Abstract
Background:
Microsurgical reconstruction is indicated for infants with brachial plexus birth palsy (BPBP) that demonstrate limited spontaneous neurological recovery. This investigation defines the demographic, perinatal, and physical examination characteristics leading to microsurgical reconstruction.
Methods:
Infants enrolled in a prospective multicenter investigation of BPBP were evaluated. Microsurgery was performed at the discretion of the treating provider/center. Inclusion required enrollment prior to six months of age and follow-up evaluation beyond twelve months of age. Demographic, perinatal, and examination characteristics were investigated as possible predictors of microsurgical reconstruction. Toronto Test scores and Hospital for Sick Children Active Movement Scale (AMS) scores were used if obtained prior to three months of age. Univariate and multivariate logistic regression analyses were performed.
Results:
365 patients from six regional medical centers met the inclusion criteria. 127 of 365 (35%) underwent microsurgery at a median age of 5.4 months, with microsurgery rates and timing varying significantly by site. Univariate analysis demonstrated that several factors were associated with microsurgery including race, gestational diabetes, neonatal asphyxia, neonatal intensive care unit admission, Horner’s syndrome, Toronto Test score, and AMS scores for finger/thumb/wrist flexion, finger/thumb extension, wrist extension, elbow flexion, and elbow extension. In multivariate analysis, four factors independently predicted microsurgical intervention including Horner’s syndrome, mean AMS score for finger/thumb/ wrist flexion <4.5, AMS score for wrist extension <4.5, and AMS score for elbow flexion <4.5. In this cohort, microsurgical rates increased as the number of these four factors present increased from zero to four: 0/4 factors = 0%, 1/4 factors = 22%, 2/4 factors = 43%, 3/4 factors = 76%, and 4/4 factors = 93%.
Conclusions:
In patients with BPBP, early physical examination findings independently predict microsurgical intervention. These factors can be used to provide counseling in early infancy for families regarding injury severity and plan for potential microsurgical intervention.
Level of Evidence: Prognostic Level I
Keywords: palsy, plexus, obstetric, brachial, birth
Introduction
Brachial plexus birth palsy (BPBP) refers to paralysis of the upper extremity secondary to a traction or compression injury sustained to the brachial plexus during birth. The incidence of BPBP is approximately 0.9 to 2.0 out of 1,000 live births.1-3 Risk factors include shoulder dystocia, macrosomia, difficult or instrumented delivery, and breech presentation.1 BPBP varies in severity and the extent of plexus involvement, ranging from transient neuropraxia to complete cervical nerve root avulsion of part or all of the brachial plexus.4-6
The natural history of BPBP remains largely unknown, due to the paucity of studies of affected patients followed from birth to maturity. Historically, 80 to 90% of patients were thought to demonstrate spontaneous recovery within the first 2 months of life, with subsequent normal upper extremity function.2,3,5,7-10 More recent investigation has suggested a much lower recovery rate, with only 66% of affected children recovering completely and 10% to 30% with considerable permanent weakness.11,12 The variation in reported recovery rates may correlate with regional differences in injury severity, referral patterns, and treatment strategies.
Patients with persistent neurologic deficits after 3 to 6 months of age are at high risk for permanent neurologic dysfunction.3,6,8,10,13-15 Microsurgical reconstruction with nerve grafting or transfers is indicated for infants that demonstrate limited spontaneous neurological recovery. Traditionally, return of antigravity biceps function by 3 to 6 months of age has been used to prognosticate long-term neurological recovery. In a small retrospective series, Michelow et al. reported that use of biceps function alone incorrectly predicted recovery in 12.8% of cases.10 When elbow flexion as well as elbow, wrist, thumb, and finger extension at 3 months were combined into a single test score (the Toronto Test score), the proportion of patients whose recovery was incorrectly predicted was reduced to 5.2 percent.10 Fisher et al. have also reported that elbow flexion alone cannot adequately predict spontaneous neurological recovery in BPBP.16
This investigation seeks to define the demographic, perinatal, and early physical examination characteristics of infants which led to microsurgical reconstruction in a large prospective multicenter study. We hypothesize that physical examination factors, but not demographic and perinatal factors, will independently predict microsurgical intervention in children with BPBP.
Materials and Methods
A prospective multicenter cohort study of infants with BPBP was performed by the TOBI (Treatment and Outcomes of Brachial Plexus Injuries) Study Group, comprised of six regional medical centers. This investigation was performed as a secondary study aim, with the primary study aim directed towards determining the optimal timing for microsurgical intervention. Inclusion criteria for this investigation required enrollment prior to six months of age and follow-up evaluation beyond twelve months of age. In this cohort, microsurgery was performed at the discretion of the treating provider at each institution. The distributions of age at microsurgery at the three sites with at least thirty microsurgical procedures were compared with the Kruskal-Wallis test.
Demographic, perinatal, and physical examination characteristics were investigated as possible predictors of microsurgical reconstruction. The demographic and perinatal factors analyzed included gender, race (white, black, other), gravidity (1, 2, ≥3), parity (1, 2, ≥3), presence of gestational diabetes, history of preeclampsia, history of previous difficult delivery, duration of labor (0-4, 5-10, 11-16, ≥17 hours), type of obstetric provider (obstetrician, midwife), fetal presentation (vertex, breech, face), type of delivery (uncomplicated, difficult, forceps/vacuum extraction, Cesarean section), gestational age (≤37, 38 40, ≥41 weeks), birth weight (≤4000, 4000-4500, >4500 grams), Apgar scores at 1 and 5 minutes, neonatal asphyxia, respiratory complications, and neonatal intensive care unit (NICU) admission. Since the rate of microsurgery, demographic, and perinatal factors were expected to vary by site of care, these demographic and perinatal factors were examined for confounding by site of care. For all demographic and perinatal factors except Apgar scores, logistic models were used to test the association between microsurgery and each characteristic, with and without adjusting for site. Factors were screened for consideration in multivariable models by identifying those with p values <0.10 either in unadjusted analysis or when adjusted only for site. The logistic models were also used to estimate odds ratios (OR) and 95% confidence intervals (CI). Apgar scores were treated as continuous factors and a two sample Wilcoxon test was used to compare the Apgar distributions between infants who did and did not undergo microsurgical reconstruction. The Van Elteren test, a stratified version of the Wilcoxon test, was subsequently used to control these comparisons for site of care. The demographic and neonatal factors were then evaluated with multivariable logistic regression.
The physical examination characteristics assessed included presence of a Horner’s syndrome, maximum Toronto Test score in the first three months of life (<3.5, ≥3.5), 10 and maximum Hospital for Sick Children Active Movement Scale (AMS) scores for finger, thumb, wrist, and elbow flexion and extension in the first three months of life.17 Of note, both a logistic regression analysis and a factor analysis of the eight studied AMS scores suggested the following five summary scores: hand/wrist flexion (average of finger, thumb, and wrist flexion scores), hand extension (average of finger and thumb extension scores), wrist extension, elbow flexion, and elbow extension. Each of these summary scores was dichotomized at <4.5 versus ≥4.5, corresponding to no motion against gravity versus motion against gravity. For all physical examination factors, logistic models were used to test the association between microsurgery and each characteristic, with and without adjusting for site. The logistic models were also used to estimate odds ratios and 95% confidence intervals. The physical examination factors were then evaluated with multivariable logistic regression.
Finally, the demographic, perinatal, and physical examination characteristics were combined in a single multivariable logistic regression model to assess whether each factor contributed unique information toward predicting which patients would undergo microsurgery. The final model was illustrated by tabulating the percentages of patients undergoing microsurgery separately for patients with 0, 1, 2, 3, or 4 of the 4 factors in the model.
In all of these analyses, patients with missing values on particular variables were excluded from analyses of those variables, two sided tests were performed, and p-values less than 0.05 were considered statistically significant.
Results
365 infants with BPBP met the inclusion criteria including 165 (45%) males and 200 (55%) females. The median age at initial evaluation for these patients was 2.2 months and 246 (67%) were examined within the first three months of life.
Rate and Timing of Microsurgery
127 (35%) infants underwent brachial plexus reconstruction, with microsurgery rates varying significantly by site (p < 0.0001) (Table 1). The median age at microsurgery was 5.4 months (interquartile range 3.8 to 7.5); however, the age distribution differed significantly between sites (p=0.003). Figure 1 shows the distribution at the three sites with at least 30 procedures. Microsurgical procedures at Site 1 were performed over a narrow age interval, generally between the fifth and eighth months of life, whereas procedures at Site 3 and Site 4 were often performed earlier, during the fourth and fifth months of life.
Table 1.
Microsurgery by Site of Care
| Site | #/N | (%) |
|---|---|---|
| 1 | 31/163 | (19%) |
| 2 | 3/10 | (30%) |
| 3 | 48/78 | (62%) |
| 4 | 41/65 | (63%) |
| 5 | 3/35 | (9%) |
| 6 | 1/14 | (7%) |
| All Sites | 127/365 | (35%) |
Figure 1.
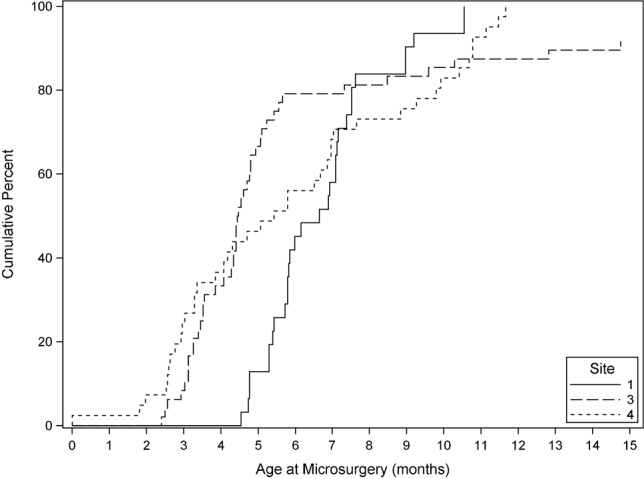
Distribution of microsurgical procedures over time. Cumulative percent of microsurgical procedures performed as a function of age by site of care. Displayed plot is truncated at 15 months, but procedures were performed at later ages at site 3. The sites not shown had three or fewer microsurgical procedures each. Microsurgical procedures at Site 1 were performed over a narrow age interval, generally between the fifth and eighth months of life, whereas procedures at Site 3 and Site 4 were often performed earlier, during the fourth and fifth months of life. Microsurgery at Site 3 was generally performed prior to 5 months although there were several procedures performed at much later ages. While some microsurgical procedures at Site 4 were performed at early ages, the timing was spread relatively evenly through 12 months of age.
Analysis of Demographic and Perinatal Factors
In preliminary analysis, we identified demographic and perinatal factors demonstrating an association with microsurgery at a significance level of α = 0.10 either in completely unadjusted analysis or adjusted only for site (Table 2). When these demographic and perinatal factors were considered in a multivariable logistic regression model, only gestational diabetes (OR 1.83, p = 0.04) and neonatal asphyxia (OR 1.83, p =0.02) were significant predictors of microsurgery (Table 3). Patients with gestational diabetes and/ or neonatal asphyxia had a microsurgical rate of 48% (62/130), compared to 29% (60/208) for patients with neither.
Table 2.
Microsurgery by Demographic and Perinatal Characteristics.
Only characteristics with unadjusted or adjusted p values <.10 are shown.
| Characteristic | #/N | (%) | Unadjusted | Adjusted for Site | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||||
| All | (N=365) | 127/335 | (35%) | ||||
| Race | (N=304) | ||||||
| White | 68/220 | (31%) | 1.0 | .002 | 1.0 | .14 | |
| Black | 33/59 | (56%) | 2.84(1.58, 5.11) | 1.86(0.95, 3.66) | |||
| Other | 8/25 | (32%) | 1.05(0.43, 2.56) | 1.75(0.62, 4.93) | |||
| Gestational Diabetes | (N=354) | ||||||
| No | 94/290 | (32%) | 1.0 | .02 | 1.0 | .02 | |
| Yes | 31/64 | (48%) | 1.96(1.13, 3.39) | 2.08(1.11, 3.90) | |||
| Hx Difficult Delivery | (N=348) | ||||||
| No | 91/281 | (32%) | 1.0 | .06 | 1.0 | .22 | |
| Yes | 30/67 | (45%) | 1.69(0.98, 2.91) | 1.48(0.80, 2.73) | |||
| Obstetric Provider | (N=328) | ||||||
| Obstetrician | 102/291 | (35%) | 1.0 | .06 | 1.0 | .05 | |
| Midwife | 7/37 | (19%) | 0.43(0.18, 1.02) | 0.40(0.16, 1.02) | |||
| Neonatal Asphyxia | (N=344) | ||||||
| No | 81/256 | (32%) | 1.0 | .007 | 1.0 | .001 | |
| Yes | 42/88 | (48%) | 1.97(1.20, 3.23) | 2.64(1.46, 4.75) | |||
| Respiratory Issues | (N=347) | ||||||
| No | 69/217 | (32%) | 1.0 | .09 | 1.0 | .25 | |
| Yes | 53/130 | (41%) | 1.48(0.94, 2.32) | 1.35(0.81, 2.25) | |||
| NICU Admission | (N=352) | ||||||
| No | 72/239 | (30%) | 1.0 | .02 | 1.0 | .01 | |
| Yes | 49/113 | (43%) | 1.78(1.12, 2.82) | 2.03(1.19, 3.47) | |||
| Birth A Weight (g) ≤4000 | (N=362) | 50/152 | (33%) | 1.0 | .39 | 1.0 | .04 |
| 4000-4500 | 43/128 | (34%) | 1.03(0.63, 1.70) | 1.11(0.63, 1.95) | |||
| >4500 | 34/82 | (41%) | 1.44(0.83, 2.52) | 2.27(1.17, 4.38) | |||
Table 3.
Multivariable Model PredictingMicrosurgery from Demographic and PerinatalCharacteristics (N=388)
| Characteristic | Unadjusted | Adjusted for Site | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Gestational Diabetes | ||||
| No | 1.0 | .04 | 1.0 | .04 |
| Yes | 1.83(1.04, 3.21) | 1.97(1.02, 3.78) | ||
| Neonatal Asphyxia | ||||
| No | 1.0 | .02 | 1.0 | .003 |
| Yes | 1.83(1.11, 3.02) | 2.64(1.36, 4.48) | ||
Analysis of Physical Examination Factors
Univariate analysis suggested that all selected physical examination factors had strong associations with microsurgery (Table 4), and remained statistically significant when adjusting for site of care. When these physical examination factors were considered in a multivariable logistic regression model, only presence of Horner’s syndrome (OR 4.43, p =0.04), AMS score for hand/wrist flexion (OR 4.57, p =0.008), AMS score for wrist extension (OR 2.77, p =0.004), and AMS score for elbow flexion (OR 11.32, p =0.02) were statistically significant predictors of microsurgery (Table 4).
Table 4.
Microsurgery by Physical Examination Factorsa
| Characteristic | #/N | (%) | Unadjusted | Multivariable Model (N=224) | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |||
| All (N=365) | 127/335 (35%) | |||||
| AMS Score Hand/Wrist Flexionb (N=226) | ||||||
| ≥4.5 | 49/194 (25%) | 1.0 | <.001 | 1.0 | 0.008 | |
| <4.5 | 27/32 (84%) | 15.98 (5.83, 43.77) | 4.57 (1.49, 14.04) | |||
| AMS Score Hand Extensionc (N=232) | ||||||
| ≥4.5 | 31/153 (20%) | 1.0 | <.001 | |||
| <4.5 | 46/79 (58%) | 5.49 (3.02, 9.96) | ||||
| AMS Score Wrist Extension (N=232) | ||||||
| ≥4.5 | 23/135 (17%) | 1.0 | <.001 | 1.0 | 0.004 | |
| <4.5 | 55/97 (57%) | 6.38 (3.49, 11.64) | 2.77 (1.38, 5.57) | |||
| AMS Score Elbow Flexion (N=234) | ||||||
| ≥4.5 | 1/39 (3%) | 1.0 | 0.002 | 1.0 | 0.02 | |
| <4.5 | 77/195 (39%) | 24.79 (3.34, 184.3) | 11.32 (1.48, 86.49) | |||
| AMS Score Elbow Extension (N=233) | ||||||
| ≥4.5 | 29/141 (21%) | 1.0 | <.001 | |||
| <4.5 | 49/92 (53%) | 4.40 (2.47, 7.85) | ||||
| Toronto Score (N=227) | ||||||
| ≥3.5 | 38/156 (24%) | 1.0 | <.001 | |||
| <3.5 | 48/73 (66%) | 5.96 (3.25, 10.93) | ||||
| Horner’s Syndrome (N=359) | ||||||
| No | 101/326 (31%) | 1.0 | <.001 | 1.0 | 0.04 | |
| Yes | 26/33 (79%) | 8.27 (3.48, 19.69) | 4.43 (1.10, 17.78) | |||
AMS and Toronto Test scores represent maximum score in the first three months of life
Average of individual AMS scores finger, thumb, and wrist flexion
Average of individual AMS scores for finger and thumb extension
Combined Analysis of Demographic, Perinatal, and Physical Examination Factors
The demographic, perinatal, and physical examination factors were combined in a single regression model to assess which factors contributed unique information toward predicting which infants underwent microsurgical reconstruction. The perinatal factors that were statistically significant when analyzed independent of the physical examination factors were not significant in this global model. This analysis suggested that only the physical examination factors presented above (multivariable model, Table 4) were statistically significant predictors of microsurgery, including presence of Horner’s syndrome, AMS score for hand/wrist flexion, AMS score for wrist extension, and AMS score for elbow flexion. Microsurgical rates increased as the number of these four factors present increased from zero to four: 0/4 factors = 0%, 1/4 factors = 22%, 2/4 factors = 43%, 3/4 factors = 76%, and 4/4 factors = 93% (Table 5).
Table 5.
Microsurgery by Number of Exam Factors
| # of Factors | #/N | (%) |
|---|---|---|
| 0 | 0/35 | (0%) |
| 1 | 21/95 | (22%) |
| 2 | 25/58 | (43%) |
| 3 | 16/21 | (76%) |
| 4 | 14/15 | (93%) |
| All | (34%) | |
Discussion
In this multicenter prospective cohort of infants with BPBP, several early physical examination findings independently predicted microsurgical brachial plexus reconstruction. Although perinatal factors, including gestational diabetes and neonatal asphyxia, were also associated with microsurgery, these factors demonstrate no independent predictive value when physical examination characteristics are available in clinical decision-making. In this cohort, nearly half of patients with a history of asphyxia and/or maternal history of gestational diabetes underwent microsurgical reconstruction. Interestingly, one-third of infants without a history of either still underwent microsurgical reconstruction. This finding is supported by an investigation performed by Al-Qattan et al which demonstrated no difference in recovery among infants of diabetic and non-diabetic mothers.18
Among physical examination characteristics, Horner’s syndrome as well as antigravity elbow flexion, wrist extension, and finger/thumb/wrist flexion prior to three months of age were the only factors to demonstrate independent predictive value. Since Horner’s syndrome is highly associated with preganglionic injury and poor spontaneous recovery,5,9,19 it logically follows that presence of a Horner’s syndrome was strongly associated with future microsurgical reconstruction. This finding corresponds with the consensus that microsurgical reconstruction should be undertaken for infants with global lesions and Horner’s syndrome, by 3 months of age.4,6,8,9,20 Interestingly, in this investigation, the Toronto Test score demonstrated no independent predictive value when considered as either a continuous or a dichotomous variable. In contrast, AMS scores for elbow flexion, wrist extension, and finger/ thumb/wrist flexion prior to three months of age did independently predict future microsurgical intervention.
Of all factors, the presence or absence of antigravity elbow flexion prior to three months of age was the most predictive of future microsurgical reconstruction, suggesting that surgeons continue to utilize early return of biceps function as a marker of plexus recovery of from birth injuries. Multiple prior investigations suggest that infants with return of biceps function after 3 months rarely experience complete recovery without some persistent limitations in strength or motion. Global shoulder function worsens with increasing delay in return of biceps function,15 as suggested by lower Mallet scores found in patients who recovered function after 6 months of life, compared with those who recovered between 4-6 months.21 Interestingly, the average AMS score for finger, thumb, and wrist flexion—functions not scored by the Toronto Test—independently predicted future microsurgical reconstruction. Since these motor functions correspond to the C7 and C8 nerve roots, lower AMS scores suggest involvement of the middle and lower trunk. As noted, the prognostic value of the average AMS score for these functions is also supported by the general consensus that infants with Horner’s syndrome or global injuries be targeted for early microsurgical reconstruction.4,6,8,9,20
The data from this cohort suggests that physical examination characteristics may be used to provide counseling in early infancy for families regarding injury severity and plan for potential microsurgical intervention. A previous investigation by Bae et al. suggests that there is excellent intraobserver and interobserver reliability for use of the Hospital for Sick Children Active Movement Scale and Toronto Test Score,22 which supports reliance on physical examination for indicating microsurgical intervention. Since these classification systems grade strength with gravity eliminated, they are particularly useful for infants who cannot follow commands and, therefore, can only be prompted and observed.
The value of early counseling for families cannot be understated. More than 80% of parents utilize the internet to learn more about treatment and surgical options.23 Counseling families as they embark on this process of self-education may improve understanding of the condition and increase the integrity of shared-decision making. Since early physical examination factors are used to indicate microsurgical intervention, referral of infants with persistent brachial plexus birth palsy to specialized treatment centers is recommended prior to two months of age.
The data analysis in this investigation was supported by prospective multicenter design, which permitted the enrollment of a large number of patients and the inclusion of 365 subjects. The large sample size permitted robust statistical analysis of broad set of demographic, perinatal, and physical examination characteristics when evaluating which factors were most predictive of microsurgical reconstruction. The multicenter design combined with a sample size large enough to be adjusted for site of care was a strength of this study, as the investigation was able to identify salient predictive characteristics across sites that varied with respect to racial composition, injury severity, and treatment paradigms.
The study had several important limitations. Although the study was designed to initiate enrollment in the first few months of life, only 66% of the subjects eligible for this analysis were enrolled prior to 3 months. As we were interested in the predictive value of physical examination characteristics present in the first three months of life, this data was not available for a substantial subset of patients. Moreover, missing data points limited comparisons, particularly when multivariate analyses were performed. We had to exclude 141 subjects from our final multivariable model because of missing data on one or more physical exam factors.
In this series, the four independently predictive physical examination characteristics were associated with a stepwise increase of about 20% to 30% in the rate of microsurgery with the addition of each additional factor. However, this result needs validation in a different set of patients to determine whether microsurgery can be predicted at three months of age. Nonetheless, awareness of perinatal and physical examination characteristics most predictive for microsurgery allows clinicians to focus on the most salient patient characteristics. This awareness can guide clinical decision-making for providers engaged with this unique, complex patient population.
One of the most controversial elements of brachial plexus management remains the timing of surgery for patients with post-ganglionic injuries, in which there are varying degrees of severity of injury and recovery. Although this investigation does not elucidate the optimal timing of microsurgical reconstruction, it does provide clinicians additional insights at an early age regarding which patients are likely candidates for microsurgical reconstruction, thereby permitting planning for microsurgical reconstruction and providing appropriate time for counseling and discussion with families.
References
- 1.DeFrancesco CJ, Shah DK, Rogers BH, et al. The epidemiology of brachial plexus birth palsy in the United States: declining incidence and evolving risk factors. J Pediatr Orthop. e-published ahead of print. [DOI] [PubMed]
- 2.Greenwald AG, Schute PC, Shiveley JL. Brachial plexus birth palsy: a 10-year report on the incidence and prognosis. J Pediatr Orthop. 1984;4(6):689–692. doi: 10.1097/01241398-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Hardy AE. Birth injuries of the brachial plexus: incidence and prognosis. J Bone Joint Surg Br. 1981;63-B(1):98–101. doi: 10.1302/0301-620X.63B1.7204481. [DOI] [PubMed] [Google Scholar]
- 4.Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg Am. 2010;35(2):322–331. doi: 10.1016/j.jhsa.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Narakas AO. [Injuries of the brachial plexus and neighboring peripheral nerves in vertebral fractures and other trauma of the cervical spine] Orthopade. 1987;16(1):81–86. [PubMed] [Google Scholar]
- 6.Waters PM. Obstetric Brachial Plexus Injuries: Evaluation and Management. J Am Acad Orthop Surg. 1997;5(4):205–214. doi: 10.5435/00124635-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Adler JB, Patterson RL. Erb’s palsy. Long-term results of treatment in eighty-eight cases. J Bone Joint Surg Am. 1967;49(6):1052–1064. [PubMed] [Google Scholar]
- 8.Gilbert A, Tassin JL. [Surgical repair of the brachial plexus in obstetric paralysis] Chirurgie. 1984;110(1):70–75. [PubMed] [Google Scholar]
- 9.Laurent JP, Lee R, Shenaq S, Parke JT, et al. Neurosurgical correction of upper brachial plexus birth injuries. J Neurosurg. 1993;79(2):197–203. doi: 10.3171/jns.1993.79.2.0197. [DOI] [PubMed] [Google Scholar]
- 10.Michelow BJ, Clarke HM, Curtis CG, et al. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93(4):675–680. discussion 681. [PubMed] [Google Scholar]
- 11.Foad SL, Mehlman CT, Foad MB, et al. Prognosis following neonatal brachial plexus palsy: an evidence-based review. J Child Orthop. 2009;3(6):459–463. doi: 10.1007/s11832-009-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeksma AF, ter Steeg AM, Nelissen RG, et al. Neurological recovery in obstetric brachial plexus injuries: an historical cohort study. Dev Med Child Neurol. 2004;46(2):76–83. doi: 10.1017/s0012162204000179. [DOI] [PubMed] [Google Scholar]
- 13.Boome RS, Kaye JC. Obstetric traction injuries of the brachial plexus. Natural history, indications for surgical repair and results. J Bone Joint Surg Br. 1988;70(4):571–576. doi: 10.1302/0301-620X.70B4.3403599. [DOI] [PubMed] [Google Scholar]
- 14.Smith NC, Rowan P, Benson LJ, et al. Neonatal brachial plexus palsy. Outcome of absent biceps function at three months of age. J Bone Joint Surg Am. 2004;86-A(10):2163–2170. [PubMed] [Google Scholar]
- 15.Waters PM. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81(5):649–659. doi: 10.2106/00004623-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Fisher DM, Borschel GH, Curtis CG, et al. Evaluation of elbow flexion as a predictor of outcome in obstetrical brachial plexus palsy. Plast Reconstr Surg. 2007;120(6):1585–1590. doi: 10.1097/01.prs.0000282104.56008.cb. [DOI] [PubMed] [Google Scholar]
- 17.Curtis C, Stephens D, Clarke HM, et al. The active movement scale: an evaluative tool for infants with obstetrical brachial plexus palsy. J Hand Surg Am. 2002;27(3):470–478. doi: 10.1053/jhsu.2002.32965. [DOI] [PubMed] [Google Scholar]
- 18.Al-Qattan MM, El-Sayed AA, Al-Zahrani AY, et al. Obstetric brachial plexus palsy in newborn babies of diabetic and non-diabetic mothers. J Hand Surg Eur Vol. 2010;35(5):362–365. doi: 10.1177/1753193410362645. [DOI] [PubMed] [Google Scholar]
- 19.Al-Qattan MM, Clarke HM, Curtis CG. The prognostic value of concurrent Horner’s syndrome in total obstetric brachial plexus injury. J Hand Surg Br. 2000;25(2):166–167. doi: 10.1054/jhsb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 20.Bain JR, Dematteo C, Gjertsen D, et al. Navigating the gray zone: a guideline for surgical decision making in obstetrical brachial plexus injuries. J Neurosurg Pediatr. 2009;3(3):173–180. doi: 10.3171/2008.12.PEDS0885. [DOI] [PubMed] [Google Scholar]
- 21.Mallet J. [Obstetrical paralysis of the brachial plexus. II. Therapeutics. Treatment of sequelae. Priority for the treatment of the shoulder. Method for the expression of results] Rev Chir Orthop Reparatrice Appar Mot. 1972;58(Suppl 1):166–168. [PubMed] [Google Scholar]
- 22.Bae DS, Waters PM, Zurakowski D. Reliability of three classification systems measuring active motion in brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85-A(9):1733–1738. doi: 10.2106/00004623-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Kuo A, Zurakowski D, et al. Use and satisfaction of the internet in obtaining information on brachial plexus birth palsies and its influence on decision-making. J Pediatr Orthop. 2006;26(6):781–784. doi: 10.1097/01.bpo.0000229971.93812.37. [DOI] [PubMed] [Google Scholar]


