Abstract
Tissue engineering is defined as the combination of biomaterials and bioengineering principles together with cell transplantation or directed growth of host cells to develop a biological replacement tissue or organ that can be a substitute for normal tissue both in structure and function. Despite early promising preclinical studies, clinical translation of tissue engineering in pediatric urology into humans has been unsuccessful both for cell-seeded and acellular scaffolds. This can be ascribed to various factors, including the use of only non-diseased models that inaccurately describe the structural and functional modifications of diseased tissue. The paper addresses potential future strategies to overcome the limitations experienced in clinical applications so far. This includes the use of stem cells of various origins (mesenchymal stem cells, hematopoietic stem/progenitor cells, urine-derived stem cells, and progenitor cells of the urothelium) as well as the need for a deeper understanding of signaling pathways and directing tissue ingrowth and differentiation through the concept of dynamic reciprocity. The development of smart scaffolds that release trophic factors in a set and timely manner will probably improve regeneration. Modulation of innate immune response as a major contributor to tissue regeneration outcome is also addressed. It is unlikely that only one of these strategies alone will lead to clinically applicable tissue engineering strategies in pediatric urology. In the meanwhile, the fundamental new insights into regenerative processes already obtained in the attempts of tissue engineering of the lower urogenital tract remain our greatest gain.
Keywords: bladder exstrophy, low-compliance bladder, regenerative medicine, spina bifida, stem cells, tissue engineering, tissue remodeling
List of abbreviations: BC, basal cells (of the urothelium); ECM, extracellular matrix; FGF, fibroblast growth factor; HSPCs, hematopoietic stem/progenitor cells; IC, intermediate cells (of the urothelium); IGF, insulin-like growth factor; IL, interleukin; iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells; PGA, polyglycolic acid; PPARγ, peroxisome proliferator-activated receptor γ; RA, retinoic acid; RALDH2, retinaldehyde dehydrogenase 2; SF, silk fibroin; SIS, small intestine submucosa; SMCs, smooth muscle cells; UDSCs, urine-derived stem cells; UP3a, uroplakin 3a; UTI, urinary tract infection; VEGF, vascular endothelial growth factor.
Introduction
Tissue engineering is defined as the combination of biomaterials and bioengineering principles with cell transplantation or directed growth of host cells to develop a biological replacement tissue or organ that can be a substitute for normal tissue both in structure and function [1], [2]. The lack of available autologous tissue, either from loss through injury or disease or from congenital absence, has driven the search for new ways to regenerate tissue. This is especially true in pediatric urology, where either malformations such as posterior urethral valves and bladder exstrophy or urologic comorbidities in patients with spina bifida come with devastating long-term sequelae, in particular renal function loss due to elevated bladder and voiding pressure because of low bladder compliance. Surgical therapies have certainly improved patients’ lives in the last decades but still inherit severe complications. Bladder replacement with enterocystoplasty has been a major advancement in this respect, and is considered the gold standard in low-compliance bladders. It allows protecting the upper urinary tract and achieves social continence in most patients [3], but is associated with complications such as urinary tract infections (UTIs), stone formation, electrolyte imbalances, mucus production, and eventually malignant transformation [4], [5], [6]. Congenital and acquired urethral diseases like hypospadias or epispadias, as well as strictures and fistulas, represent a major challenge both in adult and pediatric urology; patients often need multiple surgeries, and urethral replacement in these cases can be difficult because of limited autologous tissue.
Theoretically, the prospect of tissue engineering therefore yields a promise unmatched by conventional surgical means; however, as we will aim to show in this review, the discrepancy of early pretensions and the clinical results so far have been far from satisfying. As of yet, there is no objective evidence that tissue engineering approaches in urology can achieve equal or superior outcomes compared to traditional therapies [7], [8].
However, these failures have led to new insights into mechanisms of regenerative processes, and this raises the hope that more sophisticated strategies will lead to new directions with better results.
Tissue engineering in (pediatric) urology comprises both replacement strategies of the upper urogenital tract – i.e. kidney – and those of the lower urogenital tract. As tissue engineering of the kidney is a very distinct topic by itself [9] and most lessons were learned in the pursuit of bladder replacement and urethral grafting, this review will therefore focus on tissue engineering aspects of the lower urogenital tract.
Biomaterials
Tissue engineering requires the use of scaffolds and matrices on which to grow new tissue on. To render them useful, these scaffolds require certain biocompatibility properties: a scaffold should provide an ideal environment for cell migration, proliferation, and differentiation. It should not inhibit cell-cell interaction, while at the same time be able to fully degrade in a timely manner that leads neither to an accumulation of degradation products, which inhibit further regeneration, nor to a too early degradation while regeneration is still incomplete. Also, an ideal matrix should be immunologically inert without unwanted inflammatory response or graft rejection [10].
Acellular scaffolds
A variety of biomaterials have been described for clinical applications. They can be divided into synthetic and naturally derived extracellular matrices. Synthetic scaffolds contain biodegradable polymers such as polyglycolic acid (PGA), polylactide, poly(glycolide-co-lactide) [11], poly(ethylene) glycol [12], polycapronolactone, etc. [7]. These scaffolds have the advantage of being able to be manufactured “off the shelf” with identical characteristics in large quantities but also induce inflammatory responses.
The group of naturally derived scaffolds basically comprises either matrices made from proteins such as collagen [13] or laminin [14], or matrices made of chemically or enzymatically decellularized tissues such as porcine small intestine submucosa (SIS) [15] or bladder acellular matrix [16], [17]. These scaffolds maintain features of their underlying organ or tissue, including an environment that can contain growth factors or a microstructure that facilitates cell-matrix interaction to allow for better cell migration and repopulation. In SIS, the presence of glycosaminoglycans [18], proteoglycans, fibronectin [19], basic fibroblast growth factor (b-FGF, FGF-2) [20], [21], and vascular endothelial growth factor (VEGF) [21], [22] could be shown. Western blots and enzyme-linked immunosorbent assay procedures showed that SIS extracellular matrix (ECM) contains as much as 0.77 ng VEGF/g SIS [22]. As many of the abovementioned constituents are highly conserved proteins, they may function as bioresponse modifiers or promote such responses also in humans.
One drawback of these scaffolds is that they are inextricably linked with a considerable intrinsic variation between grafts of the same source [23]. This has been studied extensively in porcine SIS. For example, SIS harvested from proximal intestine showed inferior regenerative properties when compared to SIS from distal intestine [24]. Additionally, age of the source animals may play a pivotal role. Several studies showed that SIS used from older animals showed less muscle regeneration [25], [26]. Moreover, the need for sterilization before use in preclinical or clinical studies raises the concern that this can alter or diminish the structural or functional properties in these naturally derived scaffolds [27], [28].
Several studies have also evaluated combination of biomaterials by creating bi-layered hybrid scaffolds with the aim of optimizing biomechanical properties or creating an optimized microenvironment for different cellular layers [29], [30].
A relatively new type of biomaterial made from silk stands in between these groups. On the one hand, it is derived from a natural source instead of being a synthetic product. On the other hand, it is highly reproducible and comes with little intrinsic variability, similar to synthetic biomaterials. These scaffolds consist of silk fibroin (SF), which is one of the two components of natural silk. Fibroin fibers form the structural core, while the other component (sericin) acts as the gum-like coating between the fibers. By removing the highly antigenic sericin, SF retains the structural qualities of silk while being largely immunologically inert [31]. SF contains excellent tensile and elasticity characteristics compared to other biomaterials. SF polymers can be processed in different ways, which allows the creation of numerous matrix configurations, such as three-dimensional porous forms, nanofibers, hydrogels, films, and tubes, depending on the application. Additionally, SF contains tailorable degradation characteristics dependent on scaffold pore size and fibroin content [10]. SF, when compared to other naturally derived or synthetic scaffolds, has demonstrated less inflammatory responses and immunogenic activity [32]. Developing a bilayer SF scaffold by combining a water-tight film layer to a porous foam made by a solvent-casting/salt-leaching method led to superior results regarding histological outcome as well as urodynamic parameters in various animal models, including a large animal model with a large 6×6 cm defect size [33], [34]. Promising results have been demonstrated in a study of ventral onlay urethroplasty as well. Compared to SIS matrices, SF grafts did not produce relevant inflammatory response and supported wide urethral calibers without strictures 3 months after urethroplasty [35].
Cell-seeded scaffolds
To overcome the limitations of acellular scaffolds and to facilitate faster regeneration, the application of matrices from either group seeded with cells in vitro before implantation has been studied [36]. The obvious primary source of cells are autologous donor cells that are expanded in vitro, and then combined with the scaffold and implanted into the specific body site, because autologous cells do not inherit the risk of rejection and associated complications [37], [38]. For bladder reconstruction, this is performed usually by combining cells of urothelial and bladder smooth muscle origin. To allow cells to survive and multiply on the scaffold, they need specific metabolic and nutritional conditions, which are achieved by in vitro bioreactors [39]. Interestingly, in a meta-analysis of animal studies, a cellular graft did not lead to advantageous results when compared to acellular grafts [40]. Other, more sophisticated cell sources, which have recently gained attention in tissue engineering scenarios, like (mesenchymal) stem cells, will be discussed in a later section of the paper.
Early failures
Bladder tissue engineering
Bladder reconstruction has been named one of the major surgical challenges both in adult and pediatric urology [41]. It is therefore not surprising that the first attempt to augment bladders goes back as far as 1917, when Neuhoff used fascia as a free tissue graft in dogs [42]. In the 1950s, a plastic mold with a distensible rubber bag was used to create a fibrotic cavity in which the ureters drained after cystectomy, mostly in carcinoma patients [43]. About 10 years later, a gelatin sponge was the first biodegradable scaffold for bladder replacement used clinically in tuberculosis patients [44]. A Japanese group in the 1970s even experimented with thin resin-covered paper as bladder augment in tuberculous contracted bladder, creating fibrous pseudo-bladders [45]. Needless to say, these earliest trials in tissue-engineered bladder augmentation were prone to complications such as bladder shrinkage, and have rightfully faded into obscurity. Many other materials, both synthetic and organic (such as pericardium, dura, and placenta), have since been used without promising results. In the 1990s, after initial reports of successful preclinical studies with acellular [46] and cell-seeded [47], [48] scaffolds, first studies of clinical translation were performed in patients with end-stage low-compliance bladders [49]. In the study by Atala et al., a cell-seeded PGA-collagen composite scaffold was used for bladder reconstruction in myelomeningocele patients aged 4–19 years who suffered from end-stage bladder disease. The cells were obtained by bladder biopsy and grown ex vivo onto the scaffold. The results of the study were regarded encouraging; however, detailed analysis shows that, overall, there was only a very moderate increase of compliance and absolute bladder capacity, translating into even less age-related bladder capacity. Additionally, the surgical approach changed during the study, which also impairs the study’s significance. Three patients were operated on with an additional omentum wrap on the scaffold. These patients showed the best results in the study, which might be attributed to better angiogenesis or, even simpler, increased tightness during regeneration, therefore leading to less leakage and inflammatory response.
Consequently, a prospective phase II study with the same cell-seeded scaffold with autologous bladder smooth muscle and urothelial cells by Joseph et al. reported unfavorable outcomes in a larger cohort of patients with the same condition [50]. No significant increase in functional parameters of the bladder were documented, and adverse events, like small-bowel obstruction or even bladder rupture, were reported in all patients during 12- to 36-month follow-ups.
Similar experiences were noted in studies with acellular SIS grafts. Initial preclinical reports showed encouraging results in (healthy) rabbits [51], dogs [52], [53], and pigs [41], [54]; however, translation into use in human patients showed disappointing outcomes. In one series of five bladder exstrophy patients presenting with poor bladder capacity and compliance after complete exstrophy repair and bladder augmentation using a SIS scaffold, bladder capacity and compliance failed to increase significantly while histology showed poor muscle components [55].
In a similar study from our department with six patients suffering from low-compliance bladder mainly because of bladder exstrophy and spina bifida, similar results were obtained. After augmentation with SIS, bladder compliance and capacity failed to increase substantially in long-term outcome (mean follow-up 24 months) [56]. Bladder compliance postoperatively ranged from 0.9 to 5.6 (mean 3.0) mL/cmH2O. Histological examinations showed a complete conversion of SIS, but irregular urothelial lining and bladder wall containing relatively thick connective tissue in four patients and regular urothelium in only two patients. Furthermore, three patients experienced major complications: two experienced bladder stones and one a bladder rupture. Of note, Von Brunn cell nests, which are distinctive of pathologic bladder wall in exstrophy bladders [57], were noted in the augment – clear evidence that pathologic tissue regenerates differently and passes pathological features onto regenerative sites.
A third study by Zhang and Liao showed somewhat more promising results [58]. Bladder augmentation with a large SIS patch was performed in eight patients with neurogenic bladder (six with meningomyelocele, two after spinal trauma). They reported a significant increase in mean bladder capacity from 170.1±75.7 mL preoperatively to 385.5±52.8 mL at 12 months after surgery (p<0.01). However, it must be taken into account that the relatively short follow-up time prevented the authors from capturing any long-term bladder shrinkage. Additionally, only in three of the patients were bladder biopsies taken postoperatively, which showed incomplete urothelial lining and suboptimal bladder wall formation with little muscle and excessive connective tissue.
Most patients in these unsuccessful clinical studies suffered from spina bifida, and while it is clear that even full regeneration of the bladder cannot restore the full function in the bladder, especially voluntary voiding due to the primary condition, the failure to achieve a stable increase of storage capacity is disappointing and forbids further clinical use of these approaches.
Urethral reconstruction
The surgery of large urethral defects as in hypospadias or epispadias often requires substitute tissue such as foreskin [59] (which is often missing, especially in repeat surgery) or buccal mucosa [60] because of the lack of suitable on-site tissue. To overcome this limitation, attempts to utilize acellular as well as cell-seeded matrices haven been performed. For acellular grafts, regeneration was shown to be unsuccessful for larger defects due to contraction and, therefore, stenosis of the neourethra. Epithelial ingrowth could only be noted in areas not exceeding 0.5 cm in diameter, possibly showing the limited vascularization in these grafts [61]. Several preclinical studies showed somewhat better results in the use of foreskin- or oral mucosa-sourced cell-seeded scaffolds [62], [63]. Fu et al. used epithelial cells from the foreskin of rabbits onto a bladder submucosa scaffold. Six months after urethral replacement in contrast to unseeded grafts, persistent epithelium was noted in the cell-seeded group. Analogue results were obtained by the same group using keratinocytes from oral mucosa as a cell source [64]. Studies of a similar design using cells from oral punch biopsy and combining them with muscle-derived cells on a collagen matrix [63] or autologous corporal smooth muscle cells (SMCs) combined with lingual keratinocytes seeded on acellular porcine corpus spongiosum matrices [65] showed stable regeneration and patent urethras as well. While these results are promising, two limitations must be taken into account. First, all these studies were performed in healthy animals and results are very limited in terms of follow-up – the longest follow-up was only 6 months.
It is therefore not surprising that the limited data on early clinical translation has been disappointing as well. In a small study with six patients 14–44 months old, 50% developed severe complications such as urethrocutaneous fistula or stricture after repair of severe hypospadias using urothelial cells seeded on acellular dermis [66]. Bhargava et al. corrected urethral strictures caused by lichen sclerosus using a similar approach with buccal mucosa seeded on de-epithelialized dermis [67]. Two of five patients needed total removal of the grafted urethra due to fibrosis, while the other three needed further procedures, bringing the complication rate to 100%.
Only one study by Raya-Rivera et al. showed better results [68]. Their group used tubular PGA scaffolds, which were seeded with urothelial cells on the inner surface and with SMCs on the outer surface. This graft was then implanted into five boys with urethral defects. Stricture was reported in one boy and incontinence due to postoperative sphincteric incompetence in another boy.
Given these results, urethral replacement with tissue-engineered grafts seems to be far from ready for implementation into clinical practice.
New insights
The disillusioning results of these early clinical studies can be ascribed to several reasons. First, preceding preclinical studies relied mainly on histological and therefore structural outcomes. Thus, they are little more than feasibility studies with short follow-ups. Functional tests, i.e. urodynamic studies in animals, were, often enough, not performed. Second, healthy animal models are of limited value in tissue engineering scenarios, as they show better regeneration than diseased animals. For example, Burmeister et al. showed complete regeneration of the bladder wall in a rat model of partial cystectomy of 75% of the bladder, resulting in complete structural and functional bladder regeneration within 8 weeks, illustrating the high regenerative capacity of the rat bladder [69]. It is therefore not surprising that in healthy animal models, even incomplete remodeling of a graft can be compensated by natural regeneration, a scenario that would not be the case in diseased animals.
This principle could also be demonstrated on a cellular level. Lin et al. showed that cultured neuropathic bladder SMCs possess and maintain different characteristics in vitro compared to normal bladder SMCs [70]: SMCs from neuropathic bladders contracted significantly less than SMCs from normal bladders in in vitro contractility assays and showed less adherence. Subramaniam et al. observed that urothelial cells, when from diseased patients, contain a highly reduced proliferation and differentiation capacity in vitro than when from healthy persons [71]. Additionally, most samples in their study lacked expression of uroplakin 3a (UP3a) and cytokeratin 20, which characterize differentiation into superficial umbrella cells. In an analysis of gene expression of neuropathic bladder SMCs compared to normal SMCs, several dysregulated pathways, including fibroblast growth factor signaling, were found [72].
These studies show clearly that clinical use of diseased cells for regenerative purposes (either for cell-seeded or acellular matrices) may not be beneficial (at least without further modification), and the identification of other sources of cells may be warranted.
Thus, at least one requirement for further attempts of tissue engineering of the bladder is the use of diseased models. Models of neurogenic bladder have been developed in various animals. A rat model of spinal cord injury, for example, has been shown to produce reliable bladder dysfunction and pathologic remodeling [73]. Comparing bladder regeneration in a bladder augmentation model with SF matrix showed lower numbers of synaptic areas in the neotissue along with the presence of chronic inflammation in the diseased animals [74]. These results contradict findings in a non-diseased rat model, wherein no inflammatory reactions could be found using the same scaffold preparations [33].
Studies in rodents in which the urethra is occluded by surgical means to achieve partial bladder outlet obstruction can mimic functional and structural bladder changes, such as loss of contractility and fibrosis in humans with subvesical obstruction. These established models, which have also been characterized using urodynamic studies, have been widely used to investigate bladder remodeling and will be useful in tissue engineering studies as well [75].
While rodent models are useful for organ development and proof-of-principle studies or studies investigating signaling pathways and cell-cell interactions because of the availability of genetically engineered animals, large animal models, which mimic the human body more closely, are needed for tissue engineering with larger grafts [76]. For example, favorable outcomes after tissue engineered bladder augmentation have been reported in rats with spinal cord injuries [77], [78]; however, in a porcine model of partial bladder outlet obstruction, the diseased bladder status negatively affected regeneration [79].
A large animal model for creating bladder exstrophies exists in sheep, in which bladder exstrophy is created through fetal surgery at gestation day 79, with the lamb then delivered at full term [80]. In these exstrophic bladders, a significant increase in the ratio of collagen to smooth muscle was noted compared to normal control bladders (p<0.05). This difference was similar to that noted in neonatal human bladders. In a study of bladder augmentation with collagen scaffolds in bladder exstrophy lambs 1 week after birth, a low survival rate of 58% was noted compared to 100% in augmented healthy animals [13]. In the surviving animals, a comparable regeneration was achieved, but with a trend toward lower compliance in the bladder exstrophy group 6 months after surgery. The authors admit that improvements are still needed for larger augmentations or more severely diseased bladders.
Of course, the above also applies to urethral tissue engineering as well. Promising results with new scaffolds in healthy animals [35] will have to be tested in models of urethral strictures, e.g. in a well-described rabbit model of urethral stricture, which is produced by 10-mm-long circumferential electrocoagulation of the bulbar urethra using a 13Fr pediatric resectoscope [81].
Overall, these studies show the increased difficulties of regeneration in diseased bladders. Studies with diseased animal models are costly, time consuming, and ethically demanding [82]; however, there is no doubt that the insights that can be gained from such approaches are immense and should be mandatory before clinical translation.
Future strategies
Stem cells
Because of their ability for differentiation, plasticity, migration, and continuous self-renewal, stem cells have received particular attention in recent years for cell-seeded tissue engineering approaches [83]. Due to the ability to regulate cell signaling via their secretome, stem cells contain immunomodulatory properties that can modify the inflammatory response and improve vascularization and cellular coordination [84].
Bone marrow contains two specific cellular components with stem cell properties: mesenchymal stem cells (MSCs) and multipotent hematopoietic stem/progenitor cells (HSPCs) [85], [86]. MSCs maintain self-renewal and differentiate into various tissue lineages. They represent a heterogeneous cell population but can be isolated from multiple tissues. Of note, adult MSCs resemble bladder SMCs in regards to contractile protein furnishings [87]. In a nude rat model of MSCs seeded on a poly-1,8-octanedio-co-citrate scaffold, regeneration showed superior smooth muscle bundle formation compared to adult SMC-seeded and unseeded scaffolds. Collagen-to-muscle ratio showed a better, near-to-normal ratio of roughly 1:1 in the MSC-seeded group compared to a ratio of around 2.3:1–4:1 in the other groups; this indicates less fibrosis and the potential of higher compliance [88]. In a rabbit model of urethral reconstruction using bone marrow MSC- and SMC-seeded bladder acellular matrix, experimental animals showed normal-caliber urethra at 16 weeks after implantation, while in the control group with acellular matrix alone, 50% of the animals died because of urethral obstruction [89].
HSPCs and angiogenesis
A major problem of early tissue engineering approaches has been the promotion of adequate vascularization, especially in structurally larger grafts used for bladder reconstruction. In the center of such grafts, nutrient and oxygen supply is low [90], leading to incomplete regeneration and graft failure. It is known that a specific fraction of HSPCs (CD34+ HSPCs) has a unique ability to induce robust tissue vascularization [83]. In a study of partial cystectomy, nude rats were augmented with various combinations of cell-seeded scaffolds that contained MSCs or CD34+ HPSCs alone or MSC/CD34+ HSPC combinations [91]. Quantitative analysis revealed an up to nine-fold increase in vascularization in the MSC/CD34+ HSPC group compared with the other groups. This indicates that the combination of the two stem cell types was determinative in the vascularization of the entire graft, including the center area. The source for MSC/CD34+ HSPCs in this study were pediatric spina bifida patients as well as healthy adult and pediatric donors, and the results showed similar regeneration potential for the spina bifida donor cells compared to the cells of healthy donors. This is of particular interest, as it indicates the possibility to circumvent the drawback of the use of differentiated (urothelial or muscle) cells, which seem to take properties of the disease with them when used in tissue engineering scenarios. Developmental defects in pediatric spina bifida patients seem to have no negative effect on bladder functionality when using an autologous stem/progenitor cell source.
In general, the demand for adequate and early vascularization should get more emphasis in bladder tissue engineering research, as tissue regeneration will always be impaired by suboptimal oxygen and nutrient supply. Combination of stem cell approaches with prevascularization strategies (e.g. generation of preformed microvascular networks in grafts prior to implantation) might help circumvent this fundamental problem of the implantation of large grafts [92].
Induced pluripotent stem cells (iPSCs)
Stem cell science is a rapidly evolving field, and, in recent years, iPSCs, which can be generated directly from adult tissue by converting adult cells to stem cells using four specific genes that encode transcription factors, have been named prime candidates for tissue engineering strategies [83]. Compared to embryonic stem cells, they are relatively easy to obtain and share similar plasticity. Although the advent of iPSCs has already led to clinical use in other fields, available data in the field of bladder tissue engineering are still very limited. So far, only in vitro studies exist, which show that iPSCs can be voluntarily differentiated into different urothelial cells as well as SMCs [93], [94].
Other sources of stem cells
Recently, cells with stem cell properties have been found in human voiding urine [95]. Urine-derived stem cells (UDSCs) have been named as a potential source for tissue engineering, although the origin of these cells, which only represent a small fraction of cells in voiding urine, is still unclear (proposed to be of renal origin [96]). Even if the possibility of effortless collecting of stem cells seems intriguing, data are still very limited on this stem cells source [97]. In one study, the authors claim that in a rabbit model of a 2-cm urethral defect, a UDSC-seeded SIS graft showed better regeneration regarding urethral caliber, speed of urothelial regeneration, content of smooth muscle, and vessel density compared to an acellular SIS graft. Furthermore, inflammatory cell infiltration and fibrosis were diminished in the autologous UDSC-seeded SIS group [98].
Progenitor cells in urothelium?
Related to the use of stem cells is the question of whether progenitor cells exist in the regenerating organ itself and whether they can be utilized and directed for optimal tissue formation. This has been thoroughly investigated in the case of urothelium. Shin et al. showed that there is a source of resident multipotent progenitor cells in the basal urothelium in a model of UTI bladder urothelial damage. They identified keratin 5-positive basal cells (BCs) as the source for regeneration of the superficial urothelial layers. Since then, a substantial controversy has arisen over this question. In a subsequent study from the Mendelssohn group, UP3-positive intermediate cells (ICs) were identified as progenitors in a model of chemical injury of the bladder, mimicking chronic cystitis. These studies have in common that they investigated scenarios with mild to moderate damage of the urothelium. In tissue engineering scenarios, however, regeneration takes place after complete removal of both urothelium and stroma/muscle.
In a model of genetically engineered lineage tracing mice, one of the authors (FMS) could show that, in regeneration following bladder augmentation with an acellular graft, BCs are the progenitor source for all layers of the urothelium in a linear fashion, in contrast to less severe injury, where regeneration takes place also in a non-linear fashion utilizing intermediate cells [99]. Moreover, two previously unreported urothelial cell types (BC-2 and IC-2) were observed, which, as transient cell types, are subsequently exfoliated into the lumen as regeneration proceeds to final stages. Putatively, these cells serve as a temporary scab-like layer that shields the forming bladder wall from urine, allowing mature cell formation to occur [99].
We could also show that in the newly forming suburothelial stroma, retinaldehyde dehydrogenase 2 (RALDH2) shows a transient peak at 4 weeks after augmentation. RALDH2 is an enzyme necessary for retinoic acid (RA) synthesis, which, in turn, is necessary to drive the differentiation of intermediate cells to superficial cells, and therefore illustrates one potentially useful pathway to influence differentiation of urothelial cells during regeneration [100].
Understanding the role of signaling pathways and dynamic crosstalk between the urothelium and stroma in bladder regeneration as well as the potential of indigenous progenitor cell sources will probably also allow us to direct and influence regenerative processes. The ability to direct these complex dependencies on molecular levels will ultimately determine the results of regeneration of large tissue grafts.
Dynamic reciprocity and regulating remodeling
Not only interactions between urothelium and stroma but also bi-directional interactions between tissue-engineered grafts and the host tissue environment are important factors in achieving better outcomes in tissue remodeling. This concept has been termed “dynamic reciprocity” [101]. Understanding and directing these remodeling events, i.e. host cell adhesion and migration, differentiation, immune response, differentiation and apoptosis, formation of new ECM, etc., may help significantly optimize remodeling processes. One way to influence host-scaffold interaction is to create “smart scaffolds,” which release trophic factors in a set way to optimize host reactions toward the scaffold.
In recent years, the influence of immune response in tissue engineering has been moved into focus. In particular, macrophage reactions have been studied extensively. It is known that two types of macrophages – pro-inflammatory M1 macrophages and anti-inflammatory macrophages of M2 type – play distinct roles in tissue repair during wound healing following injury of various tissues [102]. It has been shown that pore size and fiber diameter of scaffolds alter macrophage activation, leaning to an increased presence of either M1 or M2 phenotypes [103], [104], [105].
These two types do not solely represent independent subtypes. Rather, they represent a spectrum of macrophages leaning toward one of the types with transitional phenotypes expressing markers of both M1 and M2 macrophages, up-regulation of M2 macrophages in a timely fashion may promote functional tissue remodeling and diminish scar tissue formation [106]. It seems as if both types of macrophages are needed in a dynamic sequence during tissue repair. A coordinated sequence of M1 over M2 dominance in an early period of repair seems to promote angiogenesis [107], while M2 over M1 dominance in later stages seem to induce functional tissue formation [108].
Even though limited data are available for urologic applications, these studies show that further investigation of macrophage-scaffold interaction and macrophage activation is warranted. Timely release of bioactive substances such as interleukin (IL)-4, IL-13 [109], or interferon-regulatory factors [110], which allow for an improved sequence of M1 and M2 activation, may be a promising target for smart scaffold research and promote healthy tissue regeneration. Another leverage point to modulate macrophage-dependent immune response may arise through RA-related peroxisome proliferator-activated receptor γ (PPARγ) signaling pathways by incorporation of pharmacologic modulators of PPARγ signaling [111]. This is of particular interest, as it has been shown that RA-dependent pathways are important for the final stages of urothelial differentiation as well [99].
As it is known that acellular natural-derived grafts contain growth factors, and still yield unsatisfactory results, efforts optimizing their interactions might lead to better outcomes. In one study, a modified FGF-2, which was fused with a collagen-binding domain to improve release kinetics, was incorporated into an acellular graft, and this graft promoted more vascularization, SMC ingrowth, and urodynamic parameters compared to a graft with native FGR-2 [112]. A similar principle was found superior in a rat study in which a modified insulin-like growth factor (IGF)-1 fusion protein, which permitted retaining of the growth factor at the site of tissue regeneration, was compared to wild-type IGF-1, the former promoting better smooth muscle repair in a rat model [113].
The co-administration of growth factors and the timely release from scaffolds might be a promising strategy as well. In one study, histological outcome and functional bladder contractility proved superior in a model of bladder acellular matrix tissue engineering, when the scaffold contained a mix of platelet-derived growth factor-BB and VEGF compared to a scaffold without growth factors [114]. In a similar study in a rat model of spinal cord injury and partial bladder replacement with bladder acellular matrix, the co-administration of nerve growth factor/VEGF showed better bladder capacity and compliance than a scaffold lacking growth factors [115].
Although scaffolds with reliable growth factor release are difficult to produce, and the choice of growth factors as well as concentration and timing of release need to be thoroughly investigated, these studies show that there is a high potential of optimizing results in tissue repair processes, provided that the underlying principles are properly understood. To achieve this, further research is mandatory.
Conclusion
Attempts of tissue engineering have included a large variety of synthetic and biological scaffolds and cell types in all conceivable combinations with very limited clinical success so far. The initial notion that bladder is an easy-to-replace organ, because it is of simple structure and function, has since been revised. We have learned that bladder regeneration is a challenge, given the multitude of functions the bladder has to fulfill. The bladder is an active organ with a highly specialized impermeable inner surface, is richly innervated and vascularized, and has unique structural and functional dynamics.
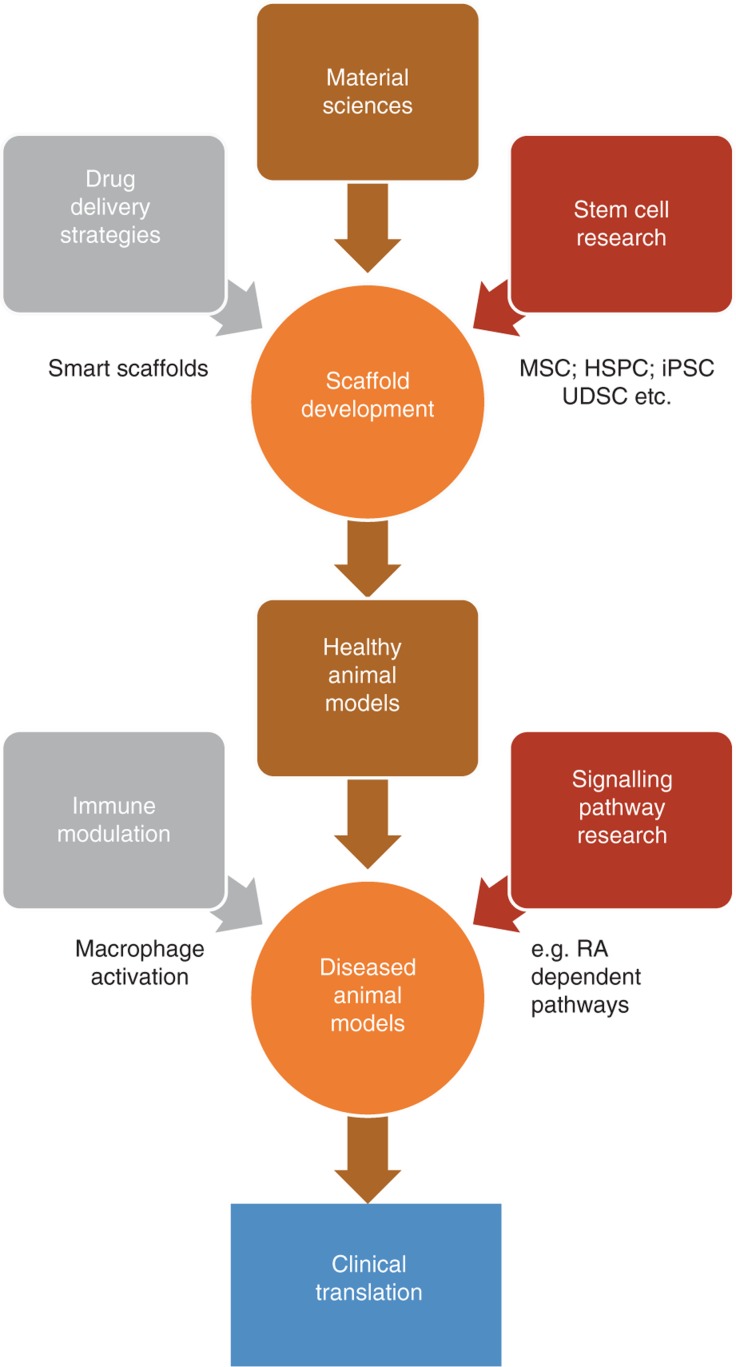
It is unlikely that one of the abovementioned strategies alone will lead to clinically applicable tissue engineering solutions. It is far more likely that only the integration of multiple approaches will lead to tissue engineering applications that meet the goal indicated in the introduction, namely to surpass the results of conventional surgical solutions (Figure 1). To achieve this, the complex relationship between tissue compartments, cell-cell and cell-stroma interaction, as well as molecular signaling pathways that determine regeneration need to be understood to a much deeper extent. Stem cell research, scaffold composition, including the implementation of pharmaceutical factors modulating repair processes and immune response (e.g. through altered macrophage activation), need to be cornerstones of further urological tissue engineering research. For the time being, the fundamental new insights into regenerative processes already obtained in the attempts of tissue engineering of the lower urogenital tract remain our greatest gain.
Figure 1:
Pathway to clinical translation of tissue engineering approaches.
Only combination of different strategies and scientific subspecialties will likely allow tissue engineering to become part of clinical routine in the future.
Supporting Information
Supplementary Material:
The article (iss-2018-0011) offers reviewer assessments as supplementary material.
Author Statement
Research funding: Authors state no funding involved. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: Ethical approval is not applicable.
Author Contributions
Frank-Mattias Schäfer: conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing – original draft; writing – review and editing. Maximilian Stehr: supervision; validation; writing – review and editing.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1].Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920–6. [DOI] [PubMed]; Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- [2].MacArthur BD, Oreffo RO. Bridging the gap. Nature 2005;433:19. [DOI] [PubMed]; MacArthur BD, Oreffo RO. Bridging the gap. Nature. 2005;433:19. doi: 10.1038/433019a. [DOI] [PubMed] [Google Scholar]
- [3].Greenwell TJ, Venn SN, Mundy AR. Augmentation cystoplasty. BJU Int 2001;88:511–25. [DOI] [PubMed]; Greenwell TJ, Venn SN, Mundy AR. Augmentation cystoplasty. BJU Int. 2001;88:511–25. doi: 10.1046/j.1464-4096.2001.001206. [DOI] [PubMed] [Google Scholar]
- [4].Kispal Z, Balogh D, Erdei O, Kehl D, Juhasz Z, Vastyan AM, et al. Complications after bladder augmentation or substitution in children: a prospective study of 86 patients. BJU Int 2011;108:282–9. [DOI] [PubMed]; Kispal Z, Balogh D, Erdei O, Kehl D, Juhasz Z, Vastyan AM. et al. Complications after bladder augmentation or substitution in children: a prospective study of 86 patients. BJU Int. 2011;108:282–9. doi: 10.1111/j.1464-410X.2010.09862.x. [DOI] [PubMed] [Google Scholar]
- [5].Obermayr F, Szavay P, Schaefer J, Fuchs J. Outcome of augmentation cystoplasty and bladder substitution in a pediatric age group. Eur J Pediatr Surg 2011;21:116–9. [DOI] [PubMed]; Obermayr F, Szavay P, Schaefer J, Fuchs J. Outcome of augmentation cystoplasty and bladder substitution in a pediatric age group. Eur J Pediatr Surg. 2011;21:116–9. doi: 10.1055/s-0030-1267223. [DOI] [PubMed] [Google Scholar]
- [6].Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep 2007;8:157–62. [DOI] [PubMed]; Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep. 2007;8:157–62. doi: 10.1007/s11934-007-0066-9. [DOI] [PubMed] [Google Scholar]
- [7].Garriboli M, Radford A, Southgate J. Regenerative medicine in urology. Eur J Pediatr Surg 2014;24:227–36. [DOI] [PubMed]; Garriboli M, Radford A, Southgate J. Regenerative medicine in urology. Eur J Pediatr Surg. 2014;24:227–36. doi: 10.1055/s-0034-1382259. [DOI] [PubMed] [Google Scholar]
- [8].Versteegden LR, de Jonge PK, IntHout J, van Kuppevelt TH, Oosterwijk E, Feitz WF, et al. Tissue engineering of the urethra: a systematic review and meta-analysis of preclinical and clinical studies. Eur Urol 2017;72:594–606. [DOI] [PubMed]; Versteegden LR, de Jonge PK, IntHout J, van Kuppevelt TH, Oosterwijk E, Feitz WF. et al. Tissue engineering of the urethra: a systematic review and meta-analysis of preclinical and clinical studies. Eur Urol. 2017;72:594–606. doi: 10.1016/j.eururo.2017.03.026. [DOI] [PubMed] [Google Scholar]
- [9].Moon KH, Ko IK, Yoo JJ, Atala A. Kidney diseases and tissue engineering. Methods 2016;99:112–9. [DOI] [PubMed]; Moon KH, Ko IK, Yoo JJ, Atala A. Kidney diseases and tissue engineering. Methods. 2016;99:112–9. doi: 10.1016/j.ymeth.2015.06.020. [DOI] [PubMed] [Google Scholar]
- [10].Sack BS, Mauney JR, Estrada Jr CR. Silk fibroin scaffolds for urologic tissue engineering. Curr Urol Rep 2016;17:16. [DOI] [PMC free article] [PubMed]; Sack BS, Mauney JR, Estrada CR Jr. Silk fibroin scaffolds for urologic tissue engineering. Curr Urol Rep. 2016;17:16. doi: 10.1007/s11934-015-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nakanishi Y, Chen G, Komuro H, Ushida T, Kaneko S, Tateishi T, et al. Tissue-engineered urinary bladder wall using PLGA mesh-collagen hybrid scaffolds: a comparison study of collagen sponge and gel as a scaffold. J Pediatr Surg 2003;38:1781–4. [DOI] [PubMed]; Nakanishi Y, Chen G, Komuro H, Ushida T, Kaneko S, Tateishi T. et al. Tissue-engineered urinary bladder wall using PLGA mesh-collagen hybrid scaffolds: a comparison study of collagen sponge and gel as a scaffold. J Pediatr Surg. 2003;38:1781–4. doi: 10.1016/j.jpedsurg.2003.08.034. [DOI] [PubMed] [Google Scholar]
- [12].Adelow CA, Frey P. Synthetic hydrogel matrices for guided bladder tissue regeneration. Methods Mol Med 2007;140:125–40. [DOI] [PubMed]; Adelow CA, Frey P. Synthetic hydrogel matrices for guided bladder tissue regeneration. Methods Mol Med. 2007;140:125–40. doi: 10.1007/978-1-59745-443-8_7. [DOI] [PubMed] [Google Scholar]
- [13].Roelofs LA, Kortmann BB, Oosterwijk E, Eggink AJ, Tiemessen DM, Crevels AJ, et al. Tissue engineering of diseased bladder using a collagen scaffold in a bladder exstrophy model. BJU Int 2014;114:447–57. [PubMed]; Roelofs LA, Kortmann BB, Oosterwijk E, Eggink AJ, Tiemessen DM, Crevels AJ. et al. Tissue engineering of diseased bladder using a collagen scaffold in a bladder exstrophy model. BJU Int. 2014;114:447–57. [PubMed] [Google Scholar]
- [14].Junka R, Valmikinathan CM, Kalyon DM, Yu X. Laminin functionalized biomimetic nanofibers for nerve tissue engineering. J Biomater Tissue Eng 2013;3:494–502. [DOI] [PMC free article] [PubMed]; Junka R, Valmikinathan CM, Kalyon DM, Yu X. Laminin functionalized biomimetic nanofibers for nerve tissue engineering. J Biomater Tissue Eng. 2013;3:494–502. doi: 10.1166/jbt.2013.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 1989;47:74–80. [DOI] [PubMed]; Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- [16].Brown AL, Farhat W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials 2002;23:2179–90. [DOI] [PubMed]; Brown AL, Farhat W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23:2179–90. doi: 10.1016/s0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- [17].Brown AL, Brook-Allred TT, Waddell JE, White J, Werkmeister JA, Ramshaw JA, et al. Bladder acellular matrix as a substrate for studying in vitro bladder smooth muscle-urothelial cell interactions. Biomaterials 2005;26:529–43. [DOI] [PubMed]; Brown AL, Brook-Allred TT, Waddell JE, White J, Werkmeister JA, Ramshaw JA. et al. Bladder acellular matrix as a substrate for studying in vitro bladder smooth muscle-urothelial cell interactions. Biomaterials. 2005;26:529–43. doi: 10.1016/j.biomaterials.2004.02.055. [DOI] [PubMed] [Google Scholar]
- [18].Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng 1996;2:209–17. [DOI] [PubMed]; Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2:209–17. doi: 10.1089/ten.1996.2.209. [DOI] [PubMed] [Google Scholar]
- [19].McPherson A, Badylak S. Characterization of fibronectin derived from porcine small intestinal submucosa. Tissue Eng 1998;4:9.; McPherson A, Badylak S. Characterization of fibronectin derived from porcine small intestinal submucosa. Tissue Eng. 1998;4:9. [Google Scholar]
- [20].Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem 1997;67:478–91. [PubMed]; Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67:478–91. [PubMed] [Google Scholar]
- [21].Hurst RE, Bonner RB. Mapping of the distribution of significant proteins and proteoglycans in small intestinal submucosa by fluorescence microscopy. J Biomater Sci Polym Ed 2001;12:1267–79. [DOI] [PubMed]; Hurst RE, Bonner RB. Mapping of the distribution of significant proteins and proteoglycans in small intestinal submucosa by fluorescence microscopy. J Biomater Sci Polym Ed. 2001;12:1267–79. doi: 10.1163/156856201753395798. [DOI] [PubMed] [Google Scholar]
- [22].Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium 2001;8:11–24. [DOI] [PubMed]; Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8:11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- [23].Korossis S, Bolland F, Southgate J, Ingham E, Fisher J. Regional biomechanical and histological characterisation of the passive porcine urinary bladder: implications for augmentation and tissue engineering strategies. Biomaterials 2009;30:266–75. [DOI] [PubMed]; Korossis S, Bolland F, Southgate J, Ingham E, Fisher J. Regional biomechanical and histological characterisation of the passive porcine urinary bladder: implications for augmentation and tissue engineering strategies. Biomaterials. 2009;30:266–75. doi: 10.1016/j.biomaterials.2008.09.034. [DOI] [PubMed] [Google Scholar]
- [24].Ashley RA, Roth CC, Palmer BW, Kibar Y, Routh JC, Fung KM, et al. Regional variations in small intestinal submucosa evoke differences in inflammation with subsequent impact on tissue regeneration in the rat bladder augmentation model. BJU Int 2010;105:1462–8. [DOI] [PubMed]; Ashley RA, Roth CC, Palmer BW, Kibar Y, Routh JC, Fung KM. et al. Regional variations in small intestinal submucosa evoke differences in inflammation with subsequent impact on tissue regeneration in the rat bladder augmentation model. BJU Int. 2010;105:1462–8. doi: 10.1111/j.1464-410X.2009.08965.x. [DOI] [PubMed] [Google Scholar]
- [25].Sicari BM, Johnson SA, Siu BF, Crapo PM, Daly KA, Jiang H, et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 2012;33:5524–33. [DOI] [PMC free article] [PubMed]; Sicari BM, Johnson SA, Siu BF, Crapo PM, Daly KA, Jiang H. et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials. 2012;33:5524–33. doi: 10.1016/j.biomaterials.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tottey S, Johnson SA, Crapo PM, Reing JE, Zhang L, Jiang H, et al. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 2011;32:128–36. [DOI] [PMC free article] [PubMed]; Tottey S, Johnson SA, Crapo PM, Reing JE, Zhang L, Jiang H. et al. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials. 2011;32:128–36. doi: 10.1016/j.biomaterials.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hodde J, Janis A, Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med 2007;18:545–50. [DOI] [PubMed]; Hodde J, Janis A, Hiles M. Effects of sterilization on an extracellular matrix scaffold: part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007;18:545–50. doi: 10.1007/s10856-007-2301-9. [DOI] [PubMed] [Google Scholar]
- [28].Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med 2007;18:537–43. [DOI] [PubMed]; Hodde J, Janis A, Ernst D, Zopf D, Sherman D, Johnson C. Effects of sterilization on an extracellular matrix scaffold: part I. Composition and matrix architecture. J Mater Sci Mater Med. 2007;18:537–43. doi: 10.1007/s10856-007-2300-x. [DOI] [PubMed] [Google Scholar]
- [29].Horst M, Madduri S, Milleret V, Sulser T, Gobet R, Eberli D. A bilayered hybrid microfibrous PLGA – acellular matrix scaffold for hollow organ tissue engineering. Biomaterials 2013;34:1537–45. [DOI] [PubMed]; Horst M, Madduri S, Milleret V, Sulser T, Gobet R, Eberli D. A bilayered hybrid microfibrous PLGA – acellular matrix scaffold for hollow organ tissue engineering. Biomaterials. 2013;34:1537–45. doi: 10.1016/j.biomaterials.2012.10.075. [DOI] [PubMed] [Google Scholar]
- [30].Horst M, Milleret V, Noetzli S, Gobet R, Sulser T, Eberli D. Polyesterurethane and acellular matrix based hybrid biomaterial for bladder engineering. J Biomed Mater Res B Appl Biomater 2017;105:658–67. [DOI] [PubMed]; Horst M, Milleret V, Noetzli S, Gobet R, Sulser T, Eberli D. Polyesterurethane and acellular matrix based hybrid biomaterial for bladder engineering. J Biomed Mater Res B Appl Biomater. 2017;105:658–67. doi: 10.1002/jbm.b.33591. [DOI] [PubMed] [Google Scholar]
- [31].Lam Van Ba O, Aharony S, Loutochin O, Corcos J. Bladder tissue engineering: a literature review. Adv Drug Deliv Rev 2015;82–83:31–7. [DOI] [PubMed]; Lam Van Ba O, Aharony S, Loutochin O, Corcos J. Bladder tissue engineering: a literature review. Adv Drug Deliv Rev. 2015;82–83:31–7. doi: 10.1016/j.addr.2014.11.013. [DOI] [PubMed] [Google Scholar]
- [32].Mauney JR, Cannon GM, Lovett ML, Gong EM, Di Vizio D, Gomez P 3rd, et al. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials 2011;32:808–18. [DOI] [PMC free article] [PubMed]; Mauney JR, Cannon GM, Lovett ML, Gong EM, Di Vizio D, Gomez P 3rd. et al. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials. 2011;32:808–18. doi: 10.1016/j.biomaterials.2010.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Seth A, Chung YG, Gil ES, Tu D, Franck D, Di Vizio D, et al. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials 2013;34:4758–65. [DOI] [PMC free article] [PubMed]; Seth A, Chung YG, Gil ES, Tu D, Franck D, Di Vizio D. et al. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials. 2013;34:4758–65. doi: 10.1016/j.biomaterials.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tu DD, Chung YG, Gil ES, Seth A, Franck D, Cristofaro V, et al. Bladder tissue regeneration using acellular bi-layer silk scaffolds in a large animal model of augmentation cystoplasty. Biomaterials 2013;34:8681–9. [DOI] [PMC free article] [PubMed]; Tu DD, Chung YG, Gil ES, Seth A, Franck D, Cristofaro V. et al. Bladder tissue regeneration using acellular bi-layer silk scaffolds in a large animal model of augmentation cystoplasty. Biomaterials. 2013;34:8681–9. doi: 10.1016/j.biomaterials.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chung YG, Tu D, Franck D, Gil ES, Algarrahi K, Adam RM, et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One 2014;9:e91592. [DOI] [PMC free article] [PubMed]; Chung YG, Tu D, Franck D, Gil ES, Algarrahi K, Adam RM. et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One. 2014;9:e91592. doi: 10.1371/journal.pone.0091592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhang Y, Frimberger D, Cheng EY, Lin HK, Kropp BP. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int 2006;98:1100–5. [DOI] [PubMed]; Zhang Y, Frimberger D, Cheng EY, Lin HK, Kropp BP. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98:1100–5. doi: 10.1111/j.1464-410X.2006.06447.x. [DOI] [PubMed] [Google Scholar]
- [37].Turner AM, Subramaniam R, Thomas DF, Southgate J. Generation of a functional, differentiated porcine urothelial tissue in vitro. Eur Urol 2008;54:1423–32. [DOI] [PubMed]; Turner AM, Subramaniam R, Thomas DF, Southgate J. Generation of a functional, differentiated porcine urothelial tissue in vitro. Eur Urol. 2008;54:1423–32. doi: 10.1016/j.eururo.2008.03.068. [DOI] [PubMed] [Google Scholar]
- [38].Cilento BG, Freeman MR, Schneck FX, Retik AB, Atala A. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J Urol 1994;152:665–70. [DOI] [PubMed]; Cilento BG, Freeman MR, Schneck FX, Retik AB, Atala A. Phenotypic and cytogenetic characterization of human bladder urothelia expanded in vitro. J Urol. 1994;152:665–70. doi: 10.1016/s0022-5347(17)32676-9. [DOI] [PubMed] [Google Scholar]
- [39].Davis NF, Mooney R, Piterina AV, Callanan A, McGuire BB, Flood HD, et al. Construction and evaluation of urinary bladder bioreactor for urologic tissue-engineering purposes. Urology 2011;78:954–60. [DOI] [PubMed]; Davis NF, Mooney R, Piterina AV, Callanan A, McGuire BB, Flood HD. et al. Construction and evaluation of urinary bladder bioreactor for urologic tissue-engineering purposes. Urology. 2011;78:954–60. doi: 10.1016/j.urology.2011.06.036. [DOI] [PubMed] [Google Scholar]
- [40].Sloff M, Simaioforidis V, de Vries R, Oosterwijk E, Feitz W. Tissue engineering of the bladder – reality or myth? A systematic review. J Urol 2014;192:1035–42. [DOI] [PubMed]; Sloff M, Simaioforidis V, de Vries R, Oosterwijk E, Feitz W. Tissue engineering of the bladder – reality or myth? A systematic review. J Urol. 2014;192:1035–42. doi: 10.1016/j.juro.2014.03.116. [DOI] [PubMed] [Google Scholar]
- [41].Caione P, Capozza N, Zavaglia D, Palombaro G, Boldrini R. In vivo bladder regeneration using small intestinal submucosa: experimental study. Pediatr Surg Int 2006;22:593–9. [DOI] [PubMed]; Caione P, Capozza N, Zavaglia D, Palombaro G, Boldrini R. In vivo bladder regeneration using small intestinal submucosa: experimental study. Pediatr Surg Int. 2006;22:593–9. doi: 10.1007/s00383-006-1705-9. [DOI] [PubMed] [Google Scholar]
- [42].Neuhof H. Fascial transplantation into visceral defects: an experimental and clinical study. Surg Gynecol Obstet 1917;1917:383–7.; Neuhof H. Fascial transplantation into visceral defects: an experimental and clinical study. Surg Gynecol Obstet. 1917;1917:383–7. [Google Scholar]
- [43].Bohne AW, Urwiller KL. Experience with urinary bladder regeneration. J Urol 1957;77:725–32. [DOI] [PubMed]; Bohne AW, Urwiller KL. Experience with urinary bladder regeneration. J Urol. 1957;77:725–32. doi: 10.1016/S0022-5347(17)66624-2. [DOI] [PubMed] [Google Scholar]
- [44].Tsuji I, Kuroda K, Fujieda J, Shiraishi Y, Kunishima K. Clinical experiences of bladder reconstruction using preserved bladder and gelatin sponge bladder in the case of bladder cancer. J Urol 1967;98:91–2. [DOI] [PubMed]; Tsuji I, Kuroda K, Fujieda J, Shiraishi Y, Kunishima K. Clinical experiences of bladder reconstruction using preserved bladder and gelatin sponge bladder in the case of bladder cancer. J Urol. 1967;98:91–2. doi: 10.1016/S0022-5347(17)62828-3. [DOI] [PubMed] [Google Scholar]
- [45].Taguchi H, Ishizuka E, Saito K. Cystoplasty by regeneration of the bladder. J Urol 1977;118:752–6. [DOI] [PubMed]; Taguchi H, Ishizuka E, Saito K. Cystoplasty by regeneration of the bladder. J Urol. 1977;118:752–6. doi: 10.1016/s0022-5347(17)58181-1. [DOI] [PubMed] [Google Scholar]
- [46].Kropp, BP, Badylak S, Thor KB. Regenerative bladder augmentation: a review of the initial preclinical studies with porcine small intestinal submucosa. Adv Exp Med Biol 1995;385:229–35. [DOI] [PubMed]; Kropp P, Badylak S, Thor KB. Regenerative bladder augmentation: a review of the initial preclinical studies with porcine small intestinal submucosa. Adv Exp Med Biol. 1995;385:229–35. doi: 10.1007/978-1-4899-1585-6_28. [DOI] [PubMed] [Google Scholar]
- [47].Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 1999;17:149–55. [DOI] [PubMed]; Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–55. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- [48].Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology 1998;51:221–5. [DOI] [PubMed]; Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998;51:221–5. doi: 10.1016/s0090-4295(97)00644-4. [DOI] [PubMed] [Google Scholar]
- [49].Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367:1241–6. [DOI] [PubMed]; Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- [50].Joseph K, Nijjar Y, Warkentin H, Schiller D, Tankel K, Usmani N, et al. Prospective phase II study of tomotherapy based chemoradiation treatment for locally advanced anal cancer. Radiother Oncol 2015;117:234–9. [DOI] [PubMed]; Joseph K, Nijjar Y, Warkentin H, Schiller D, Tankel K, Usmani N. et al. Prospective phase II study of tomotherapy based chemoradiation treatment for locally advanced anal cancer. Radiother Oncol. 2015;117:234–9. doi: 10.1016/j.radonc.2015.08.008. [DOI] [PubMed] [Google Scholar]
- [51].Ayyildiz A, Akgül KT, Huri E, Nuhoğlu B, Kiliçoğlu B, Ustün H, et al. Use of porcine small intestinal submucosa in bladder augmentation in rabbit: long-term histological outcome. ANZ J Surg 2008;78:82–6. [DOI] [PubMed]; Ayyildiz A, Akgül KT, Huri E, Nuhoğlu B, Kiliçoğlu B, Ustün H. et al. Use of porcine small intestinal submucosa in bladder augmentation in rabbit: long-term histological outcome. ANZ J Surg. 2008;78:82–6. doi: 10.1111/j.1445-2197.2007.04361.x. [DOI] [PubMed] [Google Scholar]
- [52].Badylak SF, Kropp B, McPherson T, Liang H, Snyder PW. Small intestinal submucosa: a rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng 1998;4:379–87. [DOI] [PubMed]; Badylak SF, Kropp B, McPherson T, Liang H, Snyder PW. Small intestinal submucosa: a rapidly resorbed bioscaffold for augmentation cystoplasty in a dog model. Tissue Eng. 1998;4:379–87. doi: 10.1089/ten.1998.4.379. [DOI] [PubMed] [Google Scholar]
- [53].Kropp BP. Small-intestinal submucosa for bladder augmentation: a review of preclinical studies. World J Urol 1998;16:262–7. [DOI] [PubMed]; Kropp BP. Small-intestinal submucosa for bladder augmentation: a review of preclinical studies. World J Urol. 1998;16:262–7. doi: 10.1007/s003450050064. [DOI] [PubMed] [Google Scholar]
- [54].Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology 1995;46: 396–400. [DOI] [PubMed]; Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF. et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46:396–400. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- [55].Caione P, Boldrini R, Salerno A, Nappo SG. Bladder augmentation using acellular collagen biomatrix: a pilot experience in exstrophic patients. Pediatr Surg Int 2012;28:421–8. [DOI] [PubMed]; Caione P, Boldrini R, Salerno A, Nappo SG. Bladder augmentation using acellular collagen biomatrix: a pilot experience in exstrophic patients. Pediatr Surg Int. 2012;28:421–8. doi: 10.1007/s00383-012-3063-0. [DOI] [PubMed] [Google Scholar]
- [56].Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol 2013;9:878–83. [DOI] [PubMed]; Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol. 2013;9:878–83. doi: 10.1016/j.jpurol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- [57].Eastman R Jr, Leaf EM, Zhang D, True LD, Sweet RM, Seidel K, et al. Fibroblast growth factor-10 signals development of von Brunn’s nests in the exstrophic bladder. Am J Physiol Renal Physiol 2010;299:F1094–110. [DOI] [PMC free article] [PubMed]; Eastman R Jr, Leaf EM, Zhang D, True LD, Sweet RM, Seidel K. et al. Fibroblast growth factor-10 signals development of von Brunn’s nests in the exstrophic bladder. Am J Physiol Renal Physiol. 2010;299:F1094–110. doi: 10.1152/ajprenal.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang F, Liao L. Tissue engineered cystoplasty augmentation for treatment of neurogenic bladder using small intestinal submucosa: an exploratory study. J Urol 2014;192:544–50. [DOI] [PubMed]; Zhang F, Liao L. Tissue engineered cystoplasty augmentation for treatment of neurogenic bladder using small intestinal submucosa: an exploratory study. J Urol. 2014;192:544–50. doi: 10.1016/j.juro.2014.01.116. [DOI] [PubMed] [Google Scholar]
- [59].Scuderi N, Chiummariello S, De Gado F. Correction of hypospadias with a vertical preputial island flap: a 23-year experience. J Urol 2006;175:1083–7; discussion 1087. [DOI] [PubMed]; Scuderi N, Chiummariello S, De Gado F. Correction of hypospadias with a vertical preputial island flap: a 23-year experience. J Urol. 2006;175:1083–7. doi: 10.1016/S0022-5347(05)00407-6. discussion 1087. [DOI] [PubMed] [Google Scholar]
- [60].Cruz-Diaz O, Castellan M, Gosalbez R. Use of buccal mucosa in hypospadias repair. Curr Urol Rep 2013;14:366–72. [DOI] [PubMed]; Cruz-Diaz O, Castellan M, Gosalbez R. Use of buccal mucosa in hypospadias repair. Curr Urol Rep. 2013;14:366–72. doi: 10.1007/s11934-013-0334-9. [DOI] [PubMed] [Google Scholar]
- [61].Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol 2008;26:323–6. [DOI] [PubMed]; Dorin RP, Pohl HG, De Filippo RE, Yoo JJ, Atala A. Tubularized urethral replacement with unseeded matrices: what is the maximum distance for normal tissue regeneration? World J Urol. 2008;26:323–6. doi: 10.1007/s00345-008-0316-6. [DOI] [PubMed] [Google Scholar]
- [62].Fu Q, Deng CL, Liu W, Cao YL. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int 2007;99:1162–5. [DOI] [PubMed]; Fu Q, Deng CL, Liu W, Cao YL. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007;99:1162–5. doi: 10.1111/j.1464-410X.2006.06691.x. [DOI] [PubMed] [Google Scholar]
- [63].Mikami H, Kuwahara G, Nakamura N, Yamato M, Tanaka M, Kodama S. Two-layer tissue engineered urethra using oral epithelial and muscle derived cells. J Urol 2012;187:1882–9. [DOI] [PubMed]; Mikami H, Kuwahara G, Nakamura N, Yamato M, Tanaka M, Kodama S. Two-layer tissue engineered urethra using oral epithelial and muscle derived cells. J Urol. 2012;187:1882–9. doi: 10.1016/j.juro.2011.12.059. [DOI] [PubMed] [Google Scholar]
- [64].Li C, Xu YM, Song LJ, Fu Q, Cui L, Yin S. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J Urol 2008;180:1538–42. [DOI] [PubMed]; Li C, Xu YM, Song LJ, Fu Q, Cui L, Yin S. Urethral reconstruction using oral keratinocyte seeded bladder acellular matrix grafts. J Urol. 2008;180:1538–42. doi: 10.1016/j.juro.2008.06.013. [DOI] [PubMed] [Google Scholar]
- [65].Feng C, Xu YM, Fu Q, Zhu WD, Cui L. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng Part A 2011;17:3011–9. [DOI] [PubMed]; Feng C, Xu YM, Fu Q, Zhu WD, Cui L. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng Part A. 2011;17:3011–9. doi: 10.1089/ten.TEA.2011.0061. [DOI] [PubMed] [Google Scholar]
- [66].Fossum M, Svensson J, Kratz G, Nordenskjöld A. Autologous in vitro cultured urothelium in hypospadias repair. J Pediatr Urol 2007;3:10–8. [DOI] [PubMed]; Fossum M, Svensson J, Kratz G, Nordenskjöld A. Autologous in vitro cultured urothelium in hypospadias repair. J Pediatr Urol. 2007;3:10–8. doi: 10.1016/j.jpurol.2006.01.018. [DOI] [PubMed] [Google Scholar]
- [67].Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol 2008;53:1263–9. [DOI] [PubMed]; Bhargava S, Patterson JM, Inman RD, MacNeil S, Chapple CR. Tissue-engineered buccal mucosa urethroplasty-clinical outcomes. Eur Urol. 2008;53:1263–9. doi: 10.1016/j.eururo.2008.01.061. [DOI] [PubMed] [Google Scholar]
- [68].Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 2011;377:1175–82. [DOI] [PMC free article] [PubMed]; Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–82. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Burmeister D, Aboushwareb T, Tan J, Link K, Andersson KE, Christ G. Early stages of in situ bladder regeneration in a rodent model. Tissue Eng Part A 2010;16:2541–51. [DOI] [PMC free article] [PubMed]; Burmeister D, Aboushwareb T, Tan J, Link K, Andersson KE, Christ G. Early stages of in situ bladder regeneration in a rodent model. Tissue Eng Part A. 2010;16:2541–51. doi: 10.1089/ten.tea.2009.0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lin HK, Cowan R, Moore P, Zhang Y, Yang Q, Peterson Jr. JA, et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol 2004;171:1348–52. [DOI] [PubMed]; Lin HK, Cowan R, Moore P, Zhang Y, Yang Q, Peterson A. et al. Characterization of neuropathic bladder smooth muscle cells in culture. J Urol. 2004;171:1348–52. doi: 10.1097/01.ju.0000108800.47594.8b. [DOI] [PubMed] [Google Scholar]
- [71].Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol 2011;186:2014–20. [DOI] [PubMed]; Subramaniam R, Hinley J, Stahlschmidt J, Southgate J. Tissue engineering potential of urothelial cells from diseased bladders. J Urol. 2011;186:2014–20. doi: 10.1016/j.juro.2011.07.031. [DOI] [PubMed] [Google Scholar]
- [72].Dozmorov MG, Kropp BP, Hurst RE, Cheng EY, Lin HK. Differentially expressed gene networks in cultured smooth muscle cells from normal and neuropathic bladder. J Smooth Muscle Res 2007;43:55–72. [DOI] [PubMed]; Dozmorov MG, Kropp BP, Hurst RE, Cheng EY, Lin HK. Differentially expressed gene networks in cultured smooth muscle cells from normal and neuropathic bladder. J Smooth Muscle Res. 2007;43:55–72. doi: 10.1540/jsmr.43.55. [DOI] [PubMed] [Google Scholar]
- [73].Seth A, Chung YG, Kim D, Ramachandran A, Cristofaro V, Gomez P 3rd, et al. The impact of discrete modes of spinal cord injury on bladder muscle contractility. BMC Urol 2013;13:24. [DOI] [PMC free article] [PubMed]; Seth A, Chung YG, Kim D, Ramachandran A, Cristofaro V, Gomez P 3rd. et al. The impact of discrete modes of spinal cord injury on bladder muscle contractility. BMC Urol. 2013;13:24. doi: 10.1186/1471-2490-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chung YG, Algarrahi K, Franck D, Tu DD, Adam RM, Kaplan DL, et al. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials 2014;35:7452–9. [DOI] [PMC free article] [PubMed]; Chung YG, Algarrahi K, Franck D, Tu DD, Adam RM, Kaplan DL. et al. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials. 2014;35:7452–9. doi: 10.1016/j.biomaterials.2014.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vasquez E, Cristofaro V, Lukianov S, Burkhard FC, Gheinani AH, Monastyrskaya K, et al. Deletion of neuropilin 2 enhances detrusor contractility following bladder outlet obstruction. JCI Insight 2017;2:e90617. [DOI] [PMC free article] [PubMed]; Vasquez E, Cristofaro V, Lukianov S, Burkhard FC, Gheinani AH, Monastyrskaya K. et al. Deletion of neuropilin 2 enhances detrusor contractility following bladder outlet obstruction. JCI Insight. 2017;2:e90617. doi: 10.1172/jci.insight.90617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Farhat WA. Tissue engineering of the bladder – when will we get there? J Urol 2014;192:1021–2. [DOI] [PubMed]; Farhat WA. Tissue engineering of the bladder – when will we get there? J Urol. 2014;192:1021–2. doi: 10.1016/j.juro.2014.07.079. [DOI] [PubMed] [Google Scholar]
- [77].Obara T, Matsuura S, Narita S, Satoh S, Tsuchiya N, Habuchi T. Bladder acellular matrix grafting regenerates urinary bladder in the spinal cord injury rat. Urology 2006;68:892–7. [DOI] [PubMed]; Obara T, Matsuura S, Narita S, Satoh S, Tsuchiya N, Habuchi T. Bladder acellular matrix grafting regenerates urinary bladder in the spinal cord injury rat. Urology. 2006;68:892–7. doi: 10.1016/j.urology.2006.04.030. [DOI] [PubMed] [Google Scholar]
- [78].Cayan S, Chermansky C, Schlote N, Sekido N, Nunes L, Dahiya R, et al. The bladder acellular matrix graft in a rat chemical cystitis model: functional and histologic evaluation. J Urol 2002;168:798–804. [DOI] [PubMed]; Cayan S, Chermansky C, Schlote N, Sekido N, Nunes L, Dahiya R. et al. The bladder acellular matrix graft in a rat chemical cystitis model: functional and histologic evaluation. J Urol. 2002;168:798–804. doi: 10.1016/s0022-5347(05)64746-5. [DOI] [PubMed] [Google Scholar]
- [79].Akbal C, Lee SD, Packer SC, Davis MM, Rink RC, Kaefer M. Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol 2006;176:1706–11. [DOI] [PubMed]; Akbal C, Lee SD, Packer SC, Davis MM, Rink RC, Kaefer M. Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol. 2006;176:1706–11. doi: 10.1016/j.juro.2006.04.085. [DOI] [PubMed] [Google Scholar]
- [80].Slaughenhoupt BL, Mathews RI, Peppas DS, Gearhart JP. A large animal model of bladder exstrophy: observations of bladder smooth muscle and collagen content. J Urol 1999;162:2119–22. [DOI] [PubMed]; Slaughenhoupt BL, Mathews RI, Peppas DS, Gearhart JP. A large animal model of bladder exstrophy: observations of bladder smooth muscle and collagen content. J Urol. 1999;162:2119–22. doi: 10.1016/S0022-5347(05)68137-2. [DOI] [PubMed] [Google Scholar]
- [81].Fu D, Chong T, Li H, Zhang H, Wang Z. Docetaxel inhibits urethral stricture formation, an initial study in rabbit model. PLoS One 2014;9:e112097. [DOI] [PMC free article] [PubMed]; Fu D, Chong T, Li H, Zhang H, Wang Z. Docetaxel inhibits urethral stricture formation, an initial study in rabbit model. PLoS One. 2014;9:e112097. doi: 10.1371/journal.pone.0112097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nordgren A. Moral imagination in tissue engineering research on animal models. Biomaterials 2004;25:1723–34. [DOI] [PubMed]; Nordgren A. Moral imagination in tissue engineering research on animal models. Biomaterials. 2004;25:1723–34. doi: 10.1016/s0142-9612(03)00506-4. [DOI] [PubMed] [Google Scholar]
- [83].Iannaccone PM, Galat V, Bury MI, Ma YC, Sharma AK. The utility of stem cells in pediatric urinary bladder regeneration. Pediatr Res 2018;83:258–66. [DOI] [PubMed]; Iannaccone PM, Galat V, Bury MI, Ma YC, Sharma AK. The utility of stem cells in pediatric urinary bladder regeneration. Pediatr Res. 2018;83:258–66. doi: 10.1038/pr.2017.229. [DOI] [PubMed] [Google Scholar]
- [84].Smolar J, Souzan Salemi S, Horst M, Sulser T, Eberlia D. Stem cells in functional bladder engineering. Transfus Med Hemother 2016;43:328–35. [DOI] [PMC free article] [PubMed]; Smolar J, Souzan Salemi S, Horst M, Sulser T, Eberlia D. Stem cells in functional bladder engineering. Transfus Med Hemother. 2016;43:328–35. doi: 10.1159/000447977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells 2013;31:1044–50. [DOI] [PMC free article] [PubMed]; Smith JN, Calvi LM. Concise review: current concepts in bone marrow microenvironmental regulation of hematopoietic stem and progenitor cells. Stem Cells. 2013;31:1044–50. doi: 10.1002/stem.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med 2015;4:412. [DOI] [PMC free article] [PubMed]; Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2015;4:412. doi: 10.5966/sctm.2013-0196erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sharma AK, Fuller NJ, Sullivan RR, Fulton N, Hota PV, Harrington DA, et al. Defined populations of bone marrow derived mesenchymal stem and endothelial progenitor cells for bladder regeneration. J Urol 2009;182:1898–905. [DOI] [PubMed]; Sharma AK, Fuller NJ, Sullivan RR, Fulton N, Hota PV, Harrington DA. et al. Defined populations of bone marrow derived mesenchymal stem and endothelial progenitor cells for bladder regeneration. J Urol. 2009;182:1898–905. doi: 10.1016/j.juro.2009.03.014. [DOI] [PubMed] [Google Scholar]
- [88].Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H, et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials 2010;31:6207–17. [DOI] [PubMed]; Sharma AK, Hota PV, Matoka DJ, Fuller NJ, Jandali D, Thaker H. et al. Urinary bladder smooth muscle regeneration utilizing bone marrow derived mesenchymal stem cell seeded elastomeric poly(1,8-octanediol-co-citrate) based thin films. Biomaterials. 2010;31:6207–17. doi: 10.1016/j.biomaterials.2010.04.054. [DOI] [PubMed] [Google Scholar]
- [89].Li CL, Liao WB, Yang SX, Song C, Li YW, Xiong YH, et al. Urethral reconstruction using bone marrow mesenchymal stem cell- and smooth muscle cell-seeded bladder acellular matrix. Transplant Proc 2013;45:3402–7. [DOI] [PubMed]; Li CL, Liao WB, Yang SX, Song C, Li YW, Xiong YH. et al. Urethral reconstruction using bone marrow mesenchymal stem cell- and smooth muscle cell-seeded bladder acellular matrix. Transplant Proc. 2013;45:3402–7. doi: 10.1016/j.transproceed.2013.07.055. [DOI] [PubMed] [Google Scholar]
- [90].Valentin JE, Freytes DO, Grasman JM, Pesyna C, Freund J, Gilbert TW, et al. Oxygen diffusivity of biologic and synthetic scaffold materials for tissue engineering. J Biomed Mater Res A 2009;91:1010–7. [DOI] [PubMed]; Valentin JE, Freytes DO, Grasman JM, Pesyna C, Freund J, Gilbert TW. et al. Oxygen diffusivity of biologic and synthetic scaffold materials for tissue engineering. J Biomed Mater Res A. 2009;91:1010–7. doi: 10.1002/jbm.a.32328. [DOI] [PubMed] [Google Scholar]
- [91].Sharma AK, Bury MI, Fuller NJ, Marks AJ, Kollhoff DM, Rao MV, et al. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. Proc Natl Acad Sci USA 2013;110: 4003–8. [DOI] [PMC free article] [PubMed]; Sharma AK, Bury MI, Fuller NJ, Marks AJ, Kollhoff DM, Rao MV. et al. Cotransplantation with specific populations of spina bifida bone marrow stem/progenitor cells enhances urinary bladder regeneration. Proc Natl Acad Sci USA. 2013;110:4003–8. doi: 10.1073/pnas.1220764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv 2016;34:112–21. [DOI] [PubMed]; Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol Adv. 2016;34:112–21. doi: 10.1016/j.biotechadv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- [93].Kang M, Kim HH, Han YM. Generation of bladder urothelium from human pluripotent stem cells under chemically defined serum- and feeder-free system. Int J Mol Sci 2014;15:7139–57. [DOI] [PMC free article] [PubMed]; Kang M, Kim HH, Han YM. Generation of bladder urothelium from human pluripotent stem cells under chemically defined serum- and feeder-free system. Int J Mol Sci. 2014;15:7139–57. doi: 10.3390/ijms15057139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Wang Z, Wen Y, Li YH, Wei Y, Green M, Wani P, et al. Smooth muscle precursor cells derived from human pluripotent stem cells for treatment of stress urinary incontinence. Stem Cells Dev 2016;25:453–61. [DOI] [PMC free article] [PubMed]; Wang Z, Wen Y, Li YH, Wei Y, Green M, Wani P. et al. Smooth muscle precursor cells derived from human pluripotent stem cells for treatment of stress urinary incontinence. Stem Cells Dev. 2016;25:453–61. doi: 10.1089/scd.2015.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ, et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol 2008;180:2226–33. [DOI] [PubMed]; Zhang Y, McNeill E, Tian H, Soker S, Andersson KE, Yoo JJ. et al. Urine derived cells are a potential source for urological tissue reconstruction. J Urol. 2008;180:2226–33. doi: 10.1016/j.juro.2008.07.023. [DOI] [PubMed] [Google Scholar]
- [96].Zhang D, Wei G, Li P, Zhou X, Zhang Y. Urine-derived stem cells: a novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis 2014;1:8–17. [DOI] [PMC free article] [PubMed]; Zhang D, Wei G, Li P, Zhou X, Zhang Y. Urine-derived stem cells: a novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 2014;1:8–17. doi: 10.1016/j.gendis.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther 2014;5:69. [DOI] [PMC free article] [PubMed]; Qin D, Long T, Deng J, Zhang Y. Urine-derived stem cells for potential use in bladder repair. Stem Cell Res Ther. 2014;5:69. doi: 10.1186/scrt458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Liu Y, Ma W, Liu B, Wang Y, Chu J, Xiong G, et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther 2017;8:63. [DOI] [PMC free article] [PubMed]; Liu Y, Ma W, Liu B, Wang Y, Chu J, Xiong G. et al. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther. 2017;8:63. doi: 10.1186/s13287-017-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Schafer FM, Algarrahi K, Savarino A, Yang X, Seager C, Franck D, et al. Mode of surgical injury influences the source of urothelial progenitors during bladder defect repair. Stem Cell Reports 2017;9:2005–17. [DOI] [PMC free article] [PubMed]; Schafer FM, Algarrahi K, Savarino A, Yang X, Seager C, Franck D. et al. Mode of surgical injury influences the source of urothelial progenitors during bladder defect repair. Stem Cell Reports. 2017;9:2005–17. doi: 10.1016/j.stemcr.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M, et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell 2013;26:469–82. [DOI] [PMC free article] [PubMed]; Gandhi D, Molotkov A, Batourina E, Schneider K, Dan H, Reiley M. et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Dev Cell. 2013;26:469–82. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mauney JR, Adam RM. Dynamic reciprocity in cell-scaffold interactions. Adv Drug Deliv Rev 2015;82–83:77–85. [DOI] [PMC free article] [PubMed]; Mauney JR, Adam RM. Dynamic reciprocity in cell-scaffold interactions. Adv Drug Deliv Rev. 2015;82–83:77–85. doi: 10.1016/j.addr.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Klar AS, Michalak-Mićka K, Biedermann T, Simmen-Meuli C, Reichmann E, Meuli M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int 2018;34:129–35. [DOI] [PubMed]; Klar AS, Michalak-Mićka K, Biedermann T, Simmen-Meuli C, Reichmann E, Meuli M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr Surg Int. 2018;34:129–35. doi: 10.1007/s00383-017-4179-z. [DOI] [PubMed] [Google Scholar]
- [103].Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013;34:4439–51. [DOI] [PMC free article] [PubMed]; Garg K, Pullen NA, Oskeritzian CA, Ryan JJ, Bowlin GL. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34:4439–51. doi: 10.1016/j.biomaterials.2013.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng 2014;42:1508–16. [DOI] [PubMed]; Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42:1508–16. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- [105].Bury MI, Fuller NJ, Meisner JW, Hofer MD, Webber MJ, Chow LW, et al. The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials 2014;35:9311–21. [DOI] [PMC free article] [PubMed]; Bury MI, Fuller NJ, Meisner JW, Hofer MD, Webber MJ, Chow LW. et al. The promotion of functional urinary bladder regeneration using anti-inflammatory nanofibers. Biomaterials. 2014;35:9311–21. doi: 10.1016/j.biomaterials.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol 2014;5:510. [DOI] [PMC free article] [PubMed]; Brown BN, Sicari BM, Badylak SF. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol. 2014;5:510. doi: 10.3389/fimmu.2014.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014;35:4477–88. [DOI] [PMC free article] [PubMed]; Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW. et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–88. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ley K. M1 means kill; M2 means heal. J Immunol 2017;199:2191–3. [DOI] [PubMed]; Ley K. M1 means kill; M2 means heal. J Immunol. 2017;199:2191–3. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- [109].Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One 2013;8:e81774. [DOI] [PMC free article] [PubMed]; Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013;8:e81774. doi: 10.1371/journal.pone.0081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018;223:101–11. [DOI] [PubMed]; Chistiakov DA, Myasoedova VA, Revin VV, Orekhov AN, Bobryshev YV. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology. 2018;223:101–11. doi: 10.1016/j.imbio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- [111].Bullers SJ, Baker SC, Ingham E, Southgate J. The human tissue-biomaterial interface: a role for PPARγ-dependent glucocorticoid receptor activation in regulating the CD163+ M2 macrophage phenotype. Tissue Eng Part A 2014;20: 2390–401. [DOI] [PMC free article] [PubMed]; Bullers SJ, Baker SC, Ingham E, Southgate J. The human tissue-biomaterial interface: a role for PPARγ-dependent glucocorticoid receptor activation in regulating the CD163+ M2 macrophage phenotype. Tissue Eng Part A. 2014;20:2390–401. doi: 10.1089/ten.tea.2013.0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chen W, Shi C, Yi S, Chen B, Zhang W, Fang Z, et al. Bladder regeneration by collagen scaffolds with collagen binding human basic fibroblast growth factor. J Urol 2010;183: 2432–9. [DOI] [PubMed]; Chen W, Shi C, Yi S, Chen B, Zhang W, Fang Z. et al. Bladder regeneration by collagen scaffolds with collagen binding human basic fibroblast growth factor. J Urol. 2010;183:2432–9. doi: 10.1016/j.juro.2010.02.042. [DOI] [PubMed] [Google Scholar]
- [113].Lorentz KM, Yang L, Frey P, Hubbell JA. Engineered insulin-like growth factor-1 for improved smooth muscle regeneration. Biomaterials 2012;33:494–503. [DOI] [PubMed]; Lorentz KM, Yang L, Frey P, Hubbell JA. Engineered insulin-like growth factor-1 for improved smooth muscle regeneration. Biomaterials. 2012;33:494–503. doi: 10.1016/j.biomaterials.2011.09.088. [DOI] [PubMed] [Google Scholar]
- [114].Zhou L, Yang B, Sun C, Qiu X, Sun Z, Chen Y, et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A 2013;19:264–76. [DOI] [PMC free article] [PubMed]; Zhou L, Yang B, Sun C, Qiu X, Sun Z, Chen Y. et al. Coadministration of platelet-derived growth factor-BB and vascular endothelial growth factor with bladder acellular matrix enhances smooth muscle regeneration and vascularization for bladder augmentation in a rabbit model. Tissue Eng Part A. 2013;19:264–76. doi: 10.1089/ten.tea.2011.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T, et al. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int 2009;103:1424–8. [DOI] [PubMed]; Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T. et al. Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int. 2009;103:1424–8. doi: 10.1111/j.1464-410X.2008.08129.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.