Abstract
Background
Exercise-based cardiac rehabilitation (ebCR) often includes various psychological interventions for lifestyle change or distress management. However, the additional benefit of specific psychological interventions on depression, anxiety, quality of life, cardiac morbidity and cardiovascular or total mortality is not well investigated.
Design
Systematic review and meta-analysis.
Methods
Randomized controlled trials and controlled cohort trials published between January 1995 and October 2017 comparing ebCR with or without pre-specified psychosocial interventions were selected and evaluated on the basis of predefined inclusion and outcome criteria.
Results
Out of 15,373 records, 20 studies were identified, including 4450 patients with coronary artery disease (88.5%) or congestive heart failure (11.5%), respectively. Studies were of low to moderate quality and methodological heterogeneity was high. As compared with ebCR alone, additional psychological interventions for lifestyle change or distress management showed a trend to reduce depressive symptoms (standardized mean difference –0.13, 95% confidence interval (CI) –0.30; 0.05). Furthermore, during a follow-up of five years, distress management was associated with a trend to reduce cardiac morbidity (risk ratio 0.74, 95% CI 0.51; 1.07). There was no evidence for an additional impact of either psychological lifestyle change interventions or distress management on anxiety, quality of life, cardiovascular or total mortality.
Conclusions
Specific psychological interventions offered during ebCR may contribute to a reduction of depressive symptoms and cardiac morbidity, but there remains considerable uncertainty under which conditions these interventions exert their optimal effects. (CRD42015025920).
Keywords: Cardiac, secondary prevention, rehabilitation, psychological interventions, quality of life, depression, anxiety, morbidity, mortality, systematic review
Introduction
There is robust evidence that physical exercise is effective in primary and secondary prevention of cardiovascular disease (CVD).1,2 Hence, the most prominent concept in cardiac rehabilitation in western countries is exercise-based cardiac rehabilitation (ebCR), which additionally may include education, support of individual lifestyle changes, evidence-based medication and various psychological interventions, then defined as ‘multi-component’ or ‘multimodal rehabilitation’.
Previous meta-analyses on ebCR reported reductions in cardiovascular morbidity,3–6 cardiovascular mortality3,4,7,8 and total mortality.3,4,9 One meta-analysis on ebCR also reported positive effects on depressive symptoms,5 and quality of life (QoL) also may be improved by ebCR.8 Importantly, there is strong evidence that the clinical effectiveness of ebCR depends on dose and intensity3,7–9 and moreover may be influenced by the clinical characteristics of ebCR participants. For example patients after an acute cardiovascular event may especially benefit from early ebCR participation.3,8,9 These considerations may at least partly explain neutral results on all-cause mortality in a recent reevaluation of the latest Cochrane analysis focusing on randomized controlled trials (RCTs) published in 2000 or later, and also including studies without clinical events of interest during follow-up.10 In this reevaluation, however, only seven out of N = 22 studies focused on patients after acute coronary syndrome (ACS) exclusively, but an analysis of this important subgroup has not been published.10
These uncertainties with respect to minimal requirements on ebCR content and volume to improve clinical outcomes underscore the need to critically reevaluate all therapeutic interventions delivered during ebCR. This also includes specific psychological interventions (e.g. psychologically supported lifestyle changes and various types of distress management).1,11
Previous meta-analyses have demonstrated beneficial effects of psychological interventions on symptoms of depression, anxiety, distress and QoL.12–16 However, effects on cardiovascular morbidity, cardiovascular mortality and total mortality were inconclusive, with predominantly older meta-analyses showing positive effects.12–14 One recent meta-analysis could demonstrate a significant reduction of cardiovascular mortality (risk ratio (RR) 0.79, 95% confidence interval (CI) 0.63; 0.98), while the rates of coronary revascularizations, non-fatal myocardial infarctions and total mortality were not significantly reduced.15 Another recent meta-analysis, specifically examining effects of cognitive-behavioural therapy, showed a non-significant trend to reduce cardiac events, whereas data on cardiovascular mortality were not available.16
Still, current guidelines and position papers on CVD secondary prevention1,11 univocally recommend so called ‘multimodal interventions’, combining structured exercise training not only with medical supervision, information and education but also with specific psychological interventions (e.g. stress management, coping support, or psychological interventions to facilitate lifestyle change). However, the added value of these specific psychological interventions on top of ebCR has not been thoroughly evaluated, as control groups in all previous meta-analyses on psychological interventions12–15 were heterogeneous, comprising ‘usual care’ as well as ebCR. In addition, so called ‘psychological interventions’ often were combined with some form of physical exercise. Thus, to our best knowledge, until now no meta-analysis has specifically focused on the effects of well-defined psychological interventions on top of ebCR compared with ebCR alone.
In addition, there is uncertainty as to which different kinds of psychological interventions, for example, psychologically supported lifestyle change or stress management, may result in favourable outcomes with respect to mental wellbeing, quality of life, cardiovascular morbidity and mortality. Differential effects of various psychological interventions have been addressed in only one meta-analysis, comparing ‘educational’, ‘behavioural’, ‘cognitive’, ‘relaxation’ and ‘support’ or combinations of the aforementioned interventions compared with ‘usual care’ or ebCR.13 According to this meta-analysis all psychological interventions reduced anxiety, and ‘behavioural’ and/or ‘cognitive’ interventions also reduced depressive symptoms. ‘Behavioural interventions’ additionally resulted in a tendency to reduce total mortality and non-fatal myocardial infarction.13 However, the interpretation of this meta-analysis is hampered due to an inconsistent allocation of ‘exercise training’ to either intervention or control groups.
Finally, many previous meta-analyses on ebCR or psychological interventions included studies published before 1995,6,12–15 thus, they could not control for the effects of modern pharmacotherapy and invasive interventions, and effects on morbidity/mortality might be overestimated.
The objective of this systematic review therefore was to evaluate the current efficacy of additional, well defined psychological interventions compared with ebCR alone on depression, anxiety, QoL, cardiovascular morbidity, cardiovascular mortality and total mortality in CVD patients.
Methods
The study protocol was published in advance in the Prospero registry (CRD42015025920). The meta-analysis was performed in accordance to the Cochrane handbook for systematic reviews of interventions17 and reported following the PRISMA guidance.18
Data searches and sources
We performed a systematic literature search along predefined search terms from January 1995 to October 2017 (for details see Supplementary Material Table 1 online). The following databases have been used: PubMed, Embase, Cochrane Library, Web of Science Core Collection, CINAHL, Pycinfo, Current Contents Medicine, and ClinicalTrials.gov. Moreover, reference lists of recent meta-analyses and potentially eligible studies were screened for additional publications of interest. EndNote X7 was used for literature management.
Study selection criteria
The study selection process is outlined in Figure 1. Two trained doctoral students (CK, NM, supervised by CA and CHL) independently screened all titles and abstracts for relevant trials, excluded irrelevant studies and assessed the remaining trials for eligibility using full texts. The resulting trials were included into a qualitative synthesis by two independent experts (CA, CHL), who had to agree in their judgement in order to select publications for the quantitative analysis. The final selection was performed by a consensus of the two experts. Study selection had to meet the following study selection criteria:
Study design. RCTs or controlled cohort trials (CCTs), with a minimum follow-up period of six months, were included.
Patients. Age ≥ 18 years, men and women, with coronary artery disease (CAD) including patients with stable CAD, after ACS, including myocardial infarction (MI), percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), and patients with clinically stable congestive heart failure (CHF) of ischaemic or non-ischaemic origin.
Intervention. Exercise-based cardiac rehabilitation plus any of the following specific psychological interventions: lifestyle change support, coping support, social support, distress management (e.g. psychological intervention addressing work or family stress, anxiety, depression) or relaxation therapy, or combinations of the aforementioned psychological interventions. All interventions had to be based on established psychological principles and had to be delivered by trained professionals. If there was an index cardiac event, the intervention (ebCR + psychological intervention) had to start within six months thereafter.
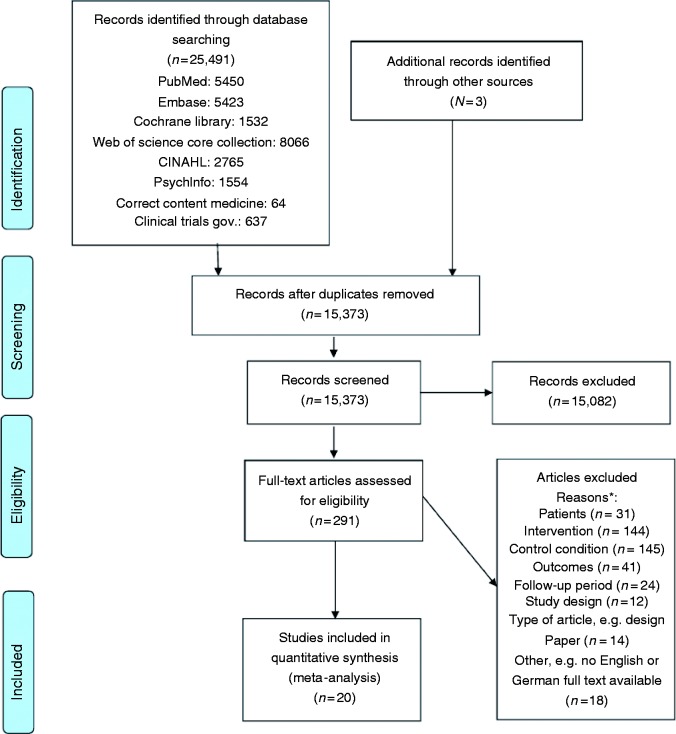
Figure 1.
PRISMA study flow chart (inclusion period January 1995 to October 2017).
*Note: articles may be excluded for more than one reason.
For specification of the psychological interventions the following subgroups were defined:
–Psychologically supported lifestyle change, that is, interventions to facilitate lifestyle change;
–Distress management, that is, interventions to specifically address stress, anxiety or depression via coping support, social support and stress management with or without relaxation therapies;
–Lifestyle change plus distress management, that is, a combination of the aforementioned interventions.
4. Control-intervention. ebCR, which may include education, medical and brief psychological advice, but no specific psychological interventions as defined above. There were no minimum requirements with respect to intensity and/or duration of ebCR.
5. Outcomes. Depression, anxiety, QoL, cardiovascular morbidity, cardiovascular mortality and total mortality. Depression, anxiety and QoL had to be assessed with validated psychometric instruments. Cardiovascular morbidity, cardiovascular mortality and total mortality had to be assessed by clinical records or official databases.
Data extraction and management
Data were extracted by two biometricians independently (MH, KJ). Disagreements were resolved by discussion. The data extraction table was optimized by using three selected trials for pilot testing. For dichotomous outcomes the number of total participants and the number of participants with an event and for continuous outcomes, the mean, standard deviation and sample size were extracted and stratified by intervention group. The following data were extracted in addition: name of the first author, year of publication, subgroup allocation, study design, sample size (randomized and analysed), intervention duration, follow-up period, time periods for data collection, measuring instruments and risk of bias.
Risk of bias assessment
The risk of bias for each study was assessed by the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials.17 In concordance with Anderson et al.,8 three further criteria for assessing the risk of bias were investigated:
Appropriate balance of participants’ baseline characteristics between intervention and control group;
Implementation of an intention-to-treat analysis;
Balance of participants’ treatment at baseline between intervention and control group (except specific psychological interventions).
Statistical analysis
Meta-analyses were performed overall but also separately with regard to the predefined interventions: psychologically supported lifestyle change, distress management, and lifestyle change plus distress management. For binary outcomes, as effect measures, RRs with their 95% CIs were chosen (RR < 1 indicating a lower risk in favour of the intervention). For continuous outcomes, Hedges’ g as the standardized mean difference (SMD) with its 95% CI was used as the effect measure.
Treatment-associated change scores from baseline and their differences between the treatment groups had been rarely reported in the included trials. Therefore, final values were used in the meta-analyses. In Focht et al.,19 QoL results for men and women were combined in order to get a mean and a standard deviation for the whole patient group, using the formulas provided by the Cochrane Handbook.17 All reported follow-up time points (one, three, six, nine, 12, 24, 36 and 60 months) were taken into consideration for data extraction.
RRs were pooled using the Mantel–Haenszel method, and SMDs using the inverse-variance method. Anticipating a relevant heterogeneity between the ‘true’ effects of the various interventions evaluated in the included studies, a random-effects model according to the Hartung–Knapp adjustment20 has been applied. As sensitivity analysis, the results of the fixed-effect model were calculated additionally.
All results were checked for statistical heterogeneity by I2 statistics with 50–75% representing substantial heterogeneity and 75–100% representing considerable heterogeneity. Potential publication bias was planned to be investigated by visual examination of funnel plots and statistical tests on asymmetry. Nevertheless, funnel plot asymmetry could not be assessed because of the low number of studies at each follow-up time point.
Sensitivity analyses were done incorporating the baseline values and apart, concentrating on RCTs. With respect to baseline values, we assumed a correlation estimate of 0.7 when deriving missing standard deviations for the changes from baseline per treatment group and used the formula provided by the Cochrane Handbook.17 In order to assess the impact of CCTs on the overall effects a sensitivity analysis with only RCTs was done for each outcome where possible.
Beyond the predefined interventions the data available did not allow to analyse additional characteristics of individual studies or patient subgroups (e.g. predefined psychosocial condition, sex).
R version 3.4.4 (R Foundation for Statistical Computing, 2018) and the R meta package version 4.9-1 (developed by Guido Schwarzer) were used for statistical analyses.
Results
Study selection
The PRISMA flow chart is shown in Figure 1. In summary, extensive database and additional hand-search resulted in 15,373 records, from which 15,082 were excluded. Two hundred and ninety-one full-text articles were assessed for eligibility and qualitative analysis, and 21 publications based on 20 studies were finally included into the quantitative synthesis.
Study characteristics
Study design
There were 17 RCTs19,21–37 and three CCTs,38–40 one of which had a cross-sectional design.38 The study size ranged from N = 70–1127 patients in total and follow-up periods ranged from six months up to five years. The only cross-sectional study covered a follow-up period from two up to 36 months. In five studies the follow-up time did not exceed the duration of the intervention.27,30,35,37,40 Two reports were based on the same cohort,27,29 but reported on different outcomes and therefore were included into the final selection. For further details see Table 1.
Table 1.
Characteristics of all studies included into the meta-analysis.
| Study | Design | N | Patients Clinical condition; gender; mean age | Intervention Content; total length and hours of specific psychosocial intervention | Control Content; total length; hours | Outcomes | Follow-up (max.) |
|---|---|---|---|---|---|---|---|
| ebCR plus psychological lifestyle change support (psyLC) | |||||||
| Chair et al., 2013 | RCT | 146 | CAD 68.5% male; 66.4 years Poor motivation | ebCR + psyLC 6 months; 3 h | ebCR 6 months; 55 h | Depression, anxiety, QoL | 12 months |
| Focht et al., 2004 | RCT | 147 | CAD (75%) 52.3% male; 64.8 years | ebCR + psyLC 9 months; 18 h | ebCR 3 months; ≥ 36 h | QoL | 12 months |
| Peerson et al., 2017 | CCT/CS | 1127 | MI, CABG, PCI 79.0% male; 61.6 years | ebCR + psyLC 6 months; n.a. | ebCR n.a. | Depression, anxiety, QoL | 2–36 months |
| Scholz et al., 2006 | RCT | 198 | MI, CABG 82.3% male; 58.5 years | ebCR + psyLC 1.5 months; n.a | ebCR 1 month; n.a. | Depression | 12 months |
| ebCR plus distress management (DM) | |||||||
| Andersson et al., 2010 | RCT | 130 | MI, PCI, CABG 100% female; 53.5 years | ebCR + DM 5 years; n.a. | ebCR 1 month; n.a. | Depression, QoL, emergency visits | 5 years |
| Barth et al, 2006 | RCT | 59 | CAD, ACS, PCI, CABG With depression 76.3% male; 58.2 years | ebCR + DM 1 month; 4–6 h | ebCR 1 month | Anxiety, depression, QoL | 24 months |
| Blumenthal et al., 2016 | RCT | 151 | CAD, ACS, PCI, CABG 63% male; 61.0 years | ebCR + DM 3 months; 18 h | ebCR 3 months; n.a. | Depression, anxiety, hospitalization, CV events, overall mortality | 5 years |
| Brügemann et al., 2007 | RCT | 137 | PCI, CABG 100% male; 57.0 years No psychosocial problems | ebCR + DM 2 months; n.a. | ebCR 1.5 months; 11 h | QoL | 9 months |
| Karlsson et al., 2007 | RCT | 224 | MI, CABG 77% male; 63.5 years | ebCR + DM 1 year; 40 h | ebCR 3 months; ≥ 27 h | Anxiety, depression, QoL | 12 months |
| Neves et al., 2009 | RCT | 81 | CAD, MI 85.5% male; 59.5 years | ebCR + DM 3 months; 36 h | ebCR 3 months; 40 h | Hospitalization, overall mortality | 2 years |
| O’Rourke et al., 1999 | CCT | 70 | MI 72.8% male; 58.5 years | ebCR + DM 1.5 months; n.a. | ebCR 2 months; n.a. | Depression, anxiety | 6 months |
| Plüss et al., 2011 | RCT | 224 | MI, CABG 77.5% male; 63 years No mental disorder | ebCR + DM 1 year; 40 h | ebCR 3 months; ≥ 27 h | Hospitalization, CV events, CV mortality, overall mortality | 5 years |
| Raghuram et al., 2014 | RCT | 250 | CABG 100% male; 53.0 years No mental disorder | ebCR + DM 12 months; n.a. | ebCR 12 months; > 100 h | Depression, anxiety | 12 months |
| Rugulies et al., 1996 | CCT | 74 | CAD, MI, CABG 88% male; 57 years | ebCR + DM 12 months; 100 h | ebCR 1 month; n.a. | Depression | 12 months |
| van Dixhoorn et al., 1999 | RCT | 156 | MI, CABG n.a. | ebCR + DM 1.5 months; 6 h | ebCR 1 month; ≥ 12.5 h | Hospitalization, CV events, CV mortality | 5 years |
| ebCR plus psychological lifestyle change support (psyLC) and distress management (DM) | |||||||
| Beckie et al., 2011 | RCT | 252 | MI, CABG, PCI 100% female; 63.5 years | ebCR + psyLC + DM 3 months; 13 h | ebCR 3 months; 36 h | Depression | 9 months |
| Black et al., 1998 | RCT | 60 | CAD, ACS, PCI, CABG 88% male; 60.2 years Psychosocial distress | ebCR + psyLC + DM Up to 2 months; 1–7 h | ebCR 2 months; n.a. | Depression, hospitalization | 12 months |
| Meng et al., 2016 | RCT | 513 | CHF 77% male; 61.5 years | ebCR + psyLC + DM 3 weeks; 5 h | ebCR 3 weeks; n.a. | QoL | 12 months |
| Pfaeffli Dale et al., 2015 | RCT | 123 | CAD, MI, PCI/CABG 81.3% male; 59.5 years | ebCR + psyLC + DM 5 months; web-based; n.a. | ebCR 1.5–6 months; n.a. | Depression, anxiety | 6 months |
| Segbregts et al., 2005 | RCT | 204 | MI, CABG 86.5% male; 56.4 years | ebCR + psyLC + DM 2 months; 20 h | ebCR 1.5 months; n.a. | Depression, CV events | 9 months |
| Vahedian-Azimi et al., 2016 | RCT | 70 | MI 67.5% male; 61.3 years | ebCR + psyLC + DM 24 months; 10 h plus 21 webinars | ebCR n.a. | Anxiety, QoL, CV events, overall mortality | 24 months |
Studies are displayed separated into three subgroups: exercise-based rehabilitation (ebCR) plus psychological lifestyle change support (psyLC) (four of 20), ebCR plus distress management (DM) (10 of 20), and ebCR plus psyLC and DM (six of 20).
RCT: randomized controlled trial; CCT: controlled cohort trial; CS: cross-sectional design; CAD: stable coronary artery disease; MI: myocardial infarction; ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; CHF: congestive heart failure; ebCR: exercise-based cardiac rehabilitation; psyLC: psychological lifestyle change support; DM: distress management; QoL: quality of life; CV: cardiovascular; n.a.: not available
Populations
In general, populations were of high heterogeneity. Most studies (16 out of 20) included mixed CAD populations (stable CAD, MI, PCI or CABG); the remaining studies included only patients with either stable CAD,19,21 after MI37,39 or after CABG.30 One study included patients with chronic systolic CHF.34 For further details see Table 1.
Interventions
There was a considerable heterogeneity with respect to duration and content of the psychological interventions (duration: 1–60 months; time actively being spent for intervention: 1–100 h). For further details see Table 1.
Controls
There was also a considerable heterogeneity with respect to duration and intensity of ebCR (duration: 1–12 months; time spent for ebCR: 11–100 h), but always being on a par with the corresponding intervention group (ebCR plus psychological intervention). For further details see Table 1.
Outcomes
Thirteen studies provided data on depression,21–25,27,30,32,33,35,36,39,40 eight studies on anxiety21,24,25,27,30,35,37,39 and nine on QoL.19,21,23,24,26,27,34,37,38 All psychological outcomes were assessed by validated instruments (for details see Table 1). For methodological reasons data on depression of five studies23,25,33,38,40 were excluded from the meta-analysis. The same was true for data on anxiety from two trials25,37 and data of two trials on QoL23,38 (for details see Supplementary Table 2).
Because of the diversity of measuring tools for evaluating QoL, neither overall effects nor intervention subgroup effects were calculated. On the basis of comparable instruments and similar follow-up periods only two studies in the lifestyle change intervention subgroup19,21 and two studies in the distress management subgroup26,27 could be pooled.
Eight studies reported data on cardiovascular morbidity.23,25,28,29,31,33,36,37 Cardiovascular morbidity was measured using different, mostly combined endpoints, ranging from any (non-fatal) acute cardiovascular event including PCI/CABG to stroke or peripheral revascularization, and emergency visits. Apart from that, some studies reported the total number of events and some counted the number of patients with at least one event. These two types of measurements cannot be combined in a meta-analysis. Supplementary Table 3 shows a detailed overview of the measurements of morbidity. Due to only few studies with morbidity data and the diversity of morbidity definitions, an overall effect and intervention subgroup effects could not been calculated.
Three studies reported total mortality,25,28,37 one study cardiovascular mortality31 and one study both.29 Because these endpoints are represented by only a low number of events an overall effect could not be calculated.
Study quality
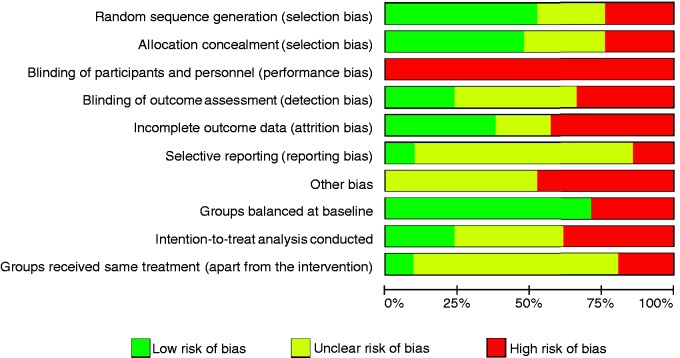
Overall, the methodological quality of studies included was moderate to low, even without taking into account performance bias, which cannot be avoided in psychological interventions. The risk of bias is summarized in Figure 2 and Supplementary Table 4. The three CCTs were graded as trials with a high risk of bias.
Figure 2.
Summary of the risk of bias in studies included into the meta-analysis.
Depression
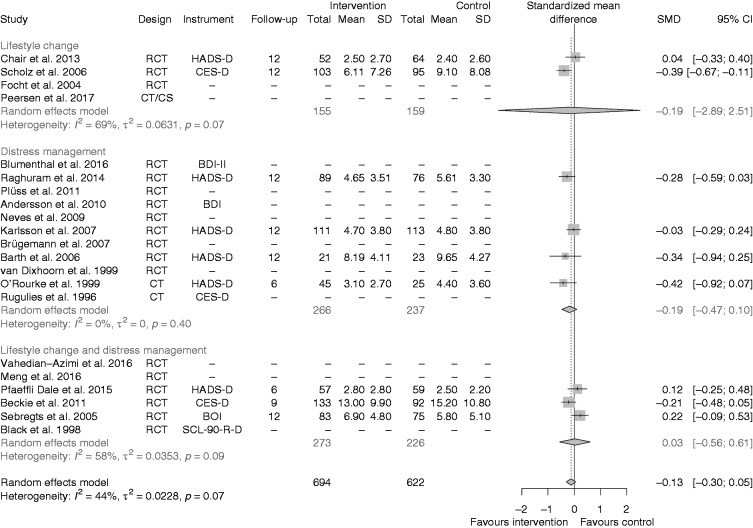
The effect on depressive symptoms of all kinds of psychological interventions was in favour of the intervention, although it did not reach statistical significance (SMD –0.13, 95% CI –0.3; 0.05 (Figure 3).
Figure 3.
Effects of the interventions on depression, all studies together, and separated for studies on lifestyle change, distress management and both interventions together.
RCT: randomized controlled trial; CT: cohort trial; CS: cross-sectional; SMD: standardized mean difference; CI: confidence interval
In the lifestyle change intervention subgroup the pooled estimate supported the intervention (SMD –0.19, 95% CI –2.89; 2.51). The CI of the random-effects model was very wide due to only two RCTs for heterogeneity estimation,21,22 resulting in substantial heterogeneity (I2 = 69%).
In the distress management intervention subgroup there were three RCTs24,27,30 and one CCT39 providing depression data for a subgroup meta-analysis and the estimated pooled effect favoured the intervention (SMD –0.19, 95% CI -0.47; 0.10). Although the effects of the CCT39 were slightly higher compared with the RCTs, no statistical heterogeneity was revealed (I2 = 0%). The three RCTs of the lifestyle change plus distress management intervention subgroup32,35,36 did not show any treatment effect (SMD 0.03, 95% CI –0.56; 0.61), but substantial statistical heterogeneity (I2 = 58%).
A sensitivity analysis was performed by including the reported baseline values for depression and then calculating the changes from baseline. The overall effect was still in favour of the intervention (see Supplementary Figure 1). In another sensitivity analysis the overall effect of RCTs only was calculated. The overall effect estimate changed only slightly to –0.10 (95% CI –0.29; 0.08) in the random-effects model and was still in favour of the intervention. The result of the fixed-effect model was similar and offered no further insight.
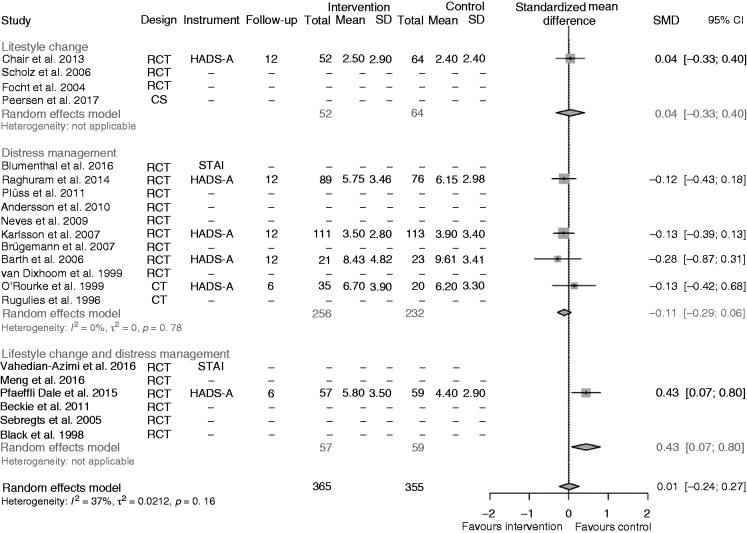
Anxiety
No difference for the overall treatment effect on anxiety was found (SMD 0.01, 95% CI –0.24; 0.27; Figure 4). The only study in the lifestyle change intervention subgroup21 showed nearly no difference between the intervention and the control condition (SMD 0.04, 95% CI –0.33; 0.40). In the distress management subgroup24,27,30,39 there was a small but insignificant effect in favour of the intervention group (SMD –0.11, 95% CI –0.29; 0.06), although the effect in the only CCT in this subgroup39 was in the opposite direction. The only trial in the lifestyle change and distress management intervention subgroup35 had a significant effect in favour of the control intervention (SMD 0.43, 95% CI 0.07; 0.80).
Figure 4.
Effects of the interventions on anxiety, all studies together, and separated for studies on lifestyle change, distress management, and both interventions together.
RCT: randomized controlled trial; CT: cohort trial; CS: cross-sectional; SMD: standardized mean difference; CI: confidence interval
The sensitivity analysis with baseline-corrected treatment effects led to a similar overall result (for further details see Supplementary Figure 2). Excluding the one CCT with data on anxiety39 led to an overall effect of only RCTs of 0.00 (95% CI –0.32; 0.32) in the random-effects model. Similarly the application of a fixed-effect model showed no effect.
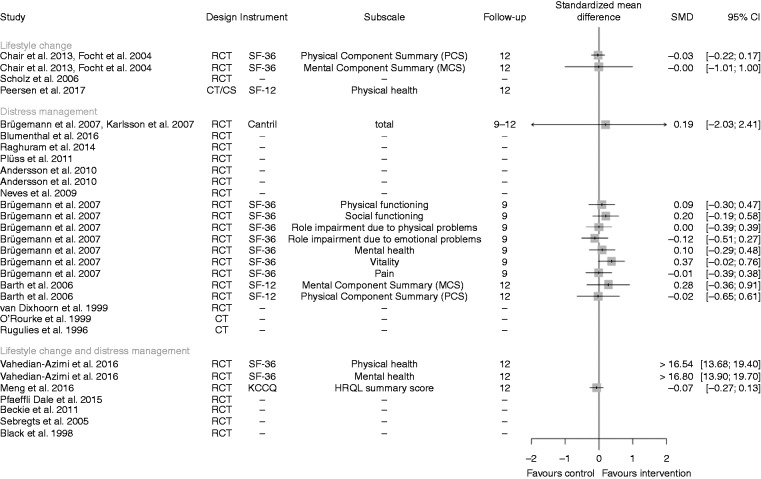
QoL
A summary of all data included on QoL is displayed in Figure 5. Seven treatment effects assessed in four RCTs 24,26,27,37 favoured the psychological interventions (SMD ≥ 0.1), one treatment effect in one RCT26 favoured the control (SMD ≤ –0.1) and seven results from four RCTs19,21,24,26 were indifferent (SMD > –0.1 and < 0.1). The extreme values of Vehedian-Azimi et al.37 are considered at high risk of bias because there was a strong and long-lasting steady improvement with regard to both SF-36 component scores for the intervention group. In contrast, the values of the control group decreased directly after starting the study and remained unchanged thereafter.
Figure 5.
Summary of all data on quality of life, separated for lifestyle change, distress management, and a combination of both interventions.
Note: The pooled effect of Brügemann et al. (2007) and Karlsson et al. (2007) is based on a random effects model.
A sensitivity analysis considering the baseline values resulted in seven studies which favoured the psychological interventions (SMD > 0.1), but now five studies favoured the control intervention (SMD < –0.1). For further details see Supplementary Figure 3.
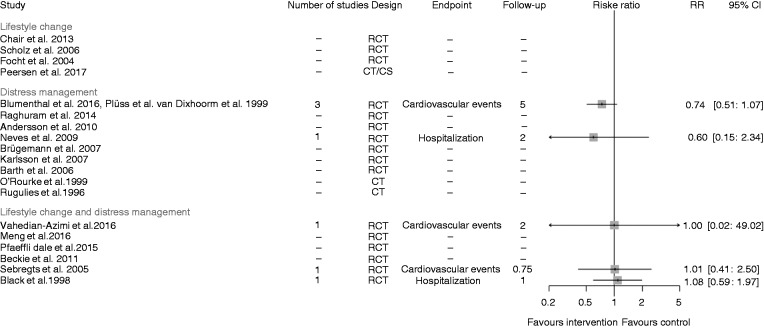
Morbidity
Figure 6 visualizes the treatment effects per study in a forest plot, calculated as ‘patients with at least one event’ under specification of the follow-up period and the endpoint under consideration. The combination of three RCTs in the distress management subgroup25,29,31 as well as another study in the distress management subgroup28 favoured the intervention with respect to cardiovascular events or hospitalization, respectively. The risk ratios of the three RCTs in the lifestyle change and distress management subgroup33,36,37 on cardiovascular events or hospitalization, respectively, were near 1. Vahedian-Azimi et al.37 reported zero events in both treatment groups. Here, the risk ratio was derived by a continuity correction.41
Figure 6.
Summary of all studies with the outcome cardiovascular morbidity, separately for distress management, and lifestyle change plus distress management.
Note: The meta-analysis of the studies of Blumenthal et al. 2016, Plüss et al. 2011 and van Dixhorn et al. 1999 is based on a random effect model. All other results are based on one study only.
RCT: randomized controlled trial; CT: cohort trial; CS: cross-sectional; RR: risk ratio; CI: confidence interval
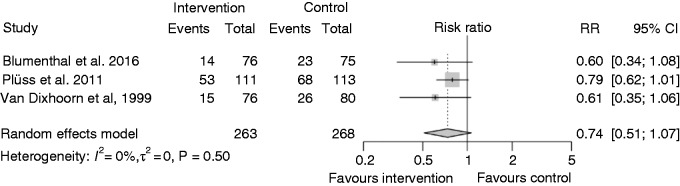
In addition to Figure 6, the effects of the three RCTs including distress management, defining morbidity as cardiovascular events and having a follow-up of five years25,29,31 are further summarized in Figure 7. Distress management results in moderately reduced cardiovascular events over five years, although the effect is not statistically significant (RR 0.74, 95% CI 0.51; 1.07).
Figure 7.
Effects of three comparable trials on distress management on morbidity.
RR: risk ratio; CI: confidence interval
Mortality
The raw data are summarized in Table 2. Over all, the mortality event rates seemed to be comparable in both treatment groups.
Table 2.
Raw data on mortality (overall and CV).
| Study | Follow-up | Overall mortality |
CV mortality |
||
|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||
| Distress management | |||||
| Blumenthal et al., 2016 | 3.2 years (median) | 0/76 | 2/75 | ||
| Plüss et al., 2011 | 5 years | 10/111 | 8/113 | 5/111 | 3/113 |
| Neves et al., 2009 | 2 years | 0/40 | 0/40 | ||
| van Dixhoorn et al., 1999 | 5 years | 5/76 | 7/80 | ||
| Lifestyle change and distress management | |||||
| Vahedian-Azimi et al., 2016 | 2 years | 1/33 | 2/30 | ||
Mortality is listed as deaths/total number of patients.
Discussion
This systematic review suggests that psychologically supported lifestyle change and distress management on top of ebCR results in a small but statistically not significant effect on depressive symptoms (SMD –0.13, 95% CI –0.30; 0.05). Furthermore, we found a small effect of distress management on cardiovascular events over five years (RR 0.74, 95% CI 0.51 to 1.07), although this effect was also not significant.
Compared with other meta-analyses on psychological interventions, our small and insignificant effects on depression and cardiovascular events might be explained by our restrictive strategy in defining control groups. It is well documented and confirmed in numerous controlled clinical trials that exercise training by itself significantly improves prognosis in patients with CVD and also may beneficially affect psychological well-being. Moreover, prognosis of patients with CVD has been improved markedly by medical treatment, which also is promoted and closely controlled in ebCR. In our study, ebCR, as defined by our inclusion criteria, often included education and brief psychological advice, which may itself contribute to the positive effects. Against this background, it might be difficult to document additional beneficial effects of specific psychological interventions as compared with ebCR alone.
Still, even in the light of these optimized control conditions, our data suggest that specific psychological interventions may reduce depressive symptoms in selected cardiovascular patients, a result which is supported by another recent meta-analysis by Richards et al.15 This study has shown a comparable but statistically significant effect of various psychological interventions on depressive symptoms (SMD –0.27, 95% CI –0.39; –0.15). In contrast, we could not confirm their small but significant effect of psychosocial interventions on anxiety (SMD –0.24, 95%- CI –0.38; –0.09).15 Moreover, our data also do not support a positive effect of specific psychological interventions on QoL, as previously described by Linden et al. (r–0.21 vs. –0.13, p < 0.05).12
The small but insignificant effect of distress management on cardiovascular events is in line with the meta-analysis presented by Richards et al.,15 who neither found significant effects on revascularization procedures (RR 0.94, 95% CI 0.81; 1.1) nor on risk reduction for non-fatal MI (RR 0.82, 95% CI 0.64; 1.05). The same is true for total mortality, which was neither positively affected in the present study nor in the meta-analysis by Richards et al. (RR 0.90, 95% CI 0.77; 1.05).15
However, the trend towards positive effects of distress management on cardiovascular morbidity as shown in this meta-analysis is in line with older meta-analyses on psychological interventions by Linden et al.12 and Cramer et al.6 Linden et al.12 especially found positive effects in studies with follow-up periods longer than two years (odds ratio (OR) 0.57, 95% CI 0.37; 0.86). Remarkably, the three studies aggregated in our meta-analyses25,29,31 had follow-up periods of five years.
Additional evidence that distress management may have a positive impact on cardiovascular prognosis was found by two RCTs with seven years of follow-up: Orth-Gomer et al.42 reported on a group intervention for women after MI, aiming at ‘stress reduction’. After seven years, total mortality was significantly reduced (OR 0.33, 95% CI 0.15; 0.74). Gulliksson et al.43 replicated this intervention in men and women with CAD and found a significant reduction in cardiovascular event rates after seven years (hazard ratio 0.59, CI 95% 0.42; 0.83). However, their control condition was defined as ‘usual care’ and thus did not necessarily include ebCR.
Indirect evidence for possible additional effects of psychological interventions on cardiovascular morbidity derives from one of the largest controlled trials on exercise training in cardiac patients with heart failure, the HF-ACTION trial.44,45 This trial found that the effect size of exercise training on depressive symptoms was small and initially depressed patients remained in the depressed range after treatment. Thus, one could hypothesize that specific psychological interventions on top of ebCR could be efficacious at least in vulnerable subgroups, for example, patients with high distress and/or depressive symptoms.
Our second research question focused on the differential efficacy of specific types of psychological interventions. Previously, Welton et al.13 reported evidence that (a) all kinds of psychological interventions (i.e. ‘educational’, ‘behavioural’, ‘cognitive’, ‘relaxation’, ‘psychosocial support’) reduce anxiety, (b) ‘behavioural’ and ‘cognitive’ interventions reduce depression, and (c) ‘behavioural interventions’ reduce total mortality and non-fatal MI. The data presented here only support the assumption that psychological lifestyle change interventions (‘behavioural intervention’ according to Welton et al.13) and distress management when added to ebCR may be able to reduce symptoms of depression. In addition, our results indicate that only distress management, most likely comparable to their definition of ‘cognitive interventions’, may reduce cardiac morbidity on top of ebCR.
Strengths
To our best knowledge, this is the first meta-analysis to specifically evaluate the added value of psychological interventions compared with ebCR alone with respect to depression, anxiety, QoL, cardiovascular morbidity and mortality. Furthermore, we only included studies published after 1995, thereby taking into account the effects of modern pharmacotherapy and interventional cardiology. Literature search, study selection, data extraction, risk of bias assessment, statistical analysis and reporting of results were in concordance with highest available standards.
Limitations
The studies included in this meta-analysis were of only low to moderate quality including predominantly small and heterogeneous populations. The psychological interventions under investigation also were heterogeneous with respect to intensity and duration, even within the subgroups analysed separately. This also applied for the control groups that should represent ebCR but very often have not sufficiently been described with respect to content and intensity. Furthermore, assessment of somatic and psychological outcomes was heterogeneous with respect to definition of endpoints and psychometric instruments selected, which in particular was relevant for subgroup analyses. Only one-third of all studies reported outcomes after a follow-up of two years or longer (maximum five years). There was almost no presentation of gender-specific results, therefore a subgroup analysis on this topic could not be performed. Only three studies explicitly included patients with psychosocial strain, for example, depression or psychosocial problems, while three others explicitly excluded patients with mental disorders or psychosocial problems. Hence, we were unable to evaluate the effects of psychological interventions in specific subgroups, for example, patients with symptoms of depression or anxiety, or chronic stress.
Consequences and future directions
Based on our findings, we conclude that psychological interventions specifically targeting lifestyle change and distress in addition to ebCR may have an additional impact on symptoms of depression and cardiovascular events, especially in vulnerable subgroups. Although our results could not confirm an additional impact on anxiety, QoL and cardiovascular mortality, our systematic review still supports the recommendation given by the European Society of Cardiology prevention and rehabilitation guidelines1,11 that ‘multimodal interventions’ like ebCR should include distinct psychological interventions adjusted to the needs of the individual patient.
However, there is considerable uncertainty regarding which specific psychological interventions may work best for whom and under which conditions. Well designed large scale trials are needed to clarify issues like gender effects, timing, and types and amount of interventions, as well as the efficacy in patients with defined psychosocial problems.
Supplemental Material
Supplemental Material for Additional effects of psychological interventions on subjective and objective outcomes compared with exercise-based cardiac rehabilitation alone in patients with cardiovascular disease: A systematic review and meta-analysis by Christian Albus, Christoph Herrmann-Lingen, Katrin Jensen, Matthes Hackbusch, Nina Münch, Catharina Kuncewicz, Maurizio Grilli, Bernhard Schwaab, Bernhard Rauch and for the German Society of Cardiovascular Prevention & Rehabilitation (DGPR) in European Journal of Preventive Cardiology
Author contribution
CA, CHL, KJ, MH, BS and BR designed this systematic review. MG performed the systematic literature search, NM, CK, CA and CHL selected and evaluated retrieved studies. KJ and MH extracted the data, assessed the risk of bias and analysed the extracted data. CA and CHL were responsible for drafting the report. All authors reviewed the manuscript, gave their final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: during the last three years, CA received grants from the German Ministry for Education and Research and lecture honoraria from Boehringer Ingelheim, Bayer Vital, Daiichi Sankyo and MSD Sharp & Dohme, outside the submitted work. CHL received grants from the German Ministry for Education and Research and lecture honoraria from Novartis, Heel and Servier, and personal fees from Hogrefe Huber Publishers, outside the submitted work. Furthermore, he is in leading functions in medical specialty societies for psychosomatic medicine. The Institute of Medical Biometry and Informatics of the University Heidelberg (MH, KJ) received grants from the German Society of Cardiovascular Prevention & Rehabilitation outside the submitted work. Outside the submitted work BR received honoraria as a consultant of ‘Abbott Medical Devices’ and lecture honoraria from the ‘Deutsche Hochdruckliga e.V.’. BS, MG, NM and CK report no conflicts of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: we thank the German Society of Cardiovascular Prevention & Rehabilitation for supporting the study by personal grants to MG and grants to the Institute of Medical Biometry and Informatics of the University Heidelberg, the Department of Psychosomatics and Psychotherapy, University of Cologne and the Department of Psychosomatic Medicine and Psychotherapy, University of Göttingen Medical Centre, all Germany.
References
- 1.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016; 23: NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Merghani A, Mont L. Exercise and the heart: The good, the bad, and the ugly. Eur Heart J 2015; 36: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 3.Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post myocardial infarction: A systematic review and meta-analysis of randomized controlled trials. Am Heart J 2011; 162: 571–584. [DOI] [PubMed] [Google Scholar]
- 4.Jansen V, De Gucht V, Dusseldorp E, et al. Lifestyle modification programmes for patients with coronary heart disease: A systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol 2012; 20: 620–640. [DOI] [PubMed] [Google Scholar]
- 5.Rutledge T, Redwine LS, Linke SE, et al. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med 2013; 75: 335–349. [DOI] [PubMed] [Google Scholar]
- 6.Cramer H, Lauche R, Paul A, et al. Mind-body medicine in the secondary prevention of coronary heart disease – a systematic review and meta-analysis. Dtsch Arztebl Int 2015; 112: 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Halewijn G, Deckers J, Tay HY, et al. Lessons from contemporary cardiovascular prevention and rehabilitation. Int J Cardiol 2017; 232: 294–303. [DOI] [PubMed] [Google Scholar]
- 8.Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016. CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauch B, Davos C, Doherty P, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized trials. The Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol 2016; 23: 1914–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell R, McGregor G, Ennis S, et al. Is exercise-based cardiac rehabilitation effective? A systematic review and meta-analysis to re-examine the evidence. BMJ Open 2018; 8: e019656–e019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pogosova N, Saner H, Pedersen SS, et al. Psychosocial aspects in cardiac rehabilitation: From theory to practice. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation of the European Society of Cardiology. Eur J Prev Cardiol 2015; 22: 1290–1306. [DOI] [PubMed] [Google Scholar]
- 12.Linden W, Phillips MJ, Leclerc J. Psychological treatments in cardiac patients: A meta analysis. Eur Heart J 2007; 28: 2972–2984. [DOI] [PubMed] [Google Scholar]
- 13.Welton NJ, Caldell DM, Adampoulos E, et al. Mixed treatment comparison meta-analysis of complex interventions: Psychological interventions in coronary heart disease. Am J Epidemiol 2009; 169: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 14.Whalley B, Thompson DR, Taylor RS. Psychological interventions for coronary heart disease: Cochrane systematic review and meta-analysis. Int J Behav Med 2014; 21: 109–121. [DOI] [PubMed] [Google Scholar]
- 15.Richards SH, Anderson L, Jenkinson CE, et al. Psychological interventions for coronary heart disease (Review). Cochrane Database Syst Rev 2017. CD002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reavell J, Hopkinson M, Clarkesmith D, et al. Effectiveness of cognitive behavioral therapy for depression and anxiety in patients with cardiovascular disease: A systematic review and meta-analysis. Psychosom Med 2018; 8: 742–753. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT and Green S (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. https://handbook-5-1.cochrane.org/.
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med 2009; 6: e1000097–e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Focht BC, Brawley LR, Rejeski WJ, et al. Group-mediated activity counseling and traditional exercise therapy programs: Effects on health-related quality of life among older adults in cardiac rehabilitation. Ann Behav Med 2004; 28: 52–61. [DOI] [PubMed] [Google Scholar]
- 20.IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014; 14: 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chair SY, Chan SW, Thompson DR, et al. Effect of motivational interviewing on the clinical and psychological outcomes and health-related quality of life of cardiac rehabilitation patients with poor motivation. Hong Kong Med J 2014; 20: 15–19. [PubMed] [Google Scholar]
- 22.Scholz U, Knoll N, Sniehotta FF, et al. Physical activity and depressive symptoms in cardiac rehabilitation: Long-term effects of a self-management intervention. Soc Sci Med 2006; 62: 3109–3120. [DOI] [PubMed] [Google Scholar]
- 23.Andersson A, Sundel KL, Unden AL, et al. A five-year rehabilitation programme for younger women after a coronary event reduces the need for hospital care. Scand J Public Health 2010; 38: 566–573. [DOI] [PubMed] [Google Scholar]
- 24.Barth J, Paul J, Englert N, et al. Brief psychotherapy for patients with coronary heart disease and comorbid depression. Research in rehabilitation. Results from a research network in southwest Germany, 2006, http://www.psychologie.uni-freiburg.de/abteilungen/Rehabilitationspsychologie/downloads/abschlussbericht_protecd (2006, accessed 23 November 2017).
- 25.Blumenthal JA, Sherwood A, Smith PJ, et al. Enhancing cardiac rehabilitation with stress management training: A randomized, clinical efficacy trial. Circulation 2016; 133: 1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brügemann J, Poels BJ, Oosterwijk MH, et al. A randomised controlled trial of cardiac rehabilitation after revascularisation. Int J Cardiol 2007; 119: 59–64. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson MR, Edstrom-Pluss C, Held C, et al. Effects of expanded cardiac rehabilitation on psychosocial status in coronary artery disease with focus on type D characteristics. J Behav Med 2007; 30: 253–261. [DOI] [PubMed] [Google Scholar]
- 28.Neves A, Alves AJ, Ribeiro F, et al. The effect of cardiac rehabilitation with relaxation therapy on psychological, hemodynamic, and hospital admission outcome variables. J Cardiopulm Rehabil Prev 2009; 29: 304–309. [DOI] [PubMed] [Google Scholar]
- 29.Pluss CE, Billing E, Held C, et al. Long-term effects of an expanded cardiac rehabilitation programme after myocardial infarction or coronary artery bypass surgery: A five-year follow-up of a randomized controlled study. Clin Rehabil 2011; 25: 79–87. [DOI] [PubMed] [Google Scholar]
- 30.Raghuram N, Parachuri VR, Swarnagowri MV, et al. Yoga based cardiac rehabilitation after coronary artery bypass surgery: One-year results on LVEF, lipid profile and psychological states – A randomized controlled study. Indian Heart J 2014; 66: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dixhoorn JJ, Duivenvoorden HJ. Effect of relaxation therapy on cardiac events after myocardial infarction: A 5-year follow-up study. J Cardiopulm Rehabil 1998; 19: 178–185. [DOI] [PubMed] [Google Scholar]
- 32.Beckie TM, Beckstead JW. The effects of a cardiac rehabilitation program tailored for women on their perceptions of health: A randomized clinical trial. J Cardiopulm Rehabil Prev 2011; 31: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black JL, Allison TG, Williams DE, et al. Effect of intervention for psychological distress on rehospitalization rates in cardiac rehabilitation patients. Psychosomatics 1998; 39: 134–143. [DOI] [PubMed] [Google Scholar]
- 34.Meng K, Musekamp G, Schuler M, et al. The impact of self-management patient education program for patients with chronic heart failure undergoing inpatient cardiac rehabilitation. Patient Educ Couns 2016; 99: 190–197. [DOI] [PubMed] [Google Scholar]
- 35.Pfaeffli-Dale L, Whittaker R, Jiang Y, et al. Text message and internet support for coronary heart disease self-management: Results from the Tex4Heart randomized controlled trial. J Med Internet Res 2015; 17: e237–e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sebregts EH, Falger PR, Appels A, et al. Psychological effects of a short behavior modification program in patients with acute myocardial infarction or coronary artery bypass grafting. A randomized controlled trial. J Psychosom Res 2005; 58: 417–24. [DOI] [PubMed] [Google Scholar]
- 37.Vahedian-Azimi A, Miller AC, Hajeiesmaieli M, et al. Cardiac rehabilitation using the Family-Centered Empowerment Model versus home-based cardiac rehabilitation in patients with myocardial infarction: A randomized controlled trial. Open Heart 2016; 3: e000349–e000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peerson K, Munkhaugen J, Gullestad L, et al. The role of cardiac rehabilitation in secondary prevention after coronary events. Eur J Prev Cardiol 2017; 13: 1360–1368. [DOI] [PubMed] [Google Scholar]
- 39.O’Rourke Á, Hampson SE. Psychosocial outcomes after an MI: An evaluation of two approaches to rehabilitation. Psychol Health Med 1999; 4: 393–402. [Google Scholar]
- 40.Rugulies R, Scherwitz L, Siegrist J, et al. Comprehensive life style changes by coronary patients - an intervention study. Gesundheitswesen 1996; 58(Suppl. 2): 149–151. [PubMed] [Google Scholar]
- 41.Pettigrew HM, Gart JJ, Thomas DG. The bias and higher cumulants of the logarithm of binomial variate. Biometrika 1986; 73: 425–435. [Google Scholar]
- 42.Orth-Gomer K, Schneiderman N, Wang HX, et al. Stress reduction prolongs life in women with coronary disease. The Stockholm women’s intervention trial for coronary heart disease (SWITCHD). Circ Cardiovasc Qual Outcomes 2009; 2: 25–32. [DOI] [PubMed] [Google Scholar]
- 43.Gulliksson M, Burell G, Vessby B, et al. Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent cardiovascular events in patients with coronary heart disease: Secondary prevention in Uppsala primary health care project (SUPRIM). Arch Intern Med 2011; 171: 134–140. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenthal JA, Babyak MA, O’Connor C, et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: The HF-ACTION randomized trial. JAMA 2012; 308: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Additional effects of psychological interventions on subjective and objective outcomes compared with exercise-based cardiac rehabilitation alone in patients with cardiovascular disease: A systematic review and meta-analysis by Christian Albus, Christoph Herrmann-Lingen, Katrin Jensen, Matthes Hackbusch, Nina Münch, Catharina Kuncewicz, Maurizio Grilli, Bernhard Schwaab, Bernhard Rauch and for the German Society of Cardiovascular Prevention & Rehabilitation (DGPR) in European Journal of Preventive Cardiology