Abstract
Vascular disease – including coronary artery disease, carotid artery disease, and peripheral vascular disease – is a leading cause of morbidity and mortality worldwide. The standard of care for restoring patency or bypassing occluded vessels involves using autologous grafts, typically the saphenous veins or internal mammary arteries. Yet, many patients who need life- or limb-saving procedures have poor outcomes, and a third of patients who need vascular intervention have multivessel disease and therefore lack appropriate vasculature to harvest autologous grafts from. Given the steady increase in the prevalence of vascular disease, there is great need for grafts with the biological and mechanical properties of native vessels that can be used as vascular conduits. In this review, we present an overview of methods that have been employed to generate suitable vascular conduits, focusing on the advances in tissue engineering methods and current three-dimensional (3D) bioprinting methods. Tissue-engineered vascular grafts have been fabricated using a variety of approaches such as using preexisting scaffolds and acellular organic compounds. We also give an extensive overview of the novel use of 3D bioprinting as means of generating new vascular conduits. Different strategies have been employed in bioprinting, and the use of cell-based inks to create de novo structures offers a promising solution to bridge the gap of paucity of optimal donor grafts. Lastly, we provide a glimpse of our work to create scaffold-free, bioreactor-free, 3D bioprinted vessels from a combination of rat vascular smooth muscle cells and fibroblasts that remain patent and retain the tensile and mechanical strength of native vessels.
Keywords: graft fabrication, vascular disease, vessel engineering
Abbreviations: EC, endothelial cell; ECM, extracellular matrix; FC, fibroblast cell; SMC, smooth muscle cell; TEVG, tissue-engineered vascular graft.
Introduction
Vascular disease is one of largest causes of morbidity and mortality globally [1], [2]. One common form of vascular disease is atherosclerosis, which involves build-up of a plaque and stiffening of the arterial wall, resulting in obstruction of the blood vessel and the induction of ischemic injury. These ischemic conditions have resulted in an ever-increasing need for vascular conduits to either restore patency to the occluded vessels or bypass the occluded regions altogether. For bypass surgery performed to treat occlusive or aneurysmal disease, autologous venous and arterial grafts are the most commonly used vascular conduits. Autologous grafts are grafts that have been harvested from another part of the patient’s body, and are usually the saphenous veins or the internal mammary arteries [3]. Because they are native and biocompatible to the patient, there is minimized risk of immune rejection. They are also immediately available and do not require prior Food and Drug Administration (FDA) approval [4]. However, the additional surgery required to harvest the autologous grafts increases morbidity, and roughly a third of patients with vascular disease lack suitable vessels that can be used as grafts due to multivessel disease [5].
Synthetic grafts such as expanded polytetrafluoroethylene (i.e. Gortex) or polyethylene terephthalate (i.e. Dacron) were first introduced in the 1950s as an alternative to autologous grafts. These have been clinically approved for large-diameter vessels, where the occurrence of thrombi is negligible due to high blood flow and low resistance. When used for the reconstruction of small-diameter vessels (<6 mm), synthetic grafts have a low patency rate, with reported patency rates being 40% after 6 months and 25% after 3 years [6]. This is due to a compliance mismatch between the graft and native vessel, which creates turbulent flow at the anastomotic site, resulting in intimal hyperplasia, platelet activation, and thrombus formation [7]. For this reason, patients with synthetic grafts require prolonged anticoagulation regimens [3].
Vascular tissue engineering offers promising approaches to generating biocompatible conduits that overcome the limitations of synthetic grafts. In this review, we will give a brief history of the approaches and advances in tissue engineering of vascular conduits, with a specific focus on the novel use of additive manufacturing methods to generate suitable grafts.
Tissue engineering
Tissue engineering is a specific domain in bioengineering that is focused on the development of artificial tissues or organs that can replace tissues in in vivo or in vitro models. Tissue-engineered vascular grafts (TEVGs) theoretically provide the promise of the low immune rejection rates seen in autologous grafts along with the commercial availability and mass reducibility of prosthetic grafts. The ideal TEVG should be biocompatible, non-thrombogenic, and have adequate mechanical strength to withstand pulsatile flow [4], [8]. Biocompatibility ensures that the grafts are non-toxic and do not induce inflammatory and/or immunogenic responses that may lead to graft rejection, while non-thrombogenicity is vital in maintaining the patency of the grafts [9].
Successful TEVGs must mimic the in vivo physiologic properties of blood vessels, including compliance, tear strength, and burst strength. Native blood vessels rely on a combination of contractile smooth muscle cells (SMCs), collagen, and elastin to establish these normal physiologic parameters. In a similar manner, TEVGs also rely on SMCs and the extracellular matrix (ECM) proteins they produce to approximate the strength and elasticity of native vasculature. They must also be hemocompatible with the host and provide long-term stability by minimizing thrombosis. Theoretically, these risks can be mitigated by seeding the graft with endothelial cells (ECs), which are important regulators of vascular wound healing, chronic inflammation, and development of atherosclerosis [10], [11]. They also block exposure of von Willebrand factor, thus blocking inappropriate primary hemostasis. However, ECs notably have poor expansion potential, making them inadequate for matrix seeding. For this reason, endothelial stem cells and progenitor cells present a more promising option [12], [13], [14], [15].
The traditional approach to fabricating TEVGs has focused on three major characteristics: a scaffolding system to support cell attachment and growth; a variety of cell types, most commonly ECs, SMCs, and fibroblast cells (FCs); and a conditioning phase that drives neo-tissue formation following exposure to physical and chemical signals [16]. While several different strategies exist for the creation of these artificial tissues, the most studied and effective approach entails overlaying cells onto preexisting matrices. This is typically achieved by seeding cells onto three-dimensional (3D) scaffolds that can be generated by electrospinning, decellularizing donor tissue, cell self-assembly, or biosynthetically via freeze drying, foaming, or rapid prototyping technologies [17].
Scaffolds for TEVGs
Electrospinning technology is a physical process that involves stretching a viscoelastic solution into a solution jet under a high electrostatic force, thus solidifying it to form thin fibers [18]. The resultant fibrous scaffold matrices have a highly interconnected porous network, a high surface-area-to-volume ratio, and nanofiber structure that is similar to native ECM [16]. The polymer fiber size and scaffold thickness can be adjusted on the electrospinning settings (voltage, speed, time, and concentration of the polymer solutions), and the mechanical and biological properties of the electrospun vascular grafts can be tailored through the composition and combination of the polymers or copolymers [19]. They can also be easily modified with a variety of bioactive molecules to stimulate SMC penetration and enhance hemocompatibility. Although they attain the mechanical strength of native blood vessels, the polymeric scaffolds need to be composed of dense layers with small pore size and low porosity, which leaves limited space for cell infiltration in vivo, thus hindering cell migration [20], [21]. Moreover, they are slow degraders, and this impairs remodeling and integration with the host environments and decreases their biologically suitability as substitutes of native blood vessels.
A second way of preparing scaffolds for TEVGs is via decellularization of native tissues [16]. Decellularized blood vessels have the capacity to preserve native ECM components that are necessary for cell adhesion, migration, and proliferation. These acellular scaffolds possess the mechanical properties to endure normal blood pressure and the required biological properties to promote EC migration and proliferation. Gui et al. decellularized human umbilical arteries and showed that they retained their mechanical strength in vitro and, when re-endothelialized with a single layer of human umbilical vein ECs, they supported endothelial adherence. The decellularized human umbilical arteries were implanted into rats as abdominal aorta interposition grafts and remained functional and patent in vivo for 8 weeks [22]. Off-the-shelf decellularized native human vascular grafts (CryoVein and CryoArtery, Kennesaw, GA, USA) are commercially available from CryoLife and range from decellularized saphenous veins to descending thoracic aorta. CryoLife uses SynerGraft Technologies, Kennesaw, GA, USA, a patented decellularization technology, to create acellular grafts, and thus far >22 published clinical studies have been reported. In one clinical study, these cryopreserved allografts have been shown to be resistant to reinfection, thrombosis, and aneurysmal dilatation at the midterm follow-up (20 months after implantation) [10], [23]. This model has the advantage of reducing immunogenic reactions by stripping the cellular components of the ECM while preserving the mechanical strength. Although very promising, there is a limited access to human vessels that are free of disease and have the appropriate dimensions.
Tissue engineering by cell self-assembly was inspired by the clinical success of the use of epithelial cell sheets in the treatment of burn patients. These self-assembling scaffolds are composed of autologous cell-derived ECM sheets that are cultured in vitro [24]. After harvesting, the sheets can by manipulated into tubular vascular grafts. L’Heureux et al. constructed TEVGs that were free of any synthetic biomaterials by wrapping human dermal FCs around a mandrel to create a tube structure [25]. These TEVGs are a good alternative to synthetic vascular grafts as they are non-immunogenic functional grafts with properties that are similar to autologous grafts. In fact, a clinical trial investigating the use of cell self-assembly TEVGs as arteriovenous shunts has demonstrated that they maintain high patency [16]. However, self-assembling vessels require 6–9 months of in vitro culture and cost >$15,000 per graft, thus limiting their use [10], [26].
To overcome these limitations, there has been a recent focus on the generation of biosynthetic vascular grafts. In this approach, cells are cultured on a biodegradable polymeric tubular scaffold that allows for cell remodeling as the scaffold degrades. The cells are typically cultured over 8–10 weeks to form the wall of the TEVG. The vessel is subsequently decellularized, leaving only the ECM, and the scaffold can then be seeded with autologous endothelial progenitor cells [27]. Shin’oka and colleagues seeded autologous bone marrow cells onto degradable composite polymer scaffolds that could be tailored for greater porosity with larger pore sizes, which enabled cellular infiltration and migration [28]. These TEVGs were used as venous grafts and remained patent for 31.6 months. However, the diameter of the grafts increased with time to 110% of the implanted size. They also had weak mechanical strength, and were thus not suitable for arterial implantations [28].
Most recently, Patel et al. described the “ring stacking method”, whereby vascular tissue rings were stacked around a post into tubular structures, seeded with fibroblasts, and then stimulated to produce collagen [29]. Within 8 days, the grafts had matured into adventitia vessels. By 2 weeks of culturing, the vessels had increased ECM deposition and tensile strength. The burst pressures, however, remained below the burst pressure values of native human saphenous vein and artery. Table 1 shows a summary of the mechanical properties of the different types of scaffolds discussed.
Table 1:
Comparison of mechanical properties of different scaffolds [22], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39].
| Scaffolds | Burst pressure (mmHg) | Ultimate tensile strength | Elastic modulus | Suture retention strength (g) |
|---|---|---|---|---|
| Human internal mammary artery | 3196±1264 | 443±55 kPa | 45.1±16.8 MPa | 138 |
| Collagen gel based | 71±4 | 58 kPa | 142 kPa | ND |
| Fibrin gel based | ND | 1.1 MPa | 4.7 MPa | 5941 |
| Cell sheet engineering | 3490±892 | >3 MPa | >20 MPa | 152 |
| Electrospinning of polymeric scaffolds | 2360±673 | 17.5 MPa | 40.4 MPa | <50 |
| Biodegradable polymeric scaffolds | ND | ND | <0.55 MPa | <50 |
| Decellularized tissue scaffolds | 2400 | 1.618 MPa | 7.41 MPa | ND |
| Sheet-based tissue engineering | >2000 | ND | ND | 162 |
| Ring stacking method | ~45 | 0.12 MPa (circumferential) | 0.461 MPa | ND |
ND, Not determined.
The above ways of generating TEVGs have been instrumental in bridging the gap between the need for vascular conduits and the scarcity of autologous grafts. Cutting through the diversity of TEVG designs, three universal development challenges exist: size limitations, time, and physiologic compatibility. Technological incapacity to adequately capillarize these artificial tissues results in insufficient nutrient and oxygen delivery, limiting sizes that can be achieved [40], [41], [42]. Furthermore, creating viable tissues can be a time-intensive process and this limits mass production. Lastly, a successful vascular graft must meet preexisting physiologic parameters necessary for blood vessels [8]. Therefore, there are still several hurdles for TEVGs to overcome before widespread dispersal and commercialization can be actualized. The advent of additive manufacturing technology promises new and exciting ways of fabricating vascular conduits, as it offers unprecedented capability to definitively deliver cells and biomaterials with precise control.
Additive manufacturing
Additive manufacturing, also known as 3D printing, is a rapid, reproducible, and accurate prototyping process whereby a printer layers successive 2D sections to construct a 3D object [43]. It was first described in 1986, when Charles W. Hull described a method called “stereolithography”, whereby he cured thin layers of a material with ultraviolet light and then used photopolymerization to solidify a liquid monomer resin layer by layer, thus printing a solid 3D structure [28], [44], [45]. 3D printing has subsequently been applied in a variety of fields and has been particularly embraced in the medical field to overcome some of the challenges associated with traditional tissue engineering methods.
In medicine, 3D printing has been used to create models that have been used both in medical education and clinical practice. 3D printed anatomical models have enabled the production of accurate anatomical structures at a relatively low cost, thus enabling learners to gain an appreciation of normal and pathological variations of several conditions [46]. It has also been used in the planning of complex surgical procedures, as it enables the visualization of complex underlying pathological structures, thus enhancing intraoperative guidance and surgical outcomes [47], [48], [49], [50]. Additionally, 3D printing has greatly advanced the production of prostheses. Due to the decreased production time and cost, it has been possible to print personalized medical implants that are specifically designed to fit the recipient’s anatomy. These have included bone plates, tracheal splints [51], heart valves, and spinal implants [46], [52], [53].
Advances in 3D printing have led to bioprinting, whereby computer-aided transfer and build-up processes are used for the precise layer-by-layer positioning of biological materials and living cells with spatial control of the placement of functional components to generate 3D structures [17], [44], [54]. This leap in tissue engineering has the potential of fabricating tissues and organs that can then be implanted in patients and maintain mechanical and physiological functions. In 2007, De Coppi et al. at Wake Forest Regeneration Medical Center used a 3D inkjet printer to print human amniotic fluid stem cells onto a scaffold, and exposed the scaffold/cell constructs to an osteogenic differentiation medium for 1 week. The 3D printed constructs subsequently differentiated into functional bone tissue that exhibited high density and strength [55]. Since then, several bioprinting strategies, cell types, and scaffolds have been employed to make 3D biological constructs.
Bioprinting strategies
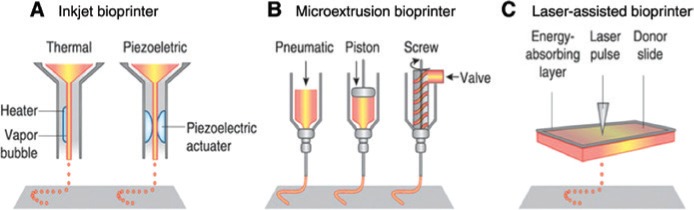
There are three main types of bioprinting techniques currently used: inkjet bioprinting, microextrusion bioprinting, and laser-assisted bioprinting (Figure 1). Inkjet bioprinting is a non-contact technique whereby picoliter droplets of bioink-containing cells or other biological factors are layered onto a substrate to generate 2D and 3D structures [56], [57]. Tissues are fabricated from the “bottom-up”, with the bottom layers being printed first and sequential layers printed on top of previous layers [58]. It has the advantage of being able to yield high-resolution structures at fairly low cost and high speed. It is also possible to engineer variations in surface concentration through overprinting at different drop densities [59]. However, the fact that bioink has to be in liquid form to enable droplet formation and then form a solid structure often compromises the structural organization and functionality of the printed structures. This can be overcome by using materials that can be cross-linked after printing using chemical, pH, or ultraviolet mechanisms [54].
Figure 1:
Schematic of inkjet, microextrusion, and laser-assisted bioprinters.
(A) Thermal inkjet printers electrically heat the printhead to produce air pressure pulses that force droplets from the nozzle, whereas acoustic printers use pulses formed by piezoelectric or ultrasound pressure. (B) Microextrusion printers use pneumatic or mechanical (piston or screw) dispensing systems to extrude continuous beads of material and/or cells. (C) Laser-assisted printers use lasers focused on an absorbing substrate to generate pressures that propel cell-containing materials onto a collector substrate. Reprinted by permission from: Murphy and Atala [54].
Microextrusion 3Dbioprinters are the most commonly used (Table 2). These work by dispensing continuous filaments of a material consisting of cells mixed with hydrogel through a micronozzle to create 3D structures [56]. As microextrusion bioprinters generate continuous beads of biomaterial (as opposed to droplets generated by inkjet printers), high-viscosity biomaterials such as hydrogels and dense cellular spheroids can be printed, thus allowing for the printing of structures with physiological cell densities that can replicate the mechanical and functional properties of tissue ECM [54], [57], [60]. One drawback of this method of bioprinting is decreased cell viability. In comparison to inkjet bioprinting, microextrusion bioprinting has cell survival rates that range from 40% to 86%, with the rate of cell survival decreasing as the extrusion pressure and nozzle gauge increase [61], [62].
Table 2:
Properties of common 3D bioprinters [57].
| Inkjet bioprinter | Microextrusion bioprinter | Laser-assisted bioprinter | |
|---|---|---|---|
| Cost of printer | Low | Medium | High |
| Printing speed | Fast (1–10,000 droplets/s) | Slow (10–50 μm/s) | Medium (200–1600 mm/s) |
| Cell density | Low (≤106 cells/mL) | High: cell spheroids | Medium: ≤108 cells/mL |
| Cell viability | Medium: 85% | Low: 40–80% | High: >95% |
Laser-assisted bioprinting is less common than inkjet and microextrusion, but its use in the field of tissue engineering has been steadily increasing. It is based on the principles of laser-induced forward transfer [63] and consists of a pulsed laser beam, a focusing system, a ribbon made from glass covered with a laser-energy-absorbing layer such as gold or titanium, a layer of biomaterial containing cells and/or hydrogel, and a receiving substrate facing the ribbon. Laser pulses are focused on the absorbing layer of the ribbon to generate a high-pressure bubble that propels the biomaterial toward the collector substrate [54], [57]. As with microextrusion bioprinting, there is the drawback of lower cell viability in the printed hydrogel in comparison to other inkjet mechanisms [57]. Because this method of bioprinting is nozzle-free, there is very little to no clogging with cells or biomaterials as in the other two bioprinting methods. Moreover, it is possible to deposit cells at a density of up to 108 cells/mL with microscale resolution of a single cell per drop [64].
Bioprinting scaffolds
As discussed above, scaffolds have thus far played an important role in tissue engineering, most notably as matrices onto which cells can be loaded. They serve as templates that not only provide mechanical support but also facilitate cell adhesion, proliferation, and expansion throughout the structure before the cells develop their own ECM [65], [66]. As such, an ideal scaffold should be temporary and be composed of a material that can disappear through dissolution or degradation as the cells produce their own ECM [59].
Scaffolds for 3D bioprinting can be made from natural polymers or synthetic polymers. Natural polymers such as alginate, gelatin, collagen, fibrin, and hyaluronic acid are often isolated from human or animal tissue. They are similar to ECM and have inherent bioactivity, thus facilitating the creation of an environment close to native ECM in which cells can be guided to create new tissue with appropriate function [66]. Synthetic polymers such as polycaprolactone, polylactic acid, polyethylene glycol, and polyglycolic acid are attractive because it is possible to optimize their chemical and physical properties to suit particular applications [67]. However, they have poor biocompatibility and often degrade into toxic products, losing their mechanical properties in the process. Nevertheless, these are both hydrophilic and absorbent, and remain commonly used due to the ease of controlling their physical properties during synthesis [54].
Bioink and hydrogels
In recent years, there has been a shift toward scaffold-free 3D printing and a focus on optimizing the bioink used for printing, mainly because the absence of a scaffold eliminates the problem that arises from degradation product biocompatibility. Bioink is essentially a biomaterial and aggregates of spheroids or cylinders composed of dense cellular slurry that can be used to print a desired structure. Ideally, the biomaterial should provide the initial mechanical and structural support for the cells, and should contain biomolecules that can provide the necessary biological cues for tissue growth. The cells used should be consistent in size, and the cell types used should be consistent with the tissue or organ to be printed. After printing, the cells usually start production of their own ECM, thus providing the support structure that an exogenous scaffold would have provided [43], [68]. As such, material characteristics to consider when choosing a bioink mixture include the printability and functionalizability of the mixture as well as degradation kinetics and by-products if degradable material is incorporated into the bioink [54], [69]. A major challenge when choosing which bioink to use for bioprinting is the limited availability of bioinks that fulfill the above criteria and simultaneously provide the required environment for cells to differentiate toward the desired lineage [53].
One way of overcoming this is through the incorporation of hydrogels into the bioink mixtures. Hydrogels possess high water content and have biocompatible and mechanical properties that are similar to those of natural tissues. Addition of hydrogel to the cellular slurry prior to the gelling process and subsequent printing allows for homogenous distribution of the cells throughout the gel [67], [70]. Shear-thinning materials such as Pluronic, polyethylene glycol, and gelatin (which is biodegradable denatured collagen) are often used as hydrogels because they possess liquid-like behavior under high shear stress during the extrusion process but can quickly recover their gel state after bioprinting, hence providing structural integrity and preventing the structure from collapsing [65]. Hydrogels that are synthesized from natural polymers such as collagen, fibrin, and hyaluronic acid are derived from the various components of the ECM and therefore serve as effective matrices for cellular growth and provide space filling for future tissue ingrowth.
Advances in bioprinting of vessels
With the advances in 3D printing discussed above, additive manufacturing has subsequently been used to fabricate a variety of organoids and tissues [53]. In the field of bioprinting vascular conduits, there have been two major parallel approaches when it comes to generating artificial blood vessels: generation of interconnected vessel systems and channels, and the generation of free-standing individual vascular conduits. The former arose from a need of perfusing bioprinted organs, and fabrication of perfusable channel systems proved to be the most direct way of enabling O2 and CO2 exchange and nutrient supply within the bulk of 3D printed tissues [17].
One such approach to generate blood vessels in artificial tissue is based on the ability of ECs to organize into blood vessels autonomously. In 2009, Cui and Boland used a modified thermal inkjet printer to deposit human ECs along micron-sized fibrin channels. The printed ECs aligned themselves inside the channels and proliferated to form a confluent lining along with the fibrin scaffold after 21 days of culture, and fabricated microvasculature showed both structural integrity and functionality [71].
Li et al. used a double-nozzle system to print a hybrid hepatocyte/hydrogel construct with a vascular-like conduit. To do this, they combined adipose-derived stromal cells with a gelatin/alginate/fibrinogen hydrogel to form a vascular network, and hepatocytes combined with gelatin/alginate/chitosan hydrogel were placed around this vascular network. The construct was stabilized in a thrombin/CaCl2/Na5P3O10 solution after assembly, after which the adipose-derived stromal cells were induced to differentiate into endothelial-like cells. After 2 weeks of culturing, the hepatocytes within the construct were observed to perform liver-like metabolic functions and the adipose-derived stromal cells at the periphery of the vascular-like network demonstrated endothelial-like cell properties [72].
Miller et al. first demonstrated the use of sacrificial 3D printing as a means of fabricating a vascular network. Their approach involved printing rigid filament networks of carbohydrate glass, which were used as a cytocompatible sacrificial template in engineered tissues to generate cylindrical networks. Once printed and hardened, the carbohydrate glass lattice was uniformly embedded in a soluble ECM that cross-links around the print. The sacrificial print was then dissolved and flushed away with water, and the residual hollow microfluidic channels were seeded with ECs, thus creating a vascular bed. The resultant cylindrical network was perfused with blood under high-pressure pulsatile flow and was able to sustain the metabolic function of primary rat hepatocytes in engineered tissue constructs. Notably, this method allows for the independent control of network geometry and is compatible with a wide variety of stromal/parenchymal cell types, ECMs, and cross-linking strategies [73], [74].
As discussed above, the classic approach to 3D bioprinting has been scaffold based, and involves employing synthetic or natural tubular scaffolds seeded with appropriate vascular cells [75], [76]. However, vascular grafts bioprinted on scaffolds have had the same limitations that face scaffold-based structures used in traditional tissue engineering. Most notably, the degradation of scaffold material often interferes with normal tissue maturation, leading to inflammation and mechanical failure of the grafts [77]. For this reason, there has been a shift toward scaffold-free bioprinting of vascular grafts.
In 2012, Marga et al. described their method of constructing scaffold-free bioprinted vascular constructs that comprised vascular SMCs, ECs, and dermal FCs. They used the Novogen MMX bioprinter (Organovo, San Diego, CA, USA), which has two printing heads, one that prints cellular material and the other prints cell-inert hydrogels. They made cylindrical bioink units comprising human aortic SMCs, human aortic ECs, and human dermal FCs, which they co-printed with cell-inert NovoGel (Organovo) onto central NovoGel rods. These grafts were matured in a bioreactor for 3 weeks and perfused with laminar and pulsatile flow for biomechanical stimulation of ECM formation. Immunohistochemical staining of the grafts showed that within 2 weeks of perfusion, the human ECs had formed networks that mimic the vaso vasorum of native vessels. These grafts also had mechanical strength that enabled them to withstand pressures of up to 773±78 mmHg at 21 days [68].
Similarly, in 2015, Kucukgul et al. used computer-aided algorithms to 3D bioprint hybrid cell and biomaterial scaffold-free macrovascular structures. They used medical imaging techniques to obtain the geometric and topological information of the targeted vascular tissue. They generated a computer model mimicking the abdominal aorta and optimized a 3D bioprinting path plan, which they used to print mouse embryonic FC aggregates and hydrogel support structure in a layer-by-layer fashion using the Novogen MMX bioprinters to form an aortic tissue construct [78].
Future directions
In light of recent advances in the field of 3D bioprinting of individual vascular conduits, the field is moving toward improving completely scaffold-free ways of bioprinting. The use of prefabricated multicellular building blocks such as cell sheets, spheroids, and tissue strands enables the fusion of these building blocks into larger cohesive constructs [70], [79], [80]. A major milestone in scaffold-free bioprinting of vascular grafts is the microneedle-based “Kenzan” method. This method employs the Regenova 3D bioprinter to print spheroids as predesigned tubular constructs using stainless-steel microneedles (“Kenzans”) as temporary support [81], [82]. Cell spheroids derived from human umbilical ECs, SMCs, and FCs have been printed using this technique. The resultant tubular structures were perfused for 7 days, after which they were implanted into the abdominal aortas of nude rats. The grafts remained patent for 5 days and had EC redistribution to an intimal layer on immunohistological staining. However, there was significant enlargement of the graft lumen and thinning of the wall, and this led to the termination of the experiment [83].
Another novel approach to scaffold-free bioprinting of vascular grafts involves the use of magnetic 3D printing. This involves assembling 3D cellular structures via magnetic levitation in the presence of paramagnetic agents, such as FDA-approved gadolinium chelates. The assembly and close interaction of cells at a certain levitation height where the magnetic force is equilibrated with gravitational force triggers the formation of complex 3D cellular structures that are capable of secreting their own ECM [84], [85]. Tseng et al. used magnetic bioprinting to print rings of vascular SMCs that mimic blood vessel segments structurally and functionally. These grafts had altered dynamics of contraction in response to a diverse set of vasodilators and vasoconstrictors, and immunohistochemical and gene expression profiling showed that their contraction properties were consistent with known vasoactive responses of blood vessels [86].
Our group is now taking advantage of these scaffold-free approaches and is bioprinting undifferentiated cylindrical vessels using a bioink containing both SMCs and FCs. To accomplish this process, we use an Organovo dual-head printer (Figure 2) to apply the appropriate cell types in a soluble support solution to create the conduit. Using this approach allows us to generate a complete cylinder in ~6 min. The lengths of the bioprinted vascular conduits can be of variable lengths, ranging from 10 cm to as short as 10 mm (Figure 3). The prints are then allowed to stabilize for 24–36 h. With this new fabrication technology, we are now able to generate a larger number of viable prints in a very short time without the need for a bioreactor, significantly reducing the time needed for the 3D bioprinted vascular grafts to mature into viable conduits. Preliminary data show that the prints remain patent after printing, and have the tensile and mechanical strength similar to native blood vessels. The lack of scaffold eliminates the toxicity associated with degradation of scaffold material and renders the prints suitable for transplantable vascular grafts in vivo. Clearly, this is just the beginning of using this new technology for printing and developing complex biological materials for biological use.
Figure 2:
Organovo MMX bioprinter.
(A) Organovo MMX bioprinter with four printheads. (B) Printheads of the Organovo MMX Bioprinter. Each printhead can print different cellular materials and/or hydrogel.
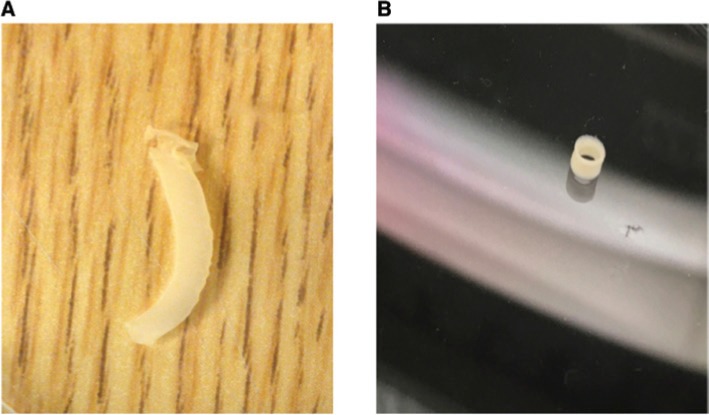
Figure 3:
Scaffold-free vascular bioprints.
(A) Full-length bioprinted vascular conduit that is scaffold free. (B) The bioprints can be sized to vascular conduits of different lengths and still remain patent.
Supporting Information
Supplementary Material
The article (iss-2018-0016) offers reviewer assessments as supplementary material.
Author Statement
Research funding: Authors state no funding involved. Conflict of interest: The authors have a collaboration with Organovo, which supplies them with a bioprinter and technical support. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animal use.
Author Contributions
Renee Muyoka Maina: conceptualization; data curation; investigation; writing – original draft; writing – review & editing. Maria J. Barahona: formal analysis; writing – original draft; writing – review & editing. Michele Finotti: conceptualization; writing – original draft; writing – review & editing. Taras Lysyy: conceptualization; data curation; writing – original draft. Peter Geibel: conceptualization; data curation; project administration. Francesco D’Amico: visualization; writing – review & editing. David Mulligan: supervision; visualization. John Geibel: conceptualization; resources; supervision; validation; writing – original draft; writing – review & editing.
References
- [1].Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation 2016;133:e38–360. [DOI] [PubMed]; Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ. et al. Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- [2].Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. [DOI] [PMC free article] [PubMed]; Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nat Rev Cardiol 2013;10:410–21. [DOI] [PubMed]; Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nat Rev Cardiol. 2013;10:410–21. doi: 10.1038/nrcardio.2013.77. [DOI] [PubMed] [Google Scholar]
- [4].Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med 2014; 6:61–76. [DOI] [PubMed]; Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med. 2014;6:61–76. doi: 10.1002/wsbm.1246. [DOI] [PubMed] [Google Scholar]
- [5].Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater 2005;74:570–81. [DOI] [PubMed]; Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74:570–81. doi: 10.1002/jbm.b.30247. [DOI] [PubMed] [Google Scholar]
- [6].Sayers RD, Raptis S, Berce M, Miller JH. Long-term results of femorotibial bypass with vein or polytetrafluoroethylene. Br J Surg 1998;85:934–8. [DOI] [PubMed]; Sayers RD, Raptis S, Berce M, Miller JH. Long-term results of femorotibial bypass with vein or polytetrafluoroethylene. Br J Surg. 1998;85:934–8. doi: 10.1046/j.1365-2168.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- [7].Desai M, Seifalian AM, Hamilton G. Role of prosthetic conduits in coronary artery bypass grafting. Eur J Cardiothorac Surg 2011;40:394–8. [DOI] [PubMed]; Desai M, Seifalian AM, Hamilton G. Role of prosthetic conduits in coronary artery bypass grafting. Eur J Cardiothorac Surg. 2011;40:394–8. doi: 10.1016/j.ejcts.2010.11.050. [DOI] [PubMed] [Google Scholar]
- [8].Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science 1999;284:489–93. [DOI] [PubMed]; Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R. et al. Functional arteries grown in vitro. Science. 1999;284:489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- [9].Li S, Henry JJ. Nonthrombogenic approaches to cardiovascular bioengineering. Annu Rev Biomed Eng 2011;13:451–75. [DOI] [PubMed]; Li S, Henry JJ. Nonthrombogenic approaches to cardiovascular bioengineering. Annu Rev Biomed Eng. 2011;13:451–75. doi: 10.1146/annurev-bioeng-071910-124733. [DOI] [PubMed] [Google Scholar]
- [10].Huang AH, Niklason LE. Engineering of arteries in vitro. Cell Mol Life Sci 2014;71:2103–18. [DOI] [PMC free article] [PubMed]; Huang AH, Niklason LE. Engineering of arteries in vitro. Cell Mol Life Sci. 2014;71:2103–18. doi: 10.1007/s00018-013-1546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med 2009;6:16–26. [DOI] [PMC free article] [PubMed]; Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang NF, Lee RJ, Li S. Chemical and physical regulation of stem cells and progenitor cells: potential for cardiovascular tissue engineering. Tissue Eng 2007;13:1809–23. [DOI] [PubMed]; Huang NF, Lee RJ, Li S. Chemical and physical regulation of stem cells and progenitor cells: potential for cardiovascular tissue engineering. Tissue Eng. 2007;13:1809–23. doi: 10.1089/ten.2006.0096. [DOI] [PubMed] [Google Scholar]
- [13].Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev 2012;18:405–25. [DOI] [PMC free article] [PubMed]; Bajpai VK, Andreadis ST. Stem cell sources for vascular tissue engineering and regeneration. Tissue Eng Part B Rev. 2012;18:405–25. doi: 10.1089/ten.teb.2011.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials 2012;33:3388–400. [DOI] [PubMed]; Krawiec JT, Vorp DA. Adult stem cell-based tissue engineered blood vessels: a review. Biomaterials. 2012;33:3388–400. doi: 10.1016/j.biomaterials.2012.01.014. [DOI] [PubMed] [Google Scholar]
- [15].Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Review: application of stem cells for vascular tissue engineering. Tissue Eng 2005;11:1535–52. [DOI] [PubMed]; Riha GM, Lin PH, Lumsden AB, Yao Q, Chen C. Review: application of stem cells for vascular tissue engineering. Tissue Eng. 2005;11:1535–52. doi: 10.1089/ten.2005.11.1535. [DOI] [PubMed] [Google Scholar]
- [16].Niu G, Sapoznik E, Soker S. Bioengineered blood vessels. Expert Opin Biol Ther 2014;14:403–10. [DOI] [PubMed]; Niu G, Sapoznik E, Soker S. Bioengineered blood vessels. Expert Opin Biol Ther. 2014;14:403–10. doi: 10.1517/14712598.2014.880419. [DOI] [PubMed] [Google Scholar]
- [17].Hoch E, Tovar GE, Borchers K. Bioprinting of artificial blood vessels: current approaches towards a demanding goal. Eur J Cardiothorac Surg 2014;46:767–78. [DOI] [PubMed]; Hoch E, Tovar GE, Borchers K. Bioprinting of artificial blood vessels: current approaches towards a demanding goal. Eur J Cardiothorac Surg. 2014;46:767–78. doi: 10.1093/ejcts/ezu242. [DOI] [PubMed] [Google Scholar]
- [18].Awad NK, Niu H, Ali U, Morsi YS, Lin T. Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes (Basel) 2018;8:1–26. [DOI] [PMC free article] [PubMed]; Awad NK, Niu H, Ali U, Morsi YS, Lin T. Electrospun fibrous scaffolds for small-diameter blood vessels: a review. Membranes (Basel) 2018;8:1–26. doi: 10.3390/membranes8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng 2006;12:1197–211. [DOI] [PubMed]; Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- [20].Kwon IK, Matsuda T. Co-electrospun nanofiber fabrics of poly(l-lactide-co-epsilon-caprolactone) with type I collagen or heparin. Biomacromolecules 2005;6:2096–105. [DOI] [PubMed]; Kwon IK, Matsuda T. Co-electrospun nanofiber fabrics of poly(l-lactide-co-epsilon-caprolactone) with type I collagen or heparin. Biomacromolecules. 2005;6:2096–105. doi: 10.1021/bm050086u. [DOI] [PubMed] [Google Scholar]
- [21].Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006;7:2796–805. [DOI] [PubMed]; Pham QP, Sharma U, Mikos AG. Electrospun poly(epsilon-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- [22].Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A 2009;15:2665–76. [DOI] [PMC free article] [PubMed]; Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009;15:2665–76. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brown KE, Heyer K, Rodriguez H, Eskandari MK, Pearce WH, Morasch MD. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J Vasc Surg 2009;49:660–6. [DOI] [PubMed]; Brown KE, Heyer K, Rodriguez H, Eskandari MK, Pearce WH, Morasch MD. Arterial reconstruction with cryopreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J Vasc Surg. 2009;49:660–6. doi: 10.1016/j.jvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- [24].Peck M, Gebhart D, Dusserre N, McAllister TN, L’Heureux N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 2012;195:144–58. [DOI] [PMC free article] [PubMed]; Peck M, Gebhart D, Dusserre N, McAllister TN, L’Heureux N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs. 2012;195:144–58. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 2006;12:361–5. [DOI] [PMC free article] [PubMed]; L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN. et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McAllister TN, Dusserre N, Maruszewski M, L’Heureux N. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen Med 2008;3:925–37. [DOI] [PubMed]; McAllister TN, Dusserre N, Maruszewski M, L’Heureux N. Cell-based therapeutics from an economic perspective: primed for a commercial success or a research sinkhole? Regen Med. 2008;3:925–37. doi: 10.2217/17460751.3.6.925. [DOI] [PubMed] [Google Scholar]
- [27].Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA 2011;108:9214–9. [DOI] [PMC free article] [PubMed]; Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA. 2011;108:9214–9. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg 2005;129:1330–8. [DOI] [PubMed]; Shin’oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T. et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–8. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- [29].Patel B, Xu Z, Pinnock CB, Kabbani LS, Lam MT. Self-assembled collagen-fibrin hydrogel reinforces tissue engineered adventitia vessels seeded with human fibroblasts. Sci Rep 2018;8:3294. [DOI] [PMC free article] [PubMed]; Patel B, Xu Z, Pinnock CB, Kabbani LS, Lam MT. Self-assembled collagen-fibrin hydrogel reinforces tissue engineered adventitia vessels seeded with human fibroblasts. Sci Rep. 2018;8:3294. doi: 10.1038/s41598-018-21681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A 2004;68:756–62. [DOI] [PubMed]; Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA. Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A. 2004;68:756–62. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- [31].Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA 2008;105:6537–42. [DOI] [PMC free article] [PubMed]; Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA. 2008;105:6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A, et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 2009;30:1542–50. [DOI] [PMC free article] [PubMed]; Konig G, McAllister TN, Dusserre N, Garrido SA, Iyican C, Marini A. et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials. 2009;30:1542–50. doi: 10.1016/j.biomaterials.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bourget JM, Gauvin R, Larouche D, Lavoie A, Labbe R, Auger FA, et al. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials 2012;33:9205–13. [DOI] [PubMed]; Bourget JM, Gauvin R, Larouche D, Lavoie A, Labbe R, Auger FA. et al. Human fibroblast-derived ECM as a scaffold for vascular tissue engineering. Biomaterials. 2012;33:9205–13. doi: 10.1016/j.biomaterials.2012.09.015. [DOI] [PubMed] [Google Scholar]
- [34].Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, et al. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater 2014;10:11–25. [DOI] [PMC free article] [PubMed]; Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR. et al. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Thomas V, Donahoe T, Nyairo E, Dean DR, Vohra YK. Electrospinning of Biosyn((R))-based tubular conduits: structural, morphological, and mechanical characterizations. Acta Biomater 2011;7:2070–9. [DOI] [PubMed]; Thomas V, Donahoe T, Nyairo E, Dean DR, Vohra YK. Electrospinning of Biosyn((R))-based tubular conduits: structural, morphological, and mechanical characterizations. Acta Biomater. 2011;7:2070–9. doi: 10.1016/j.actbio.2011.01.008. [DOI] [PubMed] [Google Scholar]
- [36].Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR, et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater 2010;6: 110–22. [DOI] [PMC free article] [PubMed]; Soletti L, Hong Y, Guan J, Stankus JJ, El-Kurdi MS, Wagner WR. et al. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010;6:110–22. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Subramanian A, Krishnan UM, Sethuraman S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed Mater 2011;6:025004. [DOI] [PubMed]; Subramanian A, Krishnan UM, Sethuraman S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed Mater. 2011;6:025004. doi: 10.1088/1748-6041/6/2/025004. [DOI] [PubMed] [Google Scholar]
- [38].Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med 2012;18:1148–53. [DOI] [PMC free article] [PubMed]; Wu W, Allen RA, Wang Y. Fast-degrading elastomer enables rapid remodeling of a cell-free synthetic graft into a neoartery. Nat Med. 2012;18:1148–53. doi: 10.1038/nm.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhao Y, Zhang S, Zhou J, Wang J, Zhen M, Liu Y, et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials 2010;31:296–307. [DOI] [PubMed]; Zhao Y, Zhang S, Zhou J, Wang J, Zhen M, Liu Y. et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010;31:296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- [40].Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 2011;63:300–11. [DOI] [PubMed]; Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- [41].Armentano RL, Levenson J, Barra JG, Fischer EI, Breitbart GJ, Pichel RH, et al. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol 1991;260:H1870–7. [DOI] [PubMed]; Armentano RL, Levenson J, Barra JG, Fischer EI, Breitbart GJ, Pichel RH. et al. Assessment of elastin and collagen contribution to aortic elasticity in conscious dogs. Am J Physiol. 1991;260:H1870–7. doi: 10.1152/ajpheart.1991.260.6.H1870. [DOI] [PubMed] [Google Scholar]
- [42].Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res 1993;73:1040–50. [DOI] [PubMed]; Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res. 1993;73:1040–50. doi: 10.1161/01.res.73.6.1040. [DOI] [PubMed] [Google Scholar]
- [43].Wengerter BC, Emre G, Park JY, Geibel J. Three-dimensional printing in the intestine. Clin Gastroenterol Hepatol 2016;14:1081–5. [DOI] [PubMed]; Wengerter BC, Emre G, Park JY, Geibel J. Three-dimensional printing in the intestine. Clin Gastroenterol Hepatol. 2016;14:1081–5. doi: 10.1016/j.cgh.2016.05.008. [DOI] [PubMed] [Google Scholar]
- [44].Vanderburgh J, Sterling JA, Guelcher SA. 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann Biomed Eng 2017;45:164–79. [DOI] [PMC free article] [PubMed]; Vanderburgh J, Sterling JA, Guelcher SA. 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann Biomed Eng. 2017;45:164–79. doi: 10.1007/s10439-016-1640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hull CW. Apparatus for production of three dimensional objects by stereolithography US 4575330 A, Google Patents; 1986.; Hull CW. Google Patents. Apparatus for production of three dimensional objects by stereolithography US 4575330 A. 1986
- [46].Yao R, Xu G, Mao SS, Yang HY, Sang XT, Sun W, et al. Three-dimensional printing: review of application in medicine and hepatic surgery. Cancer Biol Med 2016;13:443–51. [DOI] [PMC free article] [PubMed]; Yao R, Xu G, Mao SS, Yang HY, Sang XT, Sun W. et al. Three-dimensional printing: review of application in medicine and hepatic surgery. Cancer Biol Med. 2016;13:443–51. doi: 10.20892/j.issn.2095-3941.2016.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg 2017;7:668–77. [DOI] [PMC free article] [PubMed]; Perica E, Sun Z. Patient-specific three-dimensional printing for pre-surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7:668–77. doi: 10.21037/qims.2017.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Igami T, Nakamura Y, Hirose T, Ebata T, Yokoyama Y, Sugawara G, et al. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg 2014;38:3163–6. [DOI] [PubMed]; Igami T, Nakamura Y, Hirose T, Ebata T, Yokoyama Y, Sugawara G. et al. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38:3163–6. doi: 10.1007/s00268-014-2740-7. [DOI] [PubMed] [Google Scholar]
- [49].Souzaki R, Kinoshita Y, Ieiri S, Hayashida M, Koga Y, Shirabe K, et al. Three-dimensional liver model based on preoperative CT images as a tool to assist in surgical planning for hepatoblastoma in a child. Pediatr Surg Int 2015;31:593–6. [DOI] [PubMed]; Souzaki R, Kinoshita Y, Ieiri S, Hayashida M, Koga Y, Shirabe K. et al. Three-dimensional liver model based on preoperative CT images as a tool to assist in surgical planning for hepatoblastoma in a child. Pediatr Surg Int. 2015;31:593–6. doi: 10.1007/s00383-015-3709-9. [DOI] [PubMed] [Google Scholar]
- [50].Jones DB, Sung R, Weinberg C, Korelitz T, Andrews R. Three-dimensional modeling may improve surgical education and clinical practice. Surg Innov 2016;23:189–95. [DOI] [PubMed]; Jones DB, Sung R, Weinberg C, Korelitz T, Andrews R. Three-dimensional modeling may improve surgical education and clinical practice. Surg Innov. 2016;23:189–95. doi: 10.1177/1553350615607641. [DOI] [PubMed] [Google Scholar]
- [51].Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med 2013;368:2043–5. [DOI] [PubMed]; Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368:2043–5. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- [52].Chen H, Wu D, Yang H, Guo K. Clinical use of 3D printing guide plate in posterior lumbar pedicle screw fixation. Med Sci Monit 2015;21:3948–54. [DOI] [PMC free article] [PubMed]; Chen H, Wu D, Yang H, Guo K. Clinical use of 3D printing guide plate in posterior lumbar pedicle screw fixation. Med Sci Monit. 2015;21:3948–54. doi: 10.12659/MSM.895597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zadpoor AA, Malda J. Additive manufacturing of biomaterials, tissues, and organs. Ann Biomed Eng 2017;45:1–11. [DOI] [PubMed]; Zadpoor AA, Malda J. Additive manufacturing of biomaterials, tissues, and organs. Ann Biomed Eng. 2017;45:1–11. doi: 10.1007/s10439-016-1719-y. [DOI] [PubMed] [Google Scholar]
- [54].Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–85. [DOI] [PubMed]; Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- [55].De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 2007;25:100–6. [DOI] [PubMed]; De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L. et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- [56].Seol YJ, Kang HW, Lee SJ, Atala A, Yoo JJ. Bioprinting technology and its applications. Eur J Cardiothorac Surg 2014;46:342–8. [DOI] [PubMed]; Seol YJ, Kang HW, Lee SJ, Atala A, Yoo JJ. Bioprinting technology and its applications. Eur J Cardiothorac Surg. 2014;46:342–8. doi: 10.1093/ejcts/ezu148. [DOI] [PubMed] [Google Scholar]
- [57].Zhang X, Zhang Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem Biophys 2015;72:777–82. [DOI] [PubMed]; Zhang X, Zhang Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem Biophys. 2015;72:777–82. doi: 10.1007/s12013-015-0531-x. [DOI] [PubMed] [Google Scholar]
- [58].Patra S, Young V. A review of 3D printing techniques and the future in biofabrication of bioprinted tissue. Cell Biochem Biophys 2016;74:93–8. [DOI] [PubMed]; Patra S, Young V. A review of 3D printing techniques and the future in biofabrication of bioprinted tissue. Cell Biochem Biophys. 2016;74:93–8. doi: 10.1007/s12013-016-0730-0. [DOI] [PubMed] [Google Scholar]
- [59].Derby B. Printing and prototyping of tissues and scaffolds. Science 2012;338:921–6. [DOI] [PubMed]; Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338:921–6. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- [60].Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials 2009;30:2164–74. [DOI] [PMC free article] [PubMed]; Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A 2008;14:41–8. [DOI] [PubMed]; Chang R, Nam J, Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng Part A. 2008;14:41–8. doi: 10.1089/ten.a.2007.0004. [DOI] [PubMed] [Google Scholar]
- [62].Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, Kachurin AM, et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng 2004;10:1566–76. [DOI] [PubMed]; Smith CM, Stone AL, Parkhill RL, Stewart RL, Simpkins MW, Kachurin AM. et al. Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng. 2004;10:1566–76. doi: 10.1089/ten.2004.10.1566. [DOI] [PubMed] [Google Scholar]
- [63].Barron JA, Ringeisen BR, Kim H, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films 2004;453–4:383–7.; Barron JA, Ringeisen BR, Kim H, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films. 2004;453–4:383–7. [Google Scholar]
- [64].Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010;31:7250–6. [DOI] [PubMed]; Guillotin B, Souquet A, Catros S, Duocastella M, Pippenger B, Bellance S. et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials. 2010;31:7250–6. doi: 10.1016/j.biomaterials.2010.05.055. [DOI] [PubMed] [Google Scholar]
- [65].Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, Yang J, et al. 3D Bioprinting for tissue and organ fabrication. Ann Biomed Eng 2017;45:148–63. [DOI] [PMC free article] [PubMed]; Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, Yang J. et al. 3D Bioprinting for tissue and organ fabrication. Ann Biomed Eng. 2017;45:148–63. doi: 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wu GH, Hsu SH. Review: polymeric-based 3D printing for tissue engineering. J Med Biol Eng 2015;35:285–92. [DOI] [PMC free article] [PubMed]; Wu GH, Hsu SH. Review: polymeric-based 3D printing for tissue engineering. J Med Biol Eng. 2015;35:285–92. doi: 10.1007/s40846-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ou KL, Hosseinkhani H. Development of 3D in vitro technology for medical applications. Int J Mol Sci 2014;15:17938–62. [DOI] [PMC free article] [PubMed]; Ou KL, Hosseinkhani H. Development of 3D in vitro technology for medical applications. Int J Mol Sci. 2014;15:17938–62. doi: 10.3390/ijms151017938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B, et al. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012;4:022001. [DOI] [PubMed]; Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B. et al. Toward engineering functional organ modules by additive manufacturing. Biofabrication. 2012;4:022001. doi: 10.1088/1758-5082/4/2/022001. [DOI] [PubMed] [Google Scholar]
- [69].Ji S, Guvendiren M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol 2017;5:23. [DOI] [PMC free article] [PubMed]; Ji S, Guvendiren M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front Bioeng Biotechnol. 2017;5:23. doi: 10.3389/fbioe.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ovsianikov A, Khademhosseini A, Mironov V. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol 2018;36:348–57. [DOI] [PubMed]; Ovsianikov A, Khademhosseini A, Mironov V. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 2018;36:348–57. doi: 10.1016/j.tibtech.2018.01.005. [DOI] [PubMed] [Google Scholar]
- [71].Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009;30:6221–7. [DOI] [PubMed]; Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–7. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- [72].Li S, Xiong Z, Wang X, Yan Y, Liu H, Zhang R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J Bioact Compat Polym 2009;24:249–65.; Li S, Xiong Z, Wang X, Yan Y, Liu H, Zhang R. Direct fabrication of a hybrid cell/hydrogel construct by a double-nozzle assembling technology. J Bioact Compat Polym. 2009;24:249–65. [Google Scholar]
- [73].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 2012;11:768–74. [DOI] [PMC free article] [PubMed]; Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater. 2012;11:768–74. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell 2018;22:340–54. [DOI] [PMC free article] [PubMed]; Song HG, Rumma RT, Ozaki CK, Edelman ER, Chen CS. Vascular tissue engineering: progress, challenges, and clinical promise. Cell Stem Cell. 2018;22:340–54. doi: 10.1016/j.stem.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res 2006;98:25–35. [DOI] [PubMed]; Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- [76].Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg 2005;41:349–54. [DOI] [PubMed]; Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–54. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- [77].Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng 2007;35:348–55. [DOI] [PMC free article] [PubMed]; Dahl SL, Rhim C, Song YC, Niklason LE. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng. 2007;35:348–55. doi: 10.1007/s10439-006-9226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kucukgul C, Ozler SB, Inci I, Karakas E, Irmak S, Gozuacik D, et al. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol Bioeng 2015;112:811–21. [DOI] [PubMed]; Kucukgul C, Ozler SB, Inci I, Karakas E, Irmak S, Gozuacik D. et al. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol Bioeng. 2015;112:811–21. doi: 10.1002/bit.25493. [DOI] [PubMed] [Google Scholar]
- [79].Akkouch A, Yu Y, Ozbolat IT. Microfabrication of scaffold-free tissue strands for three-dimensional tissue engineering. Biofabrication 2015;7:031002. [DOI] [PubMed]; Akkouch A, Yu Y, Ozbolat IT. Microfabrication of scaffold-free tissue strands for three-dimensional tissue engineering. Biofabrication. 2015;7:031002. doi: 10.1088/1758-5090/7/3/031002. [DOI] [PubMed] [Google Scholar]
- [80].Moldovan L, Barnard A, Gil CH, Lin Y, Grant MB, Yoder MC, et al. iPSC-derived vascular cell spheroids as building blocks for scaffold-free biofabrication. Biotechnol J 2017;12:237–244. [DOI] [PubMed]; Moldovan L, Barnard A, Gil CH, Lin Y, Grant MB, Yoder MC. et al. iPSC-derived vascular cell spheroids as building blocks for scaffold-free biofabrication. Biotechnol J. 2017;12:237–244. doi: 10.1002/biot.201700444. [DOI] [PubMed] [Google Scholar]
- [81].Moldovan NI, Hibino N, Nakayama K. Principles of the Kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Eng Part B Rev 2017;23:237–44. [DOI] [PubMed]; Moldovan NI, Hibino N, Nakayama K. Principles of the Kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Eng Part B Rev. 2017;23:237–44. doi: 10.1089/ten.TEB.2016.0322. [DOI] [PubMed] [Google Scholar]
- [82].Moldovan NI. Progress in scaffold-free bioprinting for cardiovascular medicine. J Cell Mol Med 2018;22:2964–9. [DOI] [PMC free article] [PubMed]; Moldovan NI. Progress in scaffold-free bioprinting for cardiovascular medicine. J Cell Mol Med. 2018;22:2964–9. doi: 10.1111/jcmm.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K, et al. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One 2015;10:e0136681. [DOI] [PMC free article] [PubMed]; Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K. et al. Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One. 2015;10:e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Turker E, Demircak N, Arslan-Yildiz A. Scaffold-free three-dimensional cell culturing using magnetic levitation. Biomater Sci 2018;1–9. doi: 10.1039/c8bm00122g. [Epub ahead of print]. [DOI] [PubMed]; Turker E, Demircak N, Arslan-Yildiz A. Scaffold-free three-dimensional cell culturing using magnetic levitation. Biomater Sci. 2018:1–9. doi: 10.1039/c8bm00122g. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [85].Tocchio A, Durmus NG, Sridhar K, Mani V, Coskun B, El Assal R, et al. Magnetically guided self-assembly and coding of 3D living architectures. Adv Mater 2018;30:1705034. [DOI] [PMC free article] [PubMed]; Tocchio A, Durmus NG, Sridhar K, Mani V, Coskun B, El Assal R. et al. Magnetically guided self-assembly and coding of 3D living architectures. Adv Mater. 2018;30:1705034. doi: 10.1002/adma.201705034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tseng H, Gage JA, Haisler WL, Neeley SK, Shen T, Hebel C, et al. A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep 2016;6:30640. [DOI] [PMC free article] [PubMed]; Tseng H, Gage JA, Haisler WL, Neeley SK, Shen T, Hebel C. et al. A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep. 2016;6:30640. doi: 10.1038/srep30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.