Abstract
Introduction:
Lymphoedema is a worldwide pandemic causing swelling of tissues due to dysfunctional transport of lymph fluid. Present management concepts are based in conservative palliation of symptoms through manual lymphatic drainage, use of compression garments, manual lymph drainage, exercise, and skin care. Nevertheless, some curative options as autologous lymph node transplantation were shown to reduce lymphoedema in selected cases. Lately, some concern has arisen due to reports of donor site morbidity. A possible solution could be the development of artificial lymph node scaffolds as niches of lymphatic regeneration. Engineering these scaffolds has included cryopreservation of lymph node stroma. However, the effects of cryopreservation on the regeneration capacities of these organs were unknown.
Materials and methods:
Here, we used the minipig animal model to assess lymphatic regeneration processes after cryopreservation of autologous lymph nodes. Superficial inguinal lymph nodes were excised and conserved at −80°C for 1 month. Thereafter, lymph node fragments were transplanted in the subcutaneous tissue.
Results:
Regeneration of the lymph nodes was assessed five months after transplantation. We show that lymph node fragment regeneration takes place in spite of former cryopreservation. Transplanted fragments presented typical histological appearance. Their draining capacity was documented by macroscopic transport of Berlin Blue dye as well as through SPECT-CT hybrid imaging.
Discussion:
In conclusion, our results suggest that processes of cryopreservation can be used in the creation of artificial lymph node scaffolds without major impairment of lymph node fragments regeneration.
Keywords: artificial lymph node, cryopreservation, lymph node transplant, lymphoedema
Introduction
Lymphatic fluid, products of cell catabolism, antigens and debris are transported from the tissues and organs through a fine network of transparent valved vessels called lymphatics [1], [2]. These drain into lymph node chains, where lymphatic fluid is progressively thickened through selective fluid filtration into the blood capillaries of these organs [3]. This reabsorption not only promotes homeostasis of drained tissues but also allows exposure of nodal dendritic cells and macrophages to potential pathogens, promoting antigen phagocytosis. Dendritic cell-driven presentation of antigen surface molecules to lymphocytes eventually activates tissue-specific immunity [4].
Impairment of lymph node function or lymphatic transport can lead to tissue swelling called lymphoedema. This disease has multiple causes. Primary lymphoedema has a reduced overall incidence. In developing countries, secondary lymphoedema mainly occurs due to filarial worm infection. According to World Health Organization data, about 40 million patients have lymphoedema due to these parasites [5], [6]. In developed countries, secondary lymphoedema is often related to oncologic causes or trauma [7].
In malignant diseases such as breast cancer, melanoma or prostate cancer, draining lymph nodes participate in the recognition of tumour cells and have become important markers for metastatic disease and overall prognosis [8]. Surgical removal of lymph nodes is often necessary. About 20%–30% of the patients develop limb lymphoedema after axillary or inguinal lymph node removal [9], [10]. Lymphoedema is a lifelong condition that reduces the quality of life and productivity of affected patients [10]. Its continuous conservative management has high costs for the health system [11].
As the immune system and its organs show great plasticity, concepts of autologous lymph node transplantation were developed for the treatment of secondary lymphoedema [12], [13], [14]. The aim of these surgical procedures is to substitute damaged lymph nodes through functioning nodes contained in autologous tissue flaps. Clinical studies seem to show some benefit, with reported cure in 32%–40% of the patients, and improvement in the great majority [12], [13], [14], [15], [16]. However, donor site morbidity in the form of lymphoedema of the donor area was also described [17], [18], [19]. It might therefore be important to develop alternative donor solutions to prevent this major complication.
Concepts of artificial lymph node engineering originally arose to face the challenge of patients with congenital paucity of lymph nodes without autologous harvesting possibilities (primary lymphoedema). Nevertheless, present evidence of possible donor site morbidity might extend the potential utility of artificial lymph node constructions to the treatment of secondary lymphoedema.
Although still in development, artificial lymph node constructs seem to induce efficient regeneration when biocompatible scaffolds and stromal cells are used [20]. This induces migration of immune cells into the artificial constructs, resulting in the presence of dendritic cells as well as B- and T-lymphocyte clusters with typical cortical and paracortical distribution [21]. Some processes of tissue engineering associated with artificial lymph node constructs apply methods of cryopreservation to allow laboratory manipulation [21]. Nevertheless, it was unknown if such cryopreservation could permanently damage the regenerative capacity of nodal tissues [22].
The purpose of this study was to analyse lymph node fragment regeneration after cryopreservation in the minipig model. The viability of cryopreserved lymph node fragments was assessed after avascular transplantation. Here, physiological drainage capacities were analysed through SPECT-CT hybrid imaging and subsequent histologic analysis provided insights on regeneration processes.
Methods
Minipig model and experimental groups
A total of eight wild-type Göttinger minipigs weighing 6–11 kg were obtained from the animal facility of the University of Göttingen (Relliehausen, Germany). They were housed in our animal facility, according to German legal specifications, and fed mixed food and water ad libitum. Animal procedures were approved by the government of Lower Saxony (Ref. No. 509.6-42502-07/1402).
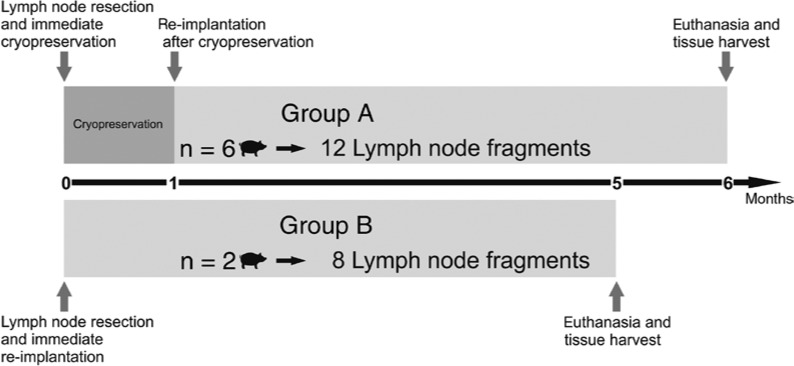
Animals were divided in two groups (Figure 1). The number of animals allocated to each group was different, as group A was prioritized on being given the possibility of intra-individual limb volume control (negative control in the non-operated left limb). Group A (n=6) was therefore submitted to resection of the right lymph node under general anaesthesia, as previously described [23], with the left side of the animal being left intact. The single superficial groin lymph node present in this species was operatively excised on the right side. Afferent and efferent lymphatics were carefully sharply resected in the immediate vicinity of the organ capsule. The blood vessels of the hilum were also resected. No ligatures were applied to the organ. The excised lymph node was then cut transversally in two fragments of about 1-cm3 size and introduced in sterile recipients. After hermetic closure, recipients were immediately submersed in a bath of nitric oxide. Soft plastic recipients were used to maximise the surface of thermal contact. No chemical cryoprotection was performed, as the maximal distance for cold penetration was about 5 mm. Therefore, the risk of water crystals formation was considered negligible, rendering additional exposure of the lymph node fragments to alcohols or sulfoxides evitable. Fragments were transported to final storage at −80°C immediately after the operation and were conserved in these conditions for 1 month. Thereafter, animals were re-operated under general anaesthesia, and the corresponding two fragments (autologous transplantation) were re-implanted in the subcutaneous fatty tissue of the groin and left to unfreeze in vivo. The left groin was used as negative control for limb swelling.
Figure 1:
Schematic overview of the study outline during the 6-month follow-up.
In both animals of group B (positive control, n=2, total of four lymph nodes), both groin lymph nodes were excised and transversally fragmented. Immediate avascular re-implantation occurred in the same operative procedure.
In group A, a degeneration of cryopreserved, avascular lymph node transplants would be plausible and could become the origin of necrosis and inflammation at the operative site with subsequent lymphoedema. Therefore, transplants were only performed unilaterally, allowing comparison of the limbs.
In control group B (positive control), based on our previous unpublished observations, we expected no lymphoedema development and therefore proceeded to bilateral transplants.
All transplantation areas were closed by atraumatic sutures and the skin was sutured. Wound healing was regularly checked. Premedication, anaesthesia and wound prophylaxis were performed, as previously described [23].
Lymph node fragment regeneration assessment through SPECT-CT hybrid imaging and histology
Five months after transplantation, animals in both groups were injected a mixture of 10 MBq 99mTc-nanocolloid in combination with Berlin blue in the hind paws, as previously described [23]. Animals were then left alive 1 h under anaesthesia to allow lymphatic drainage of the extremities. After exitus letalis, SPECT-CT hybrid imaging was performed, as previously described [23]. Shortly after, histologic probes of regenerated lymph node fragments were taken based on gamma probe signalling and macroscopic blue dye caption. A photographic register of the skin where positive samples were marked in situ allowed direct correspondence between SPECT-CT hybrid imaging and later histologic results. Lymph node samples were frozen in liquid nitrogen immediately after sampling and left to lose their radioactivity for 3 days. To avoid excessive staining due to the pre mortem administration of Berlin blue in the paws, samples were sectioned and simply stained with haematoxylin-eosin. Full slices of macroscopic regions of interest were analysed initially at ×10 magnification under the optical microscope. Samples presenting lymph node fragments were selected for further characterisation. Lymph node fragments were considered vital when they presented typical B- and T-lymphocyte distribution in the cortex and paracortex, respectively, showed uptake of Berlin blue in the sinuses and had lipomatosis and fibrotic degeneration features in less than 50% of the fragment surface on at least three sections. Histologic analysis was performed blind by one observer. Later, histologic probe numbers were associated with each SPECT-CT hybrid imaging to allow final assessment of results.
Data analysis
The experimental data generated were reported as fragment regeneration rate and expressed as proportion to the total number of transplanted fragments. The restricted number of observations in the reported big animal model allowed direct interpretation of the results without additional statistical analysis. Histologic pictures were taken, with the Panoramic Viewer (3dHistech, Budapest, Hungary) as viewing software.
Results
In this study, a total of 8 minipigs and 10 groin lymph nodes divided into 20 fragments were analysed (two animals having had a bilateral procedure). Operative procedures were well tolerated in all cases. Animals were controlled weekly for any major complications such as wound healing problems, seromata or macroscopic lymphoedema (defined for this purpose as pitting of the subcutaneous tissue after deep thumb pressure for 1 min). No complications were registered during follow-up.
Avascular lymph node fragments can regenerate in the subcutaneous tissue
Regardless of the groups, 14 avascular lymph node fragments were able to regenerate and connect to the surrounding blood and lymphatic vessels. Six fragments were histologically necrotic and/or showed no locoregional draining activity, as they did not capture 99mTc-nanocolloid or Berlin blue injected in the hind paws. Therefore, avascular lymph node fragment survival in healthy subcutaneous tissues was observed in ca. 70% of the cases.
Cryopreserved fragments have similar regeneration rates than fresh fragments
To assess the regeneration rates of transplanted lymph node fragments, blind analysis of histologic probes was performed, and results were later combined with obtained SPECT-CT hybrid imaging. A 100% correlation of both methods was observed. Regenerated fragments were clearly identifiable in SPECT-CT hybrid imaging as functional and draining the lymphatic fluid of the injected hind paws (Figure 2). Later, histologic probes were sampled based on macroscopic caption of injected Berlin blue dye, but were also located post mortem with a gamma probe. Histologic sampling occurred in the whole region of interest to account for eventual necrotic fragments not visible in the process of peripheral injection. Regional probes containing only fatty tissue or necrotic lymph nodes were considered as non-regenerated transplants (Figure 3B and C). In all cases, they corresponded to regions failing to cause “hot spots” in SPECT-CT hybrid imaging. Histologic analysis of fragments capturing Berlin blue/99mTc-nanocolloid revealed a functional organisation of the lymph node architecture, presence of a capsule and peripheral sinuses engorged with blue dye (Figure 3A and D–F). Additionally, indirect signs of immune function were given by the presence of germinal follicles (Figure 3F). According to our criteria, 8 of 12 fragments were able to regenerate for the cryopreserved lymph nodes in group A. In group B, 6 of 8 immediately transplanted fragments were regenerated after the 5-month follow-up. Therefore, the regeneration rate in group A (67%) was comparable to the regeneration in group B (75%), despite the relatively low number of total observations (Figure 4). These findings suggest no major impact of cryopreservation in the capacity of lymph node fragments to regenerate in healthy subcutaneous fatty tissue.
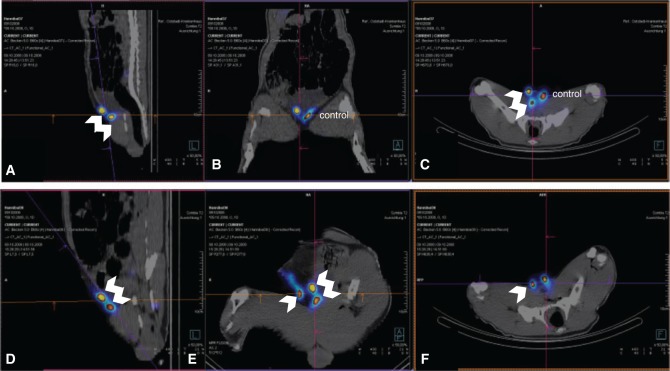
Figure 2:
Lymphatic imaging for functional analysis of transplanted lymph node fragments.
SPECT-CT hybrid imaging of a minipig in group A (A–C) with two avascular cryotransplants in the right groin (double arrow). The left groin was used as control. All images were taken after injection of 99mTc-nanocolloid bilaterally in the hind paws. (D–F) SPECT-CT hybrid imaging of a minipig in group B (bilateral transplantation). In the right groin, only one of the two transplanted fragments is vital (single arrow); in the left groin, both transplanted fragments are vital and draining the periphery (double arrow). (A, D) Sagittal plane. (B, E) Coronal plane. (C, F) Transverse plane.
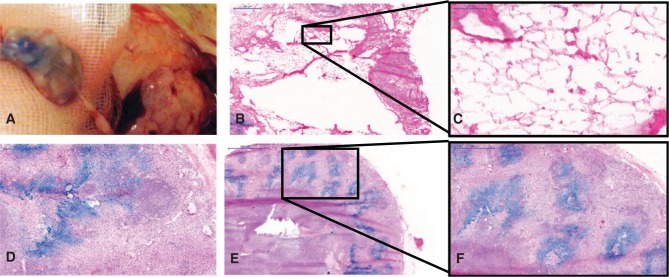
Figure 3:
Macroscopic and microscopic aspects of transplanted lymph node fragments (haematoxylin-eosin).
(A) Intraoperative macroscopic view. (B, C) Necrotic fragments presented fatty degeneration features observable within the lymph node capsule without major presence of lymphocytes. Vital fragments, in contrast, showed typical lymph node architecture (D–F) and sometimes germinal follicles (F). Here, blue-stained sinuses result from the peripheral injection of Berlin blue stain in the hind paws ante mortem. Scales: (B and F) 500 μm, (C) 100 μm, (D) 200 μm, and (E) 1000 μm.
Figure 4:
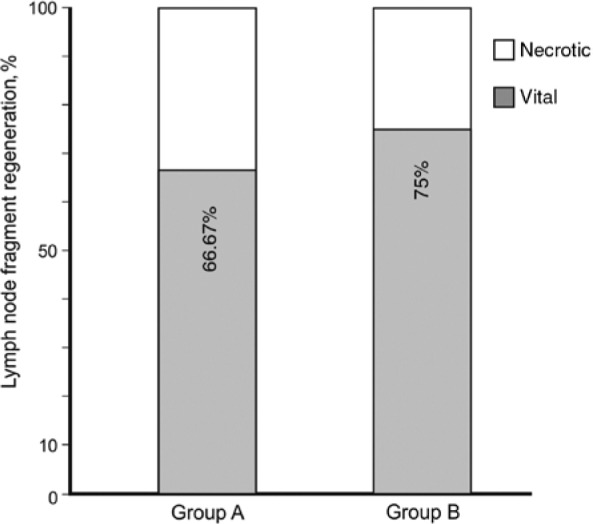
Regeneration rates (in percent) for each group.
Discussion
Lymphoedema is one of the pandemics that reached the twenty-first century without an established cure [7]. Meeting the challenges of lymphoedema treatment implies fine tuning of several management options [24], as lymphoedema has several causes. Because there is no universal cure for lymphoedema, it is paramount to thoroughly understand lymphatic regeneration and explore possible solutions to attain successful management options. The possibility of generating artificial lymph nodes might be therapeutic in specific cases when patients lack functioning donor nodes [25].
Animal model
This study aimed at a proof of concept. The animal model used is not a paradigm for lymphoedema, as none of the animals in this study developed this condition. It is still extraordinary that even bilateral resection of both superficial groin lymph nodes in group B did not generate any kind of macroscopic swelling of the hind paws. These findings suggest an intact regeneration capacity of lymphatics in these relatively young animals, a characteristic that might be lost in patients with secondary lymphoedema, e.g. after oncologic therapy including operative scars and radiotherapy of the surrounding tissues [8]. Since avascular organ transplants are initially completely dependent of the local diffusion capacities of the tissues, modelling the subcutaneous conditions in secondary lymphoedema patients is paramount for the predictive value of our findings. Although our models presented vast subcutaneous scars from radical lymphadenectomy of the groin, they were nevertheless free of tissue radiation, and therefore, the scar quality and tissue regeneration improved with time. This does not reproduce the subcutaneous evolution of scars in most irradiated oncologic patients [1], [3]. In addition, this animal model is less prone to lymphatic swelling due to the shortness of its limbs in comparison with the length of his body. The hind limb/body length ratio in an adult minipig is about 1/2, whereas this proportion in adult humans is roughly 1/1 for the arms and 5/6 for the legs [26]. Human extremities therefore possess an overproportioned terminal vessel network. Additionally, this big animal model also presents a physiological advantage against swelling by having the groin at the same level of the heart [27], a feature mostly observed in humans only during sleep. Notwithstanding all limitations, this big animal model provides a similar proportion of subcutaneous fat, mimicking the tissue environment of avascular lymph node stroma transplantations in healthy humans.
Assessment of lymph node fragment regeneration was very consistent between SPECT-CT hybrid imaging evaluation and histologic analysis. This confirms the validity of SPECT-CT hybrid imaging for functional assessment of the lymphatic system. Because the radionuclide is injected in the paws, only lymph node fragments draining the periphery could capture the signal. Additional histologic information was added to this functional characteristic by using a combination of 99mTc-nanocolloid and Berlin blue dye. It allowed macroscopic visualisation of functional fragments whilst retrieving the probes and provided histologic staining of the lymph node sinuses. The observation of lymph node sinuses and a functional lymph node capsule is paramount, as it allows the differentiation between a regenerated organ and the occurrence of tertiary lymphoid tissue [28]. Nevertheless, this strong correlation between lymph node fragment function proven by SPECT-CT hybrid imaging and histologic confirmation of the viability of radionuclide-positive, blue-stained nodes did not exclude false negatives. This possible weakness was countered by retrieving histologic probes of the subcutaneous regions of former lymph node fragment transplantation areas, even when no radionuclide signalling was visible with the gamma camera and no blue dye was present. Nevertheless, in most of these, only fatty tissue was detectable, so that a formal verification of lymph node fragment necrosis was not possible in most cases. Fatty degeneration of unviable tissues within the lymphatic tissue is a common observation within primary lymphatic organs as the thymus [29] or ageing human lymph nodes [30]. This might explain why the histologic location of unviable fragments was not feasible in most cases, and only fatty tissue could be observed in non-signalling regions of transplantation. Nevertheless, even if histologic false negatives occurred, and regenerated fragments were missed, these would have presented a local vascular connection only and therefore would have not been relevant for limb drainage or else they would have captured peripheral radionuclide.
The observed microarchitecture of regenerated lymph nodes in the histologic analysis suggests a participation in immune processes [31], although the animals were kept in a clean facility. The presence of an organ capsule, sinuses, and germinal follicles in the paracortex stressing a compartmentation of immune cells are all indirect signs of immune competence of regenerated lymph node fragments. Clear confirmation of these findings would nevertheless require immune challenge analysis as well as immunohistochemistry; thus, further studies are needed [31].
The interpretation of our regeneration rates was limited by the low number of samples. This is a common feature with most studies using big animal models. In the current study, the cryopreservation group A was bigger than the positive control group B (12 lymph node fragments in group A vs. 8 in control group B; therefore, group B was only two thirds of the size of group A). This created restrictions in the comparison of both groups, as a fragment in group A accounted for 8.3% of the results within the group, whereas in group B, each fragment represented 12.5% of the group results. Therefore, registered differences in the regeneration rates of both groups ([regeneration rate in group A] – [regeneration rate in group B]=9%) do not account for significant differences, as a single observation of fragment regeneration or necrosis within a group could be mathematically sufficient to close this gap.
A comparative analysis of the regeneration rates of vascularised lymph node flaps and avascular lymph node fragments is laborious. Vascularised flaps containing lymph nodes were mostly investigated in humans [12], [13], [14], [15], [16], and therefore, survival of the flaps was analysed indirectly by measuring the ability to improve lymphoedema. Radioisotope controls of post-operative flap draining abilities were not performed systematically [12], [13], [14], [15], [16]. Additionally, a thorough anatomical characterisation of the flaps is failing, so that the number of lymph nodes contained in each flap is a mere estimation. In humans, lymph node flaps have about three to five lymph nodes. The capacity to dramatically improve lymphoedema after transplantation procedures is established to be about 30%–40% of the cases [12], [13], [14], [15], [16], depending on lymphoedema stage, but some degree of improvement could be observed in most patients [12]. It is arguable if these partial improvement cases correspond to loss of draining function of transplanted nodes despite blood vessel micro-anastomosis. Nevertheless, it seems safe to admit that vascularised lymph node flaps present a non-negligible quantitative advantage compared with avascular lymph node fragments, as the first contain several lymph nodes and the latter only have two regeneration foci per harvested lymph node. The regeneration of autotransplanted lymph node fragments in rats can be enhanced by injection of the growth factor vascular endothelial growth factor C in the draining area [32], [33].
Meanwhile, the risk for the donor area was described for the microsurgical lymph node flaps [17], [18], [19].
Additionally, the comparison of our regeneration rates with those of artificial lymph nodes needs further studies, as current publications on artificial nodes are focussed on single cases and the success rates of the attempts were not measured or were left unmentioned [20], [21], [22].
Processes responsible for lymph node regeneration seem to be relatively independent of nutrient diffusion through the tissue. The average amount of tissue estimated to survive under diffusion conditions is about 1 cm [22], but this distance is increased in some tissues, e.g. cartilage, and decreased for highly metabolic tissues, e.g. heart muscle. Hypothetically, fragmentation, whilst multiplying regeneration foci, would improve initial lymphangiogenesis induced by the transplants when reconnecting to the surrounding tissues. Nevertheless, it could probably only partially guarantee the survival of nodal cell populations through diffusion [22].
Stroma cells seem to be the key players of lymph node regeneration [34]. Lymphocyte populations were shown to later recolonise viable transplants and recover typical architecture and immune function [33], [34]. Lymph node tissue regeneration is clinically relevant in the described lymph node transplant procedure [12], [13], [14], [15], [16]. Nevertheless, some patients present with lymphoedema combined with a paucity of potential donor lymph nodes, as in some primary conditions [25]. In such cases, creation of artificial lymph nodes based on human material could be a possible solution for inducing lymphangiogenesis and improving local immune responses [20]. Successful creation of artificial lymph nodes can use lymph node stroma cells as inducers of the whole regeneration cascade. Although migration of peripheral dendritic cells into the nodes is possible [35], this only takes place if nodal stroma cells are present for signalling the existence of this organ when it contacts its environment [34]. It was not known until now if cryopreservation processes could affect the capacity of stroma cells in inducing regeneration [22].
The present study in a large animal model shows us that cryopreservation methods do not seem to affect the regeneration of lymph node fragment function significantly. This feature might allow future laboratory use of lymph node stroma to make generation of artificial lymph nodes possible.
Supporting Information
Acknowledgments
We are grateful for the excellent laboratory technical assistance of Andrea Herden and Karin Westermann. We also thank Karl-Heinz Napierski and Paul Zerbe for their outstanding perioperative and intraoperative assistance and Claudia Dieckmann and Mrs. O. Hille for their technical assistance in the SPECT-CT hybrid imaging.
Supplementary Material:
The article (iss-2018-0003) offers reviewer assessments as supplementary material.
Author Statement
Research funding: This work was supported by the Deutsche Forschungsgemeinschaft DFG (Pa 240-10/1) and the Gesellschaft der Freunde der MHH. S.J.T. was supported by a RegSci fellowship. Conflicts of interest: The authors declare no conflicts of interest exist in relation to this study. Informed consent: Informed consent is not applicable. Ethical approval: The research related to the animal use complies with all the relevant national regulations and institutional policies. It was performed in accordance to the criteria approved by the institutional review board of the region of Lower Saxony.
Author Contributions
Catarina Hadamitzky: overall data analysis and writing of the manuscript; Hanes Perić: overall data analysis and writing of the manuscript; Sebastian Theobald: histologic data analysis; Klaus Friedrich Gratz: data analysis on SPECT-CT hybrid imaging; Hendrik Spohr: design of the study; Reinhard Pabst: design of the study and approval of the manuscript; Peter Maria Vogt: approval of the manuscript.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1].Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest 2014;124:915–21. [DOI] [PMC free article] [PubMed]; Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest. 2014;124:915–21. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rockson SG. Lymphedema. Vasc Med 2016;21:77–81. [DOI] [PubMed]; Rockson SG. Lymphedema. Vasc Med. 2016;21:77–81. doi: 10.1177/1358863X15620852. [DOI] [PubMed] [Google Scholar]
- [3].Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res 2010;87:198–210. [DOI] [PubMed]; Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- [4].Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med 2017;214:3105–122. [DOI] [PMC free article] [PubMed]; Gerner MY, Casey KA, Kastenmuller W, Germain RN. Dendritic cell and antigen dispersal landscapes regulate T cell immunity. J Exp Med. 2017;214:3105–122. doi: 10.1084/jem.20170335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J 2007;6:2. [DOI] [PMC free article] [PubMed]; Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi: 10.1186/1475-2883-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Douglass J, Graves P, Gordon S. Self-care for management of secondary lymphedema: a systematic review. PLoS Negl Trop Dis 2016;10:e0004740. [DOI] [PMC free article] [PubMed]; Douglass J, Graves P, Gordon S. Self-care for management of secondary lymphedema: a systematic review. PLoS Negl Trop Dis. 2016;10:e0004740. doi: 10.1371/journal.pntd.0004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Williams AF, Franks PJ, Moffatt CJ. Lymphoedema: estimating the size of the problem. Palliat Med 2005;19:300–13. [DOI] [PubMed]; Williams AF, Franks PJ, Moffatt CJ. Lymphoedema: estimating the size of the problem. Palliat Med. 2005;19:300–13. doi: 10.1191/0269216305pm1020oa. [DOI] [PubMed] [Google Scholar]
- [8].Linardou H, Kalogeras KT, Kronenwett R, Kouvatseas G, Wirtz RM, Zagouri F, et al. The prognostic and predictive value of mRNA expression of vascular endothelial growth factor family members in breast cancer: a study in primary tumors of high-risk early breast cancer patients participating in a randomized Hellenic Cooperative Oncology Group trial. Breast Cancer Res 2012;14:R145. [DOI] [PMC free article] [PubMed]; Linardou H, Kalogeras KT, Kronenwett R, Kouvatseas G, Wirtz RM, Zagouri F. et al. The prognostic and predictive value of mRNA expression of vascular endothelial growth factor family members in breast cancer: a study in primary tumors of high-risk early breast cancer patients participating in a randomized Hellenic Cooperative Oncology Group trial. Breast Cancer Res. 2012;14:R145. doi: 10.1186/bcr3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Disipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol 2013;14:500–15. [DOI] [PubMed]; Disipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14:500–15. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- [10].Novackova M, Halaska MJ, Robova H, Mala I, Pluta M, Chmel R, et al. A prospective study in detection of lower-limb lymphedema and evaluation of quality of life after vulvar cancer surgery. Int J Gynecol Cancer 2012;22:1081–8. [DOI] [PubMed]; Novackova M, Halaska MJ, Robova H, Mala I, Pluta M, Chmel R. et al. A prospective study in detection of lower-limb lymphedema and evaluation of quality of life after vulvar cancer surgery. Int J Gynecol Cancer. 2012;22:1081–8. doi: 10.1097/IGC.0b013e31825866d0. [DOI] [PubMed] [Google Scholar]
- [11].Chance-Hetzler J, Armer J, Van Loo M, Anderson B, Harris R, Ewing R, et al. Prospective lymphedema surveillance in a clinic setting. Pers Med 2015;5:311–25. [DOI] [PMC free article] [PubMed]; Chance-Hetzler J, Armer J, Van Loo M, Anderson B, Harris R, Ewing R. et al. Prospective lymphedema surveillance in a clinic setting. Pers Med. 2015;5:311–25. doi: 10.3390/jpm5030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313–5. [DOI] [PMC free article] [PubMed]; Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–5. doi: 10.1097/01.sla.0000201258.10304.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Becker C. Autologous lymph node transfers. J Reconstr Microsurg 2016;32:28–33. [DOI] [PubMed]; Becker C. Autologous lymph node transfers. J Reconstr Microsurg. 2016;32:28–33. doi: 10.1055/s-0035-1563393. [DOI] [PubMed] [Google Scholar]
- [14].Chen R, Mu L, Zhang H, Xin M, Luan J, Mu D, et al. Simultaneous breast reconstruction and treatment of breast cancer-related upper arm lymphedema with lymphatic lower abdominal flap. Ann Plast Surg 2014;73:12–7. [DOI] [PubMed]; Chen R, Mu L, Zhang H, Xin M, Luan J, Mu D. et al. Simultaneous breast reconstruction and treatment of breast cancer-related upper arm lymphedema with lymphatic lower abdominal flap. Ann Plast Surg. 2014;73:12–7. doi: 10.1097/SAP.0000000000000322. [DOI] [PubMed] [Google Scholar]
- [15].Lahteenvuo M, Honkonen K, Tervala T, Tammela T, Suominen E, Lähteenvuo J, et al. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation 2011;123:613–20. [DOI] [PubMed]; Lahteenvuo M, Honkonen K, Tervala T, Tammela T, Suominen E, Lähteenvuo J. et al. Growth factor therapy and autologous lymph node transfer in lymphedema. Circulation. 2011;123:613–20. doi: 10.1161/CIRCULATIONAHA.110.965384. [DOI] [PubMed] [Google Scholar]
- [16].Lin CH, Ali R, Chen SC, Wallace C, Chang YC, Chen HC, et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265–75. [DOI] [PubMed]; Lin CH, Ali R, Chen SC, Wallace C, Chang YC, Chen HC. et al. Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg. 2009;123:1265–75. doi: 10.1097/PRS.0b013e31819e6529. [DOI] [PubMed] [Google Scholar]
- [17].Vignes S, Blanchard M, Yannoutsos A, Arrault M. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg 2013;45:516–20. [DOI] [PubMed]; Vignes S, Blanchard M, Yannoutsos A, Arrault M. Complications of autologous lymph-node transplantation for limb lymphoedema. Eur J Vasc Endovasc Surg. 2013;45:516–20. doi: 10.1016/j.ejvs.2012.11.026. [DOI] [PubMed] [Google Scholar]
- [18].Viitanen TP, Maki MT, Seppanen MP, Suominen EA, Saaristo AM. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg 2012;130:1246–53. [DOI] [PubMed]; Viitanen TP, Maki MT, Seppanen MP, Suominen EA, Saaristo AM. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg. 2012;130:1246–53. doi: 10.1097/PRS.0b013e31826d1682. [DOI] [PubMed] [Google Scholar]
- [19].Sulo E, Hartiala P, Viitanen T, Mäki M, Seppänen M, Saarikko A. Risk of donor-site lymphatic vessel dysfunction after microvascular lymph node transfer. J Plast Reconstr Aesthet Surg 2015;68:551–8. [DOI] [PubMed]; Sulo E, Hartiala P, Viitanen T, Mäki M, Seppänen M, Saarikko A. Risk of donor-site lymphatic vessel dysfunction after microvascular lymph node transfer. J Plast Reconstr Aesthet Surg. 2015;68:551–8. doi: 10.1016/j.bjps.2014.11.016. [DOI] [PubMed] [Google Scholar]
- [20].Kobayashi Y, Watanabe T. Synthesis of artificial lymphoid tissue with immunological function. Trends Immunol 2010;31:422–8. [DOI] [PubMed]; Kobayashi Y, Watanabe T. Synthesis of artificial lymphoid tissue with immunological function. Trends Immunol. 2010;31:422–8. doi: 10.1016/j.it.2010.09.002. [DOI] [PubMed] [Google Scholar]
- [21].Tan JK, Watanabe T. Artificial engineering of secondary lymphoid organs. Adv Immunol 2010;105:131–57. [DOI] [PubMed]; Tan JK, Watanabe T. Artificial engineering of secondary lymphoid organs. Adv Immunol. 2010;105:131–57. doi: 10.1016/S0065-2776(10)05005-4. [DOI] [PubMed] [Google Scholar]
- [22].Cupedo T, Stroock A, Coles M. Application of tissue engineering to the immune system: development of artificial lymph nodes. Front Immunol 2012;3:343. [DOI] [PMC free article] [PubMed]; Cupedo T, Stroock A, Coles M. Application of tissue engineering to the immune system: development of artificial lymph nodes. Front Immunol. 2012;3:343. doi: 10.3389/fimmu.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Blum KS, Hadamitzky C, Gratz KF, Pabst R. Effects of autotransplanted lymph node fragments on the lymphatic system in the pig model. Breast Cancer Res Treat 2010;120:59–66. [DOI] [PubMed]; Blum KS, Hadamitzky C, Gratz KF, Pabst R. Effects of autotransplanted lymph node fragments on the lymphatic system in the pig model. Breast Cancer Res Treat. 2010;120:59–66. doi: 10.1007/s10549-009-0367-4. [DOI] [PubMed] [Google Scholar]
- [24].Hadamitzky C, Pabst R, Gordon K, Vogt PM. Surgical procedures in lymphedema management. J Vasc Surg Venous Lymphat Disord 2014;2:461–8. [DOI] [PubMed]; Hadamitzky C, Pabst R, Gordon K, Vogt PM. Surgical procedures in lymphedema management. J Vasc Surg Venous Lymphat Disord. 2014;2:461–8. doi: 10.1016/j.jvsv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- [25].Connell F, Gordon K, Brice G, Keeley V, Jeffery S, Mortimer PS, et al. The classification and diagnostic algorithm for primary lymphatic dysplasia: an update from 2010 to include molecular findings. Clin Genet 2013;84:303–14. [DOI] [PubMed]; Connell F, Gordon K, Brice G, Keeley V, Jeffery S, Mortimer PS. et al. The classification and diagnostic algorithm for primary lymphatic dysplasia: an update from 2010 to include molecular findings. Clin Genet. 2013;84:303–14. doi: 10.1111/cge.12173. [DOI] [PubMed] [Google Scholar]
- [26].Földi M, Földi E, Strößenreuther R, Kubik S. Földi’s Textbook of Lymphology: For Physicians and Lymphedema Therapists. Munich, Germany: Elsevier Health Sciences; 2012.; Földi M, Földi E, Strößenreuther R, Kubik S. Földi’s Textbook of Lymphology: For Physicians and Lymphedema Therapists. Munich, Germany: Elsevier Health Sciences; 2012. [Google Scholar]
- [27].Hadamitzky C, Pabst R. Acquired lymphedema: an urgent need for adequate animal models. Cancer Res 2008;68: 343–5. [DOI] [PubMed]; Hadamitzky C, Pabst R. Acquired lymphedema: an urgent need for adequate animal models. Cancer Res. 2008;68:343–5. doi: 10.1158/0008-5472.CAN-07-2454. [DOI] [PubMed] [Google Scholar]
- [28].Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest 2014;124:953–9. [DOI] [PMC free article] [PubMed]; Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest. 2014;124:953–9. doi: 10.1172/JCI71611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR. Thymus: the next (re)generation. Immunol Rev 2016;271:56–71. [DOI] [PMC free article] [PubMed]; Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR. Thymus: the next (re)generation. Immunol Rev. 2016;271:56–71. doi: 10.1111/imr.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hadamitzky C, Spohr H, Debertin AS, Guddat S, Tsokos M, Pabst R. Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J Anat 2010;216:556–62. [DOI] [PMC free article] [PubMed]; Hadamitzky C, Spohr H, Debertin AS, Guddat S, Tsokos M, Pabst R. Age-dependent histoarchitectural changes in human lymph nodes: an underestimated process with clinical relevance? J Anat. 2010;216:556–62. doi: 10.1111/j.1469-7580.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dimopoulos Y, Moysi E, Petrovas C. The lymph node in HIV pathogenesis. Curr HIV/AIDS Rep 2017;14:133–40. [DOI] [PubMed]; Dimopoulos Y, Moysi E, Petrovas C. The lymph node in HIV pathogenesis. Curr HIV/AIDS Rep. 2017;14:133–40. doi: 10.1007/s11904-017-0359-7. [DOI] [PubMed] [Google Scholar]
- [32].Schindewolffs L, Breves G, Buettner M, Hadamitzky C, Pabst R. VEGF-C improves regeneration and lymphatic reconnection of transplanted autologous lymph node fragments: an animal model for secondary lymphedema treatment. Immun Inflamm Dis 2014;2:152–61. [DOI] [PMC free article] [PubMed]; Schindewolffs L, Breves G, Buettner M, Hadamitzky C, Pabst R. VEGF-C improves regeneration and lymphatic reconnection of transplanted autologous lymph node fragments: an animal model for secondary lymphedema treatment. Immun Inflamm Dis. 2014;2:152–61. doi: 10.1002/iid3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sommer T, Buettner M, Bruns F, Breves G, Hadamitzky C, Pabst R. Improved regeneration of autologous transplanted lymph node fragments by VEGF-C treatment. Anat Rec 2012;295:786–91. [DOI] [PubMed]; Sommer T, Buettner M, Bruns F, Breves G, Hadamitzky C, Pabst R. Improved regeneration of autologous transplanted lymph node fragments by VEGF-C treatment. Anat Rec. 2012;295:786–91. doi: 10.1002/ar.22438. [DOI] [PubMed] [Google Scholar]
- [34].Buettner M, Bode U. Stromal cells directly mediate the re-establishment of the lymph node compartments after transplantation by CXCR5 or CCL19/21 signalling. Immunology 2011;133:257–69. [DOI] [PMC free article] [PubMed]; Buettner M, Bode U. Stromal cells directly mediate the re-establishment of the lymph node compartments after transplantation by CXCR5 or CCL19/21 signalling. Immunology. 2011;133:257–69. doi: 10.1111/j.1365-2567.2011.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 2006;24:203–15. [DOI] [PubMed]; Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M. et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.