Short abstract
Background
Chronic rhinosinusitis is a common, high-morbidity chronic inflammatory disease, and patients often experience suboptimal outcomes with current medical treatment. The exhalation delivery system with fluticasone (EDS-FLU) may improve care by increasing superior/posterior intranasal corticosteroid deposition.
Objective
To evaluate the efficacy and safety of EDS-FLU versus EDS-placebo in patients with nasal polyps (NP). Coprimary end points were change in nasal congestion and polyp grade. Key secondary end points were Sino-Nasal Outcome Test-22 (SNOT-22) and Medical Outcomes Study Sleep Scale-Revised (MOS Sleep-R). Other prespecified end points included all 4 cardinal symptoms of NP, 36-Item Short Form Health Survey (SF-36), Patient Global Impression of Change (PGIC), Rhinosinusitis Disability Index (RSDI), and key indicators for surgical intervention.
Design
Randomized, double-blind, EDS-placebo-controlled, multicenter study.
Methods
Three hundred twenty-three subjects with NP and moderate-severe congestion/obstruction, most with history of corticosteroid use (94.4%) and/or prior surgery (60.4%), were randomized to EDS-FLU 93 µg, 186 µg, or 372 µg or EDS-placebo twice daily (BID) for 24 weeks (16 double-blind + 8 single-arm extension with EDS-FLU 372 µg BID).
Results
All EDS-FLU doses produced significant improvement in both coprimary end points (P < .05) and in SNOT-22 total score (P ≤ .005). EDS-FLU significantly improved all 4 cardinal symptoms of NP (P < .05), including congestion/obstruction, facial pain/pressure, rhinorrhea/post-nasal drip, and hyposmia/anosmia. Approximately 80% of subjects reported improvement with EDS-FLU, with 65% reporting “much” or “very much” improvement by week 16. Adverse events were generally local in nature and similar to other intranasal steroids studied for similar durations in similar populations, with the most common being epistaxis.
Conclusions
In patients with chronic rhinosinusitis with NP (CRSwNP) who were symptomatic despite high rates of prior intranasal steroid use and/or surgery, EDS-FLU produced statistically significant and clinically meaningful improvements compared to EDS-placebo in multiple subjective and objective outcomes (symptoms, SNOT-22, RSDI, SF-36, PGIC, and NP grade), including all 4 cardinal symptoms of CRSwNP.
Keywords: nasal polyps, chronic rhinosinusitis, intranasal corticosteroid, fluticasone, Sino-Nasal Outcome Test-22, congestion, obstruction, polyp grade, sinus surgery, intranasal steroids, inflammatory disease
Introduction
Chronic rhinosinusitis (CRS), often accompanied by nasal polyps (CRSwNP), is a highly prevalent chronic inflammatory condition.1–3 The negative effect of CRS on overall quality of life (QoL) has been measured in multiple domains and is of comparable magnitude to other serious diseases such as congestive heart failure, chronic obstructive pulmonary disease (COPD), and Parkinson’s disease.4,5 In CRS, chronically inflamed epithelial surfaces, sometimes exacerbated by polypoid growths, impair ventilation, and drainage through sinus ostia.6 Nasal polyps (NP) are benign lesions arising from chronically inflamed tissue, typically in the region of the middle meatus, or ostiomeatal complex (OMC), a part of the deep nasal labyrinth above the inferior turbinate bone and behind the nasal valve and uncinate process, where sinus ventilation and drainage normally occur.7,8 CRS symptoms (with or without polyps) include not only the diagnostic 4 (facial pain/pressure, congestion/obstruction, rhinorrhea, and diminished/absent sense of smell) but also depression, serious sleep impairment, headaches, bodily pain, and fatigue.9,10 Several factors, including impaired mucociliary clearance and biofilm formation, predispose patients with CRS to complicating upper respiratory tract infections, such as acute sinusitis and adenotonsillitis.9 Reports suggest that nearly 70% of health-care visits with CRS as a primary diagnosis result in a prescription for an antibiotic. Antibiotic prescriptions for CRS and acute rhinosinusitis combined exceed antibiotic prescriptions for all other primary diagnoses, with obvious implications for risk of emergence of antibiotic resistance.11
The inflammation and associated NP are usually responsive to oral corticosteroid treatment, as evidenced by rapid improvement in symptoms and polyposis following treatment.12,13 However, even short courses of oral corticosteroids are associated with adverse effects, sometimes very serious.14,15 As such, clinical treatment guidelines for CRS, with or without NP, recommend initial treatment with topically acting (low bioavailability) intranasal corticosteroids (INS).9,10 Unfortunately, INS therapy may result in inadequate symptom control and limited polyp reduction.16,17 This is attributable to the inability of conventional nasal sprays and pressurized metered-dose inhalers to adequately deliver drug beyond the nasal valve and above the inferior turbinate, leaving key nasal regions obstructed due to inflammation, and polyps undertreated.18–21 As a result, most CRS patients remain symptomatic and report frustration with the symptom relief associated with medical treatment.22 For these patients, surgery may be considered.9,10,23 Although surgery has been shown to be a cost-effective treatment that improves QoL, symptoms are often either not fully or permanently resolved,24,25 and data suggest that the use of medication to control symptoms after surgery is very common.16 Substantial potential exists for the improved medical care of CRS if a reliable means were available to place high-potency topical steroid in target regions on a long-term outpatient basis.
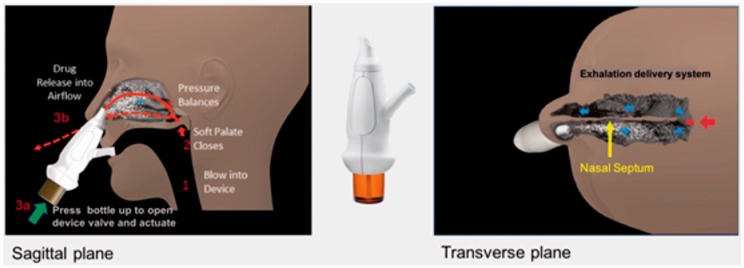
The exhalation delivery system with fluticasone (EDS-FLU) contains a suspension of fluticasone propionate.26 It uses an “exhalation delivery” mechanism that exploits a balanced closure of the soft palate and has been shown to deliver medication broadly behind the nasal valve and head of the inferior turbinate, reaching key anatomical sites in the superior and posterior aspect of the nasal cavity (Figure 1).27 The drug formulation does not contain alcohol or fragrance and has approximately twice the concentration of fluticasone as Flonase®. Prior research has shown that, even at comparable doses, EDS-FLU is not bioequivalent to Flonase and produces systemic fluticasone exposure lower than comparable doses of Flovent®.28 This randomized EDS-placebo-controlled trial was conducted to assess the safety and efficacy of EDS-FLU (93 µg, 186 µg, and 372 µg twice daily [BID]) compared to EDS-placebo in patients with moderate-severe symptoms and bilateral nasal polyposis, inclusive of those with prior steroid use and sinonasal surgery. Results from a first trial with EDS-FLU were recently reported.29
Figure 1.
- The EDS has a flexible mouthpiece and a nosepiece. The sealing nosepiece is shaped to transfer pressure from the mouth, to avoid compression of soft tissue in a way that could obstruct air flow, and to “stent” the nasal valve, particularly superiorly.
- Exhalation through the EDS (1) creates an airtight seal of the soft-palate, isolating the nose from the mouth and lungs, (2) transfers proportional air pressure into the nose, and (3) helps “float” medication around obstructions to high/deep sites in the nasal labyrinth, such as the OMC.
- The transferred intranasal pressure is proportional, across various exhalation forces, to oral pressure, counterbalancing pressure on the soft palate. This assures a patent communication behind the nasal septum and allows air to escape through the opposite nostril. “Positive-pressure” expands passages narrowed by inflammation (versus negative pressure delivery, “sniffing”).
- Use is simple and quick. A patient inserts the nosepiece into one nostril and starts blowing through the mouthpiece. This elevates and seals the soft palate, as with inflating a balloon, separating the oral and nasal cavities. The patient completes use by pressing the bottle to actuate. This causes a coordination-reducing valve to release the exhaled breath concurrently with aerosol spray in a “burst” of naturally humidified air.
- Additional information related to the EDS device can be found at https://www.optinose.com/exhalation-delivery-systems/technical-overview.
Methods
Design Overview, Setting, and Participants
This randomized, double-blind (DB), EDS-placebo-controlled, multicenter study was conducted to assess the safety and efficacy of EDS-FLU in the treatment of NP. Participants were recruited from 54 centers in the United States, Canada, the Czech Republic, South Africa, Ukraine, and United Kingdom (Online Appendix 1). Institutional Review Board/ethics committee approval was obtained at each site, and all patients provided written informed consent. The pretreatment phase included a screening visit and 7- to 14-day single-blind EDS-placebo run-in. Eligible participants were then randomized (1:1:1:1) by balanced allocation to a 16-week, DB, EDS-placebo-controlled treatment phase in which they received BID 93 µg, 186 µg, or 372 µg EDS-FLU or EDS-placebo. At the screening visit, subjects were instructed on the correct use of the EDS device. Randomization was by Interactive Voice Response/Web Response system, which provided each participant with an identifier for a blinded drug kit. Masking was accomplished with a visually identical EDS-placebo, using an identical EDS drug delivery system and otherwise identical liquid formulation of medication (except for the fluticasone). Participants completing the 16-week DB phase entered an 8-week extension, during which all received EDS-FLU 372 µg BID; prior trial allocation was not revealed to any trial participants.
Eligible participants were ≥18 years of age and required to have moderate or greater nasal congestion/obstruction as reported by the patient (morning score ≥2 [0 = none, 1 = mild, 2 = moderate, 3 = severe] for at least 5 days during the 7-day period leading up to screening) and a NP grade of 1 to 3 in each nasal cavity. Participants with comorbid allergic rhinitis were permitted, provided they did not have a “season” coinciding with the first 4 weeks of randomization. Patients with comorbid asthma or COPD were required to be stable with no exacerbations within 3 months of screening.
Exclusion criteria included complete or near-complete nasal cavity obstruction, inability to achieve bilateral nasal airflow, inability to have each nasal cavity examined for any reason (including severely obstructing nasal septum deviation), nasal septum perforation, history of >5 sinonasal surgeries, or sinonasal surgery within 6 months prior to screening. Online Appendices 2 and 3 include full inclusion/exclusion criteria. At study entry, participants were required to stop medications for nasal congestion; after week 4, nonsedating antihistamines were permitted as “rescue medication.”
Outcomes and Follow-up
Coprimary end points were (1) mean change in average morning congestion score (“instantaneous am congestion,” rated from 0 = no symptoms to 3 = severe) over 7 days prior to week 4 and (2) mean change in endoscopically assessed total polyp grade (sum of scores, rated 0–3 on each side, from both nasal cavities) at week 16.
Patients recorded congestion/obstruction symptom scores BID using electronic diaries [morning and evening, (am and pm)], both as experienced at the moment of reporting (“instantaneous”) and as recalled over the preceding 12 hours (“reflective”). Symptoms captured in the diaries included all 4 diagnostically defining symptoms of CRSwNP (congestion/obstruction, rhinorrhea, facial pain/pressure, and hyposmia). NP grade was assessed at each visit using a modified Lildholdt scale30 by the specialist (ear, nose, and throat physician or allergist) performing the nasal endoscopy. NP grade was based on one-dimensional polyp mass extension in only the vertical plane relative to anatomic landmarks as follows: 0 = no polyps; 1 = no polyp tissue below the inferior border of the middle turbinate; 2 = any polyp tissue visualized below the inferior border of the middle turbinate, but not the inferior border of the inferior turbinate; 3 = any polyp tissue below the inferior border of the inferior turbinate. Based on polyp grade, “responders” were defined as patients experiencing a ≥1-point improvement.
Key secondary efficacy end points were change from baseline to week 16 in symptoms and functioning, measured by total Sino-Nasal Outcome Test-22 (SNOT-22) score, a validated outcome measure commonly used to assess CRS,31,32 and the sleep disturbance subscale of the Medical Outcomes Study Sleep Scale-Revised (MOS Sleep-R). The MOS Sleep-R is a validated 12-item questionnaire designed to measure key aspects of sleep. The score range for the 12-item version is 12 to 71.33
Other prespecified secondary end points included objective measures of disease severity, subjective measures of symptoms and functioning, and assessment of other health outcomes, including key surgical indicators. For the purposes of this multicenter global study, a patient was considered eligible for surgical evaluation if they met standard predefined indicator criteria. These included moderate to severe congestion for ≥3 months plus topical steroids at conventional doses for ≥6 weeks plus current or previous use of saline lavage for ≥6 weeks plus endoscopically visualized bilateral nasal polyposis of at least moderate severity (NP grading score ≥2 in at least 1 nostril). These criteria were intended to serve as an indicator for patients who might be evaluated for surgical treatment, recognizing that a decision to proceed to surgery for an individual patient is complex and influenced by many factors. This report includes change in the following end points from baseline to predetermined time points: polyp grade and responder criteria, symptoms am and pm, SNOT-22, Rhinosinusitis Disability Index (RSDI),34 MOS Sleep-R, Patient Global Impression of Change (PGIC),35,36 36-Item Short-Form Health Survey (SF-36),37 and indicators for surgical evaluation.
Safety Analysis
Safety assessments included examination by a specialist performing nasal endoscopy at screening and weeks 4, 8, 12, 16, and 24, including active assessment and scoring for epistaxis, septal erosion, other erosion or ulceration, erythema, mucosal candidiasis, and atypical mucosal swelling. All subjects were assessed by an ophthalmologist with tonometry and slit-lamp examination for changes in intraocular pressure (IOP) and cataract presence at screening and weeks 16 and 24.
Statistical Methods
A sample of 80 subjects per group was estimated sufficient to detect a difference of 1.0 in each of the coprimary end points between treatments, assuming standard deviation (SD) of 1.9 using a 2-sided t test at the 5% significance level with 90% power.38–44 Safety analyses were conducted on the Safety Analysis Set (SAS), and efficacy analyses were conducted on the Full Analysis Set.
The first coprimary measure analysis (ie, congestion) was performed using an analysis of covariance (ANCOVA) model including baseline score as a covariate and treatment group and country as fixed effects. For the second coprimary measure (ie, polyp grade), a mixed-effect model for repeated measures (MMRM) was used with patients as the block factor for repeated measurements, using baseline summed bilateral polyp grade as a covariate, and treatment, country, visit, and interaction of treatment by visit as fixed effects. An unstructured covariance matrix was used for within-subject correlation modeling. For the above primary efficacy analyses, missing data were imputed using a pattern mixture model. The pattern mixture model assumed that missing assessments were either missing at random (natural fluctuation not related to a treatment allocation, MAR) or not missing at random (possibly associated with treatment allocation, NMAR). The latter possibly introduced bias. Therefore, a multiple imputation method was employed to impute both categories of missing data (MAR and NMAR) with imputation values drawn by visit from the treatment group to which the patient was assigned for MAR data and from the lowest quartile of observed values across treatment groups and visits for NMAR data, using the conservative assumption that NMAR data would be worse. Additionally, tipping-point analyses were carried out for the coprimary end points to ensure that imputation did not affect study results. For other efficacy analyses, missing values were not imputed. All analyses were conducted using SAS statistical software (Cary, NC).
Each of the 3 active treatment groups was compared to EDS-placebo on the basis of the coprimary efficacy variables. A fixed sequence multiple comparison procedure was implemented to control for study-wide type I error for tests of multiple doses across the primary and key secondary measures versus EDS-placebo. For key secondary continuous end points (SNOT-22 and MOS Sleep-R), change from baseline was analyzed with either MMRM or ANCOVA (see analysis described for primary end points). A step-down procedure, analogous to that used for the coprimary variables, was used to control for multiplicity of treatment comparisons. Key secondary end points were tested in a fixed-sequence approach in order to control for type I error, with a 2-sided significance level of .05. Using these models, the least-squares (LS) mean difference between each active treatment group and EDS-placebo for other secondary end points, the 95% confidence interval (CI), and the nominal P value associated with the difference were estimated by visit. For categorical variables, odds ratio statistics were obtained from a generalized estimating equation (GEE) model for binomial distribution that included treatment and country as fixed factors. Nominal P values were obtained from the GEE model using the χ2 test to compare each active treatment group and EDS-placebo. Onset of action was defined as the first day when the LS mean change from baseline in the am score for instantaneous nasal congestion/obstruction had a P value <.05 versus EDS-placebo and when the P value was ≤.05 at all subsequent time points were also statistically significantly different.
Results
Study Population
The first patient was randomized on November 19, 2013. A total of 323 subjects were enrolled, 322 received ≥1 dose of study drug (SAS), 292 (90.7%) completed the DB treatment phase, and 282 (87.6%) entered the 8-week extension. The last participant completed the extension phase on October 1, 2015. The EDS-placebo group had the highest drop-out rate (14.6%) versus 7.4%, 11.3%, and 5.0% in the EDS-FLU 93 µg, 186 µg, and 372 µg groups, respectively (Figure 2).
Figure 2.
Study flow diagram. DB, double-blind; EDS-FLU, exhalation delivery system with fluticasone; OL, open label; SAS, safety Analysis Set.
No meaningful differences in demographics or baseline characteristics were observed between groups (Table 1). Baseline characteristics reflected a population with moderate-to-severe disease, with ∼95% having previously used steroids to treat their nasal disease (intranasal and/or oral) and 60.4% having undergone some type of previous sinus surgery, inclusive of polypectomy. Additional baseline demographics and characteristics can be found in Online Appendix 4: Table 1.
Table 1.
Patient Baseline Clinical Characteristics.
| EDS-placebo BIDn = 82 | EDS-FLU 93 µg BIDn = 81 | EDS-FLU 186 µg BIDn = 80 | EDS-FLU 372 µg BIDn = 80 | All Patients N = 323 | |
|---|---|---|---|---|---|
| Age in years (SD) | 45.3 (12.95) | 44.9 (12.72) | 46.4 (12.65) | 43.9 (12.63) | 45.1 (12.71) |
| Minimum, maximum | 18, 74 | 18, 68 | 18, 71 | 18, 73 | 18, 74 |
| Men (%) | 36 (43.9) | 40 (49.4) | 48 (60.0) | 38 (47.5) | 162 (50.2) |
| Comorbid asthma n (%) | 33 (40.2) | 23 (28.4) | 38 (47.5) | 40 (50.0) | 134 (41.5) |
| White race (%) | 68 (82.9) | 74 (91.4) | 72 (90.0) | 69 (86.3) | 283 (87.6) |
| Any corticosteroid for nasal polyps in last 10 years (%) | 77 (93.9) | 77 (95.1) | 76 (95.0) | 75 (93.8) | 305 (94.4) |
| Any corticosteroid for nasal polyps in last 30 days (%) | 48 (58.5) | 35 (43.2) | 36 (45.0) | 40 (50.0) | 159 (49.2) |
| Previous sinus surgery and polyp removal | 52 (63.4) | 49 (60.5) | 47 (58.8) | 47 (58.8) | 195 (60.4) |
| Instantaneous am congestion scores (SD) | 2.31 (0.412) | 2.22 (0.445) | 2.24 (0.416) | 2.29 (0.438) | 2.25 (0.432) |
| Mean bilateral total polyp score (SD) | 3.8 (0.94) | 3.6 (1.07) | 3.9 (1.08) | 3.7 (0.94) | 3.7 (1.04) |
| Mean SNOT-22 total scores (SD) | 53.7 (18.12) | 46.1 (17.80) | 51.8 (20.07) | 52.4 (20.07) | 51.02 |
| Mean MOS Sleep-R Sleep disturbance subscale scores (SD) | 40.4 (22.31) | 33.6 (21.07) | 46.6 (22.01) | 41.5 (23.26) | 42.72 |
| Meet surgical evaluation criteria, n/N (%) | 53/82 (64.6) | 45/81 (55.6) | 44/80 (55.0) | 47/78 (60.3) | 58.88 |
Abbreviations: am, morning; BID, twice daily; EDS-FLU, exhalation delivery system with fluticasone; MOS, Medical Outcomes Study; N, total of number of patients randomized/enrolled/treated; n/N (%), number (%) of patients in the subset at the given time point; SD, standard deviation; SNOT-22, Sinonasal Outcome Test-22.
Baseline instantaneous am congestion, polyp grade, SNOT-22, MOS Sleep-R, and surgical evaluation data are from the Full Analysis Set, whereas other data are from the intention-to-treat population.
Efficacy End Points
All 3 doses of EDS-FLU significantly improved both coprimary end points versus EDS-placebo (P < .05, all comparisons). For instantaneous am congestion at week 4, the LS mean change from baseline was −0.49, −0.54, and −0.62, in the EDS-FLU 93 µg, EDS-FLU 186 µg, and EDS-FLU 372 µg groups, respectively, compared to −0.24 with EDS-placebo (P < .01, all comparisons; Table 2). For change in summed NP grade at week 16, the LS mean change from baseline was −0.96, −1.03, and −1.06 in the EDS-FLU 93 µg, EDS-FLU 186 µg, and EDS-FLU 372 µg groups, respectively, compared to −0.45 with EDS-placebo (P < .01, all comparisons). Increasing doses of EDS-FLU produced numerically greater improvements in congestion and polyp grade, with the 372-µg dose resulting in the largest mean reduction in both, although between-dose differences did not reach statistical significance. There was a very low percentage of missing data (Online Appendix 5), and tipping point analyses confirmed the results of the primary analyses.
Table 2.
Efficacy Outcomes.
| Measure | EDS-placebo BID n = 82 | EDS-FLU 93 µg BID n = 81 | EDS-FLU 186 µg BID n = 80 | EDS-FLU 372 µg BID n = 79 | ||
|---|---|---|---|---|---|---|
| Nasal symptoms, LS mean change from baseline (SE) | ||||||
| Coprimary end pointa | ||||||
| Congestion/obstruction, week 4 | −0.24 (0.07) | −0.49 (0.08) | −0.54 (0.08) | −0.62 (0.08) | ||
| Difference, active-placebo (95% CI) | − | −0.43, −0.06 | −0.48, −0.11 | −0.57, −0.19 | ||
| P value versus EDS-placebo | .010 | .002 | <.001 | |||
| Secondary outcomes | n = 77 | n = 78 | n = 79 | n = 78 | ||
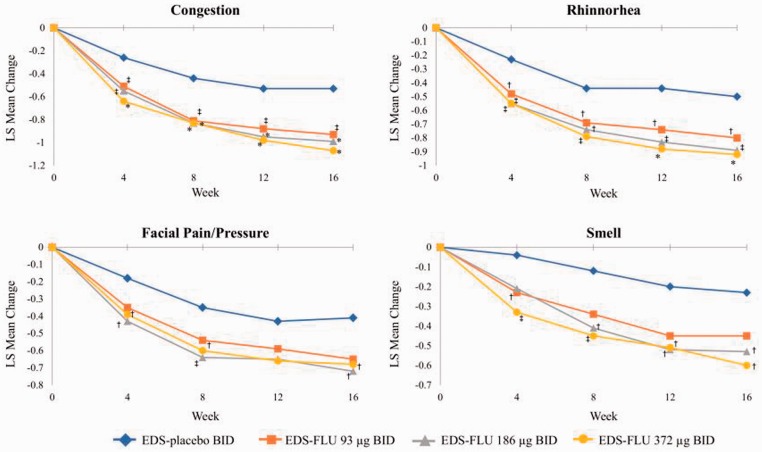
| Congestion/obstruction, week 4 | −0.26 (0.08) | −0.51 (0.08)** | −0.55 (0.08)** | −0.64 (0.08)* | ||
| Rhinorrhea, week 4 | −0.23 (0.09) | −0.48 (0.09)** | −0.55 (0.08)** | −0.55 (0.08)** | ||
| Facial pain and pressure, week 4 | −0.18 (0.08) | −0.35 (0.08) | −0.43 (0.08)** | −0.39 (0.08)** | ||
| Smell, week 4 | −0.04 (0.08) | −0.23 (0.08)** | −0.21 (0.08) | −0.33 (0.08)** | ||
| Objective assessments of nasal polyps | n = 82 | n = 81 | n = 80 | n = 79 | ||
| Coprimary end pointa | ||||||
| Total polyp grade, LS mean change (SE), week 16 | −0.45 (0.14) | −0.96 (0.14) | −1.03 (0.14) | −1.06 (0.14) | ||
| Difference, active-placebo (95% CI) | − | −0.86, −0.16 | −0.93, −0.24 | −0.96, −0.27 | ||
| P value versus EDS-placebo | − | .004 | <.001 | <.001 | ||
| Secondary outcomes | ||||||
| Total polyp grade, LS mean change (SE) | Week 24b (EDS-FLU 372 µg) | −1.08 (0.15) | −1.40 (0.15) | −1.39 (0.15) | −1.43 (0.14) | |
| ≥1-point improvement n/N (%) | Week 16 | 28/68 (41.2) | 42/75 (56.0) [OR = 1.921] |
45/68 (66.2)** [OR = 3.017] |
54/75 (72.0)* [OR = 2.783] |
|
| Week 24 (EDS-FLU 372 µg)b 6 µ | 47/64 (73.4) | 50/70 (71.4) [OR = 0.966] |
48/64 (75.0) [OR = 1.112] |
53/71 (74.6) [OR = 1.144] |
||
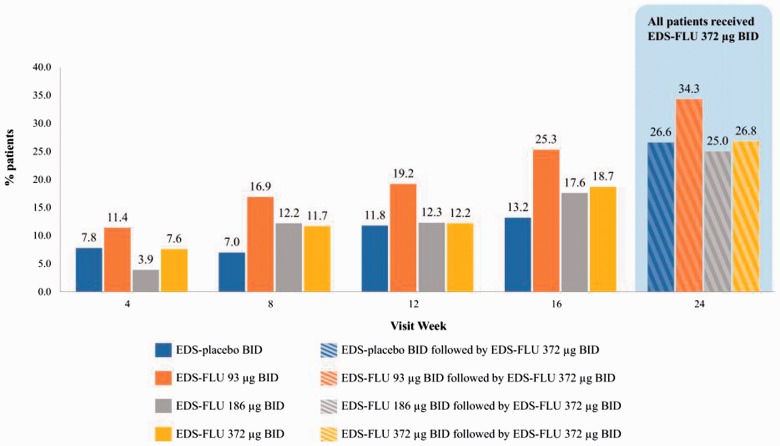
| Polyp elimination n/N (%) | Week 16 | 9/68 (13.2) | 19/75 (25.3) [OR = 2.343] |
12/68 (17.6) [OR = 1.518] |
14/75 (18.7) [OR = 1.485] |
|
| Week 24 (EDS-FLU 372 µg)b | 17/64 (26.6) | 24/70 (34.3) [OR = 1.537] |
16/64 (25.0) [OR = 0.978] |
19/71 (26.8) [OR = 1.007] |
||
| Patient reported outcome measures | ||||||
| Key secondary end pointsa | ||||||
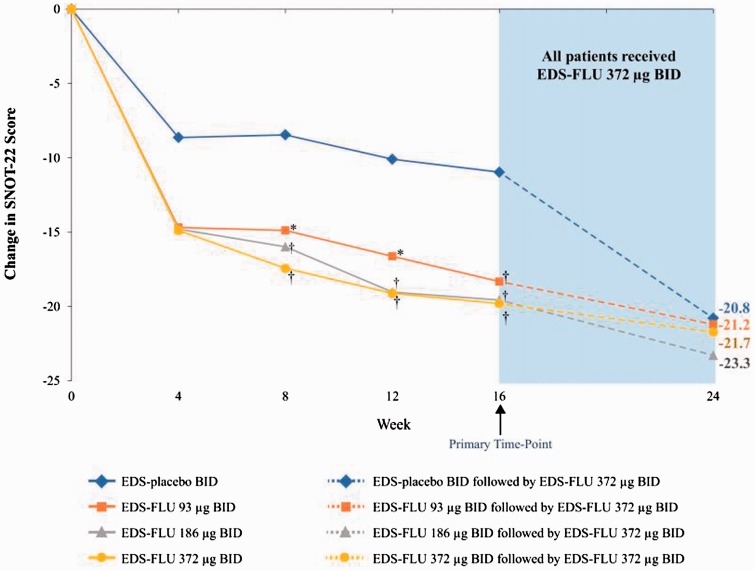
| SNOT-22, LS mean change (SE) | Week 16 | −10.96 (2.07) | −18.32 (2.05)* | −19.56 (2.04)* | −19.80 (2.05)* | |
| Week 24 (EDS-FLU 372 µg)b | −20.78 (2.11) | −21.20 (2.07) | −23.28 (2.08) | −21.72 (2.07) | ||
| MOS Sleep-R Sleep Problems Index, LS mean change (SE) | Week 16 | −8.53 (1.61) | −10.69 (1.59) | −14.24 (1.59)** | −10.66 (1.55) | |
| Other secondary end points | ||||||
| PGIC, much improved or very much improved, n/N (%) | Week 16 | 28/68 (41.2) | 45/73 (61.6)** [OR = 0.508] |
47/70 (67.1)* [OR = 0.290] |
50/75 (66.7)* [OR = 0.367] |
|
| Week 24 (EDS-FLU 372 µg)b | 52/64 (81.3) | 44/70 (62.9) [OR = 1.365] |
52/64 (81.3) [OR = 0.640] |
55/71 (77.5) [OR = 1.211] |
||
| RSDI Total Score, LS mean change (SE) | Week 16 | −10.71 (2.07) | −17.08 (1.99)** | −16.44 (2.02)** | −16.37 (1.94)** | |
| SF-36 Mental Composite, LS mean change (SE) | Week 16 | 0.7 (0.87) | 3.19 (0.84)** | 4.03 (0.85)** | 1.83 (0.82) | |
| SF-36 Physical Composite Score, LS mean change (SE) | Week 16 | 2.67 (0.76) | 4.12 (0.74)** | 5.19 (0.74) | 3.58 (0.72) | |
| Nasal polyp surgery | ||||||
| Qualified for surgical evaluation, n/N (%) | Screening | 53/82 (64.6) | 45/81 (55.6) | 44/80 (55.0) | 47/78 (60.3) | |
| Week 16 | 27/68 (39.7) | 17/74 (23.0)a,** [OR = 0.322] |
21/69 (30.4) | 19/75 (25.3)** [OR = 0.387] |
||
| Week 24b (EDS-FLU 372 µg) | 15/64 (23.4) | 14/70 (20.0) | 17/63 (27.0) | 16/70 (22.9) | ||
Abbreviations: am, morning; BID, twice daily; CI, confidence interval; EDS-FLU, exhalation delivery system with fluticasone; LS, least squares; MOS-Sleep, Medical Outcomes Study Sleep Scale; n, total of number of patients randomized/enrolled/treated; n/N (%), number (%) of patients in the subset at the given time point; OR, odds ratio; PGIC, Patient Global Impression of Change; RSDI, Rhinosinusitis Disability Index; SE, standard error; SF-36, 36-Item Short-Form Health Survey; SNOT-22, Sinonasal Outcome Test-22.
aData are from the Full Analysis Set with Imputation.
bAll patients received EDS-FLU 372 µg during the extension phase.
*P < .001 versus EDS-placebo.
**P < .05.
Key Secondary End Points
SNOT-22 improvement was substantial in all EDS-FLU groups and statistically superior to EDS-placebo (−18.3 to −19.8 for EDS-FLU vs −11.0 for EDS-placebo at week 16, P ≤ .005, all comparisons; Figure 3). MOS Sleep-R disturbance scores improved substantially in all treatment groups, including EDS-placebo, with statistical significance between groups at week 16 observed only with the EDS-FLU 186-µg group (Table 2).
Figure 3.
LS mean change in SNOT-22 total score over time. BID, twice daily; EDS-FLU, exhalation delivery system with fluticasone; LS, least squares; SNOT-22, Sinonasal Outcome Test-22. Average baseline SNOT score was 46–45. *P ≤ .05 versus EDS-placebo. †P ≤ .01 versus EDS-placebo.
Improvements in other prespecified secondary end points, including NP grade responder analysis, all 4 diagnostically defining nasal symptoms (both am and pm), and QoL measures (both SF-36 and RSDI) were statistically superior in all EDS-FLU groups versus EDS-placebo (Table 2). Numerical differences in polyp grade were evident as early as the first assessment, with statistical significance versus EDS-placebo in all dose groups by week 12 (P < .05). The proportion of patients experiencing polyp elimination increased monotonically in all EDS-FLU groups from weeks 4 through 16 (Figure 4). The percentage of responders continued to increase in the EDS-FLU 372-µg extension phase. Onset of action, defined by persistent statistically significant difference in congestion scores versus EDS-placebo, was observed within 2 weeks. EDS-FLU also produced statistically significant benefits in reflective am reports of nasal congestion/obstruction versus EDS-placebo (Online Appendix 6, Figure 1). Instantaneous am (Figure 5) and reflective am (Online Appendix 6, Figure 1) assessments of other cardinal symptoms (rhinorrhea, facial pain/pressure, and sense of smell) were also superior to EDS-placebo for the majority of comparisons at week 4 (P < .05). Evening assessments of the 4 diagnostically defining symptoms showed similar patterns of improvement (Online Appendix 6, Figures 2 and 3).
Figure 4.
Proportion of patients with polyps eliminated from ≥1 side of the nose. BID, twice daily; EDS-FLU, exhalation delivery system with fluticasone. Average baseline polyp score was 3.6–3.9. *P < .05 versus EDS-placebo. †P < .01 versus EDS-placebo. ‡P ≤ .001 versus EDS-placebo.
Figure 5.
Mean change in am instantaneous symptoms of congestion, facial pain and pressure, rhinorrhea, and sense of smell at weeks 4, 8, 12, and 16. am, morning; BID, twice daily; EDS-FLU, exhalation delivery system with fluticasone; LS, least squares. †P ≤ .05 versus EDS-placebo. ‡P ≤ .01 versus EDS-placebo. *P ≤ .001 versus EDS-placebo.
At week 16, 62% to 67% of subjects in each active treatment arm reported being “much” or “very much” improved as measured by the PGIC, compared with 41% (“much” or “very much” improved) of EDS-placebo subjects (P ≤ .05, all comparisons; Table 2).
At week 16, EDS-FLU treatment resulted in an approximately 54.1% reduction in the proportion of patients meeting predefined indicators for surgical evaluation (Table 2).
Safety Analysis
Adverse events (AEs) possibly attributable to EDS-FLU were generally local (not systemic). Specific AEs occurring more often with EDS-FLU versus EDS-placebo and reported in >5% of patients were epistaxis, nasal mucosal disorder (erythema), acute sinusitis, upper respiratory tract infection, nasal congestion, nasal septum ulceration, nasopharyngitis, and gastrointestinal disorders (Table 3).
Table 3.
Adverse Events >5% and Greater Than EDS-placebo.
| Adverse Event | EDS-Placebo n = 82 | EDS-FLU 93 µg BID n = 81 | EDS-FLU 186 µg BID n = 80 | EDS-FU 372 µg BID n = 79 |
|---|---|---|---|---|
| Epistaxis,a no. (%) | 3 (3.7) | 3 (3.7) | 7 (8.8) | 6 (7.6) |
| Nasal mucosal disorder, no. (%) | 5 (6.1) | 11 (13.6) | 6 (7.5) | 6 (7.6) |
| Acute sinusitis, no. (%) | 4 (4.9) | 5 (6.2) | 6 (7.5) | 8 (10.1) |
| URTI, no. (%) | 7 (8.5) | 1 (1.2) | 4 (5.0) | 5 (6.3) |
| Nasal congestion, no. (%) | 4 (4.9) | 3 (3.7) | 2 (2.5) | 6 (7.6) |
| Nasal septum ulceration, no. (%) | 1 (1.2) | 5 (6.2) | 5 (6.3) | 4 (5.1) |
| Nasopharyngitis, no. (%) | 4 (4.9) | 3 (3.7) | 2 (2.5) | 4 (5.1) |
| Gastrointestinal disorders, no. (%) | 4 (4.9) | 1 (1.2) | 2 (2.5) | 4 (5.1) |
Abbreviations: BID, twice daily; EDS-FLU, exhalation delivery system with fluticasone; n, total of number of patients randomized/enrolled/treated; URTI, upper respiratory tract infection.
aIncludes spontaneous adverse reaction reports.
All events of septal erosion/ulceration were “mild” (erosions with no evidence of ulceration), with the exception of 2 cases of nasal septum perforations that were endoscopically identified in EDS-FLU patients (0.8% of all EDS-FLU subjects). Both subjects (1 in the EDS-FLU 93 µg group and 1 in the EDS-FLU 186 µg group) had a history of nasal surgery. One was discovered at week 4 during nasal examination in a patient who reported a history of nasal surgery including the nasal septum (septoplasty) performed 15 months before screening. The second was observed during the week 16 nasal examination in a patient with a history of previous sinus surgery. There were no differences between active and EDS-placebo groups in mean IOP change, new identification of cataracts, or physical examination findings.
Discussion
This study of a common clinical presentation, CRSwNP patients who are symptomatic, mostly despite having previously tried steroid and/or surgical treatment, found that EDS-FLU significantly improved nasal congestion/obstruction, polyp grade, all cardinal symptoms, and a range of other outcomes including QoL and functioning, while eliminating NP in some patients, compared to EDS-placebo. EDS-FLU produced robust and statistically significant improvements in both coprimary outcome measures (congestion and polyp grade). Although the numerical effects on these measures may be difficult to interpret clinically, the magnitude of improvement with EDS-FLU on multiple outcome measures that are relatively easy to interpret (SNOT-22, PGIC, polyp elimination, and others) highlights the clinical import of these changes.
CRSwNP is a serious, common chronic inflammatory disease that produces a high burden of illness.4,5,9,10 Topically acting steroids delivered nasally by spray pump or pressurized canister are poor at accessing target sites in the OMC, such as the middle meatus,20,27,45,46 where inflammation, sometimes complicated by NP, typically obstructs normal sinus ventilation and drainage.7,8,21 Therefore, although INS are standard treatment for CRS with or without NP, they often do not provide adequate symptom relief.16–17 In a 2016 Cochrane review assessing the efficacy of conventional INS for CRS treatment, the limited available evidence (primarily from studies of patients with CRSwNP) supported benefits to QoL (very low quality evidence), moderate improvement in congestion and a small benefit in rhinorrhea (moderate quality of evidence), and small or inconsistent evidence of benefit for hyposmia/anosmia and facial pain/pressure.47 The results from this controlled trial, demonstrating improved QoL and improved symptoms, show a magnitude of effect on most symptoms above the 95% CI reported in the Cochrane review, suggesting, subject to the limitations inherent to making such comparisons, that EDS-FLU offers greater benefits than previously studied INS. Additional clinical trials will be required to substantiate these results and provide longer term data.
In patients with CRS, the presence of NP further complicates the chronic inflammation of tissues lining the nasal labyrinth and promotes swelling, impairment of sinus ventilation/drainage, blockage of nasal airflow, congestion, and hyposmia. Polyps are also prone to a greater spontaneous release of inflammatory cytokines than control mucosa, suggesting they may actively promote inflammation, rather than simply result from it.7,8 Thus, patient outcomes may be best with full or near-elimination of polyp mass, rather than modest shrinkage. Unfortunately, clinical trials in patients with baseline summed polyp scores similar to this study suggest that the improvement in bilateral polyp grade produced by conventional INS plateaus at an average score of ∼3 (0–6 scale) and does not improve further with longer treatment.13,40,42 This limited degree of maximum improvement may contribute to patient dissatisfaction and treatment failure with conventional INS. Furthermore, after conventional nasal steroids, current alternative treatment options are less attractive because they entail increased cost and/or risk and are not comparably well supported by evidence. These include oral steroids, leukotriene inhibitors, long courses of macrolide antibiotics, various types of surgical procedures, and even certain monoclonal antibodies currently in phase 3 development.9,10,48–50 In this context, EDS-FLU may offer a desirable means of improving medical treatment outcomes, before or after surgery, in patients for whom an intranasal steroid is indicated.
SNOT-22 scores, which assess a broad range of disease burden domains, improved monotonically with EDS-FLU treatment, more than doubling the reported minimal clinically important difference for the scale (8.9).32 Endoscopic sinus surgery (ESS) is an intervention recognized to produce a clinically meaningful treatment benefit, and a recent systematic review reported SNOT-22 improvements between 12.7 and 44.8 (mean = 24.4) points after ESS in a mix of studies in patients with and without NP. In this research, higher mean preoperative SNOT-22 and higher asthma prevalence predicted greater changes in SNOT-22.51 The magnitude of change with EDS-FLU in the current study was similar (∼20), supporting the conclusion that EDS-FLU produces a clinically meaningful degree of improvement. More broadly, this study found that EDS-FLU not only improved the 4 cardinal symptoms of CRS but also produced clinically significant improvements in global symptoms and both general health-related QoL and disease-specific QoL as measured by the PGIC, SF-36 mental and physical component scores, SNOT-22, and the RSDI.
Interestingly, in this study, the “placebo” group (using an EDS that delivered a liquid vehicle formulation) experienced a 10.96 improvement in SNOT-22 at 16 weeks. This is similar to the 9.2 point improvement reported with mometasone nasal spray in a recent study48 and adds to a variety of suggestive evidence that the EDS delivery mechanism of action itself may offer beneficial effects on CRS symptoms.18,26,43,44 For example, controlled trials have demonstrated that nasal delivery of CO2 has beneficial effects in allergic rhinitis, as CO2 induces a reduction in mucosal pH.52 The EDS device (without drug) has been shown to produce similar mucosal pH changes compared with studies using exogenous CO2 delivery as a therapeutic intervention.52,53 Possible mechanisms of direct device effect include CO2 in exhaled breath influencing pH, as well as inflammatory mediator and neuropeptide activity, nitric oxide removal, positive pressure, or vibration. In addition, EDS-placebo is likely not an inactive treatment comparator, as even saline alone is known to have treatment benefit and is recommended in treatment guidelines.9,10 It is plausible that the BID “high velocity, low volume” saline-like flushes of the superior/posterior nasal cavity by EDS-placebo may have offered some therapeutic benefit. Other possible explanations for the placebo response include protocol-permitted rescue medication (loratadine or alternative antihistamine) after week 4, placebo effect related to study design, or regression to the mean. Notably, a similar placebo response has been reported in prior similarly designed studies of CRSwNP.40,42
The range of fluticasone doses selected in this study exceeds the doses approved for the treatment of rhinitis, as might be expected in the context of a more chronic and severe inflammatory disease but does not exceed the range previously studied in the treatment of CRS.38,39,43 Treatment with EDS-FLU was generally well tolerated. The most common AEs occurring more often with EDS-FLU than EDS-placebo were local in nature, including epistaxis and mucosal erythema or ulceration. The incidence of spontaneously reported epistaxis in this study appears generally similar to that previously found in research with another topical steroid studied in a similar population for a similar duration, despite major differences in regional drug deposition.40–42,47 It is important to note that many patients in this 6-month trial had previously used steroids and/or undergone nasal surgery. This contrasts with the comparatively healthy allergic rhinitis populations most often studied in phase 3 trials with conventional nasal steroid sprays. Septal erosions/ulcerations are a known consequence of intranasal steroid administration. In this study, all reports were categorized as erosion (“mild”) except for one categorized as ulceration (in the EDS-FLU 186-µg group); none progressed in severity beyond mild severity, and all but one resolved with continued use of study medication. Two septal perforations were identified during the trial (1 in the EDS-FLU 93-µg group and 1 in the EDS-FLU 186-µg group). Neither was reported in the context of progression from erosion to ulceration to perforation (there were no cases of progression). It was not possible to determine if improved visualization due to reduction in nasal edema and polyps enabled new identification of the perforations, as prebaseline imaging was not available. Both patients were among the large number of patients reporting prior sinus surgery, an independent risk factor for perforation, and one had also undergone septoplasty. These 2 reports are too few to reasonably assess possible causality or dose–response; additional data from other studies should also be evaluated.29,54,55
Polyp grade assessment by endoscopic scoring in this and other trials is subject to important limitations, particularly with regard to attempting comparison across studies. The grading system in this trial and most others relies on 1-dimensional anatomic landmarks, which offers acceptable interinvestigator reproducibility.56 However, this approach uses unequal grading steps and does not capture volumetric changes, making numeric comparison of mean changes potentially misleading. Moreover, subtle-seeming differences in scoring approach (eg, 4-point adaptations that increase sensitivity to small reductions in the size of large polyps) limit the ability to compare across trials. Finally, frequent scheduled endoscopy and the active inquiry approach in this study may have increased the sensitivity of detecting certain AEs, particularly those of less clinical significance that might not otherwise be noted.
Conclusion
Effective medical management of NP is challenging, in part due to an inability to effectively deliver medication to target superior/posterior nasal regions affected by the disease. EDS-FLU uses a mechanism shown to produce superior/posterior deposition, and in this controlled trial versus EDS-placebo produced meaningful improvement in a broad range of objective (NP grade) and subjective (SNOT-22, RSDI, SF-36, and PGIC) measures in a patient population with moderate-severe disease, many of whom had previously tried steroids and/or surgery. The safety of EDS-FLU was generally comparable to previously studied conventional nasal steroids. These data, when considered in the context of additional emerging evidence, suggest that EDS-FLU may prove to be an important component of medical therapy for diseases such as CRS that are characterized by inflammation in the posterior and superior regions of the nasal cavity.
Supplemental Material
Supplemental Material for NAVIGATE I: Randomized, Placebo-Controlled, Double-Blind Trial of the Exhalation Delivery System With Fluticasone for Chronic Rhinosinusitis With Nasal Polyps by Raj Sindwani, Joseph K. Han, Daniel F.Soteres, John C.Messina, Jennifer L.Carothers, Ramy A.Mahmoud, Per G.Djupesland in American Journal of Rhinology & Allergy
Acknowledgments
The authors thank the patients who participated in this study and the principal study investigators (Online Appendix 1). Editorial assistance with drafting the report following the authors’ guidance, incorporating comments according to authors’ feedback, and providing support with submission was provided by Benjamin J. Epstein, PharmD, of ECIR Medical Communications.
Authors’ Note
This study was presented at the American Academy of Allergy, Asthma and Immunology, March 3–6, 2017, Georgia World Congress Center, Atlanta, GA, USA.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R. Sindwani, J. K. Han, and D. F. Soteres were investigators for this study and received assistance with study design, study implementation, and data analysis from OptiNose U.S., Inc. J. K. Han and D. F. Soteres have served as consultants to OptiNose U.S., Inc. R. Sindwani has served as consultant for Medtronic, Olympus, and Acclarent (not relevant to this article). D. F. Soteres is a member of the OptiNose Speaker Bureau and owns stock in OptiNose U.S., Inc. J. C. Messina, J. L. Carothers, and R. A. Mahmoud are OptiNose U.S., Inc. employees and have stock options. P. G. Djupesland is an employee of OptiNose A.S. and has stock options.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the OptiNose US, Inc.
IRB Statement
This was a randomized, double-blind trial undertaken in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Documents and procedures were approved by appropriate institutional review boards and ethics committees at each site; patients provided written informed consent before participation.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011; 128(4):693–707; quiz 708–709. [DOI] [PubMed] [Google Scholar]
- 2.Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA(2)LEN study. Allergy. 2011; 66(9):1216–1223. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AG, Stewart WF, Sundaresan AS, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017; 72(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011; 121(12):2672–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995; 113(1):104–109. [DOI] [PubMed] [Google Scholar]
- 6.Lam K, Schleimer R, Kern RC. The etiology and pathogenesis of chronic rhinosinusitis: a review of current hypotheses. Curr Allergy Asthma Rep. 2015; 15(7):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens WW, Schleimer RP, Chandra RK, Peters AT. Biology of nasal polyposis. J Allergy Clin Immunol. 2014; 133(5):1503–1503.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hull BP, Chandra RK. Refractory chronic rhinosinusitis with nasal polyposis. Otolaryngol Clin North Am. 2017; 50(1):61–81. [DOI] [PubMed] [Google Scholar]
- 9.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6(Suppl 1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012; 50(23):1–298. [PubMed] [Google Scholar]
- 11.Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern RC. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013; 132(5):1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Zele T, Gevaert P, Holtappels G, et al. Oral steroids and doxycycline: two different approaches to treat nasal polyps. J Allergy Clin Immunol. 2010; 125(5):1069–1076.e4. [DOI] [PubMed] [Google Scholar]
- 13.Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Ann Intern Med. 2011; 154(5):293–302. [DOI] [PubMed] [Google Scholar]
- 14.Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017; 357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy P, Bassiouni A, Psaltis A, Antisdel J, Brunworth J. Avascular necrosis after oral corticosteroids in otolaryngology: case report and review of the literature. Allergy Rhinol (Providence). 2016; 7(1):50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya N, Orlandi RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011; 144(3):440–445. [DOI] [PubMed] [Google Scholar]
- 17.Baguley C, Brownlow A, Yeung K, Pratt E, Sacks R, Harvey R. The fate of chronic rhinosinusitis sufferers after maximal medical therapy. Int Forum Allergy Rhinol. 2014; 4(7):525–532. [DOI] [PubMed] [Google Scholar]
- 18.Djupesland PG, Skretting A. Nasal deposition and clearance in man: comparison of a bidirectional powder device and a traditional liquid spray pump. J Aerosol Med Pulm Drug Deliv. 2012; 25(5):280–289. [DOI] [PubMed] [Google Scholar]
- 19.Weber R, Keerl R, Radziwill R, et al. Videoendoscopic analysis of nasal steroid distribution. Rhinology. 1999; 37(2):69–73. [PubMed] [Google Scholar]
- 20.Emanuel IA, Blaiss MS, Meltzer EO, Evans P, Connor A. Nasal deposition of ciclesonide nasal aerosol and mometasone aqueous nasal spray in allergic rhinitis patients. Am J Rhinol Allergy. 2014; 28(2):117–121. [DOI] [PubMed] [Google Scholar]
- 21.Leach CL, Kuehl PJ, Chand R, McDonald JD. Nasal deposition of HFA-beclomethasone, aqueous fluticasone propionate and aqueous mometasone furoate in allergic rhinitis patients. J Aerosol Med Pulm Drug Deliv. 2015; 28(5):334–340. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud R, Palmer J, Biletch R, Grosel K, Messina JC. Healthcare for chronic rhinosinusitis (CRS) symptoms—a cross-sectional population-based survey of U.S. adults meeting symptom criteria for CRS. J Allergy Clin Immunol. 2017; 139(2):AB68. [Google Scholar]
- 23.Rudmik L, Soler ZM, Hopkins C, et al. Defining appropriateness criteria for endoscopic sinus surgery during management of uncomplicated adult chronic rhinosinusitis: a RAND/UCLA appropriateness study. Int Forum Allergy Rhinol. 2016; 6(6):557–567. [DOI] [PubMed] [Google Scholar]
- 24.DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017; 127(3):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopkins C, Slack R, Lund V, Brown P, Copley L, Browne J. Long-term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope. 2009; 119(12):2459–2465. [DOI] [PubMed] [Google Scholar]
- 26.XHANCE™ (Fluticasone Propionate) [Prescribing Information]. Yardley, PA: OptiNose U.S. Inc; 2017. [Google Scholar]
- 27.Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013; 3(1):42–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messina J, Carothers J, Obaidi M, Offman E, Mahmoud R. Intranasal Fluticasone Propionate delivered by Exhalation Delivery System (FLU-EDS) versus flonase nasal spray and flovent HFA: a randomized comparison of bioavailability. J Allergy Clin Immunol. 2017; 139(2):AB253. [DOI] [PubMed] [Google Scholar]
- 29.Leopold DA, Elkayam D, Messina JC, Kosik-Gonzalez C, Djupesland PG, Mahmoud RA. NAVIGATE II: randomized, double-blind trial of the exhalation delivery system with fluticasone (EDS-FLU) for nasal polyposis. J Allergy Clin Immunol. 2019;143(1):126–134. [DOI] [PubMed] [Google Scholar]
- 30.Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double-blind, placebo-controlled study of budesonide. Clin Otolaryngol Allied Sci. 1995; 20(1):26–30. [DOI] [PubMed] [Google Scholar]
- 31.Gillett S, Hopkins C, Slack R, Browne JP. A pilot study of the SNOT 22 score in adults with no sinonasal disease. Clin Otolaryngol. 2009; 34(5):467–469. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009; 34(5):447–454. [DOI] [PubMed] [Google Scholar]
- 33.Smith MT, Wegener ST. Measures of sleep: the Insomnia Severity Index, Medical Outcomes Study (MOS) sleep scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res. 2003; 49(5S):S184–S196. [Google Scholar]
- 34.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997; 123(11):1175–1179. [DOI] [PubMed] [Google Scholar]
- 35.Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulat Physiol Ther. 2004; 27(1):26–35. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009; 10(4):S73. [Google Scholar]
- 37.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30(6):473–483. [PubMed] [Google Scholar]
- 38.Keith P, Nieminen J, Hollingworth K, Dolovich J. Efficacy and tolerability of fluticasone propionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adults. Clin Exp Allergy. 2000; 30(10):1460–1468. [DOI] [PubMed] [Google Scholar]
- 39.Penttila M, Poulsen P, Hollingworth K, Holmstrom M. Dose-related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo-controlled randomized study in adult patients. Clin Exp Allergy. 2000; 30(1):94–102. [DOI] [PubMed] [Google Scholar]
- 40.Small CB, Hernandez J, Reyes A, et al. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. J Allergy Clin Immunol. 2005; 116(6):1275–1281. [DOI] [PubMed] [Google Scholar]
- 41.Stjarne P, Mosges R, Jorissen M, et al. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2006; 132(2):179–185. [DOI] [PubMed] [Google Scholar]
- 42.Stjarne P, Blomgren K, Caye-Thomasen P, Salo S, Soderstrom T. The efficacy and safety of once-daily mometasone furoate nasal spray in nasal polyposis: a randomized, double-blind, placebo-controlled study. Acta Otolaryngol. 2006; 126(6):606–612. [DOI] [PubMed] [Google Scholar]
- 43.Vlckova I, Navratil P, Kana R, Pavlicek P, Chrbolka P, Djupesland PG. Effective treatment of mild-to-moderate nasal polyposis with fluticasone delivered by a novel device. Rhinology. 2009; 47(4):419–426. [DOI] [PubMed] [Google Scholar]
- 44.Hansen FS, Djupesland PG, Fokkens WJ. Preliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitis. Rhinology. 2010; 48(3):292–299. [DOI] [PubMed] [Google Scholar]
- 45.Merkus P, Ebbens FA, Muller B, Fokkens WJ. The ‘best method’ of topical nasal drug delivery: comparison of seven techniques. Rhinology. 2006; 44(2):102–107. [PubMed] [Google Scholar]
- 46.Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Influence of particle size and patient dosing technique on lung deposition of HFA-beclomethasone from a metered dose inhaler. J Aerosol Med. 2005; 18(4):379–385. [DOI] [PubMed] [Google Scholar]
- 47.Chong LY, Head K, Hopkins C, Philpott C, Schilder AG, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016; 26(4):CD011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachert C, Mannent L, Naclerio RM, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016; 315(5):469–479. [DOI] [PubMed] [Google Scholar]
- 49.Gevaert P, Van Bruaene N, Cattaert T, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011; 128(5):989–995.e1-8. [DOI] [PubMed] [Google Scholar]
- 50.Gevaert P, Calus L, Van Zele T, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; 131(1):110–116.e1. [DOI] [PubMed] [Google Scholar]
- 51.Soler ZM, Jones R, Le P, et al. Sino-Nasal Outcome Test-22 outcomes after sinus surgery: a systematic review and meta-analysis. Laryngoscope. 2018; 128(3):581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casale TB, Romero FA, Spierings EL. Intranasal noninhaled carbon dioxide for the symptomatic treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2008; 121(1):105–109. [DOI] [PubMed] [Google Scholar]
- 53.Djupesland PG, Messina J, Mahmoud R. New Exhalation Delivery Systems (EDS) enhance topical steroid delivery in chronic rhinosinusitis with nasal polyps (CRSwNP). Paper presented at: The AAAAI 2017 Annual Meeting; March 3–6, 2017; Atlanta, GA.
- 54.Sher M, Mair E, Carothers J, Mahmoud R, Djupesland P. EXHANCE-3: a phase 3, three-month study of safety and efficacy of Fluticasone Propionate Exhalation Delivery System (FLU-EDS) in patients with chronic rhinosinusitis with (CRSwNP) and without nasal polyps (CRSsNP). Poster presented at: AAAAI 2017 Annual Meeting, March 3–6, 2017; Atlanta, GA.
- 55.Palmer JN, Jacobson KW, Messina JC, Kosik-Gonzalez C, Djupesland PG, Mahmoud RA. EXHANCE-12: 1-year study of the exhalation delivery system with fluticasone (EDS-FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018; 8:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johansson L, Holmberg K, Melen I, Stierna P, Bende M. Sensitivity of a new grading system for studying nasal polyps with the potential to detect early changes in polyp size after treatment with a topical corticosteroid (budesonide). Acta Otolaryngol. 2002; 122(1):49–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for NAVIGATE I: Randomized, Placebo-Controlled, Double-Blind Trial of the Exhalation Delivery System With Fluticasone for Chronic Rhinosinusitis With Nasal Polyps by Raj Sindwani, Joseph K. Han, Daniel F.Soteres, John C.Messina, Jennifer L.Carothers, Ramy A.Mahmoud, Per G.Djupesland in American Journal of Rhinology & Allergy