Abstract
Background
We focused on the therapeutic effects of the stable gastric pentadecapeptide BPC 157 in spinal cord injury using a rat model. BPC 157, of which the LD1 has not been achieved, has been implemented as an anti-ulcer peptide in inflammatory bowel disease trials and recently in a multiple sclerosis trial. In animals, BPC 157 has an anti-inflammatory effect and therapeutic effects in functional recovery and the rescue of somatosensory neurons in the sciatic nerve after transection, upon brain injury after concussive trauma, and in severe encephalopathies. Additionally, BPC 157 affects various molecular pathways.
Methods
Therefore, BPC 157 therapy was administered by a one-time intraperitoneal injection (BPC 157 (200 or 2 μg/kg) or 0.9% NaCl (5 ml/kg)) 10 min after injury. The injury procedure involved laminectomy (level L2-L3) and a 60-s compression (neurosurgical piston (60–66 g) of the exposed dural sac of the sacrocaudal spinal cord). Assessments were performed at 1, 4, 7, 15, 30, 90, 180, and 360 days after injury.
Results
All of the injured rats that received BPC 157 exhibited consistent clinical improvement, increasingly better motor function of the tail, no autotomy, and resolved spasticity by day 15. BPC 157 application largely counteracted changes at the microscopic level, including the formation of vacuoles and the loss of axons in the white matter, the formation of edema and the loss of motoneurons in the gray matter, and a decreased number of large myelinated axons in the rat caudal nerve from day 7. EMG recordings showed a markedly lower motor unit potential in the tail muscle.
Conclusion
Axonal and neuronal necrosis, demyelination, and cyst formation were counteracted. The functional rescue provided by BPC 157 after spinal cord injury implies that BPC 157 therapy can impact all stages of the secondary injury phase.
Keywords: BPC 157, Injury, Rats, Spinal cord
Introduction
We focused on the application of the stable gastric pentadecapeptide BPC 157 [1–11] to improve the outcomes of spinal cord injury in rats.
Spinal cord injury generally involves the preclusion of neural relays across the lesion site and is thereby predictably associated with a lack of functional improvement [12, 13]. On the other hand, there is evidence that spinal cord injury triggers a cascade of secondary degenerative events that cause further damage to the injured area and induce local inflammation along with hemorrhage and edema [12, 13] and that the therapeutic agents imatinib (which has been shown to inhibit cytokine production and reduce hemorrhage, edema, and inflammation) [14] and ibuprofen initiate favorable axonal growth and functional recovery through Rho inhibition [15]. Likewise, there is favorable evidence to support the engraftment of neural stem cells [16] or bone marrow stromal cells [17] into the lesion site. However, there are disputes about the relevant applicability of this evidence [18, 19], particularly considering the low survival rate of bone marrow stromal cells transplanted into the contused adult rat spinal cord [20, 21] and the need to completely fill the lesion site with neural stem cells [22]. Consequently, there have been attempts to improve the therapeutic effectiveness with combined treatments (i.e., neural stem cells with fibrin and a growth factor cocktail (BDNF; NT-3; mGDNF; IGF; bFGF; EGF; PDF; aFGF; and HGF) [23] or bone marrow stromal cells with the application of cyclosporine, minocycline, and methylprednisolone [24]). Likewise, considering the beneficial effect of the deletion of the Nogo Receptor 1 (NgR1) gene, a sequential combination of Nogo-A suppression (by anti-Nogo-A antibody treatment) and treadmill rehabilitative training was examined [25].
It is generally believed that further attempts are fully justified [26]. In comparison, the stable gastric pentadecapeptide BPC 157, an emerging treatment with potential therapeutic applications, appears to be unrestricted by the limitations seen in previous therapies. The stable gastric pentadecapeptide BPC 157, an original cytoprotective antiulcer peptide that is used in ulcerative colitis and recently in a multiple sclerosis trial and that has an LD1 that has not been achieved [1–11], is known to have pleiotropic beneficial effects [1–11] and to interact with several molecular pathways [2, 27–32]. BPC 157 has beneficial effects on inflammation, hemorrhage, and edema after traumatic brain injury [33], various severe encephalopathies (which follow gastrointestinal and/or liver lesions), NSAID overdose [34–37], or insulin overdose seizures [38] and on severe muscle weakness after exposure to the specific neurotoxin cuprizone in a rat multiple sclerosis model [39] or magnesium overdose [40]. In other studies, it was shown that BPC 157 counteracts increased levels of proinflammatory and procachectic cytokines such as IL-6 and TNF-α [2]. Finally, BPC 157 improves sciatic nerve healing [41] when applied intraperitoneally, intragastrically, or locally at the site of anastomosis shortly after injury or directly into the tube after non-anastomosed nerve tubing (7-mm nerve segment resection).
Therefore, we used a model of spinal cord injury that has many characteristics found in human spastic syndrome [42] and can be used long-term to provide a realistic model of spasticity development in the tail muscle.
The administered therapy was a one-time intraperitoneal application of the stable gastric pentadecapeptide BPC 157, much like the one-time engraftment of neural stem cells [16] or bone marrow stromal cells [17] into the lesion site. This experiment will provide evidence that BPC 157 treatment can recover tail function, resolve spasticity, and improve neurologic recovery.
Materials and methods
Animals
Wistar albino male rats (aged 12 weeks, 350–400 g b.w.) were bred in-house (the animal facility at the Department of Pharmacology, School of Medicine, Zagreb, Croatia; registered by Directorate of Veterinary; Reg. No: HR-POK-007), acclimated for 5 days, and randomly assigned to experimental groups (at least 6 animals per experimental group and interval). The experiments were approved by the Local Ethics Committee. The laboratory animals were housed in PC cages in conventional laboratory conditions at a temperature of 22.4 °C, a relative humidity of 40–70%, and a noise level of 60 dB. Each cage was identified by the date, study number, group, dose, number, and sex of each animal. Fluorescent lighting provided illumination 12 h per day. A standard GLP diet and fresh water were provided ad libitum. Furthermore, all experiments were carried out under a blind protocol, and the effects were assessed by examiners who were completely unaware of the protocol. We certify that government regulations concerning the ethical use of animals were adhered to during this research.
Drugs
The pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W. 1419) (Diagen, Ljubljana, Slovenia) dissolved in 0.9% NaCl was used in all experiments [1–11]. The peptide BPC 157 is part of the sequence of the human gastric juice protein BPC and is freely soluble in water and 0.9% NaCl at pH 7.0. BPC 157 was prepared as described previously with 99% high-pressure liquid chromatography (HPLC) purification, expressing 1-des-Gly peptide as an impurity [1–11].
Surgery and spinal cord injury
Deeply anesthetized (3% isoflurane, ketamine 50 mg/kg b.w.) rats were subjected to laminectomy at lumbar level L2–L3, which corresponds to the sacrocaudal spinal cord (S2-Co1) as described previously [42]. A neurosurgical piston with a graduated force of 60–66 g was placed over the exposed dura and left for 60 s to induce a compression injury. After the piston was removed, the muscle and skin incisions were closed. A single injection (0.9% NaCl 5 ml/kg b.w.; pentadecapeptide BPC 157 200 μg/kg b.w. or 2 μg/kg b.w.) was administered intraperitoneally 10 min postinjury. Thereafter, the animals were returned to their cages in pairs, and food and water were provided ad libitum. According to previously assigned interval groups (7, 15, 30, 90, 180, and 360 days), the animals were sacrificed with an overdose of 3% isoflurane. To establish secondary spinal cord injury, four animals were sacrificed 10 min after spinal injury immediately prior to the administration of therapy. Four animals were subjected only to laminectomy without spinal cord injury and sacrificed after 360 days.
Clinical evaluation
Tail motor function was scored 8 h and 1, 4, 7, 15, 30, 90, 180, and 360 days after injury (0—autotomy; 1—complete loss of tail function; 2—maximum elevation of 1/4 of the tail length; 3—maximum elevation of 1/2 of the tail length; 4—maximum elevation of 3/4 of the tail length; 5—normal function). At the same intervals, the tails were observed for spasticity; after manual stimulation with the standardized stretch/rub maneuver, the tails were scored according to the Bennett scale [42]: 0—normal phenotype; 1—flaccid tail; 2—hypertonic flexor muscle with coiled and stiff tail; 3—hyperreflexia, e.g., coiling flexor spasm and clonus in response to light touch or stretch; and 4—hypertonic flexor and extensor muscles, clonus and hyperreflexia, the latter including a positive curling reaction.
Electrophysiology recordings
Before sacrifice, the animals from the 30-, 90-, 180-, and 360-day postspinal cord injury interval groups were placed in a wooden box with their tails exposed. Three pairs of monopolar needles were stabbed 3 mm deep into the tail 10, 60, and 100 mm caudal to the tail base. Using a TECA 15 electromyography apparatus with a signal filter between 50 Hz and 5 kHz, voluntary muscle activity was recorded from the most caudal pair of electrodes, and the average motor unit potential (MUP) was recorded. Thereafter, the compound motor action potential (CMAP) was recorded from the same pair of electrodes after stimulating the first and second electrodes (a repetition of 1 Hz and a stimulus duration of 0.05 ms). The amplitude, polyphasic changes, and the proximal and distal CMAP latencies were recorded, and the nerve conduction velocity was calculated according to previous studies [41, 43].
Histology
A 10-mm long piece of the spinal column (the L2–L3 vertebral body) and the surrounding muscle were collected from each sacrificed animal and fixed in 4% formaldehyde in phosphate buffer (pH 7.4). Upon fixation, the spinal cord was decalcinated, dehydrated in graded ethanol solutions, and embedded in paraffin. Serial 5-μm cross-sections were deparaffinated in xylene, rehydrated in graded ethanol solutions, and stained with hematoxylin/eosin and toluidine blue (Kemica, Croatia). Part of the spinal cord gray and white matter was used for analysis under light microscopy (magnification × 300). According to previous studies [13, 33], the intensity and distribution of the following pathological spinal cord changes were evaluated semiquantitatively (0—no changes; 1—small or focal changes; 2—moderate changes; 3—numerous confluent changes): (a) the hemorrhagic zone, (b) edema, (c) the loss of neurons in anterior horn and intermediate gray matter, (d) vacuoles, and (e) the loss of lateral and posterior spinal column tracts.
For peripheral nerve analysis, a 5-mm-long piece of tail 15 mm distal from the tail base was collected from each sacrificed animal, fixed in 4% formaldehyde in phosphate buffer (pH 7.4), decalcinated, and impregnated with 1% osmium tetroxide for a few days. The specimens were dehydrated in graded ethanol solutions, embedded in paraffin, cut into 5-μm sections, deparaffinated in xylene, rehydrated in graded ethanol solutions, and mounted on glass slides. Representative field images of four caudal nerves from each tail were taken using light microscopy (magnification × 500) with a CCD camera using ISSA 3.1 software (VamStec, Zagreb) according to a previous study [41]. Axonal myelination was analyzed according to the following quantifications: (a) the total number of myelinated axons per 10,000 μm2; (b) the number of myelinated axons with a diameter ≥ 7 μm per 10,000 μm2; and (c) the average axonal diameter.
Statistical analyses
Scoring data are expressed as the median, min, and max and were analyzed by Kruskal–Wallis ANOVA (P values < 0.05 were considered significant) followed by the Mann–Whitney U test (P values < 0.025 were considered significant) with Bonferroni correction; these tests are considered nonparametric alternatives to one-way ANOVA and Student’s t test. Numeric data are expressed as the mean ± standard deviation (SD) and were analyzed by one-way ANOVA followed by LSD test. The statistical program Statistica for Windows, ver. 12.1 (StatSoft Inc. Tulsa, OK, USA) was used for statistical analysis. P values < 0.05 were considered significant.
Results
Clinical examinations
Tail motor function score
As expected, the tail motor function scores demonstrated persistent debilitation in the rats that underwent spinal cord injury and received saline postinjury.
In contrast, after initial disability, the rats that underwent spinal cord injury and received BPC 157 exhibited consistent improvement in motor function compared to that in the corresponding controls (Fig. 1). In particular, from day 180, autotomy was noted in the rats that underwent spinal cord injury but not in those that had been treated with BPC 157 (Fig. 2).
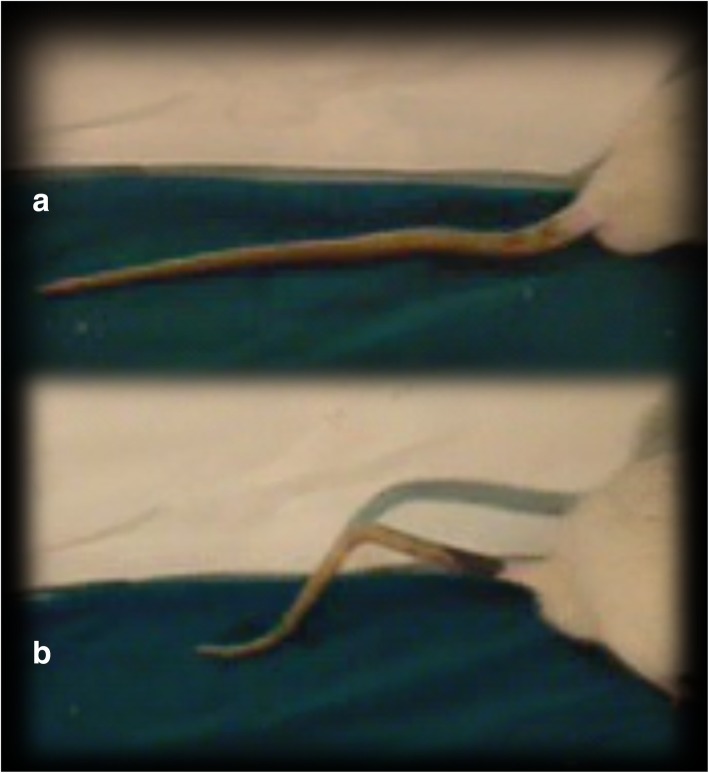
Fig. 1.
Tail motor function (a, b) in rats that underwent spinal cord injury. Thirty days following injury. Debilitated rats underwent spinal cord injury that received saline post-injury (a). Contrarily, rats that had received BPC 157 (b) exhibit tail motor function rescue and consistently better motor function than the corresponding controls (a)
Fig. 2.
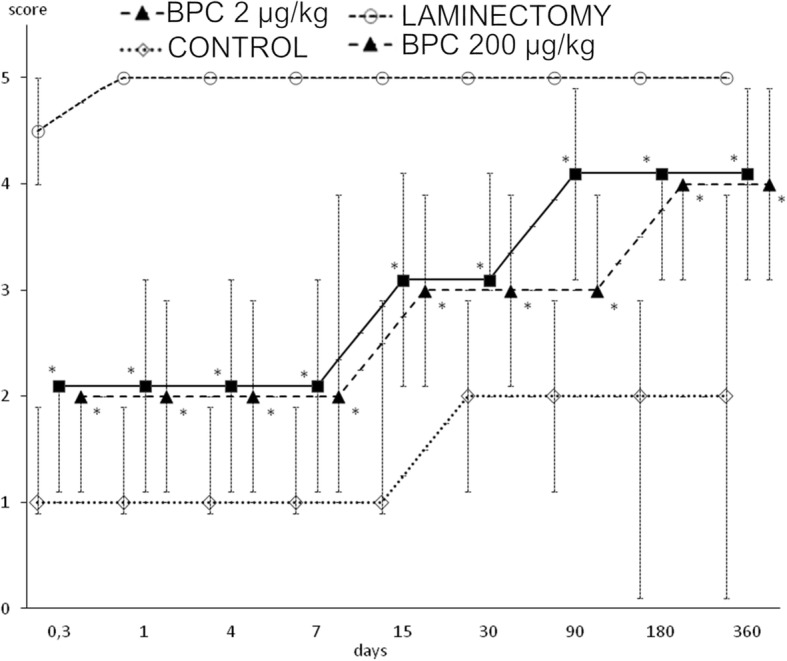
In rats that underwent spinal cord injury, debilitated and rescued tail motor function (BPC 157 (200 or 2 μg/kg) or saline 5 ml/kg intraperitoneally at 10 min after injury) presented by tail motor function score. Mark presents median score, and vertical bars correspond to maximum and minimum score. *P < 0.025 vs. control
Tail spasticity
Interestingly, the development of spasticity began earlier in the rats that underwent spinal cord injury and had been treated with BPC 157 than in the corresponding controls. However, the controls exhibited sustained spasticity until the end of the experiment (day 360) while the BPC 157 rats exhibited resolved spasticity by day 15 (Fig. 3).
Fig. 3.
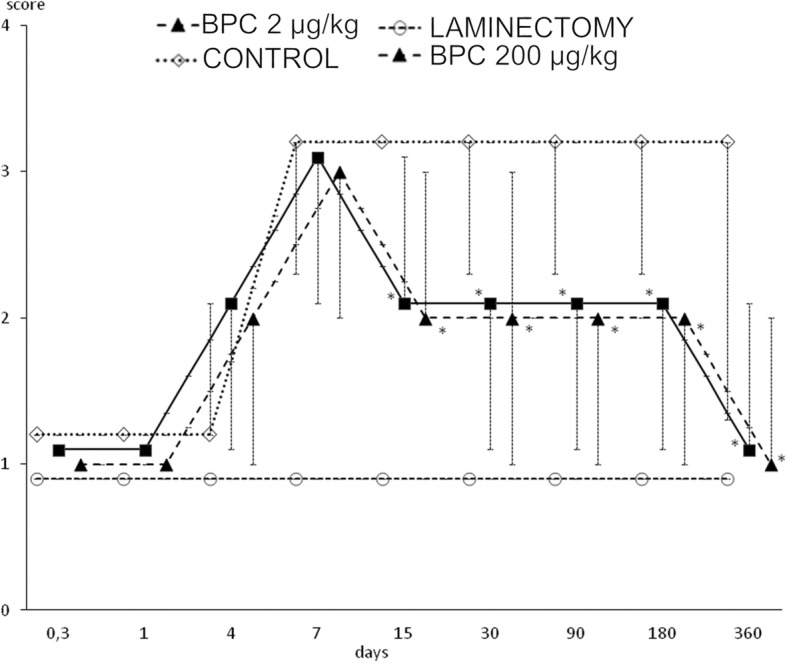
In rats that underwent spinal cord injury, debilitated and rescued tail spasticity (BPC 157 (200 or 2 μg/kg) or saline 5 ml/kg intraperitoneally at 10 min after injury) according to Bennett scoring. Mark presents median, and vertical bars correspond to maximum and minimum score. *P < 0.025 vs. control
Histology results
Before the initiation of therapy, at 10 min after injury induction, a large hemorrhagic zone was present over the lateral and posterior white columns in all of the rats, but there were no changes in the gray matter. Notably, after the application of saline or BPC 157, the injury progression in the rats from the different experimental groups was fundamentally different. Beginning on day 7, vacuoles and the loss of posterior and lateral spinal column tracts were observed instead of hemorrhagic areas in all controls, disturbances that were largely counteracted in the BPC 157-treated rats (Table 1 and Fig. 4). Likewise, beginning on day 7, the controls exhibited edema and the loss of neurons in the anterior horn and intermediate gray matter, disturbances that were largely counteracted the in BPC 157-treated rats (Table 2 and Fig. 5).
Table 1.
Histology of lateral and posterior columns of spinal cord in 7 different time points, from 10 min till 360 days, after spinal cord injury
| Treatment group | Time after spinal cord injury (min/med/max) | |||||||
|---|---|---|---|---|---|---|---|---|
| 10 min. | 7 days | 15 days | 30 days | 90 days | 180 days | 360 days | ||
|
Control saline 5 ml/kg b.w. i.p. |
Hemorrhage | 2/2.5/3 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Vacuoles | 0/0/0 | 2/2.5/3 | 1/3/3 | 2/2/2 | 2/2/2 | 2/2.5/3 | 2/2.5/3 | |
| Necrosis | 0/0/0 | 1/2.5/3 | 2/3/3 | 2/3/3 | 2/3/3 | 1/3/3 | 1/3/3 | |
| Pentadecapeptide BPC 157 200 μg/kg b.w. i.p. | Hemorrhage | 2/2.5/3 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Vacuoles | 0/0/0 | 1/1/1* | 1/1/1* | 0/1/2* | 1/1/1* | 1/1/2* | 1/2/2* | |
| Necrosis | 0/0/0 | 0/2/2 | 0/0/2* | 1/1/2* | 0/0/1* | 1/1/1* | 0/0/1 | |
| Pentadecapeptide BPC 157 2 μg/kg b.w. i.p. | Hemorrhage | 2/2.5/3 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Vacuoles | 0/0/0 | 1/1/1* | 1/1/1* | 0/1/2* | 1/1/1* | 1/1/2* | 1/2/2* | |
| Necrosis | 0/0/0 | 0/2/2 | 0/0/2* | 1/1/2* | 0/0/1* | 1/1/1* | 0/0/1 | |
| Laminectomy | Hemorrhage | – | – | – | – | – | – | 0/0/0 |
| Vacuoles | – | – | – | – | – | – | 0/0/0 | |
| Necrosis | – | – | – | – | – | – | 0/0/0 | |
Semiquantitatively evaluated intensity of (a) hemorrhagic zone, (b) vacuoles, and (c) loss of tracts in lateral and posterior columns of spinal cord (0—no changes; 1—small or focal changes; 2—moderate changes; 3—numerous confluent changes)
*Statistically significant difference BPC 157 groups vs control group, P < 0.025
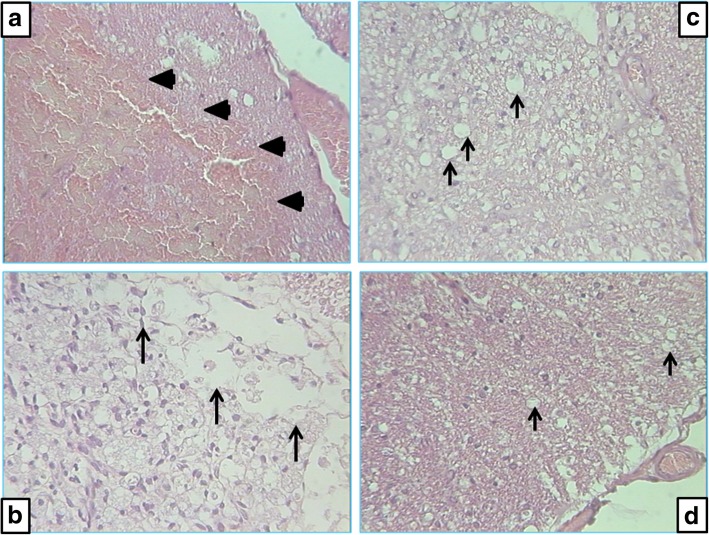
Fig. 4.
Microscopic presentation of lateral columns of rat spinal cord at the lesion site. a Ten minutes after injury, all animals have numerous fields of hemorrhage—arrowheads. b–d Histology 30 days after injury: b control animals, numerous confluent vacuoles—arrows; c BPC 157 2 μg/kg, few small vacuoles—arrows; d BPC 157200 μg/kg, only occasionally small vacuoles—arrows. Staining H&E, magnification × 300
Table 2.
Histology of anterior horn and intermediate gray matter of spinal cord in 7 different time points, from 10 min till 360 days, after spinal cord injury
| Treatment group | Time after spinal cord injury (min/med/max) | |||||||
|---|---|---|---|---|---|---|---|---|
| 10 min. | 7 days | 15 days | 30 days | 90 days | 180 days | 360 days | ||
|
Control saline 5 ml/kg b.w. i.p. |
Hemorrhage | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Edema | 0/0/0 | 1/1.5/2 | 1/3/3 | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 | |
| Necrosis | 0/0/0 | 1/1.5/2 | 1/3/3 | 3/3/3 | 3/3/3 | 3/3/3 | 3/3/3 | |
| Pentadecapeptide BPC 157 200 μg/kg b.w. i.p. | Hemorrhage | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Edema | 0/0/0 | 0/1/1 | 0/1/1* | 0/1/3* | 1/1/1* | 1/1/1* | 2/2/2* | |
| Necrosis | 0/0/0 | 0/1/1 | 1/1/1 | 0/1/3* | 0/0/2* | 3/3/3 | 3/3/3 | |
| Pentadecapeptide BPC 157 2 μg/kg b.w. i.p. | Hemorrhage | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 |
| Edema | 0/0/0 | 0/1/1 | 0/0/2* | 1/2/2* | 1/1/1* | 1/1/1* | 2/2/2* | |
| Necrosis | 0/0/0 | 1/1/1 | 0/0/3 | 1/2/2* | 0/0/2* | 3/3/3 | 3/3/3 | |
| Laminectomy | Hemorrhage | – | – | – | – | – | – | 0/0/0 |
| Edema | – | – | – | – | – | – | 0/0/0 | |
| Necrosis | – | – | – | – | – | – | 0/0/0 | |
Semiquantitatively evaluated intensity of (a) hemorrhagic zone, (b) edema, and (c) loss of neurons in anterior horn and intermediate gray matter of spinal cord (0—no changes; 1—small or focal changes; 2—moderate changes; 3—numerous confluent changes)
*Statistically significant difference between both pentadecapeptide BPC 157 groups and control group, P < 0.025
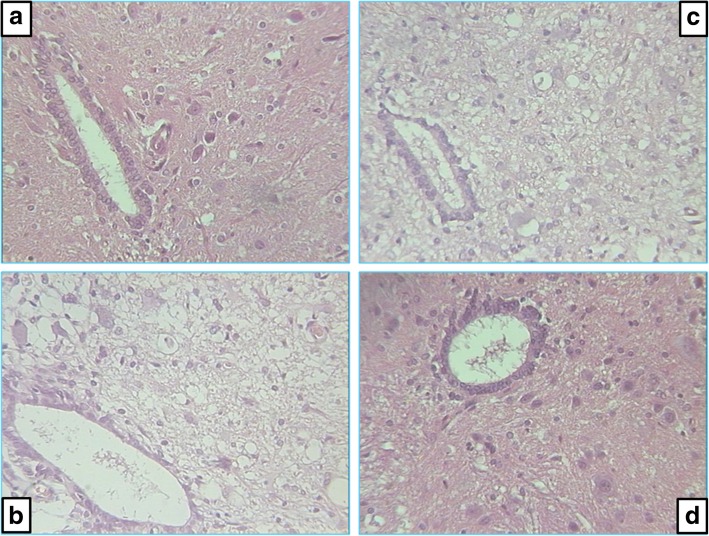
Fig. 5.
Microscopic presentation of posttraumatic spinal cord changes in the intermediate gray matter at the lesion site. a Ten min after injury, all animals have no difference compared to healthy animals. b–d Histology 30 days after injury: b control animals, huge edema and loss of neurons; c BPC 157 2 μg/kg, minimal edema changes and occasionally loss of neurons; d BPC 157 200 μg/kg, no difference to healthy animal. Staining H&E, magnification × 300
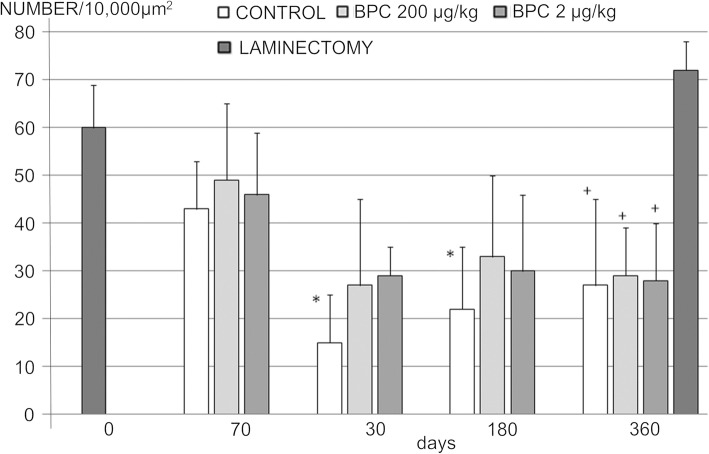
While the significance of this finding remains to be determined, it is probably worth mentioning that a decrease in the number of large myelinated axons in rat caudal nerves was observed in all animals until day 30, with a markedly greater number in controls and fewer in injured rats that received BPC 157 treatment. Interestingly, after 180 days, recovery occurred, and the number of large myelinated axons in the controls reached that in the BPC 157-treated rats, and this finding persisted through the end of the experiment (Fig. 6).
Fig. 6.
A number of myelinated axons with diameter ≥ 7 μm in rat caudal nerve per 10,000 μm2 in corresponding days. Columns present mean and vertical bars standard deviation. P value *P < 0.05 BPC 157 groups vs. saline. +P < 0.05 saline and BPC 157 groups vs. laminectomy animals
Electrophysiology results
Based on a well-known phenomenon in peripheral nerve injury (i.e., as the number of preserved motoneurons decreases, the MUP (giant potential) in the tail muscle increases), it is conceivable that the BPC 157-treated rats that underwent spinal cord injury and were subjected to EMG recordings exhibited a markedly lower MUP in the tail muscle than that in the corresponding controls (Table 3). Consistently, the motor nerve conduction study confirmed the absence of demyelinated processes in the tail caudal nerves after spinal cord injury (the CMAP showed normal biphasic potentials, similar amplitudes, and similar conduction velocities in all of the rats) (Table 4).
Table 3.
Voluntary electromyography (EMG) of rat segmental tail muscle, in 4 different time points, from 7 days till 360 days, after spinal cord injury
| Group | Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Time of EMG recording after spinal cord injury | ||||||
| 7 days | 30 days | 180 days | 360 days | |||
| Healthy control | Amp./mV | 100 ± 40 | ||||
| Saline 5 ml/kg b.w. i.p. | Amp./mV | 180 ± 55 | 370 ± 52 | 575 ± 300 | 460 ± 175+ | |
| BPC 157 200 μg/kg b.w. i.p. | Amp./mV | 200 ± 50 | 200 ± 80* | 290 ± 150* | 300 ± 65*+ | |
| BPC 157 2 μg/kg b.w. i.p. | Amp./mV | 210 ± 25 | 170 ± 25* | 270 ± 110* | 280 ± 70*+ | |
| Laminectomy | Amp./mV | 125 ± 30 | ||||
Voluntary electromyography of rat segmental tail muscle. Amp. amplitude of motor unit potential (MUP) in millivolts (mV), Mean arithmetic mean, SD standard deviation
*P value < 0.05 BPC 157 groups vs. saline
+P value < 0.05 saline and BPC 157 groups vs. laminectomy animals
Table 4.
Stimulated electromyography (EMG) of rat segmental tail muscle, in 4 different time points, from 7 days till 360 days, after spinal cord injury
| Group | Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Time of EMG recording after spinal cord injury | ||||||
| 30 days | 90 days | 180 days | 360 days | |||
| Healthy control |
Amp./mV NCV/m/s |
3.0 ± 0.8 37 ± 6 |
||||
| Saline 5 ml/kg b.w. i.p. |
Amp./mV NCV/m/s |
3.8 ± 1.2 38 ± 14 |
3.0 ± 1.0 36 ± 12 |
3.0 ± 1.8 35 ± 11 |
2.9 ± 1.2 36 ± 10 |
|
| BPC 157200 μg/kg b.w. i.p. |
Amp./mV NCV/m/s |
3.3 ± 1.3 33 ± 4 |
4.3 ± 3.0 36 ± 6 |
2.8 ± 2.2 31 ± 10 |
2.6 ± 1.5 31 ± 9 |
|
| BPC 157 2 μg/kg b.w.. i.p. |
Amp./mV NCV/m/s |
2.9 ± 0.9 37 ± 8 |
1.9 ± 0.8 37 ± 7 |
3.2 ± 2.1 30 ± 9 |
2.4 ± 1.0 31 ± 8 |
|
| Laminectomy |
Amp./mV NCV/m/s |
3.3 ± 1.3 41 ± 11 |
||||
Stimulated electromyography of rat segmental tail muscle. Compound muscle action potential amplitude (amp.) and nerve conduction velocity (NCV). Mean arithmetic mean, SD standard deviation. No statistical difference using one-way ANOVA analysis
Discussion
This study attempted to demonstrate that the application of the stable gastric pentadecapeptide BPC 157 (by either of the used regimens) can improve the symptoms of spinal cord injury and lead to functional recovery in rats. In general, the one-time intraperitoneal application of the stable gastric pentadecapeptide BPC 157 is much like the engraftment of neural stem cells [16] or bone marrow stromal cells [17] into the lesion site. One should consider the primary phase lesion and hemorrhaging that results from mechanical damage during SCI as well as the secondary phase lesion that lasts several hours or even several months and is accompanied by edema, hemorrhage, inflammation, and cytotoxic edema [44–47] and may extend to the white matter area and lead to white matter degeneration and damage [48, 49]. This substantiates the evidence that the spared white matter holds the key to the functional motor recovery of the hind limbs after SCI and is closely correlated with the functional restoration of the paralyzed hind limbs [50–52]. On the other hand, spontaneous and often substantial functional improvements [53–55] after partial lesioning of the spinal cord are associated with the spontaneous sprouting of axons in the corticospinal tract [56–58] and the formation of neural circuits by spared spinal cord tissue [26]; these processes lead to partial functional recovery [59] or the formation of the neural fiber connection between the central pattern generator (CPG) and interneurons in the spinal cord, which can enable rhythmic movement [60–62].
Thus, to illustrate these combining points (i.e., [13, 44, 63]), considering that white matter injury is the major cause of functional loss after SCI [45, 52], it is important to note that cysts and the loss of axons instead of hemorrhagic areas were observed in the white matter in all of the controls beginning on day 7 and that the rats exhibited a tail motor score that persisted with only small improvements, sustained debilitation, sustained tail spasticity until the end of the experiment (day 360), a decrease in the number of large myelinated axons in the caudal nerve, a higher MUP (giant potential) in the tail muscle, and a group of atrophic fibers that likely represented a large unit that acquired many fibers through collateral reinnervation and then degenerated. Autotomy that occurs long after injury may appear as pain that occurs below the level of the injury (below-level pain) [64, 65], and the late spontaneous worsening may be the result of complete deafferentation of one or several spinal segments the stimulation of the nerve plexus, or dorsal root injury [66]. Together, these findings illustrate definitive spinal cord injury with very small spontaneous improvements in functional loss.
In contrast, it is possible that the administration of BPC 157 counteracts these disturbances to lead to considerable functional recovery. The vacuoles and the loss of axons in the white matter were largely counteracted in BPC 157-treated rats (Table 1 and Fig. 3). This result suggests that BPC 157-treated rats exhibit continual improvement in motor function even before tissue recovery, as observed by microscopy assessment. The resolution of spasticity by day 15 (Fig. 2) suggests that BPC 157 administration prevents the chain of events after spinal cord injury that is mediated by the loss of local segmental inhibition and/or by an increased sensory afferent drive that results in the exacerbation of α-motoneuron activity [66]. These findings substantiate the number of large myelinated axons in the caudal nerve and the lower MUP in the tail muscle.
Likewise, autotomy was completely prevented, much like in a previous study that showed recovery in BPC 157-treated rats that underwent traumatic nerve injury [41]; this suggests the counteraction of the chain of events that otherwise leads to painful sensations and refers to denervated regions and the preservation of one or more spinal segments [41].
It is possible that BPC 157 may affect voltage-gated sodium channels (VGSCs), which play a major role in the generation and propagation of action potentials in primary afferents [67].
The abnormal processing of sensory inputs in the CNS [68]. Moreover, evidence that the compromised white matter integrity of specific spinal pathways has been linked to clinical disability [69–71], and cortical reorganization [72] should be considered in relation to the pleiotropic beneficial effect of BPC 157 administration observed in distinctive brain areas and lesions [32–40]. These beneficial effects include the counteractions of traumatic brain injury and severe encephalopathies after NSAID overdose, insulin overdose, magnesium overdose, and exposure to the neurotoxin cuprizone in a rat model of multiple sclerosis [33–41]. These beneficial effects may be due to the formation of detour circuits—which encompass spared tissue surrounding the lesion—and could reconnect locomotor circuits [69], thus enabling afferent inputs to be processed and conveyed to the cortex [73] and improving spinal reflexes, even below the injury [74].
Much like in the rats that underwent spinal cord injury recovery, rats with other disorders that are treated with BPC 157 maintain functional abilities that are otherwise impaired; for example, consciousness is maintained after brain trauma, and BPC 157 counteracts seizures, catalepsy akinesia, and severe muscle weakness [33–41, 75, 76]. The effect of BPC 157 on muscle function is combined with the counteraction of increased levels of pro-inflammatory and pro-cachectic cytokines and of downstream pathways to abolish muscle cachexia [2]. Likewise, BPC 157 ameliorates healing and recovers the impaired function of severely injured muscles that otherwise fail to spontaneously heal and plays a role after complete transection, crush, and denervation injuries [77–80] and after succinylcholine intramuscular application, muscle lesion, neuromuscular junction failure, fasciculations, paralysis, and hyperalgesia [81]. Likewise, given that the gray matter is particularly vulnerable during the primary phase [44, 63], we should note that, from day 7, the controls presented with edema and the loss of motoneurons in the gray matter, disturbances that were largely counteracted in BPC 157-treated rats (Table 2 and Fig. 4).
In summary, this effect may be the cause or a consequence of the beneficial effects of BPC 157 on related disturbances [1–11]. As demonstrated, BPC 157 counteracts free radical formation and free radical-induced lesions [32, 82–84]. An interesting point would be the use of the same dose range in BPC 157 studies [1–11]. Finally, further studies should clarify the molecular pathways involved and extend the one-time application (much like the engraftment of neural stem cells [16] or bone marrow stromal cells [17] into the lesion site) to the continuous application for the recovery of pre-existing spinal cord injury.
In conclusion, this manuscript tried to prove the therapeutic effects of BPC 157 in spinal cord injury using a rat model. Spinal cord injury recovery was achieved in BPC 157-treated rats, meaning that this therapy affects the acute, subacute, subchronic, and chronic stages of the secondary injury phase. Thus, despite the limitations of rat studies, the results showed that treatment with BPC 157 led to the recovery of tail function and the resolution of spasticity and improved the neurologic recovery; thus, BPC 157 may represent a potential therapy for spinal cord injury.
Acknowledgements
Not applicable
Abbreviations
- CMAP
Compound motor action potential
- CNS
Central nervous system
- ECM
Cone-extracellular matrix
- egr-1 gene
Early growth response-1 gene
- EMG
Electromyography
- FAK
Focal adhesion kinase
- GLP
Good laboratory practice
- HNE
4-Hydroxynonenal
- HPLC
High-pressure liquid chromatography
- JAK-2
Janus kinase 2
- LD1
Lethal dose 1
- LTB4
Leukotriene B4
- MPO
Myeloperoxidase
- MPTP
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MUP
Motor unit potential
- naB2
Nerve growth factor 1-A binding protein-2
- NSAIDs
Non-steroid anti-inflammatory drugs
- SCI
Spinal cord injury
- TXB2
Thromboxane B2
Authors’ contributions
DP, DK, DD, GB, SS, and PS performed the experiments. DP, DK, VB, NS, DD, EE, GB, SS, and PS collected and analyzed data. DP, DK, GB, SS, and PS wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Ministry of Science, Education and Sports, Republic of Croatia (grant number 108-1083570-3635). There was no role of the funding body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript should be declared.
Availability of data and materials
All data are included in the manuscript.
Ethics approval and consent to participate
Approved by Ethics Committee, School of Medicine, University of Zagreb, 04-1121-2006 for experiments with rats.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Darko Perovic, Email: dperovic@kbd.hr.
Danijela Kolenc, Email: danijela.kolenc@mef.hr.
Vide Bilic, Email: vide.bilic@gmail.com.
Nenad Somun, Email: nenad.somun1@gmail.com.
Domagoj Drmic, Email: iddrmic@mef.hr.
Esmat Elabjer, Email: elabjer@gmail.com.
Gojko Buljat, Email: gbuljat@gmail.com.
Sven Seiwerth, Email: sven.seiwerth@mef.hr.
Predrag Sikiric, Phone: 385-1-4566-833, Email: sikiric@mef.hr.
References
- 1.Seiwerth S, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr Pharm Des. 2018;24(18):1972–1989. doi: 10.2174/1381612824666180712110447. [DOI] [PubMed] [Google Scholar]
- 2.Kang EA, Han YM, An JM, Park YJ, Sikiric P, Kim DH, et al. BPC157 as potential agent rescuing from cancer cachexia. Curr Pharm Des. 2018;24(18):1947–1956. doi: 10.2174/1381612824666180614082950. [DOI] [PubMed] [Google Scholar]
- 3.Sikiric P, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr Pharm Des. 2018;24(18):1990–2001. doi: 10.2174/1381612824666180608101119. [DOI] [PubMed] [Google Scholar]
- 4.Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, et al. Stress in gastrointestinal tract and stable gastric pentadecapeptide BPC 157. Finally, do we have a solution? Curr Pharm Des. 2017;23(27):4012–4028. doi: 10.2174/1381612823666170220163219. [DOI] [PubMed] [Google Scholar]
- 5.Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, et al. Brain-gut axis and pentadecapeptide BPC 157: theoretical and practical implications. Curr Neuropharmacol. 2016;14(8):857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, et al. BPC 157 and blood vessels. Curr Pharm Des. 2014;20(7):1121–1125. doi: 10.2174/13816128113199990421. [DOI] [PubMed] [Google Scholar]
- 7.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20(7):1126–1135. doi: 10.2174/13816128113190990411. [DOI] [PubMed] [Google Scholar]
- 8.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19(1):76–83. doi: 10.2174/13816128130111. [DOI] [PubMed] [Google Scholar]
- 9.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19(1):126–132. doi: 10.2174/092986712803414015. [DOI] [PubMed] [Google Scholar]
- 10.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17(16):1612–1632. doi: 10.2174/138161211796196954. [DOI] [PubMed] [Google Scholar]
- 11.Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, et al. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16(10):1224–1234. doi: 10.2174/138161210790945977. [DOI] [PubMed] [Google Scholar]
- 12.Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Mod Mech. 2016;9:1125–1137. doi: 10.1242/dmm.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B, et al. Pathological changes in the white matter after spinal contusion injury in the rat. PLoS One. 2012;7(8):e43484. doi: 10.1371/journal.pone.0043484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L, et al. Imatinib enhances functional outcome after spinal cord injury. PLoS One. 2012;7(6):e38760. doi: 10.1371/journal.pone.0038760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp MA, Liebscher T, Niedeggen A, Laufer S, Brommer B, Jungehulsing GJ, et al. Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res. 2012;349(1):119–132. doi: 10.1007/s00441-012-1334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritfeld GJ, Nandoe Tewarie RD, Vajn K, Rahiem ST, Hurtado A, Wendell DF, et al. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 2012;21(7):1561–1575. doi: 10.3727/096368912X640484. [DOI] [PubMed] [Google Scholar]
- 18.Sharp KG, Yee KM, Steward O. A re-assessment of treatment with a tyrosine kinase inhibitor (imatinib) on tissue sparing and functional recovery after spinal cord injury. Exp Neurol. 2014;254:1–11. doi: 10.1016/j.expneurol.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Sharp KG, Yee KM, Stiles TL, Aguilar RM, Steward O. A re-assessment of the effects of treatment with a non-steroidal anti-inflammatory (ibuprofen) on promoting axon regeneration via RhoA inhibition after spinal cord injury. Exp Neurol. 2013;248:321–337. doi: 10.1016/j.expneurol.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandoe Tewarie RDS, Hurtado A, Ritfeld GJ, Rahiem ST, Wendell DF, Barroso MMS, et al. Bone marrow stromal cells elicit tissue sparing after acute but not delayed transplantation into the contused adult rat thoracic spinal cord. J Neurotrauma. 2009;26(12):2313–2322. doi: 10.1089/neu.2009.0987. [DOI] [PubMed] [Google Scholar]
- 22.Sharp KG, Yee KM, Steward O. A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury. Exp Neurol. 2014;257:186–204. doi: 10.1016/j.expneurol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu P, Graham L, Wang Y, Wu D, Tuszynski M. Promotion of survival and differentiation of neural stem cells with fibrin and growth factor cocktails after severe spinal cord injury. J Vis Exp. 2014;27(89):e50641. 10.3791/50641 [DOI] [PMC free article] [PubMed]
- 24.Ritfeld GJ, Nandoe Tewarie RD, Rahiem ST, Hurtado A, Roos RA, Grotenhuis A, et al. Reducing macrophages to improve bone marrow stromal cell survival in the contused spinal cord. Neuroreport. 2010;21(3):221–226. doi: 10.1097/WNR.0b013e32833677cd. [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Marsh BC, Cowan M, Al'Joboori YD, Gigout S, Smith CC, et al. Sequential therapy of anti-Nogo-A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp Neurol. 2017;292:135–144. doi: 10.1016/j.expneurol.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Filli L, Schwab ME. Structural and functional reorganization of propriospinal connections promotes functional recovery after spinal cord injury. Neural Regen Res. 2015;10(4):509–513. doi: 10.4103/1673-5374.155425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh M-J, Liu H-T, Wang C-N, Huang H-Y, Lin Y, Ko Y-S, et al. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med. 2017;95:323–333. doi: 10.1007/s00109-016-1488-y. [DOI] [PubMed] [Google Scholar]
- 28.Chang C-H, Tsai W-C, Lin M-S, Hsu Y-H, Pang J-HS. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol. 2011;110:774–780. doi: 10.1152/japplphysiol.00945.2010. [DOI] [PubMed] [Google Scholar]
- 29.Chang C-H, Tsai W-C, Hsu Y-H, Pang J-HS. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066–19077. doi: 10.3390/molecules191119066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485–2499. doi: 10.2147/DDDT.S82030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkalčević VI, Čužić S, Brajša K, Mildner B, Bokulić A, Šitum K, et al. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212–221. doi: 10.1016/j.ejphar.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 32.Vukojević J, Siroglavić M, Kašnik K, Kralj T, Stanćić D, Kokot A, et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vasc Pharmacol. 2018;106:54–66. doi: 10.1016/j.vph.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Tudor M, Jandric I, Marovic A, Gjurasin M, Perovic D, Radic B, et al. Traumatic brain injury in mice and pentadecapeptide BPC 157 effect. Regul Pept. 2010;160(1–3):26–32. doi: 10.1016/j.regpep.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Drmic D, Kolenc D, Ilic S, Bauk L, Sever M, Zenko Sever A, et al. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol. 2017;23(29):5304–5312. doi: 10.3748/wjg.v23.i29.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ilic S, Drmic D, Franjic S, Kolenc D, Coric M, Brcic L, et al. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88(11–12):535–542. doi: 10.1016/j.lfs.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Ilic S, Drmic D, Zarkovic K, Kolenc D, Brcic L, Radic B, et al. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol. 2011;667(1–3):322–329. doi: 10.1016/j.ejphar.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 37.Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, et al. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736) J Physiol Pharmacol. 2010;61(2):241–250. [PubMed] [Google Scholar]
- 38.Ilic S, Brcic I, Mester M, Filipovic M, Sever M, Klicek R, et al. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J Physiol Pharmacol. 2009;60(Suppl 7):107–114. [PubMed] [Google Scholar]
- 39.Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, et al. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64(5):597–612. [PubMed] [Google Scholar]
- 40.Medvidovic-Grubisic M, Stambolija V, Kolenc D, Katancic J, Murselovic T, Plestina-Borjan I, et al. Hypermagnesemia disturbances in rats, NO-related: pentadecapeptide BPC 157 abrogates, L-NAME and L-arginine worsen. Inflammopharmacology. 2017;25(4):439–449. doi: 10.1007/s10787-017-0323-6. [DOI] [PubMed] [Google Scholar]
- 41.Gjurasin M, Miklic P, Zupancic B, Perovic D, Zarkovic K, Brcic L, et al. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept. 2010;160(1–3):33–41. doi: 10.1016/j.regpep.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Bennett DJ, Gorassini M, Fouad K, Sanelli L, Han Y, Cheng J. Spasticity in rats with sacral spinal cord injury. J Neurotrauma. 1999;16(1):69–84. doi: 10.1089/neu.1999.16.69. [DOI] [PubMed] [Google Scholar]
- 43.Tanimoto K, Khoury B, Feng K, Cavanaugh JM. Evaluation of sciatic nerve function after ultrasonic and electrocautery muscle dissection: an electromyographic study. J Neurol Surg A Cent Eur Neurosurg. 2015;76(2):93–98. doi: 10.1055/s-0033-1349333. [DOI] [PubMed] [Google Scholar]
- 44.Song W, Song G, Zhao C, Li X, Pei X, Zhao W, et al. Testing pathological variation of white matter tract in adult rats after severe spinal cord injury with MRI. Biomed Res Int. 2018;2018:4068156. doi: 10.1155/2018/4068156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozlowski P, Raj D, Liu J, Lam C, Yung AC, Tetzlaff W. Characterizing white matter damage in rat spinal cord with quantitative MRI and histology. J Neurotrauma. 2008;25(6):653–676. doi: 10.1089/neu.2007.0462. [DOI] [PubMed] [Google Scholar]
- 46.Borgens RB, Liu-Snyder P. Understanding secondary injury. Q Rev Biol. 2012;87(2):89–127. doi: 10.1086/665457. [DOI] [PubMed] [Google Scholar]
- 47.Donnelly J, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu J, Stoica BA, Dinizo M, Pajoohesh-Ganji A, Piao C, Faden AI. Delayed cell cycle pathway modulation facilitates recovery after spinal cord injury. Cell Cycle. 2012;11(9):1782–1795. doi: 10.4161/cc.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu W, Wang P, Cheng JX, Xu XM. Assessment of white matter loss using bond-selective photoacoustic imaging in a rat model of contusive spinal cord injury. J Neurotrauma. 2014;31(24):1998–2002. doi: 10.1089/neu.2014.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schucht P, Raineteau O, Schwab OE, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176(1):143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- 51.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139(2):244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 52.Ward RE, Huang W, Kostusiak M, Pallier PN, Michael-Titus AT, Priestley JV. A characterization of white matter pathology following spinal cord compression injury in the rat. Neuroscience. 2014;260:227–239. doi: 10.1016/j.neuroscience.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 53.Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res. 1999;123:349–365. doi: 10.1016/S0079-6123(08)62870-8. [DOI] [PubMed] [Google Scholar]
- 54.Wernig A, Müller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992;30(4):229–238. doi: 10.1038/sc.1992.61. [DOI] [PubMed] [Google Scholar]
- 55.Dietz V, Wirz M, Curt A, Colombo G. Locomotor pattern in paraplegic patients: training effects and recovery of spinal cord function. Spinal Cord. 1998;36(6):380–390. doi: 10.1038/sj.sc.3100590. [DOI] [PubMed] [Google Scholar]
- 56.Li X, Yang Z, Zhang A, Wang T, Chen W. Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials. 2009;30(6):1121–1132. doi: 10.1016/j.biomaterials.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 57.Fouad K, Pedersen V, Schwab ME, Brösamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11(22):1766–1770. doi: 10.1016/S0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- 58.Raineteau O, Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2(4):263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 59.Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13(12):1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cazalets JR, Borde M, Clarac F. Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci. 1995;15(7):4943–4951. doi: 10.1523/JNEUROSCI.15-07-04943.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kremer E, Lev-Toy A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. J Neurophysiol. 1997;77(3):1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- 62.Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res Bull. 1993;30(3–4):387–393. doi: 10.1016/0361-9230(93)90270-l. [DOI] [PubMed] [Google Scholar]
- 63.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41(7):369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 64.Wieseler J, Ellis AL, McFadden A, Brown K, Starnes C, Maier SF, et al. Below level central pain induced by discrete dorsal spinal cord injury. J Neurotrauma. 2010;27(9):1697–1707. doi: 10.1089/neu.2010.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/S0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
- 66.Kupcova Skalnikova H, Navarro R, Marsala S, Hrabakova R, Vodicka P, Gadher SJ, et al. Signaling proteins in spinal parenchyma and dorsal root ganglion in rat with spinal injury-induced spasticity. J Proteome. 2013;91:41–57. doi: 10.1016/j.jprot.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 67.Persson AK, Thun J, Xu XJ, Wiesenfeld-Hallin Z, Ström M, Devor M, et al. Autotomy behavior correlates with the DRG and spinal expression of sodium channels in inbred mouse strains. Brain Res. 2009;1285:1–13. doi: 10.1016/j.brainres.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 68.Zhang SH, Blech-Hermoni Y, Faravelli L, Seltzer Z. Ralfinamide administered orally before hindpaw neurectomy or postoperatively provided long-lasting suppression of spontaneous neuropathic pain-related behavior in the rat. Pain. 2008;139(2):293–305. doi: 10.1016/j.pain.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 69.Freund P, Curt A, Friston K, Thompson A. Tracking changes following spinal cord injury: insights from neuroimaging. Neuroscientist. 2013;19(2):116–128. doi: 10.1177/1073858412449192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen-Adad J, El Mendili MM, Lehéricy S, Pradat PF, Blancho S, Rossignol S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55(3):1024–1033. doi: 10.1016/j.neuroimage.2010.11.089. [DOI] [PubMed] [Google Scholar]
- 71.Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y, et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma. 2012;29(8):1556–1566. doi: 10.1089/neu.2011.2027. [DOI] [PubMed] [Google Scholar]
- 72.Freund P, Wheeler-Kingshott CA, Nagy Z, Gorgoraptis N, Weiskopf N, Friston K, et al. Axonal integrity predicts cortical reorganisation following cervical injury. J Neurol Neurosurg Psychiatry. 2012;83(6):629–637. doi: 10.1136/jnnp-2011-301875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hubli M, Dietz V, Bolliger M. Spinal reflex activity: a marker for neuronal functionality after spinal cord injury. Neurorehabil Neural Repair. 2012;26:188–196. doi: 10.1177/1545968311420844. [DOI] [PubMed] [Google Scholar]
- 75.Jelovac N, Sikiric P, Rucman R, Petek M, Marovic A, Perovic D, et al. Pentadecapeptide BPC 157 attenuates disturbances induced by neuroleptics: the effect on catalepsy and gastric ulcers in mice and rats. Eur J Pharmacol. 1999;379(1):19–31. doi: 10.1016/S0014-2999(99)00486-0. [DOI] [PubMed] [Google Scholar]
- 76.Sikiric P, Marovic A, Matoz W, Anic T, Buljat G, Mikus D, et al. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson’s disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J Physiol Paris. 1999;93(6):505–512. doi: 10.1016/S0928-4257(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 77.Staresinic M, Petrovic I, Novinscak T, Jukic I, Pevec D, Suknaic S, et al. Effective therapy of transected quadriceps muscle in rat: gastric pentadecapeptide BPC 157. J Orthop Res. 2006;24:1109–1117. doi: 10.1002/jor.20089. [DOI] [PubMed] [Google Scholar]
- 78.Novinscak T, Brcic L, Staresinic M, Jukic I, Radic B, Pevec D, et al. Gastric pentadecapeptide BPC 157 as an effective therapy for muscle crush injury in the rat. Surg Today. 2008;38:716–725. doi: 10.1007/s00595-007-3706-2. [DOI] [PubMed] [Google Scholar]
- 79.Pevec D, Novinscak T, Brcic L, Sipos K, Jukic I, Staresinic M, et al. Impact of pentadecapeptide BPC 157 on muscle healing impaired by systemic corticosteroid application. Med Sci Monit. 2010;16:81–88. [PubMed] [Google Scholar]
- 80.Mihovil I, Radic B, Brcic I, Drmic D, Vukoja I, Boban Blagaic A, et al. Beneficial effect of pentadecapeptide BPC 157 on denervated muscle in rats. Int Congress Myol Myol. 2008;431:26–30. [Google Scholar]
- 81.Stambolija V, Stambolija TP, Holjevac JK, Murselovic T, Radonic J, Duzel V, et al. BPC 157: the counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur J Pharmacol. 2016;781:83–91. doi: 10.1016/j.ejphar.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, et al. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: new insights. World J Gastroenterol. 2017;23(48):8465–8488. doi: 10.3748/wjg.v23.i48.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Belosic Halle Z, Vlainic J, Drmic D, Strinic D, Luetic K, Sucic M, et al. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology. 2017;25(5):511–522. doi: 10.1007/s10787-017-0358-8. [DOI] [PubMed] [Google Scholar]
- 84.Luetic K, Sucic M, Vlainic J, Halle ZB, Strinic D, Vidovic T, et al. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology. 2017;25(2):255–264. doi: 10.1007/s10787-017-0330-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included in the manuscript.