Abstract
Supplemental O2 (hyperoxia; 30–90% O2) is a necessary intervention for premature infants, but it contributes to development of neonatal and pediatric asthma, necessitating better understanding of contributory mechanisms in hyperoxia-induced changes to airway structure and function. In adults, environmental stressors promote formation of senescent cells that secrete factors (senescence-associated secretory phenotype), which can be inflammatory and have paracrine effects that enhance chronic lung diseases. Hyperoxia-induced changes in airway structure and function are mediated in part by effects on airway smooth muscle (ASM). In the present study, using human fetal ASM cells as a model of prematurity, we ascertained the effects of clinically relevant moderate hyperoxia (40% O2) on cellular senescence. Fetal ASM exposed to 40% O2 for 7 days exhibited elevated concentrations of senescence-associated markers, including β-galactosidase; cell cycle checkpoint proteins p16, p21, and p-p53; and the DNA damage marker p-γH2A.X (phosphorylated γ-histone family member X). The combination of dasatinib and quercetin, compounds known to eliminate senescent cells (senolytics), reduced the number of hyperoxia-exposed β-galactosidase–, p21-, p16-, and p-γH2A.X–positive ASM cells. The senescence-associated secretory phenotype profile of hyperoxia-exposed cells included both profibrotic and proinflammatory mediators. Naive ASM exposed to media from hyperoxia-exposed senescent cells exhibited increased collagen and fibronectin and higher contractility. Our data show that induction of cellular senescence by hyperoxia leads to secretion of inflammatory factors and has a functional effect on naive ASM. Cellular senescence in the airway may thus contribute to pediatric airway disease in the context of sequelae of preterm birth.
Keywords: asthma, reactive airway disease, senescence, neonatal
Administration of supplemental O2 (hyperoxia; 30–90% O2) is often a necessary intervention for premature infants. However, prolonged exposure to hyperoxia is believed to contribute to development of neonatal and pediatric airway disease, involving recurrent wheezing and asthma that start early and can persist into school age and adulthood (1–6). Such symptoms involve airway hyperresponsiveness (AHR) and obstruction, which are mediated through airway smooth muscle (ASM) hypercontractility and remodeling (7–9). Epidemiological studies demonstrate increased risk of asthma among neonates born in the early term and moderate-late preterm period even after the introduction of steroids and surfactant into clinical practice (10–12). Conversely, practice changes have resulted in a new bronchopulmonary dysplasia involving hypoalveolarization or pruned pulmonary vasculature, but this pathology does not address the bronchial aspects of airway disease. Instead, recent studies have highlighted the detrimental effects of hyperoxia on the bronchial airway with clinically relevant levels of moderate hyperoxia (<60% O2) resulting in enhanced intracellular calcium regulation, cell proliferation, and extracellular matrix (ECM) deposition in developing human ASM (13). In newborn mice, exposure to 40–50% O2 increases bronchial wall diameter and amplifies response to methacholine challenge (14). Although these studies support a role for neonatal hyperoxia exposure in contributing to airway disease, little is known about why such early exposures in neonatal life can continue to impact airway function into adulthood. Thus, identification of underlying mechanisms is important for early intervention during and/or immediately after exposure to hyperoxia.
In aging and chronic ailments, including those of the lung, senescent cells are now recognized as significant contributors to disease pathophysiology (15–18). Senescent cells are in G1 cell cycle phase arrest and secrete factors (senescence-associated secretory phenotype [SASP]) that promote inflammation and fibrosis via paracrine effects on adjacent naive cells (19, 20). Oxidative stress, inflammation, and drugs used in cancer therapy are several factors that can induce cellular senescence (19). Several markers, including increased expression of β-galactosidase, activation of the p53-p21/p16 survival pathways, and phosphorylation of γH2A.X (γ-histone family member X), can be used to identify senescent cells (21). Studies in the lung suggest that secretion of SASP factors by senescent cells is a key mechanism promoting chronic lung diseases such as chronic obstructive pulmonary disease and pulmonary fibrosis (17, 22–24). Senolytics are a novel class of agents that selectively eliminate senescent cells (18, 25, 26). Among these is the combination of dasatinib and quercetin (D+Q), which has recently been shown to reduce bleomycin-induced airway fibroblast senescence and improve lung function in mice (20). This study highlights the potential role for senescent cells in promoting lung disease.
There is currently limited data about hyperoxia and senescence in the context of the developing lung. In neonatal mice, greater than 60% O2 has been shown to induce alveolar senescence within the framework of neonatal chronic lung disease (bronchopulmonary dysplasia) (27), whereas in vitro exposure of fibroblasts to 70% O2 also activates senescence pathways (28). However, the clinical relevance of prolonged exposure to high levels of O2 is less clear. Infants who receive supplemental oxygen for a short duration and/or at lower levels (<60% O2) also remain at increased risk of developing bronchial airway disease (3), but they are less likely to develop bronchopulmonary dysplasia. Whether moderate levels of O2 also activate senescence pathways in the developing airway, which are the relevant cells involved, and what are the effects of SASP in this regard are all unknown. In the present study, we examined the effect of clinically relevant moderate hyperoxia (40% O2) on cellular senescence in fetal ASM cells to test the hypothesis that cellular senescence and its effects on bronchial airways are relevant to hyperoxia-induced recurrent wheezing and asthma in preterm infants.
Methods
Detailed descriptions of the methods are provided in the data supplement.
Cell Culture
Fetal ASM cells were isolated from human tracheobronchial tissue of 18–22-week gestation fetuses (exempt per the Mayo Clinic Institutional Review Board [IRB] because tissue was deidentified and cells were used in vitro only). Cells were characterized to ensure smooth muscle phenotype and responsiveness to bronchoconstrictor agonists (13, 29).
Oxygen exposure
Cells were grown under otherwise standard conditions for 7 days with 21% O2 (normoxia) or 40% O2 (hyperoxia). Cell lysates and concentrated media (21% and 40% O2-conditioned media [CM]) were collected.
Etoposide exposure
Cells were grown in normoxia with 10 μM etoposide for 48 hours, and cell lysates and concentrated media were collected at 7 days.
Human samples
Autopsy paraffin-embedded airway specimens were obtained after human subjects research approval was provided by the Mayo Clinic IRB (15-007416). A total of 15 cases and 15 control subjects were sought; however, after selection, specimens for only 14 cases could be located. Control subjects were defined as neonates who died within 24 hours of birth, regardless of oxygen exposure. Cases were defined as neonates who died within 2 weeks of birth with at least 96 hours of oxygen exposure. No differentiation was made between term or preterm neonates. For each patient, a maximum of eight airways per sample were selected randomly in a blinded fashion by the individual performing the image analysis.
qRT-PCR
Standard techniques were used for measuring mRNA concentrations of cell cycle and senescence markers (see data supplement). RT-PCR was performed in triplicate with normalization to GAPDH, and fold changes were calculated using the comparative cycle threshold method.
Western Blot Analysis
Standard techniques were used with normalization to GAPDH. Results were reported as fold change from control (29).
Cellular Senescence-associated β-Galactosidase Activity
Cells were plated at known density and exposed to normoxia or hyperoxia for 7 days, and senescence-associated β-galactosidase (SA β-Gal) was detected by colorimetric assay (DAPI counterstain), followed by microscopic image analysis of 25 high-power fields per experiment.
Senolytic Exposure
On Day 5, cells were treated with vehicle (0.1% DMSO) or combinations of dasatinib (5 nM–5 μM) and quercetin (7.5 nM–7.5 μM) followed by assay of cellular SA β-Gal activity.
Traction Force Microscopy
Cellular contractility was measured using traction force microscopy (30). Briefly, naive fetal ASM cells were plated on modified 25 kD coated hydrogel plates embedded with fluorescent microspheres and collagen type I that provided a growth surface (30). Samples were treated with 1% FBS media (vehicle) or CM from cells exposed to 21% O2 or 40% O2 for 48 hours (adjusted for protein from 7-d hyperoxia-exposed cells), imaged using bright-field (cells) and fluorescence (beads) microscopy to assess bead displacement before and after cell lysis, and analyzed to determine peak and root mean square tractions (20).
ELISA
Media from cells exposed for 7 days to 21%, 40% O2 with and without treatment with D+Q (500 nM/750 nM) were collected and concentrated for analysis by customized ELISA to assess the SASP profile (PAI-1, IL-1α, IL-1β, IL-6, IL-8, TNF-α, and transforming growth factor [TGF]-β) (23) with standard procedures to determine concentrations.
ECM deposition
Naive fetal ASM cells plated at fixed density were treated with 1% FBS media (vehicle) or CM from cells exposed to 21% O2, 40% O2, or 40% O2 with D+Q treatment for 48 hours. Cell number was determined as previously described (8). The cells were then lysed, and deposition of collagen type I and fibronectin was determined using semiquantitative immunofluorescence expressed as fold change from normal and adjusted for cell numbers (31).
Immunofluorescence
ASM cells exposed to 21% O2 or 40% O2 for 7 days were fixed with 4% paraformaldehyde, permeabilized, immunostained using standard procedures (nuclei counterstained with DAPI), and imaged using laser confocal microscopy and fluorescence microscopy (32, 33). Similar procedures were used to stain human samples.
Statistical Analysis
All experiments involved at least five individual fetal ASM samples. Data were analyzed using one-way ANOVA with Holm-Sidak correction, two-tailed t test, and Wilcoxon rank-sum or Mann-Whitney U test. Values are expressed as mean ± SEM, and P values less than 0.05 were considered statistically significant.
Results
Hyperoxia Induces Markers of Senescence in Developing ASM
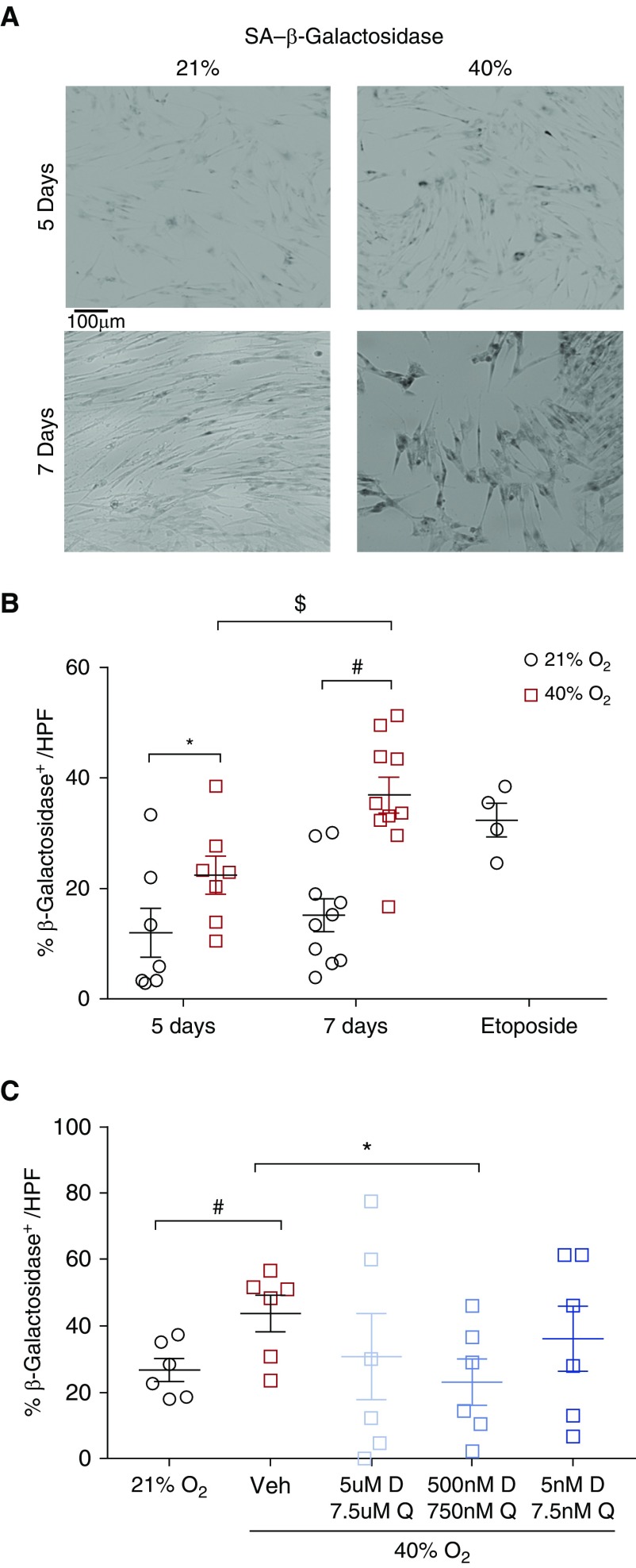
Increased expression of SA β-Gal is commonly used to identify senescent cells (21). Exposure to 40% O2 significantly increased the percentage of SA β-Gal–positive cells after 5 and 7 days of exposure (Figures 1A and 1B) (P < 0.05). Because exposure to 40% O2 for 7 days led to a greater percentage of SA β-Gal–positive cells and pilot experiments did not show further increases in percentages of such cells with more prolonged exposure, all subsequent experiments were completed using the 7-day time point.
Figure 1.
Effect of hyperoxia on senescence in fetal airway smooth muscle (ASM) cells. (A) Forty percent O2 exposure increased cellular senescence–associated β-galactosidase (SA β-Gal) activity (blue; bright-field microscopy) at 5 days and 7 days of exposure. Scale bar: 100 μm. (B) These effects of hyperoxia were comparable to the senescence induced by etoposide (positive control). Data are presented as mean ± SEM (n = 7 individual fetal ASM samples). P < 0.05. *Significant difference from control at 5 days. #Significant difference at 7 days. $Significant difference between 5 and 7 days of exposure. (C) Dasatinib (D) and quercetin (Q) reduced the percentage of β-Gal–positive fetal ASM cells after exposure to 40% O2 compared with vehicle-treated cells (0.1% DMSO). Treatment with 500 nM D and 750 nM Q starting on Day 4 of hyperoxia until Day 7 was particularly effective. Other combinations did not demonstrate a similar effect. n = 3–5 independent experiments, with analysis of 25 high-power fields (HPF). P < 0.05. *#$Significant differences. Veh = vehicle.
Senolytics Reduce Senescent Cells Induced by Hyperoxia
We treated cells with D+Q (20) during the hyperoxia exposure. Fetal ASM was treated with different dosing combinations of D+Q upon exposure to 40% O2. Cells treated with 500 nM dasatinib and 750 nM quercetin demonstrated significant decreases in cellular SA β-Gal activity (Figure 1C) (P < 0.05), but not at other doses.
Markers of Senescence Induced by Hyperoxia
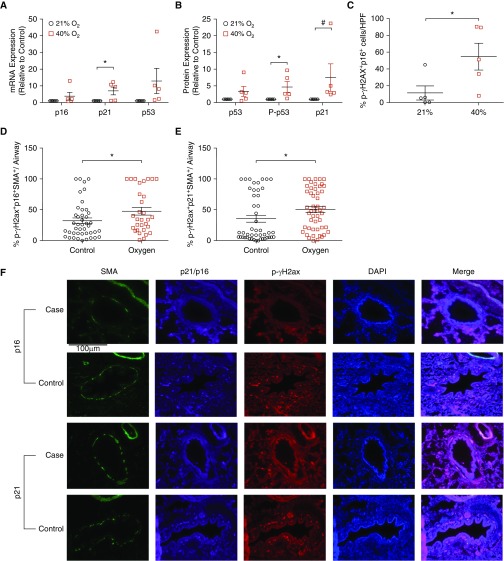
Real-time quantitative PCR analysis of cell cycle checkpoint genes demonstrated significantly elevated mRNA concentrations of p53 and p21 (Figure 2A) (P < 0.05); however, mRNA concentrations of p16 were not altered. Western blot analysis demonstrated significantly increased concentrations of cell cycle checkpoint proteins p21 and p-p53 (Ser15); however, total p53 concentrations were not increased (Figure 2B) (P < 0.05).
Figure 2.
Effect of hyperoxia on cell cycle and DNA damage markers. (A) Cell cycle checkpoint mRNA and (B) protein expression increased after 40% O2 for 7 days. p21 and p53 were affected (but not p16). Forty percent O2 elevated p-p53 (Ser15; B). Data are presented as mean ± SEM (n = 5 fetal ASM samples). P < 0.05. *#Significant differences from control. (C) Confocal microscopy of immunofluorescently stained fetal ASM cells showed increased nuclear colocalization of p16 and p-γH2A.X after exposure to hyperoxia for 7 days (relative to total DAPI-counterstained cells). n = 5 ASM samples with analysis of 10 high-power fields. P < 0.05. *#Significant differences from control. (D–F) Fluorescence microscopy of stained human autopsy samples demonstrated increased triple localization of both p21 and p16 with p-γH2A.X and SMA within airways from neonates exposed to at least 96 hours of oxygen compared with those who died within 24 hours of birth. *Significant differences between cases (n = 14) and control subjects (n = 15); P < 0.05. Scale bar: 100 μm. p-γH2A.X = phosphorylated γ-histone family member X; SMA = smooth muscle actin.
Increased p16 expression and phosphorylation of (p-γH2A.X) are additional established cellular senescence markers (21). Using immunofluorescence analysis, we measured p16 and p-γH2A.X in fetal ASM exposed to 21% or 40% O2 and found p16 and p-γH2A.X colocalized to the nucleus after hyperoxia (Figure 2C) (P < 0.05), with a significantly increased percentage of cells that coexpress p16 and p-γH2A.X (see Figure E 2A in the data supplement).
Similar findings were noted in autopsy specimens of neonates exposed to hyperoxia for at least 96 hours compared with those who died within 24 hours of birth. Elevated concentrations of p16 and p-γH2A.X colocalization (Figure 2D) (P < 0.05) and p21 and p-γH2A.X colocalization (Figure 2E) (P < 0.05) were noted in the ASM layer of airways denoted using ACTA2 triple-positive costaining (Figure 2F).
SASP Factors Include Inflammatory Cytokines and ECM Proteins
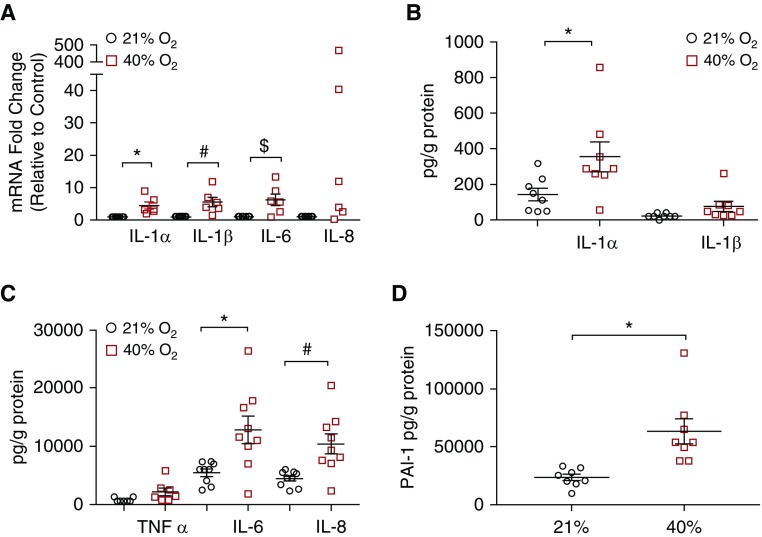
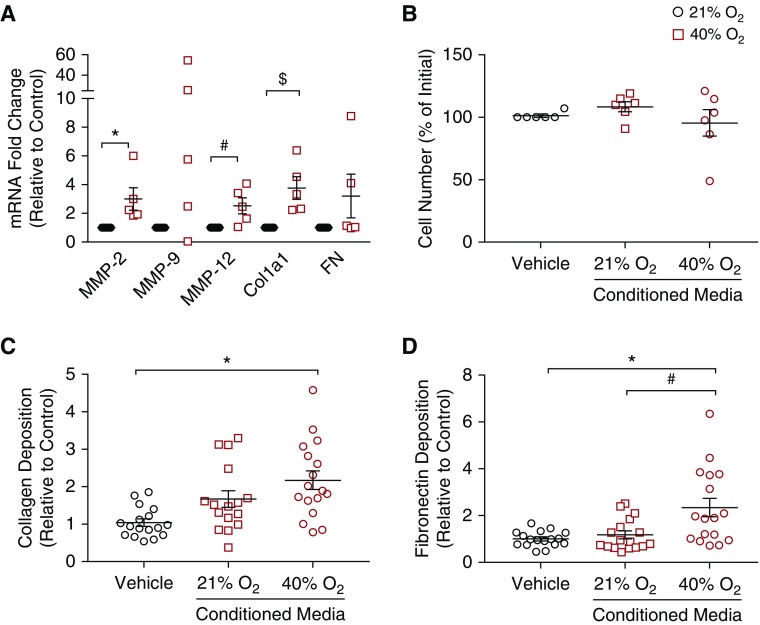
RT-PCR demonstrated increases in mRNA expression of several cytokine genes (IL-1α, IL-1β, IL-6) (P < 0.05) and a trend toward significance with IL-8 (Figure 3A). Using multiplex ELISA, protein secretion of inflammatory cytokines was measured. Exposure to 40% O2 significantly increased IL-1α, IL-6, and IL-8 protein concentrations in media (Figures 3B and 3C) (P < 0.05); however, IL-1β (Figure 3B) and TNF-α (Figure 3C) were unchanged. In addition, PAI-1, a marker of senescence, was significantly higher in cells exposed to 40% O2 (Figure 3D) (P < 0.05). mRNA of ECM-related genes matrix metalloproteinase 2 (MMP2), MMP12, and ECM structural protein collagen type I, α1 chain (ColIa1), was noted to be higher (Figure 4A) (P < 0.05); however, MMP9 and fibronectin mRNA transcripts were not increased in 40% O2-exposed cells (Figure 4A).
Figure 3.
Effect of hyperoxia on senescence-associated secretory phenotype. (A) mRNA transcript concentrations of inflammatory cytokines (IL-1α, IL-1β, and IL-6) were significantly elevated, and although IL-8 concentrations were not statistically different, there was a trend toward elevation. (B and C) ELISA of conditioned media from senescent airway smooth muscle cells (adjusted for protein content) revealed increased concentrations of secreted inflammatory cytokines and (D) the senescence marker PAI-1. IL-1α, IL-6, and IL-8 were significantly elevated, whereas TNF-α and IL-1β concentrations were not. n = 8 airway smooth muscle samples. P < 0.05. *#$Significant differences from control. PAI-1 = plasminogen activator inhibitor-1.
Figure 4.
Effect of hyperoxia and senescence-associated secretory phenotype on extracellular matrix (ECM) markers and proliferation. (A) Exposure to 40% O2 significantly increased the ECM-modifying genes matrix metalloproteinase 2 (MMP2) and MMP12 and structural element Col1a1, but MMP2 and fibronectin (FN) were not significantly affected. n = 5 ASM samples. P < 0.05. *#$Significant differences from control. (B) Exposure of naive cells to conditioned media from senescent cells (21% or 40% O2 for 7 d) did not increase proliferation. ECM deposition (measured using semiquantitative immunofluorescence; see Methods) was significantly altered, with increased concentrations of (C) collagen type I and (D) FN after 48 hours of exposure to conditioned media from senescent cells from the 40%/7-day group. n = 17 airway smooth muscle samples. P < 0.05. *#Significant differences.
Effect of SASP on Naive ASM
Senescent cells can have profound effects on surrounding cells by secreting proinflammatory factors (i.e., SASP), leading to impaired tissue function and disease (24). To investigate the effect of the SASP on fetal ASM parameters, we treated naive ASM with media from cells exposed to 40% O2 for 7 days and assessed remodeling and contractility. Cells treated with CM from 21% or 40% O2 cells did not have altered proliferation compared with those treated with fresh 1% FBS media (Figure 4B). There was more collagen I deposition by cells exposed to 40% O2 CM than by those treated with fresh media (Figure 4C) (P < 0.05). However, cells treated with 21% O2 CM were not different from those treated with either 40% O2 CM or fresh media in this regard (Figure 4C). Furthermore, ECM modification was noted with an increase in fibronectin deposition in those cultures exposed to 40% O2 CM compared with fresh media or 21% O2 CM (P < 0.05) (Figure 4D).
Traction force microscopy was used to assess the effect of SASP on ASM contractility (20). In contrast to cells exposed to fresh media or 21% O2 CM, ASM treated with 40% O2 CM demonstrated greater baseline contractile forces as expressed by the peak and root mean square traction (P < 0.05) (Figure 5).
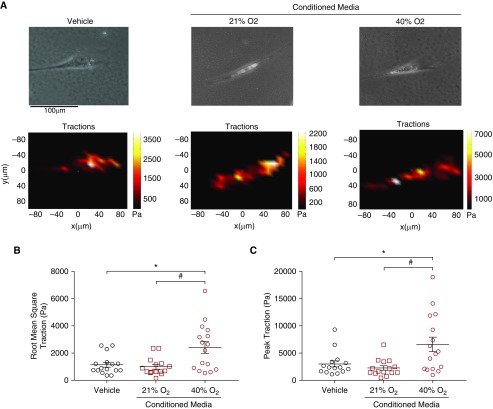
Figure 5.
Effect of the senescence-associated secretory phenotype on naive fetal ASM cell contractility. Cellular contractility was measured using traction force microscopy based on bead displacements of ASM exposed to 40% O2 conditioned media (CM), 21% O2 CM, or fresh 1% FBS media (vehicle) for 48 hours (representative samples shown in A). Scale bar: 100 μm. CM from senescent cells significantly enhanced (B) root mean square and (C) peak contractility of naive ASM. Peak traction was defined as the highest point of traction per individual cell, and root mean square traction was defined as average traction over the entire cell surface. n = 5 independent experiments with ASM, with analysis of at least three cells per well. P < 0.05. *#Significant differences.
Effect of D+Q Treatment on Reversal of Senescence Markers, ECM, and SASP Profile
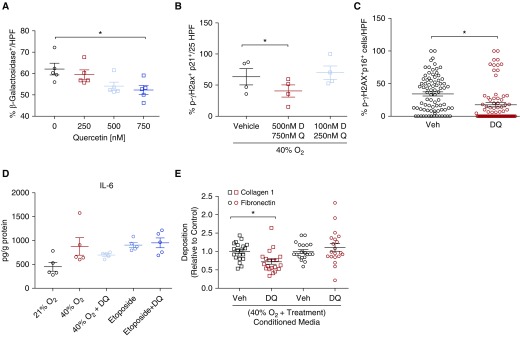
Dose–response studies using SA β-Gal staining as an endpoint demonstrated trends toward reduction with doses of quercetin of greater than 500 nM (Figure E1B) and several doses of dasatinib (Figure E1A). The combination of Q+D was noted to be synergistic, with a dose–response curve demonstrating significant decrease in SA β-Gal staining at a combination dose of 750 nM quercetin with 500 nM dasatinib (Figure 6A) (P < 0.05). Cell counts were not affected significantly by use of either medication or in combination (Figures E1C–E1E). Treatment with D+Q did reduce other molecular markers, including p21 and p-γH2A.X colocalization (Figure 6B) (P < 0.05) and p16 and p-γH2A.X colocalization (Figure 6C) (P < 0.001).
Figure 6.
Treatment with Q and D reverses senescence and associated effects. (A) Treatment with combination quercetin and dasatinib resulted in dose-dependent reduction in senescence-associated β-galactosidase activity. Immunofluorescence analysis of stained fetal ASM cells showed decreased nuclear colocalization of (B) p21 and p-γH2A.X and (C) p16 and p-γH2A.X. n = 4 independent experiments with ASM with analysis of at least 10 random HPF. (D) ELISA analysis of IL-6 secretion demonstrated a trend toward reduction in cells exposed to 40% O2/7 days and no change in those treated with etoposide to induce senescence. n = 4 independent experiments with ASM. (E) Extracellular matrix deposition (measured using semiquantitative immunofluorescence; see Methods) was significantly altered, with decreased concentrations of collagen type I but not fibronectin after 48 hours of exposure to conditioned media from drug-treated senescent cells compared with vehicle. n = 5 independent experiments with four replicates per group. *Significant difference from control.
The SASP profile did trend toward reduction in inflammatory mediators IL-6 (Figure 6D), IL-8, TNF-α, PAI-1, and TGF-β (Figures E2B–E2E). In addition, trends toward increased secretion of TGF-β, TNF-α, IL-8, and PAI-1 were noted when treated with etoposide; however, D+Q treatment did not appear to have an effect on reducing secretion of these proinflammatory and profibrotic mediators. Last, exposure to CM from D+Q-treated cells, compared with vehicle, resulted in reduced collagen I deposition (Figure 6E) (P < 0.01) with no change in fibronectin concentrations.
Discussion
Neonatal hyperoxia exposure has significant effects on airway structure and function in preterm infants that unfortunately persist several years after the initial exposure (2–6). The mechanisms responsible for airway dysfunction and thickening in preterm infants that lead to subsequent recurrent wheezing and asthma remain incompletely understood. Although hyperoxia can be expected to induce oxidant stress and thus activate a number of pathways relating to inflammation (13), hypercontractility (9), and fibrosis (8), antioxidants per se have not been shown to be effective (34). Furthermore, the extent of hyperoxia exposure (O2 concentration and/or duration) clearly colors the picture of which cell types are affected and what structure/function changes occur, with the understanding that high levels of O2 promote bronchopulmonary dysplasia (14, 34). Yet, wheezing and asthma continue to occur even with moderate hyperoxia exposure, underlining the fact that oxidant stress per se is an incomplete explanation. In this regard, senescent cells, which have recently been identified as significant contributors to chronic disease in aging (17–19), may have a role in facilitating the pathogenesis of airway disease (20, 27, 35, 36). Oxidative stress via environmental particles or cigarette smoke exposure has been shown to induce cellular senescence in multiple cell types in the lung (37, 38). Similar findings have also been suggested in the context of high levels of O2 in newborn mice, albeit within the alveolar compartment (27). To investigate this concept in the context of neonatal bronchial airway disease that persists even with moderate hyperoxia (3), we exposed human fetal ASM, a cell type that models the developing bronchial airway, to 40% O2 (vs. 21% O2; normoxia) for 7 days. Our results indicate that moderate hyperoxia induces senescence in human fetal ASM, as evidenced by expression of several senescence markers and enhanced concentrations of SASP factors. We also found that senescent cells influence the function of naive ASM, as indicated by increased ECM deposition and contractile forces in response to CM from ASM exposed to hyperoxia. Importantly, we found that senolytic drugs such as D+Q that can remove senescent cells may turn out to have alleviating effects on hyperoxia-induced promotion of ECM in developing ASM.
Senescence in the context of normal embryonic growth is an essential process driven by various signaling mechanisms leading to the orchestration of developmental events that result in the normal organ structure necessary for postnatal function (21). As such, we would expect to see low levels of senescence in premature lung tissue because processes such as airway, alveolar, and pulmonary vascular development have not completely matured. Our data do demonstrate a low level of senescence at baseline, with significant increases in cells exposed to hyperoxia. Indeed, the enhanced senescence in response to moderate hyperoxia should be interpreted bearing in mind that even normal room oxygen represents relative hyperoxia for the premature lung. Thus, if compensatory pathways are absent, moderate hyperoxia could result in an exaggerated (perhaps accelerated) senescence. What is relevant in our study is the paracrine effect of senescence inducing excessive formation of ECM and contractility, which, if it occurs in vivo (to be tested in future studies), would promote an asthmatic phenotype.
There are several markers of senescence that are related to the distinct characteristics of senescent cells, including lysosomal biogenesis (SA β-Gal), cell cycle arrest (p53-p21, p16), and DNA damage (p-γH2A.X) (19). Exposure to 40% O2 for 7 days increased cellular SA β-Gal activity, a histological marker of senescence. Furthermore, there are several pathways that lead to cell cycle arrest associated with senescence, depending on the type of insult (19, 21). Oxidative stress is known to induce senescence through p53-p21– or p16-mediated cell cycle arrest (19, 20, 27). Our studies show that expression of p-p53, p21, and p16 is increased both in our in vitro model and in autopsy samples (15, 21, 39, 40). We also observed increased p-γH2A.X, a DNA damage marker and upstream activator of the p53-p21/p16 pathways. Previous studies using much higher oxygen levels of 95% O2 have also reported increases in p53 and p21, resulting in cell cycle arrest in lung epithelial cell lines (41, 42) and bronchial smooth muscle cells (43). However, the induction of senescence or the resultant effect of senescence in naive cells has not been explored.
An important characteristic of senescent cells is that they can secrete a variety of inflammatory and profibrotic factors that can have detrimental paracrine effects and promote disease pathogenesis (23, 24, 44, 45). Exposure to 40% O2 increased expression of several proinflammatory mediators in fetal ASM, including IL-1α, IL-6, IL-8, and PAI-1. These inflammatory mediators have previously been demonstrated to exert both autocrine and paracrine effects on adult ASM contractility and remodeling (46), although their roles in developing airways have not been systematically explored. Expression of MMP2, COL1A1, and fibronectin was also increased by hyperoxia, which is consistent with our previous observation of oxygen’s effects on human fetal ASM (8).
Our results suggest that the SASP profile in developing ASM appears to be both inflammatory and fibrotic, which can adversely affect airway function and structure. Several inflammatory mediators that are part of the SASP are highly expressed during adult airway disease, and senescent cells have been detected in lungs from patients with cystic fibrosis, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease (20, 24, 45). Although senescent cells are believed to contribute to the overall inflammatory milieu in the airway, a causal role for this cell population has yet to be established. Even less is known about the presence of senescent cells and secretion of SASP factors during and after moderate hyperoxia.
Autocrine and paracrine effects of inflammatory mediators released by senescent cells in the airway could impact airway hypercontractility and remodeling by affecting surrounding cells. To model the effects of secretory factors from senescent cells on surrounding nonsenescent cells, we applied CM to naive cells and measured proliferation, ECM deposition, and contractile forces. Although ASM proliferation was not altered by 40% O2 CM, collagen I and fibronectin deposition was increased. CM from cells exposed to 40% O2 also increased baseline peak and root mean square traction. These results suggest an increase in baseline contractility; however, further investigation will be needed to determine if effects on responses to a bronchoconstrictor agent such as acetylcholine are also exaggerated. Furthermore, it is likely that hyperoxia effects and senescence involve other cell types, including epithelial cells and fibroblasts. Future studies should focus on the interaction between different airway cell types in the context of SASP effects, and in that setting an optimal methodology may be organoids exposed to insults such as supplemental oxygen and stretch as occur in the human neonatal lung in the context of intensive care.
Given their persistence and contribution to chronic inflammatory states, senescent cells have become an appealing cell population to target therapeutically. In recent years, several pharmacological strategies to selectively eliminate senescent cells in aging and chronic disease have been reported (17, 18). The combined use of D+Q was recently shown to eliminate senescent cells and reduce fibrosis in a bleomycin-induced lung fibrosis mouse model (35). We found that addition of D+Q to hyperoxia-exposed cells reduced the number of senescent cells, suggesting that this strategy may also be useful for targeting senescent cells in the developing airway.
There are some limitations of our work in that the in vitro specimens were obtained from deidentified abortuses at 18–22 weeks of gestation. Survival ex utero even at the latter end of that gestational period is rare. However, this does encapsulate the canalicular period of lung development and a period of rapid bronchial airway growth providing an adequate understanding of airway and ASM biology that can be extrapolated. In addition, we did not complete genetic studies on our specimens, because this was not within the scope of this study or our IRB approval. Although there remains a risk of bias in our data because of this, the experiments were completed with a variety of different donor cell lines (at least 12), thus reducing this risk in terms of the overall conclusions.
Overall, our data demonstrate that moderate levels of hyperoxia (30–50% O2), which is often administered to preterm infants, enhance cellular senescence with the factors secreted by such senescent cells promoting ECM formation and contractility of naive ASM. The relevance of these findings lies in understanding how early hyperoxia has long-standing influences on the growing lung in the context of wheezing and asthma observed in premature infants exposed to hyperoxia (Figure 7). Given the cell cycle arrest and metabolic characteristics of senescent cells, this population may be a contributor to the long-term effects of neonatal hyperoxia exposure even after the initial oxidant insults. Accordingly, developing strategies to identify enhanced (abnormal) cellular senescence in oxygen-exposed neonatal airways and approaches to selectively eliminate senescent cells are novel avenues for future research in the field of pediatric pulmonary medicine.
Figure 7.
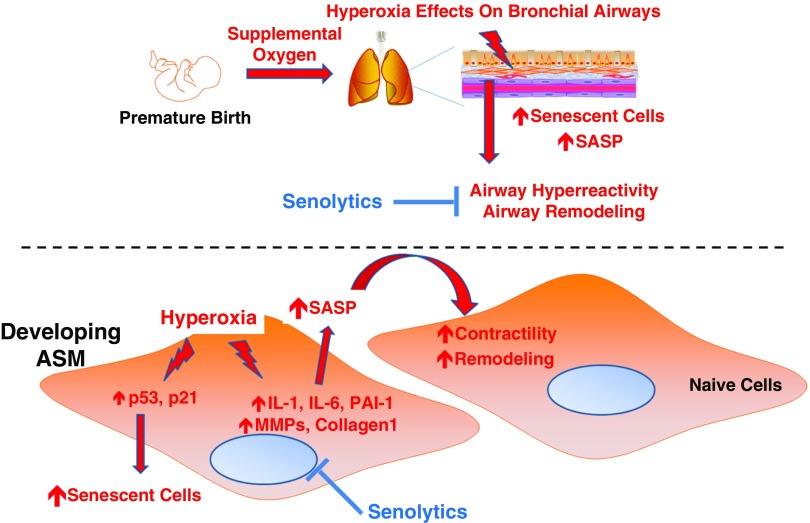
Proposed model. In developing ASM, insults such as hyperoxia (as would occur with premature infants requiring supplemental oxygen to maintain oxygenation) induce cellular senescence (involving increased markers of DNA damage and cell cycle arrest) with resultant increase in the secretion of proinflammatory and profibrotic factors (senescence-associated secretory phenotype [SASP]) that promote contractility and remodeling of the developing airway with detrimental effects. Removal of senescent cells using senolytic drugs may alleviate the effects of hyperoxia in the rapidly growing airway.
Footnotes
Supported by the Mayo Clinic Department of Obstetrics and Gynecology (P.P.); Mayo Clinic Clinical and Translational Science Award grant number UL1TR002377 from the National Center for Advancing Translational Sciences (R.D.B. and C.M.P.); National Institutes of Health grants K99 131682 (R.D.B.), R01 HL056470 (Y.S.P.), R01 HL138402 (C.M.P.), and R37 AG13925 (J.L.K.); and the Connor Group and the Noaber Foundation (J.L.K.).
Author Contributions: P.P., R.D.B., G.C.S., J.L.K., N.L., D.J.T., C.M.P., and Y.S.P. developed the concepts and experimental design; P.P., R.D.B., L.J.M., S.A.W., A.R., J.R., J.T., and M.A.T. performed experiments and analyzed data; P.P., L.J.M., and S.A.W. created figures/tables; P.P., R.D.B., and Y.S.P. wrote the first version of the manuscript; and all authors reviewed or provided edits to the final version, which was finalized by P.P., R.D.B., and Y.S.P.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0176OC on December 3, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Britt RD, Jr, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Expert Rev Respir Med. 2013;7:515–531. doi: 10.1586/17476348.2013.838020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vollsæter M, Clemm HH, Satrell E, Eide GE, Røksund OD, Markestad T, et al. Adult respiratory outcomes of extreme preterm birth: a regional cohort study. Ann Am Thorac Soc. 2015;12:313–322. doi: 10.1513/AnnalsATS.201406-285OC. [DOI] [PubMed] [Google Scholar]
- 5.Landry JS, Tremblay GM, Li PZ, Wong C, Benedetti A, Taivassalo T. Lung function and bronchial hyperresponsiveness in adults born prematurely: a cohort study. Ann Am Thorac Soc. 2016;13:17–24. doi: 10.1513/AnnalsATS.201508-553OC. [DOI] [PubMed] [Google Scholar]
- 6.Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J. 2014;43:808–816. doi: 10.1183/09031936.00039513. [DOI] [PubMed] [Google Scholar]
- 7.Thompson MA, Britt RD, Jr, Kuipers I, Stewart A, Thu J, Pandya HC, et al. cAMP-mediated secretion of brain-derived neurotrophic factor in developing airway smooth muscle. Biochim Biophys Acta. 2015;1853:2506–2514. doi: 10.1016/j.bbamcr.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel ER, Britt RD, Jr, Faksh A, Kuipers I, Pandya H, Prakash YS, et al. Moderate hyperoxia induces extracellular matrix remodeling by human fetal airway smooth muscle cells. Pediatr Res. 2017;81:376–383. doi: 10.1038/pr.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faksh A, Britt RD, Jr, Vogel ER, Kuipers I, Thompson MA, Sieck GC, et al. Effects of antenatal lipopolysaccharide and postnatal hyperoxia on airway reactivity and remodeling in a neonatal mouse model. Pediatr Res. 2016;79:391–400. doi: 10.1038/pr.2015.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harju M, Keski-Nisula L, Georgiadis L, Räisänen S, Gissler M, Heinonen S. The burden of childhood asthma and late preterm and early term births. J Pediatr. 2014;164:295–299.e1. doi: 10.1016/j.jpeds.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 11.McEvoy C, Venigalla S, Schilling D, Clay N, Spitale P, Nguyen T. Respiratory function in healthy late preterm infants delivered at 33-36 weeks of gestation. J Pediatr. 2013;162:464–469. doi: 10.1016/j.jpeds.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrijlandt EJ, Kerstjens JM, Duiverman EJ, Bos AF, Reijneveld SA. Moderately preterm children have more respiratory problems during their first 5 years of life than children born full term. Am J Respir Crit Care Med. 2013;187:1234–1240. doi: 10.1164/rccm.201211-2070OC. [DOI] [PubMed] [Google Scholar]
- 13.Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, et al. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L711–L719. doi: 10.1152/ajplung.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Jafri A, Martin RJ, Nnanabu J, Farver C, Prakash YS, et al. Severity of neonatal hyperoxia determines structural and functional changes in developing mouse airway. Am J Physiol Lung Cell Mol Physiol. 2014;307:L295–L301. doi: 10.1152/ajplung.00208.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17:324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 17.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkland JL, Tchkonia T. Cellular senescence: a translational perspective. EBioMedicine. 2017;21:21–28. doi: 10.1016/j.ebiom.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Childs BG, Durik M, Baker DJ, van Deursen JM. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Dong F, Wang RA, Wang J, Zhao J, Yang M, et al. Central role of cellular senescence in TSLP-induced airway remodeling in asthma. PLoS One. 2013;8:e77795. doi: 10.1371/journal.pone.0077795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maciel-Barón LA, Morales-Rosales SL, Aquino-Cruz AA, Triana-Martínez F, Galván-Arzate S, Luna-López A, et al. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr) 2016;38:26. doi: 10.1007/s11357-016-9886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar M, Seeger W, Voswinckel R. Senescence-associated secretory phenotype and its possible role in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2014;51:323–333. doi: 10.1165/rcmb.2013-0382PS. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Londhe VA, Sundar IK, Lopez B, Maisonet TM, Yu Y, Aghai ZH, et al. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res. 2011;69:371–377. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimova TA, Bell EL, Shroff EH, Weinberg FD, Snyder CM, Dimri GP, et al. Hyperoxia-induced premature senescence requires p53 and pRb, but not mitochondrial matrix ROS. FASEB J. 2009;23:783–794. doi: 10.1096/fj.08-114256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Britt RD, Jr, Thompson MA, Kuipers I, Stewart A, Vogel ER, Thu J, et al. Soluble guanylate cyclase modulators blunt hyperoxia effects on calcium responses of developing human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2015;309:L537–L542. doi: 10.1152/ajplung.00232.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinković A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am J Physiol Lung Cell Mol Physiol. 2012;303:L169–L180. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogel ER, VanOosten SK, Holman MA, Hohbein DD, Thompson MA, Vassallo R, et al. Cigarette smoke enhances proliferation and extracellular matrix deposition by human fetal airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;307:L978–L986. doi: 10.1152/ajplung.00111.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linkert M, Rueden CT, Allan C, Burel JM, Moore W, Patterson A, et al. Metadata matters: access to image data in the real world. J Cell Biol. 2010;189:777–782. doi: 10.1083/jcb.201004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pabelick CM, Thompson MA, Britt RD., Jr Effects of hyperoxia on the developing airway and pulmonary vasculature. Adv Exp Med Biol. 2017;967:179–194. doi: 10.1007/978-3-319-63245-2_11. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, et al. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight. 2016;1:e87732. doi: 10.1172/jci.insight.87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jobe AH, Kallapur SG. Long term consequences of oxygen therapy in the neonatal period. Semin Fetal Neonatal Med. 2010;15:230–235. doi: 10.1016/j.siny.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, et al. Impaired mitophagy leads to cigarette smoke stress-induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Büchner N, Ale-Agha N, Jakob S, Sydlik U, Kunze K, Unfried K, et al. Unhealthy diet and ultrafine carbon black particles induce senescence and disease associated phenotypic changes. Exp Gerontol. 2013;48:8–16. doi: 10.1016/j.exger.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Johmura Y, Nakanishi M. Multiple facets of p53 in senescence induction and maintenance. Cancer Sci. 2016;107:1550–1555. doi: 10.1111/cas.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Georgakilas AG, Martin OA, Bonner WM. p21: a two-faced genome guardian. Trends Mol Med. 2017;23:310–319. doi: 10.1016/j.molmed.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Rancourt RC, Staversky RJ, Keng PC, O’Reilly MA. Hyperoxia inhibits proliferation of Mv1Lu epithelial cells independent of TGF-β signaling. Am J Physiol. 1999;277:L1172–L1178. doi: 10.1152/ajplung.1999.277.6.L1172. [DOI] [PubMed] [Google Scholar]
- 42.Rancourt RC, Hayes DD, Chess PR, Keng PC, O’Reilly MA. Growth arrest in G1 protects against oxygen-induced DNA damage and cell death. J Cell Physiol. 2002;193:26–36. doi: 10.1002/jcp.10146. [DOI] [PubMed] [Google Scholar]
- 43.Shenberger JS, Dixon PS. Oxygen induces S-phase growth arrest and increases p53 and p21WAF1/CIP1 expression in human bronchial smooth-muscle cells. Am J Respir Cell Mol Biol. 1999;21:395–402. doi: 10.1165/ajrcmb.21.3.3604. [DOI] [PubMed] [Google Scholar]
- 44.Özcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 2016;8:1316–1329. doi: 10.18632/aging.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damera G, Panettieri RA., Jr Does airway smooth muscle express an inflammatory phenotype in asthma? Br J Pharmacol. 2011;163:68–80. doi: 10.1111/j.1476-5381.2010.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]