Abstract
Background
Aging is a complex physiological phenomenon, intricately associated with cardiovascular pathologies, where platelets play a central pathophysiological role. Although antiplatelets are commonly employed to prevent and treat major adverse cardiovascular events, aging associated intraplatelet changes remain largely unexplored.
Methods
Platelets were studied in high cardiovascular risk patients (aged 40–100 years) comparing them to younger healthy subjects. This was followed by cross sectional and longitudinal mice studies. Flow cytometry, biochemical and molecular assays were used to study platelets comprehensively.
Findings
CVD Patients were categorized in the age groups 40–59, 60–79, and 80–100 years. Progressive decline in platelet health was observed in the 40–79 years age cohort, marked by increase in oxidative stress, hyperactivation and apoptotic markers. Paradoxically, this was reversed in patients aged above 79 years and the improved platelet phenotype was associated with lower oxidative damage. The platelets from the very old (80–100 year) group were found to be preloaded with increased antioxidants, which also contributed to higher resistance against induced redox insults. Cross sectional mouse studies excluded the effect of comorbidities and medications. Longitudinal mouse studies implicate an adaptive increase in antioxidant levels as the mechanism.
Interpretation
We report a novel age associated, non-linear redox regulation in platelets in both humans and mice. In advanced age, there occurs an adaptive increase in platelet antioxidants, reducing the intracellular ROS and leading to a healthier platelet phenotype. Clinically, our results advocate the use of less aggressive antiplatelet therapies for CVD in the elderly population.
Fund
Keywords: Platelets, Oxidative stress, Aging, Antioxidants, Apoptosis, Bleeding in elderly, Platelet ROS, Antiplatelet therapy
Research in context.
Evidence before this study
Old age is an independent risk factor for the development of cardiovascular diseases (CVD), with platelet mediated thrombosis playing a central role in major adverse cardiovascular events (heart attacks and strokes). Advanced age has been proposed to be the consequence of accumulation of cellular oxidative damage. While oxidative stress has been shown to directly affect platelet activation, the precise effects of advanced age on intraplatelet redox environment remains largely unexplored, particularly in very elderly (>80 years old).
Added value of this study
A comprehensive study of platelet phenotype at molecular and cellular levels in 183 individuals (with or without CVD) aged 20 to 100 yrs., demonstrates that platelet redox homeostasis follows a non-linear pattern of regulation with age. Platelet oxidative damage and hyperreactivity increases linearly until the late 70s; interestingly, those older than 80 years of age, show markedly reduced oxidative damage with stabilized platelet reactivity. The improved platelet phenotype can be attributed to the upregulated intraplatelet antioxidant enzymes in the elderly (80 and above). Cross sectional and longitudinal mouse studies reveal that the distinct platelet phenotype is consistent in both human and mouse platelets, and may be an adaptive response to counter the increasing redox insults with age. The study thus reveals an age associated bimodal regulation of platelet redox homeostasis which accordingly determines platelet reactivity.
Implications of all the available evidence
Antiplatelet therapies have been associated with an increased risk of bleeding in the elderly patients. Our present findings provide a possible mechanism behind the differences in platelet activation phenotypes with advancing age, and advocates use of less aggressive antiplatelet therapy in the elderly. Our mice platelet studies suggest that this non-linear platelet redox regulation is not restricted to human subjects. Further larger clinical studies are needed to decipher the likely complex molecular basis for these changes.
Alt-text: Unlabelled Box
1. Introduction
Aging is associated with a progressive decline and deterioration of physiological and cellular health, leading to disease. The current life expectancy in USA is estimated to be 78.8 years [1], with cardiovascular disease (CVD) being the major age related illness that affects lifespan [2]. Platelets, the megakaryocyte derived circulating anucleate cells, play a central role in the development of CVD. Previous studies have shown the key role of reactive oxygen species (ROS) in platelet stress induced signaling mechanisms, involved in prothrombotic cardiovascular events [3].
Aging has been linked with an altered redox homeostasis and considered a consequence of the accumulation of cellular oxidative damage [4,5]. Several studies have correlated the levels of antioxidants and markers of oxidative stress (OS) to CVD risk [[6], [7], [8]]. Despite the known role of platelets in CVD, few studies have investigated the consequence of age mediated OS on the platelet phenotype. Platelet studies have mostly focused on broad age groups (e.g. >65 years old), with little discriminatory value for the very elderly. In older adults, platelet activation promotes pro-thrombotic phenotype and contributes to an elevated risk of CVD [9]. In mice, platelet activation and thrombus formation increases with age [10]. Most studies have focused on counts/mean platelet volume or traditional platelet assays [[11], [12], [13]]. The molecular changes in platelets during aging and particularly in the very elderly (≥80 years old), remain poorly understood.
Our present study was designed to elucidate differences in platelet redox phenotype associated with age, in a high cardiovascular risk cohort. We observed an expected progressive increase in platelet OS and a subsequent increase in platelet apoptosis up to 79 years of age. Intriguingly, in the above 79 year group, there was a significant elevation of baseline platelet antioxidants, likely leading to the lower ROS levels and thus an improved platelet phenotype. Our observations provide insights into the dynamics of platelet function and phenotype with advancing age in both humans and mice. To our knowledge, this is the first report delineating the distinct platelet redox changes with age mediated by an adaptive rise in platelet antioxidants, as evident by the longitudinal mouse studies. These results are relevant in understanding how aggressive antiplatelet therapies can lead to increased risk of bleeding in the elderly patients.
2. Materials and methods
2.1. Study approval
All human blood studies were approved by the Yale Human Investigation Committee (#1005006865). Informed consent was obtained from each subject and conform to the principles set out in the WMA Declaration of Helsinki and the Belmont Report. These are requirements for the Yale HIC. All mice procedures were approved under Institutional Animal Care and Use Committee at Yale (#2017-11413) and all guidelines duly followed.
2.2. Antibodies and reagents
Monoclonal antibodies: against Bax (Human: Cat No. 5023, Mouse: Cat No. 14796), Bcl-2 (Human: Cat No. 15071, Mouse: 3498), Bcl-xl (Cat No. 2764), Catalase (Cat No. 14097), SOD1 (Cat No. 4266), SOD2 (Cat No. 13194), Gpx1 (Cat No. 3206), Prdx6 (Cat No. 95336) and GAPDH (Cat No. 5174) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). Polyclonal antibodies: against active caspase-3 (Cat No. Ab2302) from Abcam (Cambridge, MA); Mouse antibodies against GPX1 (Cat No. PA5–30593) and SOD1 (Cat No. LF-PA0013) from Invitrogen (Life Technologies, Carlsbad, CA, USA). FITC conjugated CD41 (Cat No. 303703) was obtained from Biolegend (San Diego, CA). FITC conjugated PAC1 antibody (Cat No. 340507) was obtained from BD Biosciences (Rockville, MO). Reagents used were as follows: Tetramethylrhodamine methyl ester (TMRE) (Invitrogen), Ac-DEVD-pNA (Enzo Life Sciences), H2DCF-DA (Molecular Probes, Gottingen, Germany), TRIzol reagent (Invitrogen). Kits used: soluble P-selectin and soluble CD40L (Affymetrix eBioscience, CA, USA), OxiSelect™ TBARS Assay (Cell Biolabs Inc., USA), Protein Carbonyl Content Assay kit and GSH/GSSG Radio Detection Assay kit (Abcam, Cambridge Science Park, UK), Catalase activity and SOD activity kit (Invitrogen, Life Technologies, Carlsbad, CA, USA). For RNA studies, Direct-Zol RNA mini prep kit (ZymoResearch), Human Oxidative Stress and Antioxidant Defense RT2 Profiler™ PCR array (SABiosciences Corp., Valencia, CA), iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) were used.
2.3. Isolation of human platelets
Venous blood was drawn from patients or healthy adult volunteers (Yale HIC approval #1005006865). Platelet-rich plasma or PRP was prepared from citrated freshly drawn blood by centrifugation at 220g for 12 min at 25 °C. WBC exclusion was confirmed by viewing under phase contrast microscope. Washed platelets were prepared by centrifugation of PRP (upper 2/3 of supernatant) at 700g for 8 min. To avoid activation due to processing, PGI2 (20 ng/ml) and apyrase were added to the PRP. Platelet pellet thus obtained was gently resuspended in calcium free Platelet washing buffer (103 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l MgCl2, 5 mmol/l glucose, 36 mmol/l citric acid, 0.35% BSA, pH 6.5). This step was repeated to get washed platelets. The washed platelets were then used for biochemical assays, flow cytometry based assays or for confocal microscopy.
2.4. ELISA
Soluble platelet activation markers were assayed by commercially available enzyme immunoassays specific for soluble P-selectin and soluble CD40L (Affymetrix, CA, USA) as per the manufacturer's guidelines.
2.5. Platelet activation based adhesion assay
Platelet microscopy was performed as previously described [14] with some modifications. The assay was optimized to visualize the platelet hyperreactivity ex-vivo using glass surface as the activator. Briefly, washed platelets (equal counts between comparison groups) were incubated on a glass bottomed dish at 37 °C for 1 h to allow adhesion and activation. The incubation at 37 °C causes activation of the washed platelets. This was followed by fixation by 4% paraformaldehyde (Santa cruz Biotechnology). FITC conjugated antibody for CD41 (Biolegend) was used to stain platelets. After washing excess antibody, the stained platelets were imaged using Nikon Eclipse-Ti confocal microscope with 100× oil immersion objective. The images were captured and analyzed using Volocity software.
2.6. TMRE assay
For apoptosis assay, the pretreated washed platelets were incubated with 1 μM tetramethylrhodamine methyl ester (TMRE) reagent (Invitrogen) at 37 °C for 20 min [15]. This assay quantifies the cells with depolarized mitochondrial membrane. The mitochondrial depolarization has been found to precede the other apoptotic events like caspase 3 activation [16]. For anucleate platelets, unlike nucleated cells, this is considered a reliable method to measure platelet apoptotic tendency. For both the assays, 3 × 10 [4] events were acquired using special order BD LSR II Cell Analyzer and data were analyzed by FlowJo v10.
2.7. Platelet surface activation assay
For human platelets, the PAC1 assay was used to analyze activation of αIIbβ3 receptor on platelet surface. The PAC1 antibody binds specifically to the activated conformation of human αIIbβ3 receptor and not to its resting form [17].The FITC conjugated PAC1 antibody (BD Biosciences) was added to washed platelets and after 20 min incubation the events were acquired on LSRII. The absence of contaminating WBCs in platelet population was further confirmed at this step.
2.8. Caspase-3 activity assay
Caspase-3 activity in the platelets was measured colorimetrically at 405 nm by degradation of the specific substrate (Ac-DEVD-pNA). The specific caspase-3 activity, normalized to total protein content of platelet lysates, is expressed as μmol pNA/min/mg protein.
2.9. Detection of intracellular ROS
Levels of platelet-derived intracellular H2O2 were measured using 2′,7′ dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular Probes). Washed Platelets were incubated H2DCF-DA and the fluorescence intensity was detected by a microplate reader (Biotek) using excitation at 492 nm and emission at 517 nm wavelengths. DCFH-DA is cell permeable and diffuses across the platelet cytomembrane. It is hydrolyzed in platelet cytoplasm to form non-fluorescent DCFH, which loses the ability to diffuse across the cytomembrane. ROS rapidly induces DCFH and undergoes one-electron oxidation to generate DCF, which is a fluorescent compound. Therefore, the reactive oxygen species production is reflected by an increase of DCF radicals in platelets. Results are expressed as mean fluorescence intensity.
2.10. Thiobarbituric acid reactive substances (TBARS) assay
Malondialdehyde (MDA) is a natural by-product of lipid peroxidation of polyunsaturated fatty acids caused by ROS and is commonly used as a marker for oxidative stress. The production of MDA was quantified by using the OxiSelect™ TBARS Assay kit according to manufacturer's protocol (Cell Biolabs Inc., USA). The concentrations of MDA in each treatment were calculated based on the standard curve of absorbance against MDA concentration. The experiments were performed in duplicate and results expressed as μM MDA per mg protein.
2.11. Protein carbonylation assay
Protein Carbonyl content is the most common indicator of protein oxidation. The levels of protein carbonyl in platelets were determined using a Protein carbonyl content Assay Kit (Abcam, US) and all the procedures were performed as per the manufacturer's instruction. Results are expressed as nmol carbonyl/mg protein.
2.12. Catalase activity
The Catalase activity was assayed using the Catalase colorimetric activity kit from Invitrogen (Life Technologies, Carlsbad, CA, USA). Briefly the platelet lysate was diluted 1:10 in the Assay Buffer provided and assayed as per the manufacturer's instructions. One unit of catalase (U) decomposes 1.0 μmol of H2O2 per min at pH 7.0 and 25 °C. The results are expressed as U/mg protein.
2.13. SOD activity
Superoxide Dismutase activity was measured using the SOD Colorimetric Activity kit from Invitrogen (Life Technologies, Carlsbad, CA, USA). Briefly the platelet lysate was diluted 1:5 in the Assay Buffer provided and the activity assayed as per the manufacturer's instructions. One Unit (U) of SOD is the amount of enzyme causing half the maximum inhibition of 1.5 mM nitroblue tetrazolium reduction in the presence of riboflavin at pH 7.8 and 25 °C. The results are expressed as U/mg protein.
2.14. Determination of glutathione levels
To measure the levels of glutathione in the washed platelets, a GSH/GSSG Radio Detection Assay kit (Abcam) was used according to the manufacturer's protocol. The kit uses a non-fluorescent dye that becomes strongly fluorescent when it binds to reduced glutathione (GSH). A GSSG probe was used to determine the GSSG levels. Fluorescence was measured in multi-well plate reader (Biotek) by exciting samples at 490 nm and detecting at an emission wavelength of 520 nm.
2.15. RNA extraction
Total platelet RNA was extracted using TRIzol reagent (Invitrogen). RNA was isolated from platelets using the Zymograph Direct-zol kit (with DNase treatment and A260/A280 > 1.8). The purity of each platelet RNA preparation was assessed by RT-PCR analysis of platelet (GPIb) and leukocyte (CD45) markers, as described previously [18].
2.16. Pathway focused PCR array
The Human Oxidative Stress and Antioxidant Defense RT2 Profiler™ PCR array (SABiosciences Corp., Valencia, CA) was used to measure the alteration of gene expression in the platelet RNA isolated from the different age cohorts. The RT2 Profiler™ PCR Array (SABiosciences Corp.) is a commercially available set of optimized qPCR primer assays on a 96-well plate. Total RNA was treated with DNase I, reverse-transcribed using an RT2 First Strand Kit (Qiagen), and brought to a final volume of 120 μl. cDNA was pre-amplified using RT2 Nano PreAmp cDNA Synthesis Primer Mix Human Oxidative Stress kit according to manufacturer's instructions (SabBioscience, Qiagen). Amplified cDNA from individual samples was used as a template for the PCR array, according to the array instructions, using SYBR green on CFX96 Real-Time PCR Detection system (Bio-Rad Laboratories). Data was analyzed using SABiosciences RT2 Profiler PCR Data Analysis software, available at http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php, and were considered significant at >2-fold change. Relative quantitation for each gene was determined by normalization to the housekeeping genes included with the array by using the 2-ΔΔCt method and represented as fold change over the Young Control group. The Array was performed in n = 3 samples for each age cohort.
2.17. Quantitative RT-PCR
Quantitative Real-time PCR (qPCR) was used to measure mRNA expression levels of selected genes as a validation of the array data. 1 μg of RNA isolated from platelets was reverse-transcribed using an iScript cDNA synthesis kit (Bio-Rad). qPCR was performed with Bio-Rad SYBR green supermix and sequence-specific primers (purchased from IDT) using a Bio-Rad CFX Connect real-time PCR machine.
Primers used were as follows: CAT 5’-ACCGAGAGAGAATTCCTGAGA-3′ and 5-GCCTTGGAG TATTTGGTAATGTC-3′; SOD1 5′- CCTCGGAACCAGGACCT-3′ and 5’-TTAATGCTTCCCCACACCTT-3′; GPX1 5’-GAGAACGCCAAGAACGAAGA- 3′ and 5′- TCTCGAAGAGCATGAAGTTGG-3′; PRDX6 5′- CCACGCAGCTTTCAGTCAT-3′ and 5′- AGGACCACGTACTTCCCTT-3′; TRXN 5′- TACAGCCGCTCGTCAGA-3′ and 5′- GCTGAGAAGTCAACTACTACAAGT-3′; GAPDH 5’-ACATCGCTCAGACACCATG-3′ and 5’-TGTAGTTGAGGTCAATGAAGGG-3′. Purity of the platelet RNA was validated using CD45 as a marker for leucocyte contamination. The primers used were as follows: CD45 5′- TGCATGCTCGATTATTCCCTGT-3′ and 5′- TGGGGCCTGTAAAAGTGTCC-3′; GPIbα 5′- AAGGCCATGACCTCTAACGT-3′ and 5′- GCAGTGGACCATGAGTAGAATAG-3′. Fold changes were calculated relative to the young healthy control, using the ΔΔCT method by normalizing to the average of a subset of housekeeping genes included on each plate [19]. Values were normalized to GAPDH and relative to young healthy control samples are shown. Error bars are mean ± SEM from a minimum of n = 6 independent experiments.
2.18. Mice
C57BL/6 mice were purchased from Jackson Laboratory at the age of 3 months and the animals were housed at the Yale Animal facility 300 George St. New Haven, CT, under the supervision of YARC and Rita Weber (Animal facility manager, Yale CVRC). All animal protocols were approved by the Yale University Animal Care and Use Committee. Both male and female mice were included in the study. Mice at 6, 12, and 24 months of age were used for the studies. These age groups were chosen in accordance with accepted principles for experiments on the biology of aging in mice [20]. Because the mean life span of C57BL/6 mice is ≈27 to 29 months, the 6-, 12-, and 24-month-old mice are roughly equivalent to young adult (20–30 years of age), middle-aged (≈45–50 years of age), and older (>70 years of age) humans, respectively. For longitudinal studies, randomly selected wild type mice, n = 8, were followed from the age of 6 months till 18 months.
2.19. Isolation of mouse platelets
Blood (0.7–1 ml) was directly aspirated from the right cardiac ventricle into 1.8% sodium citrate (pH 7.4). The blood was centrifuged at 200g for 8 min at room temperature to obtain PRP. The PRP was again centrifuged at 100g for 5 min to settle the contaminating WBCs. The purity was checked manually under microscope using hemocytometer. The PRP was diluted with Tyrodes-Hepes buffer (calcium free) and was either used for further assays or centrifuged at 700g after addition of PGI2 to obtain pellet. The pellet was resuspended in 0.5 to 1 ml Tyrodes-Hepes buffer to obtain washed platelets and appropriately diluted to equate counts between study groups. For longitudinal studies, retero-orbital blood collection was done once a month for over a period of 12 months. Blood (100 μl) was collected directly into citrate loaded tubes. Platelets were isolated as described above and checked for the absence of leucocyte and RBC contamination by flow cytometry.
2.20. Mice platelet aggregation
Mice platelet aggregation was tested in whole blood using aggregometer (Chrono-log model 700) by a modified method. The citrated whole blood from each mouse was prediluted with saline (1:1) and prewarmed at 37°C and recalcified before adding agonist. The ADP (40 μM) was used as the agonist. The rest of the steps were performed as per manufacturer's instructions. The aggregation was measured as impedance (ohms) which was converted to % aggregation for quantitation comparisons between groups. For comparative quantitation, the maximum level of 20 Ω was taken as 100% aggregation.
2.21. SDS PAGE and western blotting
Washed platelets were lysed in RIPA buffer with the addition of protease and phosphatase inhibitors (Sigma Aldrich). Western blot analysis was performed using 20 μg samples of protein to evaluate expression of apoptosis associated proteins and antioxidant enzyme levels. The cell lysates were subjected to 10% SDS-polyacrylamide gel electrophoresis. The protein was electrophoretically transferred to nitrocellulose membrane and then blocked with 5% milk in Tris-buffered saline solution containing 0.5% Tween-20. The membranes were probed using the respective antibodies and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG or anti mouse IgG. Antibody against Active caspase-3 were purchased from Abcam. Antibodies against Bcl-2, Bax, Bcl-xl, Catalase, SOD1, SOD2, Prdx6, Gpx1 were purchased from Cell Signaling and mouse specific GPX1 and SOD1 were from Invitrogen. Bands were developed using the enzyme-linked chemiluminescence detection reagents. GAPDH was used as a loading control.
2.22. Ex vivo oxidative stress induction
Washed Platelets from the respective age cohorts were exposed to H2O2 (Sigma Aldrich) at the specified concentration for 30 min at 37 °C. Following treatment, platelets were immediately used for biochemical assays and flow cytometry as described.
2.23. Statistical analysis
Values are presented as mean ± standard error of the mean (SEM). The sample size or experiments used for each study is specified for each Fig. legend. Differences between group means were assessed using the non-parametric t-test for continuous variables and χ2 or Fischer exact tests for categorical variables where appropriate. Two-sided P < .05 was considered statistically significant. Comparisons between two samples were performed using the Student's t-test. Data sets with three or more groups were analyzed by one-way ANOVA with a Bonferroni post hoc test. Analysis was performed with Prism 8.0 software (GraphPad Software, Inc., La Jolla, CA). Flow cytometry and western data were analyzed using FlowJo and ImageJ, as indicated. For correlation, Pearson's test was used to determine significance and the trend line was determined by curve fitting spline using Prism 8.0. P values <.05 were considered significant.
3. Results
3.1. The study cohort represents a typical aging cardiovascular population
Our cohort represents a typical aging cardiovascular population, as nearly all elderly patients visit a cardiologist for at least one cardiovascular risk factor [3]. Fig. 1 shows the schematic for the study design. Assays were all performed blinded to age as depicted in the flow diagram (Fig. 1). As shown in Table 1, there were expected differences between the three recruited age groups (male %,hypertension, documented CAD and statin use) with only gender (more females in VOA compared to OA) being significantly different between the VOA and OA groups (Table 1).
Fig. 1.
Schematic Overview of the study. Flow diagram outlining procedures taken for the study from patient screening and enrollment to assays, analysis, and interpretation. * Indicates samples excluded due to hemolysis, insufficient volumes of blood drawn and delayed arrival to the lab for processing for platelets. See also Table 1.
Table 1.
Basic demographics and clinical information for the recruited patients.
| Middle Age (40–59 years) |
Old Age (60–79 years) |
Very Old (80–100 years) |
P-values* | P-values Old vs Very Old | |
|---|---|---|---|---|---|
| n = 23 | n = 50 | n = 30 | |||
| Male, n % | 6 (26) | 35 (70) | 14 (47) | 0.003 | 0.039 |
| Body Mass Index, kb/m2 | 35 ± 6 | 30 ± 5 | 28 ± 5 | 0.713 | 0.789 |
| PLT Count (x 103per μl) | 233 ± 27 | 214 ± 81 | 210 ± 72 | 0.925 | 0.973 |
| HbA1c (%) | 5.9 ± 0.4 | 7.0 ± 2.0 | 6.7 ± 1.7 | 0.941 | 0.917 |
| BSL (mg/dl) | 109 ± 17 | 136 ± 63 | 119 ± 33 | 0.003 | 0.839 |
| Diabetes, n (%) | 4 (17.4) | 22 (44.0) | 9 (30) | 0.071 | 0.243 |
| Hypertension, n (%) | 14 (60.8) | 43 (86.0) | 28 (93.3) | 0.006 | 0.315 |
| Hyperlipidemia, n (%) | 9 (39.1) | 30 (60.0) | 21 (70) | 0.073 | 0.368 |
| Current Smoker, n (%) | 3 (13.0) | 9 (18.0) | 2 (6.66) | 0.3573 | 0.154 |
| Documented CAD, n (%) | 4 (17.3) | 30 (60.0) | 15 (50) | 0.003 | 0.383 |
| Aspirin use, n (%) | 10 (43.4) | 36 (72.0) | 21 (70) | 0.06 | 0.999 |
| Statin use, n (%) | 13 (56.5) | 41 (82.0) | 27 (90) | 0.009 | 0.332 |
| β-blocker use, n (%) | 17 (73.9) | 39 (78.0) | 25 (83.3) | 0.701 | 0.564 |
| Diuretics use, n (%) | 12 (52.2) | 22 (44.0) | 16 (53.3) | 0.182 | 0.459 |
| Cancer diagnosis, n (%) | 0 (0) | 6 (12.0) | 2 (6.66) | 0.198 | 0.441 |
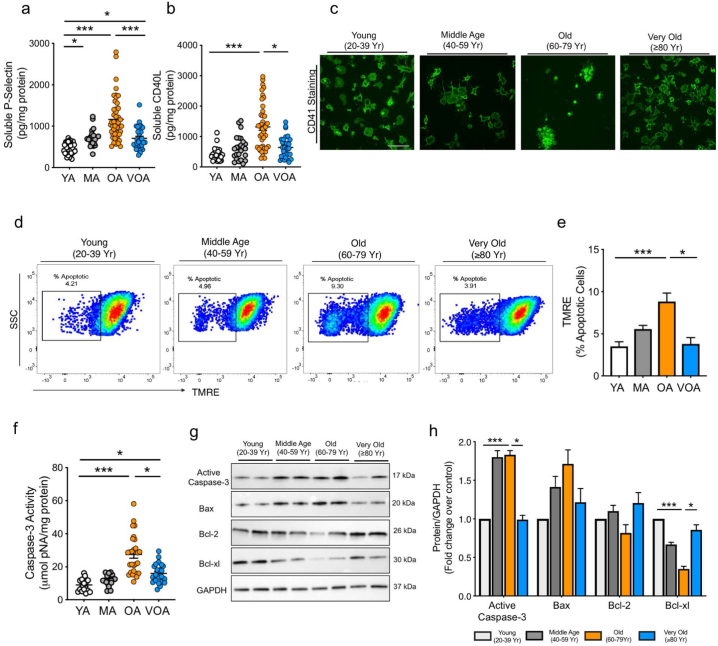
3.2. Platelets from above 80 year old patients show decreased activation and apoptosis
Large cohort studies assessing age associated changes (or response) often classify the elderly as >60, or > 65 with little discriminatory value between those less than or >80 years of age [3]. More detailed evaluation is critical, as life expectancy past 80 years of age is increasing [1]. Random, consecutive consenting patients were recruited from a cardiovascular outpatient clinic without consideration for age or gender (Fig. 1; Table 1). There were no significant differences in platelet count (Supplementary Fig. S1a) the mean platelet volume (Supplementary Fig. S1b), and platelet protein concentration (Supplementary Fig. S1c). We initially found that soluble markers of platelet activation, sP-Selectin and sCD40L, increased progressively from the 20–39 cohort (Young Age, YA) to the 60–79 cohort (Old Age, OA), but were significantly reduced in the 80 years and older cohort (Very Old Age, VOA) (Fig. 2a-b; Supplementary Fig. S1d-e). The elevation in activated αIIbβ3 levels, a surface marker of platelet activation was highest in the OA group (Supplementary Fig. S1f). This activation pattern was reproduced using confocal microscopy in a platelet activation adhesion assay. The OA platelets were more activated and aggregated ex vivo in comparison to YA and VOA cohorts (Fig. 2c).
Fig. 2.
Platelets from above 80 year old patients (Very Old cohort) show decreased platelet activation and apoptosis as compared to Old (60–79 year). Freshly isolated platelets from the citrated bloods of the patients were used for all experiments. a-b. Soluble markers of platelet activation measured in plasma. c. Platelet surface adhesion and morphology assessed using FITC conjugated CD41. Magnification 100×. Scale bar =10 μm. d-e. Representative image and quantification of mitochondrial apoptosis, expressed as the % apoptotic cells. f. Intraplatelet caspase-3 activity as measured using Ac-DEVD-pNA as substrate. g-h. Representative immunoblotting images and respective quantitation for expression of key apoptotic and pro-survival proteins in platelets. Patient data represents results from independent experiments in n = 20 to 40 patients per group. Immunoblotting was performed in 3 to 6 independent experiments with a minimum of n = 10 per group. YA: Young Age; MA: Middle Age; OA: Old Age; VOA: Very Old Age. Values expressed as Mean ± SEM. *p < .05; **p < .005; ***p < .001 determined by one-way ANOVA with Bonferroni post hoc analysis. See also Supplementary Fig. 1.
Platelet activation and apoptotic events are highly complex due to their overlapping and distinct nature. In the presence of prolonged/continued stress, platelets display mitochondrial depolarization and caspase 3 activation leading to apoptosis [21]. In the OA platelets, platelet hyperactivation was marked with a loss of mitochondrial membrane potential and a distinct increase in apoptotic platelets (Fig. 2d-e). Increased caspase-3 activity (Fig. 2f) and reduced expression of pro-survival markers Bcl-2 and Bcl-xl in the OA versus VOA was also observed, supporting the mitochondrial depolarization based apoptosis data (Fig. 2g-h). In contrast, the VOA platelets demonstrated “healthier” mitochondria and increased levels of pro-survival proteins (Fig. 2d-h, Supplementary Fig. S1). Thus, the VOA cohort platelets appear to be better protected against hyperactivation and apoptosis. We hypothesize that with advanced age (VOA), platelets either become less susceptible to the pathophysiological burden or develop adaptive strategies to combat the age associated cardiovascular risk factors. This unexpected result led us to pursue further functional biochemical analysis to delineate the possible mechanism behind the reduced platelet activation in the VOA cohort.
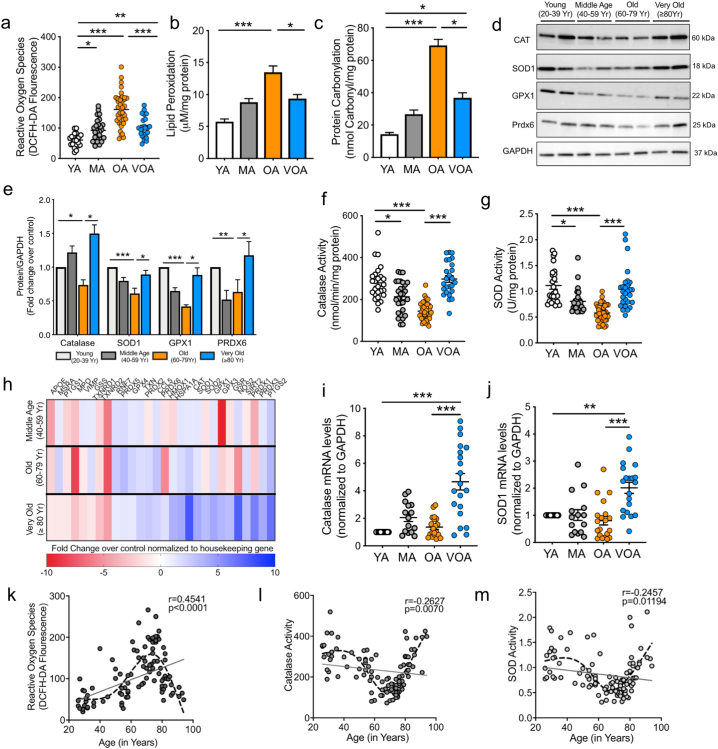
3.3. Platelets from VOA patients have elevated antioxidants and show a subsequent decline in oxidative stress
Aging is associated with accumulation of oxidative damage [4], with a recognized link between OS and platelet activation [22]. Platelets from the middle age (40–59 yrs.; MA) and OA groups revealed an expected increase in intra-platelet ROS. However, here again, there was an intriguing decline in ROS levels in the VOA (p < .001 vs OA; one way ANOVA with Bonferroni test; Fig. 3a). This deviation from a linear increase in ROS mediated modifications was also observed with lipid peroxidation and protein carbonylation levels, which was highest in OA cohort (Fig. 3b-c). A significant decline in the GSH/GSSG ratio was also observed in the OA cohort as compared to the other age sub-groups (Supplementary Fig. S2a).
Fig. 3.
Platelets from the Very Old cohort (≥ 80 years) possess significantly higher levels of antioxidant enzymes and improved redox homeostasis. a. Intracellular ROS in platelets. b-c. Intraplatelet lipid peroxidation and protein carbonylation levels. d-e. Representative immunoblot images and quantification showing expression of platelet enzymatic antioxidants. f-g. Intraplatelet Catalase and SOD activity. h. Heat map showing the differential expression of key oxidative stress genes. Heat map represents fold change in each age subset normalized to the housekeeping genes and expressed over the Young Healthy controls. Array performed in n = 3 platelet samples per group. i-j. mRNA expression levels of Cat, Sod1 as measured by qPCR. k-m. Correlation curves between the respective parameters and individual ages of the patient population. Solid line represents linear regression and the r and p values for correlation. Dash line represents the trendline generated using a curve fitting spline, n = 114. Patient data represents results from independent experiments in n = 20 to 40 patients per group. Immunoblotting was performed in 3 to 6 independent experiments with a minimum of n = 10 per group. Values expressed as Mean ± SEM. *p < .05; **p < .005; ***p < .001 determined by one-way ANOVA with Bonferroni post hoc analysis. See also Supplementary Fig. 2.
Lower platelet ROS level in the VOA group could be due to two major possibilities. First, a decreased OS burden, which is unlikely since, in addition to a longer lifetime exposure to oxidative stressors in this oldest age group, there are comorbidities, cardiovascular risk factors and medications similar to OA cohort (Table 1). Secondly, and more likely, there are increased antioxidants in this elderly group. Little is known about levels of platelet antioxidants in the aging population. Evaluation of the key antioxidant enzymes in the platelets demonstrated elevated expression of Catalase (CAT), Superoxide Dismutase (SOD), Glutathione Peroxidase (GPX1) and Peroxiredoxin 6 (Prdx6) in the VOA cohort as compared to OA and MA cohorts (Fig. 3d-e). Consistent with protein expression, a decline was observed in the intraplatelet enzyme activity of the CAT and SOD in the OA cohort with the activity of both enzymes being significantly higher in the VOA cohort (p < 0.001vs OA; one way ANOVA, Fig. 3f-g). This is not without precedence as previous studies have shown elevated antioxidant levels in the plasma of the centenarians [7,[23], [24], [25]]. To delineate the contribution of platelet antioxidants in the plasma measurements, we measured the catalase and SOD activity in both the platelet rich (PRP) and platelet poor plasma (PPP) fractions. Our observations demonstrate that plasma levels of key enzymatic antioxidants, particularly CAT, arise mostly from platelets (Supplementary Fig. S2b-c).
To examine whether there is any difference in the pre-translational landscape of the platelets in the context of OS genes, we performed a gene expression array. For RNA studies, absence of leuckocyte contamination in platelets was ensured by RT-PCR (Supplementary Fig. S3A). A heat map, generated of the selected genes (based on expression quality/consistency), showed altered gene expression in the platelets. (Fig. 3h). A distinctly higher expression of several antioxidant genes including Cat, Sod1, Sod2, Gsr, Hspa5 was observed in the VOA patients. Further validation by qPCR showed significant increases in the mRNA levels of Cat and Sod1 as well other key intracellular antioxidants, in platelets from the VOA group (p < .001 vs OA; one way ANOVA with Bonferroni test; Fig. 3i-j, Supplementary Fig. S3b-d). Thus, the platelets from the VOA cohort are prepackaged with a repertoire of antioxidant enzymes, which contributes to the reduced ROS and lower platelet hyperactivation.
To further highlight antioxidant changes associated with age, we analyzed absolute values versus individual ages at the time of patient recruitment. Correlation analysis revealed weak values of linear correlation. However, generating a curve fitting trendline using a smoothing spline curve (Prism 8.0, GraphPad, La Jolla, CA) defined the decline in ROS and increase in antioxidant levels at the age of 80 years and above (Fig. 3k-m). The significant differences in the levels of platelet antioxidants across the old and very old cohorts were independent of gender (Supplementary Fig. S4).
An important question arises whether the observed redox changes in the advanced age (VOA) cohort, are related to differences in demographics, comorbidities, or medications. The non-linear changes with age in the platelet phenotype were found to be independent of the presence of co-morbidities such as hypertension, diabetes (Supplementary Fig. S5) and use of medications such as aspirin and statins (Supplementary Fig. S6). As shown in Supplementary Fig. S7, intraplatelet antioxidant levels were observed to be higher in both the CVD and non-CVD advanced age populations as compared to the 60–79 year old group, thus highlighting these changes as a consequence of aging and not the presence of underlying diseases. To further address the concerns about the observed changes being the concurrent effects of aging and/or CVD, we investigated the platelet phenotype in mice of different ages.
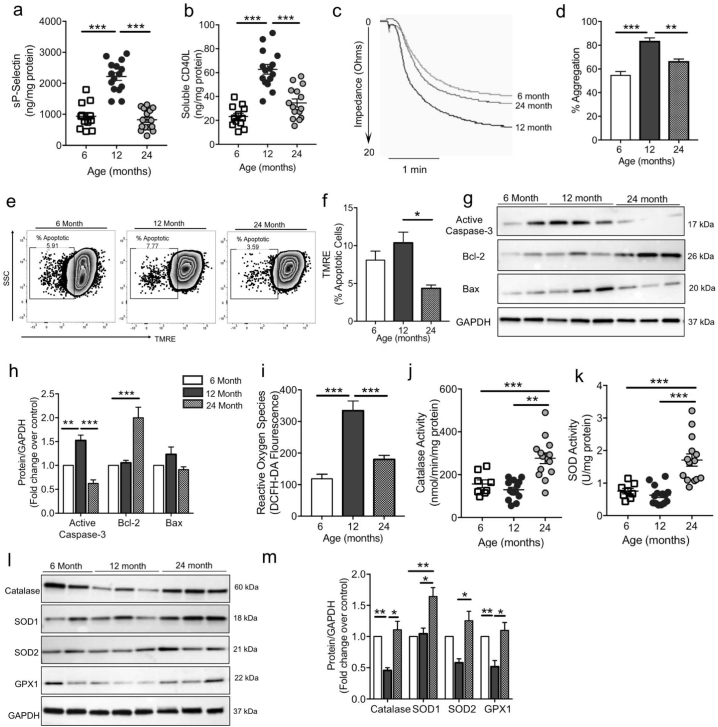
3.4. Aging modulates redox homeostasis in mice platelets
To exclude the effects of concurrent factors in the clinical studies, we used cross sectional studies to analyze platelets from mice in three different age groups (6, 12 and 24 months of age). These age groups were chosen in accordance with the accepted principles for experiments on the biology of aging in mice [20]. Because the mean life span of C57BL/6 mice is ≈27 to 29 months, the 6-, 12-, and 24-month-old mice are roughly equivalent to young adult (18–20 years of age), middle-aged (≈45–50 years of age), and older (>70 years of age) humans, respectively. Consistent with the human studies, the levels of soluble platelet activation markers, sP-selectin and sCD40L (Fig. 4a-b) were significantly lower in the 24-month mice as compared to the 12 month mice. Conversely, agonist induced platelet aggregation in 12 month mice was found to be highest as compared to the 6- and 24-month mice (p < .001 and p < .005, respectively), when tested in whole blood (Fig. 4c-d). This confirms the higher prothrombotic propensity in the 12 month mice. The platelets from the 12 month mice also showed increase in apoptotic platelets (TMRE negative; Fig. 4E-F) and intracellular apoptotic markers (Fig. 4g-h). Interestingly, in concurrence with the human results, the platelets from 24 month mice (very old) had improved mitochondrial status and higher expression of pro-survival Bcl-2 (Fig. 4e-h). ROS levels were highest and comparable between the 6- and 12-month mice and significantly lower in the mice that had survived till 24 months (Fig. 4i). Platelet CAT and SOD activity were increased in the 24-month mice (Fig. 4j-k) and this was further confirmed by the enhanced expression of key antioxidants by immunoblotting (Fig. 4l-m). Taken together this mouse data supports the observed changes as being intrinsic to aging rather than arising from factors such as concurrent morbidities, diet and/or medications.
Fig. 4.
Platelets from 24 month mice are less active, and richer in antioxidant enzymes as compared to platelets from 6- and 12-month old mice. a-b. Levels of sP-selectin and sCD40L in the mice plasma (6 m n = 12, 12 m n = 15, 24 m n = 15). c. Representative platelet aggregation tracings in response to ADP. d. Quantitation of aggregation shown taking 20 Ω (maximal impedance) as the 100% response (n = 4 mice per group). e-f. Representative zebra plots and quantitation showing the percentage of apoptotic cells as measured by the dye TMRE in the platelets. g-h. Representative immunoblot and respective densitometric analysis showing expression of apoptotic proteins in the platelets. i. Intracellular ROS levels measured in freshly isolated platelets (6 m n = 10, 12 m n = 14, 24 m n = 14). j-k. Activity of the enzymatic antioxidants, catalase and SOD (6 m n = 10, 12 m n = 14, 24 m n = 14). l-m. Representative western blotting images and densitometric quantification for the expression of the key antioxidant proteins. Expression is normalized to GAPDH and expressed as fold change over 6 month mice. Experiments unless otherwise specified, performed in a minimum of n = 10 samples per group. Values are expressed as Mean ± SEM. *p < .05; **p < .005; ***p < .001 *p < .05; **p < .005; ***p < .001 determined by one-way ANOVA with Bonferroni post hoc analysis.
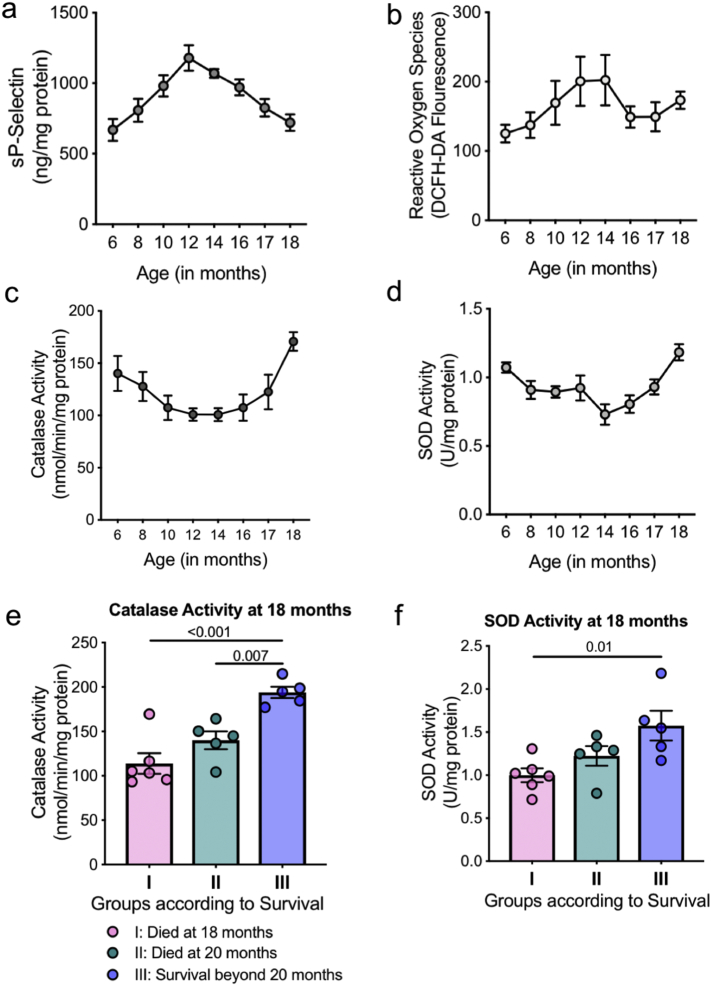
3.5. Longitudinal mice studies reveal an age associated adaptive response in platelet antioxidants
To further determine whether the platelet phenotype was a consequence of normal aging, we conducted longitudinal mice studies. Randomly selected wild type mice were bled every month, from 6 months of age to 18 months and platelets isolated from the small volume of blood collected. In a first of its kind studies, analysis of mice platelet antioxidants over time revealed a similar trend as observed in human platelets. As shown in Fig. 5a-d, there was a decline in CAT and SOD activity, with a concomitant increase in sP-selectin and ROS levels until ~14 months of age. With further aging, there was an increase in antioxidant enzyme activity, leading to a subsequent decline in ROS levels and platelet activation as the mice aged to 18 months. Thus the adaptive increase in intraplatelet antioxidants with advanced age, likely contributes to the decline in platelet reactivity. Further, we randomly selected a small group of mice and followed them from age 16 months onwards (based on longitudinal group data). Although previously documented lifespan studies [20] and our own mice data shows the average age of mice to be 20–28 months, the median survival of mice in this small group was around 20 months. This could be attributed to the stress of bleeding which was however consistent for all the randomly selected mice. We observed that the mice which survived longer had higher CAT and SOD activity at 18 months of age (Fig. 5e-f). Thus consistent with our human study cohort, the age associated non-linear redox changes in the platelets are also observed in cross sectional and longitudinal mice studies. As shown by the longitudinal mice data, the observed age associated platelet phenotype is likely an adaptive consequence of aging.
Fig. 5.
Longitudinal mice studies show adaptive changes in platelet antioxidants levels. Randomly selected mice (n = 8) were bled consecutively for 12 months and their a. soluble P-selectin levels. b. ROS levels. c. Catalase activity and d. SOD activity measured in platelets. e-f. Randomly selected mice (n = 15) were bled from age 16 months onwards till their death and divided into groups based on their lifespan, Group I: Death at 18 months, Group II: Death at 20 months and Group III: Survival beyond 20 months. Data shows (e) Catalase and (f) SOD activity at 18 months of age. Values are expressed as Mean ± SEM. Significance determined by one-way ANOVA with Bonferroni post hoc analysis for each group.
3.6. Platelets from the VOA patients are more resistant to the damaging consequences of induced oxidative stress
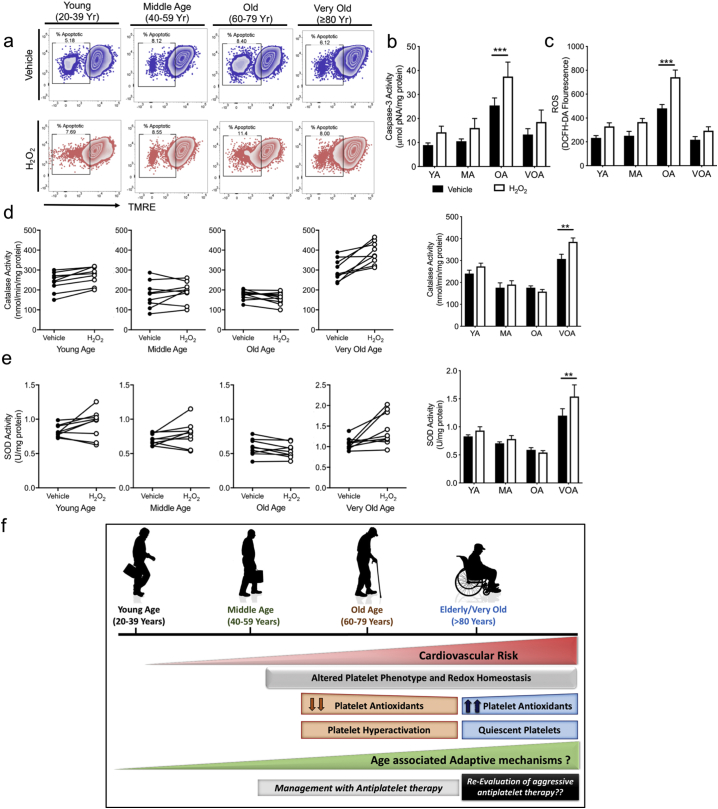
Based on our insights from the mice longitudinal studies, we wanted to assess whether the platelets from our human cohort age groups are equipped to respond to ex vivo stressors, differentially. We performed a platelet stress test by exposing freshly isolated platelets from the randomly selected subjects of different age groups to exogenous H2O2. As shown in Fig. 6a, the mitochondrial membrane potential was better preserved under OS induction in the VOA cohort compared to the OA group. The change in the activity of pro-apoptotic caspase-3 and the intraplatelet ROS levels was maximal in the OA cohort (Fig. 6b–c). Conversely, the CAT activity in the VOA platelets was highest at both the baseline and upon OS induction. In contrast, the OA baseline platelet CAT activity was low and the presence of exogenous H2O2 had little effect on the enzyme activity (Fig. 6d). The SOD response was more blunted and variable with the least response in the OA group (Fig. 6e). This suggests that platelets themselves can respond to external oxidative stress challenge and this response is blunted in the MA and OA groups as compared to the YA and VOA subjects. This is consistent with the mRNA results, demonstrating platelets in the elderly population (≥ 80 years old) are not only pre-packaged with more antioxidant defense mechanisms, but appear to respond more actively to external oxidative stress.
Fig. 6.
Platelets from the Very Old Age cohort (≥ 80 years) are more resistant to induced oxidative stress. Washed platelets (1 × 106 cells/ml) from each age subset from the human cohort, were incubated for 30 min with 50 μM H2O2. a. Representative Zebra plots represent the changes in mitochondrial permeability in isolated platelets for each group following OS induction. b. Caspase-3 activity following hydrogen peroxide treatment. c. ROS levels. d-e. Panel A and B depict the changes in the catalase and SOD activity, respectively, following ex vivo OS. Each age subset is represented by a separate graph and represents the before-after levels of antioxidant activity in individual subjects. f. Central Illustration depicting the key findings of the study: Platelet phenotype differs distinctly with age and is likely due to the age associated adaptive changes in platelet antioxidant levels. Experiments unless otherwise specified, performed in a minimum of n = 9 samples per group. Values are expressed as Mean ± SEM. *p < .05; **p < .005; ***p < .001 determined by one-way ANOVA with Bonferroni post hoc analysis for each group.
4. Discussion
We report that age is associated with a non-linear regulation of redox homeostasis in the anucleate platelet. We demonstrate that there is a progressive increase in ROS and platelet hyperreactivity up to 79 years of age; interestingly in patients 80 years of age and older, there is a decline in platelet ROS and reactivity. Cross-sectional and longitudinal studies on mice followed from 6 to 18 months of age revealed this to most likely be a consequence of biological aging and an adaptive process. We provide strong evidence that there is an increased protection against redox imbalance in the platelets from the elderly group as shown by the ex vivo stress induction studies. The adaptive increase in antioxidants likely forms the basis for altered prothrombotic phenotype with age, thus supporting careful reconsideration of aggressive anti- platelet therapy for the very elderly (summarized in Fig. 6f).
Lower platelet ROS levels in the 80 years and over group, could arise from a decreased oxidative stress burden. However, this is unlikely as, in addition to a longer lifetime exposure to oxidative stressors, the comorbidities, cardiovascular risk factors and medications are similar to Old Age and Middle Age (below 80 years) cohort (Table 1). Indeed, the non-linear redox regulation in platelets was observed even in age-matched healthy donors (60–79 and ≥80 years old) who do not have CVD (Supplementary Fig. S7). Moreover, the levels of platelet antioxidants across the old and very old human cohorts are independent of gender and comorbidities (Supplementary Fig. S5–6). This indicates that the upregulation of antioxidants in the platelets from the advanced age (≥80 years) cohort, is likely a consequence of biological aging and not the effect of CVD or other disease pathologies that occur with age. This is not without precedence as previous studies have shown elevated antioxidant levels in the plasma of the centenarians [7,23].
We hypothesize that platelets from the advanced age patients as well as the old 24 month mice are pre-equipped with antioxidant enzymes that combat the increased ROS, preserve the platelet function and thus reduce the cardiovascular risk. As confirmed by our longitudinal studies on mice, in which platelets were isolated from the same mice over a period of several months, there likely occurs an age associated adaptive increase in intraplatelet antioxidant levels beyond a certain age. More research is needed to explore the contribution of the increased platelet antioxidants, hyperactivation and lower apoptosis in the very elderly, to survival, mediated by the upregulation of cytoprotective mechanisms. While their roles have not yet been defined in the anucleate platelets, signaling cascades mediated via Nrf2, IGF-1, sirtuins and Foxo3 could all contribute to the distinct differences in platelet oxidative stress with age [[26], [27], [28], [29], [30]]. There is likely an interplay of several complex adaptive as well as selective mechanisms which could each in part contribute to the elevated platelet antioxidants with age in both humans and mice and thus affect the prothrombotic phenotype.
Antiplatelet agents such as aspirin (cyclooxygenase inhibitor) and Clopidogrel (P2Y12 inhibitor) are used to prevent major adverse cardiovascular events (MACE), reducing cardiovascular morbidity and mortality [31,32]. The clinical guidelines for the recommended anti-platelet therapy are currently based on results from a younger population. Few platelet studies have focused on aging, and particularly patients ≥80 years of age. However, there is a recognized increased risk of bleeding associated with antiplatelet treatment, particularly in the patients older than 75 years [[33], [34], [35], [36], [37]]. There is a mounting need to carefully investigate the risk and benefits of antiplatelet drugs in the elderly patients. Our study, provides a possible explanation behind the increased bleeding risk associated with antiplatelet agents, likely due to the reduced platelet activity in the very elderly. Less aggressive antiplatelet therapies should therefore be considered in the very elderly, particularly in the presence of lower ROS and less reactive platelets. While further studies are needed in independent larger cohorts, our results provide mechanistic insights into the dilemma faced by physicians regarding the administration of antiplatelet agents to the elderly [35,38].
There were a number of limitations to our studies. Our main concern was whether our observations were affected by variations in medications or clinical history or comorbidities between study groups. This concern was largely addressed by our results on the age-matched healthy donors (60–79 and > 80 years old) who do not have CVD as well as the mouse studies. Clearly, long term follow-up studies assessing morbidity and mortality will provide more detailed insight into the role of platelet antioxidants in major adverse cardiovascular events and lifespan. While our results are statistically significant and our human findings are validated by the mouse studies, further studies are needed in independent larger cohorts of cardiovascular diseases and other pathological conditions. Although our studies were performed in freshly isolated platelets, it would be interesting to investigate whether this phenotype is unique to platelets or observed in other circulating cells as well. The precise molecular mechanism behind the increase in intraplatelet antioxidants, especially Catalase and SOD, in advanced age remains undefined.
Thus, we present our results on the effects of aging on platelet health and observe an unexpected improved platelet phenotype in patients >79 years old. The reduced platelet hyper-reactivity in the VOA (>79 years) is due to the enhanced levels of platelet antioxidant enzymes and improved redox homeostasis. This age associated change in platelet phenotype is conceivably due to adaptive mechanisms as evident by the aged mouse studies. The presence of this novel phenomenon in the platelets mandates careful consideration of the risks and benefits when prescribing antithrombotic therapy in older patients.
Acknowledgments
Acknowledgements
We wish to thank Rita Webber for care of the mice in the Yale Cardiovascular Research Center. The graphical abstract was created using BioRender and Adobe Creative Cloud.
Funding sources
These studies have been supported by grants from NIH-NHLBI, RO1-HL122815 and RO1-HL115247. The funders of this work had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
Conflict of interest statement
Dr. Hwa reports grants from NIH-NHLBI, during the conduct of the study.
Author contributions
Kanika Jain and Tarun Tyagi designed and performed the experiments and analyzed the data. Kanchi Patell, Costin N. Ionescu, Anis John Kadado recruited the patients and provided clinical details. Yi Xie, Timur Yarovinsky, Seung Hee Lee and Jing Du assisted with the mouse studies and RNA experiments. Kanika Jain and John Hwa interpreted the data and wrote the original draft. Tarun Tyagi, Janice Hwang, Kathleen A. Martin, Jeffrey Testani and Costin N. Ionescu reviewed and provided critical insights and edited the manuscript. John Hwa and Costin N. Ionescu supervised the study. John Hwa provided all the funding and resources. All authors approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.05.022.
Contributor Information
Costin N. Ionescu, Email: costin.ionescu@yale.edu.
John Hwa, Email: john.hwa@yale.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.National Center for Health S. Health, United States . 2016. With chartbook on long-term trends in health, 2017. [PubMed] [Google Scholar]; National Center for Health S. Health, United States, 2016: With Chartbook on Long-term Trends in Health, 2017. [PubMed]
- 2.Wang J.C., Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012;111(2):245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]; Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res 2012; 111(2): 245-59. [DOI] [PubMed]
- 3.Benjamin E.J., Virani S.S., Callaway C.W. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]; Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018; 137(12): e67-e492. [DOI] [PubMed]
- 4.Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev. 2002;1(3):397–411. doi: 10.1016/s1568-1637(02)00008-9. [DOI] [PubMed] [Google Scholar]; Barja G. Endogenous oxidative stress: relationship to aging, longevity and caloric restriction. Ageing Res Rev 2002; 1(3): 397-411. [DOI] [PubMed]
- 5.Franco R., Vargas M.R. Redox biology in neurological function, dysfunction, and aging. Antioxid Redox Signal. 2018;28(18):1583–1586. doi: 10.1089/ars.2018.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]; Franco R, Vargas MR. Redox Biology in Neurological Function, Dysfunction, and Aging. Antioxid Redox Signal 2018; 28(18): 1583-6. [DOI] [PMC free article] [PubMed]
- 6.Mecocci P., Polidori M.C., Troiano L. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28(8):1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]; Mecocci P, Polidori MC, Troiano L, et al. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med 2000; 28(8): 1243-8. [DOI] [PubMed]
- 7.Blankenberg S., Rupprecht H.J., Bickel C. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349(17):1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]; Blankenberg S, Rupprecht HJ, Bickel C, et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 2003; 349(17): 1605-13. [DOI] [PubMed]
- 8.Pastori D., Pignatelli P., Farcomeni A. Aging-related decline of glutathione peroxidase 3 and risk of cardiovascular events in patients with atrial fibrillation. J Am Heart Assoc. 2016;5(9) doi: 10.1161/JAHA.116.003682. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pastori D, Pignatelli P, Farcomeni A, et al. Aging-Related Decline of Glutathione Peroxidase 3 and Risk of Cardiovascular Events in Patients With Atrial Fibrillation. J Am Heart Assoc 2016; 5(9). [DOI] [PMC free article] [PubMed]
- 9.Mohebali D., Kaplan D., Carlisle M., Supiano M.A., Rondina M.T. Alterations in platelet function during aging: clinical correlations with thromboinflammatory disease in older adults. J Am Geriatr Soc. 2014;62(3):529–535. doi: 10.1111/jgs.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mohebali D, Kaplan D, Carlisle M, Supiano MA, Rondina MT. Alterations in platelet function during aging: clinical correlations with thromboinflammatory disease in older adults. J Am Geriatr Soc 2014; 62(3): 529-35. [DOI] [PMC free article] [PubMed]
- 10.Dayal S., Wilson K.M., Motto D.G., Miller F.J., Chauhan A.K., Lentz S.R. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation. 2013;127(12):1308–1316. doi: 10.1161/CIRCULATIONAHA.112.000966. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dayal S, Wilson KM, Motto DG, Miller FJ, Chauhan AK, Lentz SR. Hydrogen peroxide promotes aging-related platelet hyperactivation and thrombosis. Circulation 2013; 127(12): 1308-16. [DOI] [PMC free article] [PubMed]
- 11.Fuentes E., Palomo I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci. 2016;148:17–23. doi: 10.1016/j.lfs.2016.02.026. [DOI] [PubMed] [Google Scholar]; Fuentes E, Palomo I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci 2016; 148: 17-23. [DOI] [PubMed]
- 12.Fuentes F., Palomo I., Fuentes E. Platelet oxidative stress as a novel target of cardiovascular risk in frail older people. Vascul Pharmacol. 2017;93-95:14–19. doi: 10.1016/j.vph.2017.07.003. [DOI] [PubMed] [Google Scholar]; Fuentes F, Palomo I, Fuentes E. Platelet oxidative stress as a novel target of cardiovascular risk in frail older people. Vascul Pharmacol 2017; 93-95: 14-9. [DOI] [PubMed]
- 13.Balduini C.L., Noris P. Platelet count and aging. Haematologica. 2014:953–955. doi: 10.3324/haematol.2014.106260. [DOI] [PMC free article] [PubMed] [Google Scholar]; Balduini CL, Noris P. Platelet count and aging. Haematologica. 2014:953-5. [DOI] [PMC free article] [PubMed]
- 14.Lee S.H., Du J., Stitham J. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Mol Med. 2016;8(7):779–795. doi: 10.15252/emmm.201506046. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee SH, Du J, Stitham J, et al. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. Embo Molecular Medicine 2016; 8(7): 779-95. [DOI] [PMC free article] [PubMed]
- 15.Wang Z., Cai F., Chen X., Luo M., Hu L., Lu Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0075044. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang Z, Cai F, Chen X, Luo M, Hu L, Lu Y. The Role of Mitochondria-Derived Reactive Oxygen Species in Hyperthermia-Induced Platelet Apoptosis. PLoS ONE 2013; 8(9). [DOI] [PMC free article] [PubMed]
- 16.Leytin V., Allen D.J., Mykhaylov S. Pathologic high shear stress induces apoptosis events in human platelets. Biochem Biophys Res Commun. 2004;320(2):303–310. doi: 10.1016/j.bbrc.2004.05.166. [DOI] [PubMed] [Google Scholar]; Leytin V, Allen DJ, Mykhaylov S, et al. Pathologic high shear stress induces apoptosis events in human platelets. Biochemical and Biophysical Research Communications 2004; 320(2): 303-10. [DOI] [PubMed]
- 17.Shattil S.J., Hoxie J.A., Cunningham M., Brass L.F. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260(20):11107–11114. [PubMed] [Google Scholar]; Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. Journal of Biological Chemistry 1985; 260(20): 11107-14. [PubMed]
- 18.Landry P., Plante I., Ouellet D.L., Perron M.P., Rousseau G., Provost P. Existence of a microRNA pathway in anucleate platelets. Nature Structural and Molecular Biology. 2009;16(9):961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]; Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nature Structural and Molecular Biology 2009; 16(9): 961-6. [DOI] [PMC free article] [PubMed]
- 19.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods (San Diego, Calif) 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]; Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001; 25(4): 402-8. [DOI] [PubMed]
- 20.Flurkey K., Currer J.M., Harrison D.E. Chapter 20 - mouse models in aging research. In: Fox J.G., Davisson M.T., Quimby F.W., Barthold S.W., Newcomer C.E., Smith A.L., editors. The mouse in biomedical research. 2nd ed. Academic Press; Burlington: 2007. pp. 637–672. [Google Scholar]; Flurkey K, Currer J.M, Harrison DE. Chapter 20 - Mouse Models in Aging Research. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, eds. The Mouse in Biomedical Research (Second edition). Burlington: Academic Press; 2007: 637-72.
- 21.Leytin V., Allen D.J., Mutlu A., Mykhaylov S., Lyubimov E., Freedman J. Platelet activation and apoptosis are different phenomena: evidence from the sequential dynamics and the magnitude of responses during platelet storage. Br J Haematol. 2008;142(3):494–497. doi: 10.1111/j.1365-2141.2008.07209.x. [DOI] [PubMed] [Google Scholar]; Leytin V, Allen DJ, Mutlu A, Mykhaylov S, Lyubimov E, Freedman J. Platelet activation and apoptosis are different phenomena: evidence from the sequential dynamics and the magnitude of responses during platelet storage. Br J Haematol 2008; 142(3): 494-7. [DOI] [PubMed]
- 22.Tang W.H., Stitham J., Jin Y. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation. 2014;129(15):1598–1609. doi: 10.1161/CIRCULATIONAHA.113.005224. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tang WH, Stitham J, Jin Y, et al. Aldose reductase-mediated phosphorylation of p53 leads to mitochondrial dysfunction and damage in diabetic platelets. Circulation 2014; 129(15): 1598-609. [DOI] [PMC free article] [PubMed]
- 23.Barbieri M., Rizzo M.R., Manzella D. Glucose regulation and oxidative stress in healthy centenarians. Exp Gerontol. 2003;38(1–2):137–143. doi: 10.1016/s0531-5565(02)00153-5. [DOI] [PubMed] [Google Scholar]; Barbieri M, Rizzo MR, Manzella D, et al. Glucose regulation and oxidative stress in healthy centenarians. Exp Gerontol 2003; 38(1-2): 137-43. [DOI] [PubMed]
- 24.Mecocci P., Polidori M.C., Troiano L. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radical Biology and Medicine. 2000;28(8):1243–1248. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]; Mecocci P, Polidori MC, Troiano L, et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radical Biology and Medicine 2000; 28(8): 1243-8. [DOI] [PubMed]
- 25.Paolisso G., Tagliamonte M.R., Rizzo M.R., Manzella D., Gambardella A., Varricchio M. Oxidative stress and advancing age: results in healthy centenarians. J Am Geriatr Soc. 1998;46(7):833–838. doi: 10.1111/j.1532-5415.1998.tb02716.x. [DOI] [PubMed] [Google Scholar]; Paolisso G, Tagliamonte MR, Rizzo MR, Manzella D, Gambardella A, Varricchio M. Oxidative stress and advancing age: results in healthy centenarians. J Am Geriatr Soc 1998; 46(7): 833-8. [DOI] [PubMed]
- 26.Nguyen T., Nioi P., Pickett C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009; 284(20): 13291-5. [DOI] [PMC free article] [PubMed]
- 27.Webster B.R., Lu Z., Sack M.N., Scott I. The role of sirtuins in modulating redox stressors. Free Radic Biol Med. 2012;52(2):281–290. doi: 10.1016/j.freeradbiomed.2011.10.484. [DOI] [PMC free article] [PubMed] [Google Scholar]; Webster BR, Lu Z, Sack MN, Scott I. The role of sirtuins in modulating redox stressors. Free Radic Biol Med 2012; 52(2): 281-90. [DOI] [PMC free article] [PubMed]
- 28.Higashi Y., Pandey A., Goodwin B., Delafontaine P. Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: implications for atheroprotective actions of insulin-like growth factor-1. Biochim Biophys Acta. 2013;1832(3):391–399. doi: 10.1016/j.bbadis.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Higashi Y, Pandey A, Goodwin B, Delafontaine P. Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: Implications for atheroprotective actions of insulin-like growth factor-1. Biochim Biophys Acta 2013; 1832(3): 391-9. [DOI] [PMC free article] [PubMed]
- 29.Morris B.J., Willcox D.C., Donlon T.A., Willcox B.J. FOXO3: a major gene for human longevity—a mini-review. Gerontology. 2015;61(6):515–525. doi: 10.1159/000375235. [DOI] [PMC free article] [PubMed] [Google Scholar]; Morris BJ, Willcox DC, Donlon TA, Willcox BJ. FOXO3: A Major Gene for Human Longevity—A Mini-Review. Gerontology 2015; 61(6): 515-25. [DOI] [PMC free article] [PubMed]
- 30.Klotz L.O., Sanchez-Ramos C., Prieto-Arroyo I., Urbanek P., Steinbrenner H., Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Klotz LO, Sanchez-Ramos C, Prieto-Arroyo I, Urbanek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol 2015; 6: 51-72. [DOI] [PMC free article] [PubMed]
- 31.Patrono C., Garcia Rodriguez L.A., Landolfi R., Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]; Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005; 353(22): 2373-83. [DOI] [PubMed]
- 32.Baigent C., Blackwell L., Collins R. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009; 373(9678): 1849-60. [DOI] [PMC free article] [PubMed]
- 33.Sørensen R., Hansen M.L., Abildstrom S.Z. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. The Lancet. 2009;374(9706):1967–1974. doi: 10.1016/S0140-6736(09)61751-7. [DOI] [PubMed] [Google Scholar]; Sørensen R, Hansen ML, Abildstrom SZ, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. The Lancet 2009; 374(9706): 1967-74. [DOI] [PubMed]
- 34.Riva N., Smith D.E., Lip G.Y.H., Lane D.A. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing. 2011:653–655. doi: 10.1093/ageing/afr128. [DOI] [PubMed] [Google Scholar]; Riva N, Smith DE, Lip GYH, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age and Ageing. 2011:653-5. [DOI] [PubMed]
- 35.Li L., Geraghty O.C., Mehta Z., Rothwell P.M. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. The Lancet. 2017;390(10093):490–499. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li L, Geraghty OC, Mehta Z, Rothwell PM. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. The Lancet 2017; 390(10093): 490-9. [DOI] [PMC free article] [PubMed]
- 36.McNeil J.J., Wolfe R., Woods R.L. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509–1518. doi: 10.1056/NEJMoa1805819. [DOI] [PMC free article] [PubMed] [Google Scholar]; McNeil JJ, Wolfe R, Woods RL, et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N Engl J Med 2018; 379(16): 1509-18. [DOI] [PMC free article] [PubMed]
- 37.Melkonian M., Jarzebowski W., Pautas E., Siguret V., Belmin J., Lafuente-Lafuente C. Bleeding risk of antiplatelet drugs compared with oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-analysis. J Thromb Haemost. 2017;15(7):1500–1510. doi: 10.1111/jth.13697. [DOI] [PubMed] [Google Scholar]; Melkonian M, Jarzebowski W, Pautas E, Siguret V, Belmin J, Lafuente-Lafuente C. Bleeding risk of antiplatelet drugs compared with oral anticoagulants in older patients with atrial fibrillation: a systematic review and meta-analysis. J Thromb Haemost 2017; 15(7): 1500-10. [DOI] [PubMed]
- 38.Pugh D., Pugh J., Mead G.E. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683. doi: 10.1093/ageing/afr097. [DOI] [PubMed] [Google Scholar]; Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40(6): 675-83. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material