Abstract
Rationale
The benefit of thrombectomy in patients with intracranial large vessel occlusion of the anterior circulation has been shown in selected patients in previous randomized controlled trials, but patients with extended ischemic lesions were excluded in the majority of these trials. TENSION aims to demonstrate efficacy and safety of thrombectomy in patients with extended lesions in an extended time window (up to 12 h from onset or from last seen well).
Design
TENSION is an investigator-initiated, randomized controlled, open label, blinded endpoint, European, two-arm, postmarket study to compare the safety and effectiveness of thrombectomy as compared to best medical care alone in stroke patients with extended stroke lesions defined by an Alberta Stroke Program Early Computed Tomography Scan score of 3–5 and in an extended time window. In an adaptive design study, up to 665 patients will be randomized.
Outcomes
Primary efficacy endpoint will be clinical outcome defined by the modified Rankin Scale at 90-day poststroke. The main safety endpoint will be death and dependency (modified Rankin Scale 4–6) at 90 days. Additional effect measures include adverse events, health-related quality of life, poststroke depression, and costs utility assessment.
Discussion
TENSION may make effective treatment available for patients with severe stroke in an extended time window, thereby improving functional outcome and quality of life of thousands of stroke patients and reducing the individual, societal, and economic burden of death and disability resulting from severe stroke. TENSION is registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier NCT03094715).
Keywords: Acute stroke therapy, intervention, ischemic stroke, protocols, radiology, stroke, therapy, treatment
Introduction and rationale
Thrombectomy in acute ischemic stroke (AIS) patients with intracranial large vessel occlusion in the anterior circulation results in clear clinical benefits without increased adverse events. However, previous randomized clinical trials (RCTs) have limited this treatment option to patients with good prognostic factors (i.e. short time interval between stroke onset and endovascular treatment as well as small size of ischemic lesion prior to treatment).1–5 Of 1287 patients randomized in five large RCTs less than 5% were treated beyond 6 h of symptom onset. In four of these five trials, patients with early ischemic signs seen as an Alberta Stroke Program Early Computed Tomography Scan score (ASPECTS) below 6 or 7 were excluded. Only MR CLEAN did not specify pretreatment ASPECTS values as an exclusion criterion, but even in this study only small numbers of patients with low ASPECTS values were included. In 28 patients with ASPECTS 0–4 no treatment effect was evident with an OR of 1.1 (95% CI 0.1–8.4) for benefit in the thrombectomy group, while in 92 patients with ASPECTS 5–7 with an OR of 2.0 (0.9–4.4) a clear trend for a treatment benefit was observed.1 In a meta-analysis of individual patient data from the five positive thrombectomy trials, no treatment effect was observed for patients with ASPECTS 0–5 with an OR of 1.24 (95% CI 0.62–2.49).6 A recently published bicentric registry study which included 218 patients with a diffusion-weighted imaging (DWI)-ASPECTS ≤ 6 found an increased rate of favorable outcomes (modified Rankin Scale (mRS)≤2 at 90 days) and a decreased rate of mortality in reperfused patients (TICI score ≥2b) compared to nonreperfused patients.7 However, the rate of favorable outcomes did not differ significantly and mortality increased in patients with a DWI-APECTS <5. Additionally, this study did not evaluate outcomes in patients with a low ASPECTS who did not undergo any endovascular procedure. Thus, insufficient evidence is available to judge whether mechanical thrombectomy is also safe and effective in patients with an extended time window or signs of early ischemia, or a combination of both. This was also acknowledged by experts from the European Stroke Organisation, the European Society for Minimally Invasive Neurological Therapy (ESMINT), and the European Society of Neuroradiology in a consensus statement on thrombectomy.8 In this statement the experts recommended further RCTs addressing open issues such as “treatment in a late and unknown time windows, treating patients with imaging findings not sufficiently covered in recent trials.…” Recently, the efficacy of thrombectomy in an extended time window has been shown in the DAWN and DEFUSE 3 trials.9,10 However, these studies only included patients with an initial small infarct core. The question remains if treatment is still beneficial and safe with an extended ischemic lesion prior to treatment. The TENSION (efficacy and safety of ThrombEctomy iN Stroke with extended leSION and extended time window: a randomized, controlled trial) trial aims to close this gap. As it is estimated that about 25% of acute stroke patients with LVO present with an ASPECTS of 0–5 this issue is highly relevant in clinical practice. The primary objective of TENSION is to test efficacy and safety of thrombectomy in acute stroke patients with large vessel occlusion and extended ischemic lesion size (ASPECTS 3–5) in an extended time window (up to 12 h or unknown onset).11
Methods
Design
TENSION is an European investigator-initiated, randomized controlled, open label, blinded endpoint (PROBE), two-arm, randomized, postmarket study to compare the safety and effectiveness of endovascular thrombectomy as compared to best medical care alone in the treatment of AIS in patients with extended stroke lesions defined by an ASPECTS of 3–5 and in an extended time window (up to 12 h from onset or last known well). The study will apply an adaptive design study with interim analyses with prespecified stopping rules allowing for the possibility of early termination based on either a determination of study success or futility.
As of January 2018, 40 centers in eight European countries (Austria, Czech Republic, Denmark, France, Germany, Norway, Slovakia, Sweden) have agreed to participate.
Patient population
Patients presenting with AIS based on focal occlusion in the M1 segment of the middle cerebral artery, and/or the intracranial segment of the distal internal carotid artery (ICA), determined by magnetic resonance angiography (MRA) or computed tomography angiography (CTA), and who meet all eligibility criteria will be considered for study enrolment. Table 1 lists the inclusion and exclusion criteria. Up to 665 subjects, 333 per treatment group, will be enrolled and randomized for the intent to treat (ITT) analysis. A screening log of all potential patients will be kept locally at each center.
Table 1.
TENSION inclusion and exclusion criteria
| Clinical inclusion criteria | Clinical exclusion criteria |
|---|---|
| Moderate to severe stroke (NIHSS score <26) | Patient is an active participant in another drug or device treatment trial |
| Premorbid modified Rankin Scale (mRS) score 0–2 | Patient has preexisting neurological or psychiatric disease that could impede the study results or would confound the neurological or functional evaluations |
| Life expectancy >6 months | Patient has vascular disease preventing endovascular treatment (e.g. aortic dissection or aneurysm, no arterial transfemoral access) |
| Age >18–80 years | Patient has history of contraindication for contrast medium |
| Treatment can be accomplished within 12 h after stroke onset (if known), i.e. randomization within 11 h after ictus | Patient is known to have infective endocarditis |
| Informed consent by the patient, legal guardian, or inclusion of patient under presumptive will, in accordance with national regulations after consultation of an independent physician and statement of investigator | Patient's anticipated life expectancy is less than six months |
| Imaging inclusion criteria | Imaging exclusion criteria |
| Occlusion of the M1 segment of the middle cerebral artery (MCA) and/or the intracranial segment of the distal internal carotid artery (ICA), determined by MRA or CTA | CT scan or MRI with evidence of mass effect or intracranial tumor, or hypodensity on unenhanced CT and cerebral blood volume (CBV) drop on CBV maps on CT perfusion, or, alternatively as per institutional standard, restricted diffusion on DWI with an ASPECT score of 0–2, or above 5 |
| CT (noncontrast CT) or DWI with an ASPECT score of 3–5 | Any other finding on brain CT or MRI considered as indicative of a high risk of SICH related to potential thrombectomy treatment in the judgment of the investigator |
ASPECTS: Alberta Stroke Program Early CT Score; CT: computed tomography; CTA: computed tomography angiography; DWI: diffusion-weighted imaging; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; NIHSS: National Institutes of Health Stroke Scale; SICH: symptomatic intracranial hemorrhage; TENSION: efficacy and safety of ThrombEctomy iN Stroke with extended leSION and extended time window: a randomized, controlled trial.
Randomization
Patients are randomized in the two treatment arms using a web-based system with a 1:1 ratio. Stratified randomization by time from symptom onset/last known well (0–6 and >6 h) and stroke severity (National Institutes of Health Stroke Scale (NIHSS) ≤18, NIHSS >18) may minimize imbalances that might affect outcome and bias results. Due to the differences between treatment regimens blinding is not possible, but final assessment will be blinded to treatment.
Treatment or intervention
Treatment Arm A: Best medical care: Medical treatment will be performed as detailed in established Standard Operating Procedures, following regional guidelines (AHA, EROICAS, DSG, local country, etc.). The reason for iv tPA ineligibility will be documented on the eCRF.
Treatment Arm B: Endovascular thrombectomy and best medical care: In the TENSION trial, CE-marked devices for thrombectomy will be used within their intended use according to their instruction for use. If a subject is randomized to thrombectomy and subsequently fails the angiographic screening or is not treated due to rapidly improving neurologic symptoms prior to procedure, the subject will remain in the ITT population.
Clinical assessment
Baseline disease characteristics include prestroke mRS, presenting symptom(s) and results of pretreatment imaging with a description of the occluded vessel. Neurological deficit will be assessed using the NIHSS by certified investigators at baseline, at 24–36 h, at seven days or at hospital discharge, and at 90 ± 14 days. At 90 ± 14 days and 12 months (±14 days), outcome assessment will also comprise the mRS, health-related quality of life (EQ-5D, PROMIS-10), and poststroke depression (PHQ-4).
Imaging protocol
Baseline imaging, either MRA with DWI or CTA should demonstrate a new focal occlusion (in the M1 segment and/or the intracranial ICA) accessible to the thrombectomy device. Baseline imaging should depict all supra-aortic vessels (head and neck). Angiographic imaging before, during, and after the endovascular procedure as well as follow-up imaging at 30 (−6/+6) hours to assess for intracranial hemorrhage will be sent to the Imaging Core Lab. All investigators should be qualified to assess images according to ASPECTS. ASPECTS training for TENSION will be performed using a web-based “reading academy” consisting of two modules: a training module and a rating module. Only physicians who pass the test are allowed to enroll patients.
Primary outcomes
The primary endpoint of TENSION is the patient's mRS at 90-day poststroke analyzed with a shift analysis.
Secondary outcomes
Secondary endpoints will comprise independent functional outcome (mRS ≤ 2), health-related quality of life (EQ-5D. PROMIS-10), survival, symptomatic intracranial hemorrhage at 30 h, new ischemic stroke, space-occupying infarction (malignant brain edema), AE, SAE, infarct volume at 30 h, infarct growth, rates of hemicraniectomy, treatment effect by device, and poststroke depression (PHQ-4). Cost-utility assessment will include health-related quality of life assessment at 90 (±14) days and 12 months (±14 days) and assessment of costs from the time of randomization to the 12-month follow-up, including costs of hospitalization, institutionalized living, outpatient care, informal care provided by relatives, and cost of lost productivity.
Data and Safety Monitoring Board (DSMB)
To ensure that appropriate ethical consideration is given to the welfare of the patients enrolled in the study, an independent Ethics Advisory Board as well as a DSMB was formed. The members of the DSMB are not participants of the TENSION consortium and not involved in the clinical trial in any other way. The DSMB will meet every 12 months during the study to review trial group data in partially unblinded fashion on the baseline and safety parameters. In addition, the trial will undergo two interim analyses with possible premature stopping for futility or early success with control of the overall type one error rate using a Lan–Demets alpha-spending function. Interim data analysis is planned after the primary endpoint has been obtained for one-third and two-thirds of the patients. At each of these sample sizes, the available 90-day mRS data for each treatment arm will be evaluated. The tasks and operating procedures of the DSMB will be described in detail in a separate DSMB charter.
Sample size
Simulations were carried out using an assumption for the possible true distribution of mRS from the literature (Goyal et al.,5 Supplement: distribution of mRS in patients with an ASPECT score of 7 or less) and a proportional odds alternative with an odds ratio of 1.5 to be assessed using the primary endpoint mRS shift analysis. Under the assumption of these distributions, a total of 620 patients is required to achieve a power of 80% for a one-sided test at the 0.025 level. Assuming a 7% dropout rate of patients to assess the primary endpoint obtained three months after inclusion an effective sample size of 665 is necessary to obtain 620 complete observations.
Also, as a consequence of the sequential monitoring of the trial, the total sample size needs to be increased according to the characteristics of the alpha-spending function that will be chosen. With a power function of parameter ρ = 2, up to a maximum of 714 patients may be required if the trial does not stop early.
Statistical analyses
Summary tables for subject demographics and baseline characteristics will be provided and comparisons will be made between study arms for the ITT and PP analysis sets. Procedural characteristics unique to usage of devices for thrombectomy will be described for subjects in the best medical care plus thrombectomy treatment group (within the ITT analysis set). The primary and secondary effectiveness endpoints will be summarized and compared between study groups for the ITT and PP analysis sets. Safety endpoints will be summarized and compared between study groups for the safety analysis set.
In general, summaries will be presented by treatment group pooled across occlusion location and investigators/sites, and by occlusion location within treatment group in the relevant analysis populations. Descriptive statistics for dichotomous/categorical variables will include number and percent of subjects in each category (including missing), by treatment group. Descriptive statistics for continuous variables will include number of nonmissing and missing subjects, minimum, lower quartile, median, upper quartile, maximum, mean, and standard deviation, stratified by treatment group. Regarding comparisons between treatment groups, Chi-squared test (Fisher's exact test where appropriate) will be utilized for the comparison of categorical variables and the t-test (or the Mann–Whitney U test when appropriate) will be utilized for the comparison of continuous variables. Data for the primary endpoint will be presented by treatment group (pooled over all sites) and by treatment group within study site. The primary effectiveness endpoint analysis will be carried out in an ordinal logistic regression model with the mRS ordinal scale as response variable and treatment group, stroke severity (NIHSS 8–18 and NIHSS >18), and time from symptom onset until randomization (0–6 h and 6–11 h) as explanatory variables. Aside from protocol-specified hypothesis tests, confidence intervals will be presented to facilitate clinical judgment of the secondary safety and effectiveness endpoints, and not to test hypotheses. Confidence intervals for dichotomous or ordinal endpoints will be reported on the odds ratio scale.
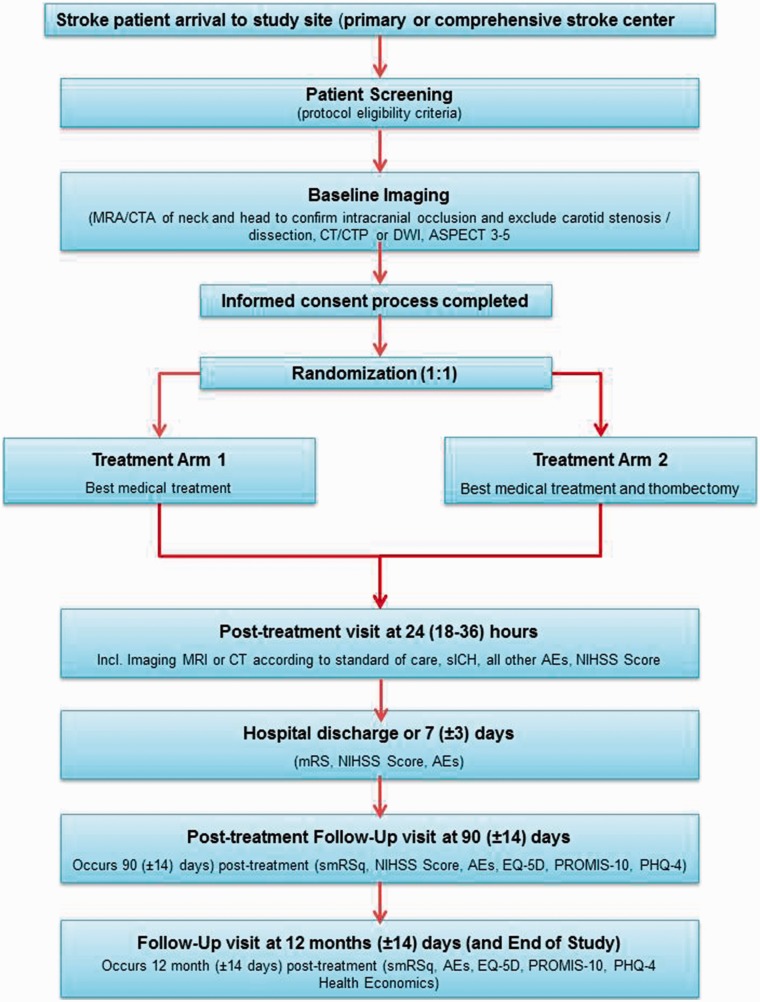
Figure 1.
Study flow chart. AE: adverse event; ASPECTS: Alberta Stroke Program Early CT Score; CT: computed tomography; CTA: computed tomography angiography; CTP: computed tomography perfusion; DWI: diffusion-weighted imaging; EuroQol 5D; MRA: magnetic resonance angiography; MRI: magnetic resonance imaging; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; PHQ-4: Patient Health Questionnaire-4; PROMIS-10: Patient-Reported Outcomes Measurement Information System-10; SICH: symptomatic intracranial hemorrhage.
Figure 2.
The concept of treatment response in stroke patients with extended stroke lesions. ASPECTS: Alberta Stroke Program Early CT Score.
Study organization and funding
TENSION is an EU-funded, investigator-initiated and conducted trial. Coordination and project management will be provided by Prof. Götz Thomalla (Department of Neurology, University Hospital Hamburg Eppendorf, Germany). The principal investigator Prof. Martin Bendszus (Department of Neuroradiology, University Hospital Heidelberg, Germany) will organize the trial together with input from International Consortium for Health Outcomes Measurement (ICHOM), SAFE (European patients organization), and the national principal investigators: E. Gizweski (AUT), A. Krajina (CZE), C. Simonsen (DEN), L. Pierot (FRA), E. Berge (NOR), K. Zeleňák (SVK), P. Brouwer (SWE). Central trial management at the Koordinierungszentrum Klinische Studien (Coordination Center for Clinical Trials) at the University Hospital Heidelberg and European Clinical Research Infrastructure Network (ECRIN-ERIC) will perform the submission to ethics committees and competent authorities, trial management, data management, monitoring, and pharmacovigilance. Prof. Jens Fiehler (Eppdata GmbH and Department of Neuroradiology, University Hospital Hamburg Eppendorf, Germany) will be responsible for the Imaging Core Lab.
TENSION is registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier NCT03094715).
Conclusion
TENSION is a European PROBE, European two-arm, postmarket study to compare the safety and effectiveness of endovascular thrombectomy as compared to best medical care alone in the treatment of AIS patients with extended stroke lesions defined by an ASPECT score of 3–5 and in an extended time window (up to 12 h or unknown time of symptom onset). Up to 714 subjects will be randomized. Primary endpoint will be functional outcome assessed by the mRS at 90-day poststroke (“mRS shift analysis”). By this, TENSION will provide evidence of efficacy and safety of thrombectomy in an acute stroke population with uncertain benefit of endovascular stroke treatment so far and may greatly increase the proportion of patients eligible for treatment.
Acknowledgments
TENSION boards and institutions: Data and Safety Monitoring Board, Ethics Advisory Board, Innovation and Exploitation Management Board, Steering Committee, ECRIN, ICHOM, ESMINT.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: TENSION receives funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. 754640.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Diener HC. SWIFT PRIME Investigators. Stent-retriever thrombectomy for stroke. N Engl J Med 2015; 373: 1077. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BCV, Donnan GA, Lees KR, et al. Endovascular stent thrombectomy: the new standard of care for large vessel ischaemic stroke. Lancet Neurol 2015; 14: 846–854. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Hill MD. Endovascular therapy for ischemic stroke. N Engl J Med 2015; 372: 2366. [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 7.Desilles JP, Consoli A, Redjem H, et al. Successful reperfusion with mechanical thrombectomy is associated with reduced disability and mortality in patients with pretreatment diffusion-weighted imaging-Alberta Stroke Program early computed tomography score </=6. Stroke 2017; 48: 963–969. [DOI] [PubMed] [Google Scholar]
- 8.Fiehler J, Cognard C, Gallitelli M, et al. European Recommendations on Organisation of Interventional Care in Acute Stroke (EROICAS). Int J Stroke 2016; 11: 701–716. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 11.McMeekin P, White P, Martin AJ, Christopher IP, Flynn D, Gary AF. Estimating the number of UK stroke patients eligible for endovascular thrombectomy. Eur Stroke J 2017; 2: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]