Abstract
Background:
The purpose of this study was to analyze clinical, radiographic and intraoperative disease characteristics of patients with symptomatic acetabular dysplasia in which periacetabular osteotomy (PAO) was contraindicated due to advanced intraarticular findings at the time of disease staging hip arthroscopy (HA).
Methods:
A prospective cohort was used to identify all patients who were scheduled for a PAO and concomitant hip arthroscopy for the treatment of symptomatic acetabular dysplasia. From a total of 286 patients (286 hips), 11 patients (11 hips) were identified in whom the PAO was contraindicated due to the intraarticular findings of a disease-staging hip arthroscopy. Clinical characteristics, radiographic and intraoperative findings were analyzed and compared to those patients in whom the joint was judged to be appropriate for PAO surgery.
Results:
11 patients (11/286 or 4%), in whom a PAO was contraindicated after joint assessment with HA, were identified and included in this study. There were nine females and two males. All patients were potential candidates for PAO surgery. The PAO was contraindicated in these cases due to severe articular cartilage damage on both the femoral head and acetabulum. The patients when compared to those in which the PAO was performed, were significantly older (42.3 years (IQR, 38.1-46.8) vs. 24 years (IQR, 19-34)) (p<0.001) and had more severe dysplasia with a lower median lateral center-edge angle (LCEA, 12.9° vs. 17.7°, p=0.001) and lower anterior center-edge angle (ACE, 14.4° vs. 20.3°, p=0.021).
Conclusions:
Patients in which the PAO was contraindicated, compared to those in which PAO was performed, were older and had significant more severe dysplasia. The main cause of intraoperative disqualification for PAO was advanced articular cartilage disease.
Level of Evidence: IV
Keywords: acetabular dysplasia, PAO, hip arthroscopy, developmental dysplasia
Introduction
The Bernese periacetabular osteotomy (PAO) is a well-established technique for the treatment of symptomatic acetabular dysplasia.1 With only a few prospective, longterm studies of PAO outcomes, proper patient selection remains challenging.2,3 PAO is indicated for those patients who are symptomatic, yet have relatively preserved articular cartilage. A combination of clinical history and physical examination, along with plain radiography, computed tomography and magnetic resonance imaging (MRI) are normally utilized to establish a predictable indication.1,4-6
The use of imaging studies aids in the qualification process, but imaging may not completely define the severity of the articular cartilage and labral lesions.7,8 Severe cartilage damage as determined by radiographic joint space narrowing is a significant predictor of failure and relative contraindication for PAO.9-12 Since cartilage damage is common in patients with symptomatic DDH,13,14 MRI has been established as part of preoperative imaging modality for patient selection. However, the diagnosis of cartilage damage through noninvasive imaging modalities can have limitations relative to determining the exact size and thickness of a given articular cartilage lesion in the hip. 8,15,16
In patients with equivocal clinical presentation and imaging studies relative to indicating PAO surgery, hip arthroscopy (HA) provides an excellent tool to directly visualize and determine articular cartilage integrity. 17 Additionally, in patients with symptomatic acetabular dysplasia and associated intra-articular abnormalities (labral tears, ligamentum teres tears, and chondral lesions) concurrent hip arthroscopy can provide improved access, visualization and technical precision in the central compartment compared to an open anterior arthrotomy.18 Over the past several years, hip arthroscopy has been used in combination with the PAO (HA+PAO) to stage articular cartilage degeneration and diagnose and treat associated intraarticular abnormalities.13,19-21 However, the superiority of hip arthroscopy concomitantly with the PAO has not been established.
Uncommonly, despite the use of strict clinical parameters and advanced imaging for patient selection, the indication for PAO may not be completely clear due to uncertainty regarding the health of the femoral head and acetabular articular cartilage. Since intra-articular lesions and more advanced articular cartilage disease have been described as a risk factor for poor outcomes and osteoarthritis progression following PAO,9-12 it is important to determine joint health prior to indication for PAO treatment. In cases with equivocal health of the articular cartilage, a staging hip arthroscopy can provide important information regarding joint health and appropriateness of PAO surgery. The purpose of this study was to analyze the clinical and radiographic characteristics of patients with symptomatic acetabular dysplasia in whom PAO surgery was contraindicated due to the findings of advanced articular cartilage disease at the time of a disease-staging hip arthroscopy.
Materials and Methods
Selection criteria
This study was performed under an institutional review board–approved protocol. An institutional prospectively longitudinal database of PAOs was reviewed and 286 consecutive patients (286 hips) who were scheduled for HA+PAO by the senior surgeon (JCC) from April 2007 to April 2017, were identified. Throughout the study period, the indications for the HA+PAO were (1) symptomatic acetabular dysplasia22 as diagnosed by the senior author (center-edge angle of Wiberg of less than 25° and skeletal maturity) (2) failed nonsurgical treatment (minimum three months, including physical therapy, activity modification, NSAIDs and variable intra-articular corticosteroid injection), (3) mechanical hip symptoms, (4) MRA diagnosed labral detachment and/ or (5) concern regarding the integrity of the femoral head and acetabular articular cartilage. The contraindications for PAO were (1) osteoarthritis (Tönnis Grade 2 and higher), (2) poor hip congruence on plain radiographs, (3) BMI greater than 35 and/or (4) limited hip range of motion (flexion less than 90°, abduction less than 20°). After all patients scheduled for HA+PAO were identified, all of their operative notes were searched manually to identify those, who were intraoperatively disqualified from continuing with the PAO. This left 11 patients (11 hips) in the study group (contraindicated for the PAO) and 275 in a comparison group (HA+PAO). The process is presented in Fig. 1.
Figure 1.
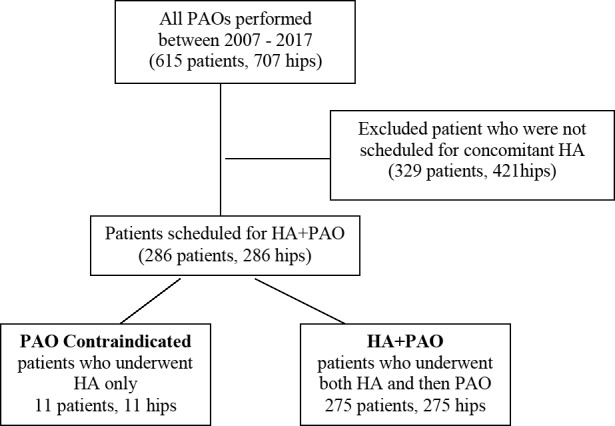
Flowchart shows final cohort and reason for exclusion. PAO = periacetabular osteotomy, HA = hip arthroscopy
Outcomes
Demographic and clinical data regarding age, sex, height, weight and previous surgeries (Table 1) were obtained. Additionally, the preoperative clinical scores including modified Harris Hip Score (mHHS),23 HOOS23 and UCLA score24 were recorded.
Table 1.
Demographic and History Data for the 286 Patients
| Parameter | PAO Contraindicated (n=11) | HA + PAO (n=275) | Significance level |
|---|---|---|---|
| Age (years) | 42.3 (38.1 - 46.8) | 24 (19 - 34) | p<0.001 |
| Sex (M: F ratio) | 2: 9 | 30: 245 | p=0.453 |
| BMI (kg/m2) | 23.5 (21.8 - 25.1) | 22.7 (20.6-25.2) | p=0.32 |
| Previous hip surgery (Y: N ratio) | 1: 10 | 39: 236 | p=0.633 |
| Pain chronicity | p=0.637 | ||
| < 6 months | 1 | 14 | |
| 6 months - 1 year | 1 | 56 | |
| 1 - 3 years | 5 | 120 | |
| 3 - 5 years | 1 | 44 | |
| > 5 years | 3 | 39 |
BMI - body mass index; M - male; F - female; Y - yes; N - no The Data are Median (IQR) for Age and BMI and Ratios for Sex and Previous Hip Surgery.
Radiographic analysis
One author (MKW), who was not aware of the status of the hip, assessed all conventional radiographs (Table 2). All patients had preoperative radiographs, which included an antero-posterior pelvis (AP), frog-lateral, false profile, and 45° Dunn lateral view.4,25-27
Table 2.
Patient-Reported Outcomes for Both Groups at Baseline
| Parameter | PAO Contraindicated (n=11) | HA+PAO (n=275) | Significance level |
|---|---|---|---|
| UCLA | 4 (3 - 6) | 6 (4 - 10) | p=0.080 |
| HOOS Symptoms | 60 (50 - 75) | 50 (35 - 65) | p=0.165 |
| HOOS Pain | 57.5 (50 - 70) | 47.5 (35 - 65) | p=0.141 |
| HOOS ADL | 69.9 (57.4 - 82.4) | 63.2 (47.1 - 80.9) | p=0.422 |
| HOOS S&R | 53.1 (25 - 75) | 37.5 (18.8 - 56.3) | p=0.133 |
| HOOS QoL | 34.4 (18.8 - 37.5) | 25 (12.5 - 43.8) | p=0.617 |
| WOMAC | 68.8 (57.3 - 79.2) | 61.5 (45.8 - 76) | p=0.324 |
The following radiographic parameters were measured: the center-edge angle of Wiberg,28 acetabular index angle29 and minimal joint space width.30 The minimal joint space width was measured as the smallest distance between the acetabular sclerotic zone and the femoral head. The degree of osteoarthritis was graded preoperatively according to the Tönnis classification (Grades 0–3).29 All of the measurements above were performed using the OrthoStudio plug-in (Boston Children’s Hospital, Boston, MA) for the OsiriX Lite DICOM viewer (Pixmeo Sarl, Bernex, Switzerland). The congruence of the hip was evaluated by identifying the center of the femoral head using the best-fitted circle; then another best-fitted circle of the acetabulum was drawn with a digital software (BJC Clinical Desktop, BJC Healthcare, St. Louis, MO). We considered the hip congruent if the centers of the femoral head and the acetabulum were concentric within one millimeter.
All patients had preoperative MRI with a 1.5-T magnetic A resonance system (Avanto; Siemens, Erlangen, Germany) with T1, T2 and PD fat sat sequences. The following parameters were used: slice thickness 0.82 mm, Repetition time (TR) 15.96 ms, Echo time (TE) 6.2 ms, Field of view (FOV) 400 mm at the hip joint, 512 512 matrix. MRI images were read by a fellowship-trained musculoskeletal radiologists and their reports were abstracted and findings analyzed.
Operative findings
The operative data was collected prospectively from a standardized data worksheet that was filled out by the operating surgeon (JCC) at the completion of the surgery. It described the status of the labrum and the articular surfaces (both acetabulum and femoral head). Labral tears were classified by involvement of anterior, superolateral and posterior regions of the acetabulum.31 For the chondromalacia, Beck scoring was used.32 Acetabular chondromalacia was identified in each one of the sextants of the acetabulum: anterior central, anterior peripheral, superolateral central, superolateral peripheral, posterior central and posterior peripheral. Femoral chondromalacia was graded in each one of the quadrants of the femoral head: anterolateral, anteromedial, posterolateral and posteromedial (Fig. 2). The results are analyzed as either the presence of chondromalacia or not. Then the grading according to Beck (ref) was clustered in two subgroups for every region and grades 2-3 vs. 4-5 were compared.
Figure 2.
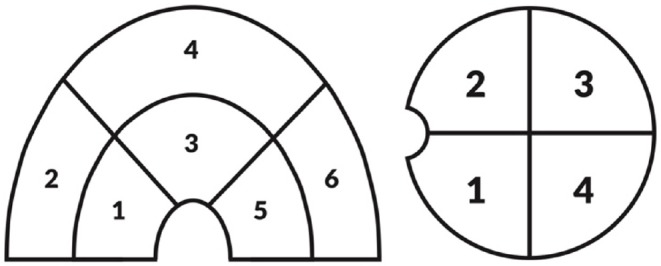
For each zone, the surgeon was requested to describe the worst chondromalacia for each section involved. Six anatomic locations were defined in the acetabulum: anterior central (1) anterior peripheral (2), superolateral central (3), superolateral peripheral (4), posterior central (5) and posterior peripheral (6). For the femoral head 4 quadrants were established as 1: posterolateral, 2: anterolateral, 3: anteromedial and 4: posteromedial.
Statistical analysis
Data are presented as means with 95% CIs when normally distributed and as medians with interquartile ranges (IQRs) when not normally distributed. Shapiro-Wilk test was used for normality testing. For comparing medians, t-test and for medians, U Mann-Whitney test was used. Occurrences were compared with a chi-square test with appropriate corrections, when necessary. The p value of < 0.05 was considered statistically significant. All calculations were performed in Statistica 13.1 software package (StatSoft Inc., Tulsa, OK).
Results
Demographics and history
From a total of 286, 11 patients (11 hips, 4%) were disqualified from the PAO due to the findings of hip arthroscopy. These included advanced articular cartilage lesions in all cases. There were nine females and two males with median age of 42.3 years (IQR, 38.1-46.8). The HA +PAO comparison group was comprised of 245 females and 30 males with a median age of 24 years (IQR 19-34). Further demographic data and data from patients’ history, including BMI, previous hip surgery and pain chronicity, are provided in Table 1. Patients in the study group (PAO contraindicated) were significant older than patients in the HA+PAO group (U Mann Whitney test, p<0.001).
Patient reported outcomes
No significant difference was observed in pre-operative clinical scores. Mean preoperative mHHS for the HA group was 61.1 (95% CI, 55.2-67) vs 57.5 (95% CI, 13.2-100) for HA+PAO (p=0.44). Other baseline patient-reported outcomes also did not differ statistically (Table 2).
Radiological characteristics
The study group had significantly more severe acetabular deformity than the comparison. Additionally the study group had a lower median lateral center-edge angle of 12.9° (IQR 5.6° - 14.7°), compared to the median 17.7° (IQR 14.7° - 20.4°) in the comparison group (p=0.001). When comparing IQRs, only 25% of the study patients and the majority (75%) of the comparison group patients had LCEA larger than 12.9°. Significantly less anterior coverage (median ACE 14.4°; IQR 8.5° - 21.4°) was observed in the study group, compared to the comparison group (median ACE of 20.3°, IQR 14.5° - 25.6°; p=0.021). Other radiographic parameters did not differ significantly and are provided in Table 3.
Table 3.
Radiographic Data for the 286 Patients
| Parameter | PAO Contraindicated (n=11) | HA+PAO (n=275) | Significance level |
|---|---|---|---|
| AP min. JSW | 4.2 (3.2 - 4.9) | 4 (3.6 - 4.5) | p=0.674 |
| LCEA | 12.9 (5.6 - 14.7) | 17.7 (14.7-20.4) | p=0.001 |
| AI | 18.6 (9.1 - 22.8) | 13.6 (10.9 - 16.2) | p=0.070 |
| ACE | 14.4 (8.5 - 21.4) | 20.3 (14.5 - 25.6) | p=0.021 |
| Tönnis grade | p=0.087 | ||
| 0 | 5 | 192 | |
| 1 | 6 | 83 |
AP min. JSW – minimal joint space width on the AP radiographic view; LCEA – lateral center-edge angle; AI – acetabular index; ACE – anterior center-edge angle
Magnetic resonance imaging
The prevalence of labral tears, paralabral cysts and chondrosis in the MRI did not differ between two groups. Details are provided in Table 4 and 5.
Table 4.
Magnetic Resonance Imaging Findings
| parameter | PAO Contraindicated (n=11) | HA+PAO ( n=275) | significance level |
|---|---|---|---|
| labral tear | p=0.295 | ||
| present | 6 | 191 | |
| absent | 5 | 84 | |
| paralabral cysts | p=0.519 | ||
| present | 0 | 10 | |
| absent | 11 | 265 | |
| chondrosis any location in | p=0.265 | ||
| present | 3 | 22 | |
| absent | 8 | 253 | |
| chondrosis of the femoral head | p=0.640 | ||
| present | 2 | 14 | |
| absent | 9 | 261 | |
| chondrosis of the acetabulum | p=0.232 | ||
| present | 2 | 22 | |
| absent | 9 | 253 |
Table 5.
Acetabular Cartilage Disease in 286 Patients
| location / compartment | PAO Contraindicated (n=11) | HA+PAO (n=275) | significance level |
|---|---|---|---|
| acetabular cartilage | |||
| in any region: | p=0.329 | ||
| chondromalacia present | 11 | 253 | |
| chondromalacia absent | 0 | 22 | |
| anterior central | p=0.022 | ||
| 2-3 | 0 | 24 | |
| 4-5 | 3 | 6 | |
| anterior peripheral | p=0.013 | ||
| 2-3 | 2 | 133 | |
| 4-5 | 4 | 39 | |
| superolateral central | p=0.347 | ||
| 2-3 | 1 | 13 | |
| 4-5 | 2 | 3 | |
| superolateral peripheral | p=0.041 | ||
| 2-3 | 5 | 187 | |
| 4-5 | 5 | 53 | |
| posterior central | N/A | ||
| 2-3 | 0 | 4 | |
| 4-5 | 0 | 1 | |
| posterior peripheral | p=0.783 | ||
| 2-3 | 3 | 28 | |
| 4-5 | 1 | 2 | |
For Each Location, the Number of Patients with Grades 1-3 and Grades 4-5 are Provided. Occurrences were Compared with Chi-Square Test, and Chi-Square Test with Yates Correction for Continuity when any of the Expected Counts was Smaller than 5
Intraoperative findings
All of the acetabula in the study group showed chondromalacia, which was also present in 92% of the hips in the comparison group (p=0.329). The study group cases had more advanced cartilage disease (grades 4 and 5, representing cleavage and defects) compared to milder lesions (grades 2 and 3, representing malacia and debonding), in anterior central, anterior peripheral and superolateral peripheral regions of the acetabulum. Seven (64%) out of 11 patients in the study group had femoral head chondromalacia, compared to 52 (17%) of the comparison group patients (p<0.001). Detailed comparisons are given in Table 6.
Table 6.
Femoral Cartilage Disease in 286 Patients
| location / compartment | PAO Contraindicated (n=11) | HA+PAO (n=275) | significance level | |
|---|---|---|---|---|
| femoral head cartilage | ||||
| in any region: | p<0.001 | |||
| chondromalacia present | 7 | 52 | ||
| chondromalacia absent | 4 | 223 | ||
| posterolateral | p=0.283 | |||
| 2-3 | 1 | 10 | ||
| 4-5 | 5 | 15 | ||
| anterolateral | p=0.684 | |||
| 2-3 | 1 | 15 | ||
| 4-5 | 3 | 15 | ||
| anteromedial | p=0.769 | |||
| 2-3 | 1 | 9 | ||
| 4-5 | 4 | 14 | ||
| posteromedial | p=0.467 | |||
| 2-3 | 1 | 7 | ||
| 4-5 | 5 | 15 | ||
Labral tear was diagnosed intraoperatively in all study group patients and in 99% (271/275) of the comparison group patients (p=0.854). Labrum morphology was normal in only two disqualified PAO patients and 140 HA+PAO patients, in the remaining it was either hypertrophic or ossified (p=0.033 ).
Discussion
The most significant finding of this study was that findings at hip arthroscopy rarely provide a contraindication to PAO surgery. Only 4% of patients scheduled for combined HA/PAO were disqualified. These patients were significantly older and had radiographically more severe dysplasia. Disqualification was secondary to severe articular cartilage damage on both femoral and acetabular side.
Advanced age33 and severity of dysplasia34,35 have previously been proposed as a risk factor for advanced cartilage disease and failure following PAO.11,36,37 Still, in our cohort there were older patients that were not disqualified from PAO. Therefore, qualification for PAO cannot be based on the age alone. Additionally, Millis et al. showed that PAO would give satisfactory functional and pain scores in patients over age 40 having dysplastic hips with mild or no arthrosis.2 During mean follow-up of five years, they had to convert 12% of patients with preoperative Tönnis grade 1 and 27% of patients with preoperative Tönnis grade 2. Different from our study, none of their patients had preoperative MRI and none were treated with a concomitant HA. Therefore, it is possible that some of those failures were attributable to the articular cartilage damage already present at the time of PAO. Thus, in patients over 40, Garbuz et al. recommended direct articular cartilage assessment for older patients.38 Based on the results of our study, this recommendation seems well-founded.
Clinical results of PAO for the treatment of severe dysplasia have been promising, although in populations younger than in our study group.39 Unfortunately, neither hip arthroscopy, nor MRI results were available for analysis in those patients. Despite the fact that most of the patients in the study group displayed significant acetabular undercoverage, it is difficult to attribute disqualification to the increased severity of acetabular dysplasia. Additionally, patients in both groups had satisfactory congruence and joint space width, making them suitable candidates for PAO.1,11 Lastly, neither hip arthroscopy, nor MRI were performed before PAO in that study.
When considering both age and degree of acetabular dysplasia, no distinct pattern of those two risk factors could be eluded from the present study. Neither age, nor lateral undercoverage, nor combination of those two factors could predict the arthroscopic findings.
Most of the patients who were disqualified displayed femoral head chondromalacia. This might be an important threshold for aborting PAO. The subchondral bone exposure on the femoral head has been previously identified as a risk factor for progression of osteoarthritis after pelvic osteotomy.9 Horisberger et al. showed that if subchondral bone exposure is found in addition to the acetabular lesions, failure of hip arthroscopy ensues quickly.40 Streich et al. found chondral defects to be prognostic of failure in arthroscopic labral repair.41 Unfortunately, many of the studies available do not give any information about the cartilage damage at the femoral head.40
Success of PAO depends on the amount of preoperative osteoarthritis (OA), even though there is little evidence to guide the choice of cut-off point.10 In general, patients with no to little radiographic evidence of OA are considered the best candidate for PAO.3,42 There are currently three imaging modalities available for staging intraarticular damage prior to PAO. These include plain radiography, conventional MRI, and compositional MRI techniques.
Radiographs assess cartilage loss indirectly by measuring apparent joint space loss or morphologic changes caused by arthritis. Thus, they cannot detect early cartilage injuries. In this study, all preoperative radiographs of aborted cases were judged to be Tönnis grade 0 or 1. Therefore, plain radiography underestimated the extent of cartilage damage in the aborted cases.43
Conventional MRI allows visualization of the cartilage morphology. Even though it remains a good tool to identify labral damage, MRI has known limitations in detecting cartilage lesions.16 Importantly, a negative MRI studydoes not exclude important intra-articular pathology thatcan be identified and treated arthroscopically.43 This wasalso the case in this study, as the MRI results did notdiscern disqualified patients from the rest of the cohort.
Compositional MRI techniques analyze the content ofhyaline cartilage and may one day become biomarkers that would influence patient selection. In the last decade,multiple studies using delayed gadolinium-enhancedMRI of cartilage (dGEMRIC) suggest this type of MRImay be a better diagnostic tool to assess early OA in thedysplastic hip.45-46 Coronal dGEMRIC index44 and thenanterior index calculated based on anterior part of selectedsaggital cuts45 were proposed as a predictors of failure.These results can be considered preliminary at best, asthey both come from the same population. There is a needfor rigorous, multicenter prospective studies to establishthe usefulness of compositional MRI techniques in prediction of failure and/or patient selection for conservative orsurgical treatment.46
Since the preoperative imaging is not fully reliable forpatient selection, some surgeons perform PAO with hip arthrotomy to address intraarticular lesions and femoral head-neck deformities.47 Other surgeons consider performing diagnostic arthroscopy before PAO to address intraarticular damage.3,48 Good results have also been reported with hip arthroscopy, followed immediately by PAO during the same procedure.19,49 A concomitant arthroscopy at the time of a PAO allows more complete visualization of the intraarticular hip structures plus improved ability to address intraarticular pathology.9,14,50 However, it is not clear whether the added surgical time and risks result in improved outcomes,51 although short-term studies show no adverse outcomes and equivalent or improved results.49,52 Disadvantages of combined HA+PAO include increased operative time (by adding HA, but also by making PAO more difficult), possibility for fluid extravasation, and increased risk of capsular adhesions from intra-articular work.19,49,53,54
Our study has limitations. One is that the decision to abort the PAO was made at the discretion of the operating surgeon. Secondly, we could not provide exact cut-off values for the factors predicting intraoperative disqualification. We believe, though, that the recorded differences in patients’ characteristics have the potential to improve selection of patients who will benefit from PAO and help in the future identify patients who might otherwise be subjected to unnecessary hip-preserving surgery, instead of being offered a primary total hip replacement.
To conclude, our data indicates that intraoperative disqualification from PAO is rare, with only 4% of the whole cohort disqualified. Patients that have been disqualified from the PAO were significantly older and presented radiographically higher grades of dysplasia. In cases with strong suspicion of substantial intra-articular damage or questionable indications, HA performed before PAO helps identify hips in which advanced articular cartilage disease may diminish the predictability of the PAO.
Acknowledgments
Work by Marcin K. Wasko was partially supported by the Kosciuszko Foundation. The authors thank Mr. Richard Wimmer-Brown for his valuable comments.
References
- 1.Clohisy J., et al. Periacetabular osteotomy: a systematic literature review. Clinical orthopaedics and related research. 2009;467(8):2041–2052. doi: 10.1007/s11999-009-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millis M, et al. Periacetabular osteotomy for acetabular dysplasia in patients older than 40 years: a preliminary study. Clinical orthopaedics and related research. 2009;467(9):2228–2234. doi: 10.1007/s11999-009-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matheney T, et al. Intermediate to long-term results following the bernese periacetabular osteotomy and predictors of clinical outcome: surgical technique. The Journal of bone and joint surgery. American volume. 2010;92:115–129. doi: 10.2106/JBJS.J.00646. [DOI] [PubMed] [Google Scholar]
- 4.Clohisy J, et al. A systematic approach to the plain radiographic evaluation of the young adult hip. The Journal of bone and joint surgery. American volume. 2008;90(Suppl 4):47–66. doi: 10.2106/JBJS.H.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris-Hayes M, et al. Bony abnormalities of the hip joint: a new comprehensive, reliable and radiation-free measurement method using magnetic resonance imaging. Journal of hip preservation surgery. 2014;1(2):62–70. doi: 10.1093/jhps/hnu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klaue K, Wallin A., Ganz R. CT evaluation of coverage and congruency of the hip prior to osteotomy. Clinical orthopaedics and related research. 1988. pp. 15–25. [PubMed]
- 7.Papalia R, et al. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? International orthopaedics. 2012;36(5):903–914. doi: 10.1007/s00264-011-1443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid M, et al. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382–386. doi: 10.1148/radiol.2262020019. [DOI] [PubMed] [Google Scholar]
- 9.Fujii M, et al. Effect of intra-articular lesions on the outcome of periacetabular osteotomy in patients with symptomatic hip dysplasia. The Journal of bone and joint surgery. British volume. 2011;93(11):1449–1456. doi: 10.1302/0301-620X.93B11.27314. [DOI] [PubMed] [Google Scholar]
- 10.Siebenrock K, M. Leunig, R. Ganz. Peri-acetabular osteotomy: the Bernese experience. Instructional course lectures. 2001;50:239–245. [PubMed] [Google Scholar]
- 11.Steppacher S, et al. Mean 20-year followup of Bernese periacetabular osteotomy. Clinical orthopaedics and related research. 2008;466(7):1633–1644. doi: 10.1007/s11999-008-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasunaga Y, et al. The state of the articular cartilage at the time of surgery as an indication for rotational acetabular osteotomy. The Journal of bone and joint surgery. British volume. 2001;83(7):1001–1004. doi: 10.1302/0301-620x.83b7.12171. [DOI] [PubMed] [Google Scholar]
- 13.Ross J, et al. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. The American journal of sports medicine. 2011;39:72S–8S. doi: 10.1177/0363546511412320. [DOI] [PubMed] [Google Scholar]
- 14.Domb B.G., et al. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clinical Orthopaedics and Related Research. 2014;472(2):674–680. doi: 10.1007/s11999-013-3140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishii T, et al. Articular cartilage evaluation in osteoarthritis of the hip with MR imaging under continuous leg traction. Magnetic resonance imaging. 1998;16(8):871–875. doi: 10.1016/s0730-725x(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 16.Mintz D, et al. Magnetic resonance imaging of the hip: detection of labral and chondral abnormalities using noncontrast imaging. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2005;21(4):385–393. doi: 10.1016/j.arthro.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Safran M, S. Hariri. Hip arthroscopy assessment tools and outcomes. Operative techniques in orthopaedics. 2010;20(4):264–277. [Google Scholar]
- 18.Domb B.G., et al. Concomitant Hip Arthroscopy and Periacetabular Osteotomy. Arthroscopy - Journal of Arthroscopic and Related Surgery. 2015;31(11):2199–2206. doi: 10.1016/j.arthro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Domb B, et al. Concomitant Hip Arthroscopy and Periacetabular Osteotomy. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2015;31(11):2199–2206. doi: 10.1016/j.arthro.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Domb B, LaReau J., Redmond J. Combined hip arthroscopy and periacetabular osteotomy: indications, advantages, technique, and complications. Arthroscopy techniques. 2014;3(1):e95–e100. doi: 10.1016/j.eats.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, et al. Peri-acetabular rotational osteotomy with concomitant hip arthroscopy for treatment of hip dysplasia. The Journal of bone and joint surgery. British volume. 2011;93(6):732–737. doi: 10.1302/0301-620X.93B6.25809. [DOI] [PubMed] [Google Scholar]
- 22.Nunley R, et al. Clinical presentation of symptomatic acetabular dysplasia in skeletally mature patients. The Journal of bone and joint surgery. American volume. 2011;93:17–21. doi: 10.2106/JBJS.J.01735. [DOI] [PubMed] [Google Scholar]
- 23.Byrd J, Jones K. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2000;16(6):578–587. doi: 10.1053/jars.2000.7683. [DOI] [PubMed] [Google Scholar]
- 24.Amstutz H, et al. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. The Journal of bone and joint surgery. American volume. 1984;66(2):228–241. [PubMed] [Google Scholar]
- 25.Dunn D. Anteversion of the neck of the femur; a method of measurement. The Journal of bone and joint surgery. British volume. 1952;34(2):181–186. doi: 10.1302/0301-620X.34B2.181. [DOI] [PubMed] [Google Scholar]
- 26.Lequesne M. Le Faux profile du bassin. Nouvelle incidence radiographique pour l’etude de la hanche. Son utilite dans les dysplasies et les differentes coxopathies. Rev Rheum. 1961;28:643–652. [Google Scholar]
- 27.Clohisy J, et al. The frog-leg lateral radiograph accurately visualized hip cam impingement abnormalities. Clinical orthopaedics and related research. 2007;462:115–121. doi: 10.1097/BLO.0b013e3180f60b53. [DOI] [PubMed] [Google Scholar]
- 28.Wiberg G. Studies on dysplastic acetabula and congenital subluxation of the hip joint: with special reference to the complication of osteoarthritis. Acta Chir Scand. 1939;83(58):53–68. [Google Scholar]
- 29.Tönnis D. Congenital dysplasia and dislocation of the hip in children and adults. Springer Science & Business Media; 2012. [Google Scholar]
- 30.Altman R, et al. Measurement of structural progression in osteoarthritis of the hip: the Barcelona consensus group. Osteoarthritis and cartilage. 2004;12(7):515–524. doi: 10.1016/j.joca.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Byrd J. Hip arthroscopy in athletes. Instructional course lectures. 2002;52:701–709. [PubMed] [Google Scholar]
- 32.Beck M, et al. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. The Journal of bone and joint surgery. British volume. 2005;87(7):1012–1018. doi: 10.1302/0301-620X.87B7.15203. [DOI] [PubMed] [Google Scholar]
- 33.Anderson S, Loeser R. Why is osteoarthritis an age-related disease? Best practice & research. Clinical rheumatology. 2010;24(1):15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadley N, Brown T., Weinstein S. The effects of contact pressure elevations and aseptic necrosis on the long-term outcome of congenital hip dislocation. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1990;8(4):504–513. doi: 10.1002/jor.1100080406. [DOI] [PubMed] [Google Scholar]
- 35.Maxian T, Brown T., Weinstein S. Chronic stress tolerance levels for human articular cartilage: two nonuniform contact models applied to long-term follow-up of CDH. Journal of biomechanics. 1995;28(2):159–166. doi: 10.1016/0021-9290(94)00054-8. [DOI] [PubMed] [Google Scholar]
- 36.Ross J, et al. Patient and disease characteristics associated with hip arthroscopy failure in acetabular dysplasia. The Journal of arthroplasty. 2014;29(9 Suppl):160–163. doi: 10.1016/j.arth.2014.03.054. [DOI] [PubMed] [Google Scholar]
- 37.Hartig-Andreasen C, et al. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clinical Orthopaedics and Related Research. 2012;470(11):2978–2987. doi: 10.1007/s11999-012-2386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garbuz D, Awwad M., Duncan C. Periacetabular osteotomy and total hip arthroplasty in patients older than 40 years. The Journal of arthroplasty. 2008;23(7):960–963. doi: 10.1016/j.arth.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Clohisy J, et al. Periacetabular osteotomy for the treatment of severe acetabular dysplasia. The Journal of bone and joint surgery. American volume. 2005;87(2):254–259. doi: 10.2106/JBJS.D.02093. [DOI] [PubMed] [Google Scholar]
- 40.Horisberger M, Brunner A., Herzog R. Arthroscopic treatment of femoral acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy: the journal of arthroscopic & related surgery: official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2010;26(5):623–629. doi: 10.1016/j.arthro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Streich N, et al. Prognostic value of chondral defects on the outcome after arthroscopic treatment of acetabular labral tears. Knee surgery, sports traumatology, arthroscopy: official journal of the ESSKA. 2009;17(10):1257–1263. doi: 10.1007/s00167-009-0833-x. [DOI] [PubMed] [Google Scholar]
- 42.Murphy S, Deshmukh R. Periacetabular osteotomy: preoperative radiographic predictors of outcome. Clinical orthopaedics and related research. 2002. pp. 168–174. [PubMed]
- 43.Keeney J, et al. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clinical orthopaedics and related research. 2004. pp. 163–169. [DOI] [PubMed]
- 44.Cunningham T, et al. Delayed gadolinium-enhanced magnetic resonance imaging of cartilage to predict early failure of Bernese periacetabular osteotomy for hip dysplasia. The Journal of bone and joint surgery. American volume. 2006;88(7):1540–1548. doi: 10.2106/JBJS.E.00572. [DOI] [PubMed] [Google Scholar]
- 45.Kim S, et al. Anterior delayed gadolinium-enhanced MRI of cartilage values predict joint failure after periacetabular osteotomy. Clinical orthopaedics and related research. 2012;470(12):3332–3341. doi: 10.1007/s11999-012-2519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zilkens C, et al. Current knowledge and importance of dGEMRIC techniques in diagnosis of hip joint diseases. Skeletal radiology. 2015;44(8):1073–1083. doi: 10.1007/s00256-015-2135-3. [DOI] [PubMed] [Google Scholar]
- 47.Nassif N, et al. Periacetabular osteotomy and combined femoral head-neck junction osteochondroplasty: a minimum two-year follow-up cohort study. The Journal of bone and joint surgery. American volume. 2012;94(21):1959–1966. doi: 10.2106/JBJS.K.01038. [DOI] [PubMed] [Google Scholar]
- 48.Vendittoli P, et al. Acetabular rim lesions: arthroscopic assessment and clinical relevance. International orthopaedics. 2012;36(11):2235–2241. doi: 10.1007/s00264-012-1595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricciardi B, et al. Patient Characteristics and Early Functional Outcomes of Combined Arthroscopic Labral Refixation and Periacetabular Osteotomy for Symptomatic Acetabular Dysplasia. The American journal of sports medicine. 2016;44(10):2518–2525. doi: 10.1177/0363546516651829. [DOI] [PubMed] [Google Scholar]
- 50.Jackson T.J., et al. Periacetabular osteotomy and arthroscopic labral repair after failed hip arthroscopy due to iatrogenic aggravation of hip dysplasia. Knee Surgery, Sports Traumatology, Arthroscopy. 2014;22(4):911–914. doi: 10.1007/s00167-013-2540-x. [DOI] [PubMed] [Google Scholar]
- 51.Kim K.I., et al. Peri-acetabular rotational osteotomy with concomitant hip arthroscopy for treatment of hip dysplasia. Journal of Bone and Joint Surgery - Series B. 2011;93(B(6)):732–737. doi: 10.1302/0301-620X.93B6.25809. [DOI] [PubMed] [Google Scholar]
- 52.Ginnetti J, et al. Prevalence and treatment of intraarticular pathology recognized at the time of periacetabular osteotomy for the dysplastic hip. Clinical orthopaedics and related research. 2013;471(2):498–503. doi: 10.1007/s11999-012-2602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters C.L., Sierra R.J. Report of breakout session: Intraarticular work during periacetabular osteotomy-simultaneous arthrotomy or hip arthroscopy? Clinical Orthopaedics and Related Research. 2012;470(12):3456–3458. doi: 10.1007/s11999-012-2414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinzpeter J, et al. Fluid Extravasation Related to Hip Arthroscopy: A Prospective Computed Tomography-Based Study. Orthopaedic journal of sports medicine. 2015;3(3):2325967115573222–2325967115573222. doi: 10.1177/2325967115573222. [DOI] [PMC free article] [PubMed] [Google Scholar]


