Dr. Pradeep K. Singh at UW for a critical review of the manuscript and for providing support and encouragement. They also thank Ellen Wilhem for assistance with specimen collection, and M. E. Long, B. Staudinger, L. Becker, and M. Aitken for insightful discussions.
Footnotes
Supported by grants from the Cystic Fibrosis Foundation (Leroy Matthews Award) and the National Institutes of Health (1K08HL136786-01A1) (K.B.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Cystic Fibrosis Foundation or the National Institutes of Health.
Author Contributions: Concept and design of the study, and acquisition and analysis of data: K.B.H. Interpretation of data, and drafting and critical revision of the manuscript: K.B.H., W.C.L., and A.M.M.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0236MA on February 11, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 2.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 3.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parisi L, Gini E, Baci D, Tremolati M, Fanuli M, Bassani B, et al. Macrophage polarization in chronic inflammatory diseases: killers or builders? J Immunol Res. 2018;2018:8917804. doi: 10.1155/2018/8917804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruscia EM, Bonfield TL. Cystic fibrosis lung immunity: the role of the macrophage. J Innate Immun. 2016;8:550–563. doi: 10.1159/000446825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy BS, Bush HM, Sundareshan V, Davis C, Hagadone J, Cory TJ, et al. Characterization of macrophage activation states in patients with cystic fibrosis. J Cyst Fibros. 2010;9:314–322. doi: 10.1016/j.jcf.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Wright AK, Rao S, Range S, Eder C, Hofer TP, Frankenberger M, et al. Pivotal advance: expansion of small sputum macrophages in CF. Failure to express MARCO and mannose receptors. J Leukoc Biol. 2009;86:479–489. doi: 10.1189/jlb.1108699. [DOI] [PubMed] [Google Scholar]
- 11.Boorsma CE, Draijer C, Melgert BN. Macrophage heterogeneity in respiratory diseases. Mediators Inflamm. 2013;2013:769214. doi: 10.1155/2013/769214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankenberger M, Menzel M, Betz R, Kassner G, Weber N, Kohlhäufl M, et al. Characterization of a population of small macrophages in induced sputum of patients with chronic obstructive pulmonary disease and healthy volunteers. Clin Exp Immunol. 2004;138:507–516. doi: 10.1111/j.1365-2249.2004.02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garratt LW, Wright AK, Ranganathan SC, Grigg J, Sly PD behalf of AREST CF. Small macrophages are present in early childhood respiratory disease. J Cyst Fibros. 2012;11:201–208. doi: 10.1016/j.jcf.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Cousin JM, Hughes J, Van Damme J, Seckl JR, Haslett C, et al. Glucocorticoids promote nonphlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–3646. [PubMed] [Google Scholar]
- 15.Meyer M, Huaux F, Gavilanes X, van den Brûle S, Lebecque P, Lo Re S, et al. Azithromycin reduces exaggerated cytokine production by M1 alveolar macrophages in cystic fibrosis. Am J Respir Cell Mol Biol. 2009;41:590–602. doi: 10.1165/rcmb.2008-0155OC. [DOI] [PubMed] [Google Scholar]
- 16.Heslet L, Bay C, Nepper-Christensen S. The immunomodulatory effect of inhaled granulocyte-macrophage colony-stimulating factor in cystic fibrosis: a new treatment paradigm. J Inflamm Res. 2012;5:19–27. doi: 10.2147/JIR.S22986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;187:621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 18.Lavelle GM, White MM, Browne N, McElvaney NG, Reeves EP. Animal models of cystic fibrosis pathology: phenotypic parallels and divergences. BioMed Res Int. 2016;2016:5258727. doi: 10.1155/2016/5258727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henig NR, Tonelli MR, Pier MV, Burns JL, Aitken ML. Sputum induction as a research tool for sampling the airways of subjects with cystic fibrosis. Thorax. 2001;56:306–311. doi: 10.1136/thorax.56.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, et al. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA. 2012;109:13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, Obermayer A, et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros. 2012;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Cory TJ, Birket SE, Murphy BS, Hayes D, Jr, Anstead MI, Kanga JF, et al. Impact of azithromycin treatment on macrophage gene expression in subjects with cystic fibrosis. J Cyst Fibros. 2014;13:164–171. doi: 10.1016/j.jcf.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattison SH, Gibson DS, Johnston E, Peacock S, Rivera K, Tunney MM, et al. Proteomic profile of cystic fibrosis sputum cells in adults chronically infected with Pseudomonas aeruginosa Eur Respir J 201750pii: 1601569. [DOI] [PubMed] [Google Scholar]
- 24.Keating SM, Taylor DL, Plant AL, Litwack ED, Kuhn P, Greenspan EJ, et al. Opportunities and challenges in implementation of multiparameter single cell analysis platforms for clinical translation. Clin Transl Sci. 2018;11:267–276. doi: 10.1111/cts.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc. 2007;4:406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFarlane H, Holzel A, Brenchley P, Allan JD, Wallwork JC, Singer BE, et al. Immune complexes in cystic fibrosis. BMJ. 1975;1:423–428. doi: 10.1136/bmj.1.5955.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexis N, Soukup J, Ghio A, Becker S. Sputum phagocytes from healthy individuals are functional and activated: a flow cytometric comparison with cells in bronchoalveolar lavage and peripheral blood. Clin Immunol. 2000;97:21–32. doi: 10.1006/clim.2000.4911. [DOI] [PubMed] [Google Scholar]
- 29.Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T, et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung-draining lymph nodes. Am J Respir Crit Care Med. 2016;193:614–626. doi: 10.1164/rccm.201507-1376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lay JC, Peden DB, Alexis NE. Flow cytometry of sputum: assessing inflammation and immune response elements in the bronchial airways. Inhal Toxicol. 2011;23:392–406. doi: 10.3109/08958378.2011.575568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan M, Li WC, Vlahos R, Maxwell MJ, Anderson GP, Hibbs ML. Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. J Immunol. 2012;189:946–955. doi: 10.4049/jimmunol.1200660. [DOI] [PubMed] [Google Scholar]
- 32.Guth AM, Janssen WJ, Bosio CM, Crouch EC, Henson PM, Dow SW. Lung environment determines unique phenotype of alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2009;296:L936–L946. doi: 10.1152/ajplung.90625.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 34.Watt AP, Courtney J, Moore J, Ennis M, Elborn JS. Neutrophil cell death, activation and bacterial infection in cystic fibrosis. Thorax. 2005;60:659–664. doi: 10.1136/thx.2004.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 36.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kreda SM, Mall M, Mengos A, Rochelle L, Yankaskas J, Riordan JR, et al. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol Biol Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulrich M, Worlitzsch D, Viglio S, Siegmann N, Iadarola P, Shute JK, et al. Alveolar inflammation in cystic fibrosis. J Cyst Fibros. 2010;9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann JJ. Neutrophil CD64: a diagnostic marker for infection and sepsis. Clin Chem Lab Med. 2009;47:903–916. doi: 10.1515/CCLM.2009.224. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Sanders DB, Rock MJ, Kosorok MR, Collins J, Green CG, et al. Regional differences in the evolution of lung disease in children with cystic fibrosis. Pediatr Pulmonol. 2012;47:635–640. doi: 10.1002/ppul.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer KC, Sharma A. Regional variability of lung inflammation in cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1536–1540. doi: 10.1164/ajrccm.156.5.9701098. [DOI] [PubMed] [Google Scholar]
- 42.Bennett WD, Zeman KL, Laube BL, Wu J, Sharpless G, Mogayzel PJ, Jr, et al. Homogeneity of aerosol deposition and mucociliary clearance are improved following ivacaftor treatment in cystic fibrosis. J Aerosol Med Pulm Drug Deliv. 2018;31:204–211. doi: 10.1089/jamp.2017.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.