Abstract
The endovascular technique has led to a revolution in the care of patients with vascular disease; however, acquiring and maintaining proficiency over a broad spectrum of procedures is challenging. Three-dimensional (3D) printing technology allows the production of models that can be used for endovascular training. This article aims to explain the process and technologies available to produce vascular models for endovascular training, using 3D printing technology. The data are based on the group experience and a review of the literature. Different 3D printing methods are compared, describing their advantages, disadvantages and potential roles in surgical training. The process of 3D printing a vascular model based on an imaging examination consists of the following steps: image acquisition, image post-processing, 3D printing and printed model post-processing. The entire process can take a week. Prospective studies have shown that 3D printing can improve surgical planning, especially in complex endovascular procedures, and allows the production of efficient simulators for endovascular training, improving residents’ surgical performance and self-confidence.
Keywords: 3D printing, endovascular, patient-specific, simulations, training
Abbreviations: 3D printing, three-dimensional printing; angioCT scan, computed angio-tomography scan; CT, computed tomography; DICOM, Digital Imaging and Communication in Medicine; MRI, magnetic resonance imaging; STL, stereolithography.
Introduction
The endovascular technique has led to a revolution in the care of patients with vascular disease; however, acquiring and maintaining proficiency over a broad spectrum of procedures is challenging for experienced surgeons and surgeons under training [1], [2], as the rapid innovation process of the endovascular material demands frequent update and technical training [3]. Although the importance of training based on simulations is well documented in several studies [4], [5], [6], [7], [8], [9], [10], the use of simulators is still limited [3], and the cost of the simulator is probably the main reason [10], [11], [12]. Therefore, affordable simulators are the key to make simulations a routine [11].
Three-dimensional (3D) printing, also known as additive manufacturing or rapid prototyping, is a growing technology that is changing the manufacturing industry [13]. The process consists of creating 3D objects through deposition of successive layers of different materials, based on a computer file. 3D printing offers many advantages over traditional manufacturing, including the ability to create objects with complex internal structures, improved versatility, and customisation and lower space requirements [12]. The cost of 3D printers has recently decreased, and the availability of 3D printing services has increased. Itagaki [13] showed the feasibility of producing a 3D-printed splenic aneurysm using Internet-based services (www.shapeways.com, www.imaterialise.com) at a low cost.
The combination of 3D printing and imaging examinations offers a great opportunity for the progress of medical science [14], [15], [16], as it allows the visualisation of diseases with complex anatomy [12] and the creation of models in different materials, which can be used for surgical planning and training [7], [17], [18]. Furthermore, 3D printing allows a patient-specific simulation, which is more efficient than generic simulation [8], [19].
Methods
We performed electronic searches in PubMed to collect studies on 3D printing technology and its applications in endovascular training. Several searches were performed using the combination of different terms: 3D printing and/or endovascular training and/or surgical planning. The only limit defined was language: only reports in Portuguese or English were considered. Titles and abstracts were screened to exclude irrelevant or duplicate abstracts. Then, the included articles underwent a full-text review. We initially found 409 results, which were narrowed down to 190 after the analysis of abstracts (duplicated articles and researches deemed irrelevant to surgical practice were excluded). This set of results was then reviewed to form a set of 58 full-text articles used for the final analysis. In addition, the websites of the 3D printers were searched for technical information.
Process to create 3D-printed vascular models for endovascular training
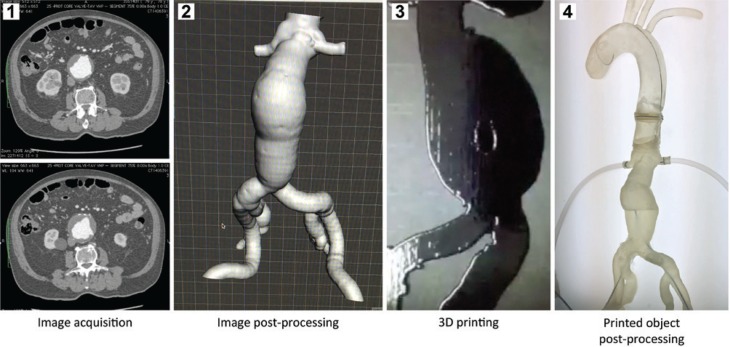
The process to produce 3D-printed vascular models based on image examinations consists of four steps: image acquisition, image post-processing, 3D printing and printed object post-processing (Figure 1). Depending on the size and complexity of the vascular model, it can take up to 48 h just to 3D print it. It is reasonable to expect 1 week for the completion of the entire process.
Figure 1:
Steps to produce a 3D-printed aneurysm.
1, Images from an angioCT; 2, abdominal aorta after image post-processing; 3, 3D printing process; 4, post-processed 3D-printed aneurysm.
Image acquisition
Data for generating medical 3D-printed models are typically acquired with computed angio-tomography scan (angioCT) or magnetic resonance imaging (MRI) [12], [15]. For vascular models, image post-processing is less complex for angioCT scan data [15]. An ideal computed tomography (CT) acquisition should be free of image artefacts and have isotropic voxel resolution, high image contrast between the anatomy of interest and neighbouring tissues, and low noise. A slice thickness of 0.5–1.5 mm is adequate. Acquired data are saved in Digital Image and Communication in Medicine (DICOM) format.
Image post-processing
Image post-processing can be performed using several different software programs, as shown in Table 1.
Table 1:
Software programs available for image post-processing.
| Software programs for DICOM file processing | References |
|---|---|
| Mimics® (Materialise NV, Leuven, Belgium) | Biglino et al. [20], Wilasrusmee et al. [21], Håkansson et al. [22], Yuan et al. [23], Mafeld et al. [18], Dong et al. [24], Koleilat et al. [25], Taher et al. [26] |
| OsiriX (Pixmeo SARL, Bernex, Switzerland) | Marro et al. [12], Tam et al. [27], Takao et al. [28] |
| Vitrea 3D Station (Vital Images, Inc., Minnetonka, MN, USA) | O’Hara et al. [29], Russ et al. [30] |
| iNtuition software (TeraRecon Inc., Foster City, CA, USA) | Koleilat et al. [25] |
| Vascular Modeling Toolkit (VMTK, Orobix, Bergamo, Italy) | Meess et al. [31] |
|
Software programs for STL file processing | |
| 3-matic® (Materialise NV, Leuven, Belgium) | Biglino et al. [20], Mafeld et al. [18], Koleilat et al. [25] |
| MeshLab (Visual Computing Lab – ISTI-CNR, Rome, Italy) | Marro et al. [12] |
| Blender (Blender Foundation, Amsterdam, the Netherlands) | Itagaki [13] |
| Google SketchUp (Trimble Inc., CA, USA) | Govsa et al. [32] |
| Magics (Materialise NV, Leuven, Belgium) | Yuan et al. [23] |
| Meshmixer software (Autodesk, San Rafael, CA, USA) | O’Hara et al. [29], Takao et al. [28], Russ et al. [30], Meess et al. [31] |
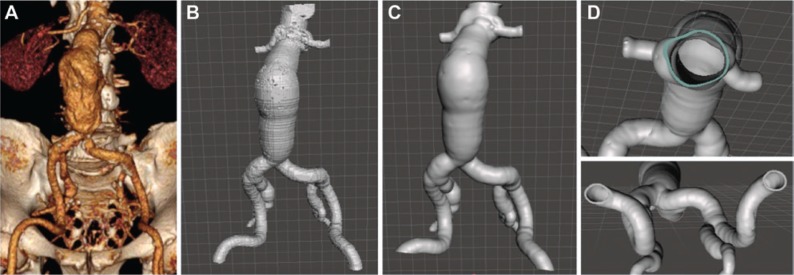
For DICOM post-processing, the authors’ choices were iNtuition Unlimited software (Aquarius, TeraRecon, San Mateo, CA, USA), OsiriX software (Pixmeo, Geneva, Switzerland), or Horos software (The Horos Project, sponsored by Nimble Co LLC d/b/a Purview, Annapolis, MD, USA). The first step was to generate a reconstruction of the region of interest based on the contrast inside the arterial lumen. After that, the vascular reconstruction was extracted from the surrounding tissue (manually or using specific tools, such as extracting the central line in TeraRecon) and exported as a stereolithography (STL) file. Thereafter, we chose Mesh Mixer (Mesh Mixer 2.8; Autodesk Inc., San Rafael, CA, USA) or Magics Software (Magics, 3-matic®; Materialise NV, Leuven, Belgium) for STL processing. These programs allow the user to smooth the surface of the vascular structure and to correct errors in the mesh. A wall thickness was digitally produced, and the space occupied by the lumen was subtracted to create the primary hollow models, as shown in Figure 2.
Figure 2:
Image post-processing.
(A) Reconstruction of the aorta based on the contrast inside the arterial lumen – DICOM file. (B) Aorta after conversion of DICOM to STL file. (C) Surface of the aorta smoothed. (D) Wall of the aorta digitally thickened to 1.5 mm.
3D printing
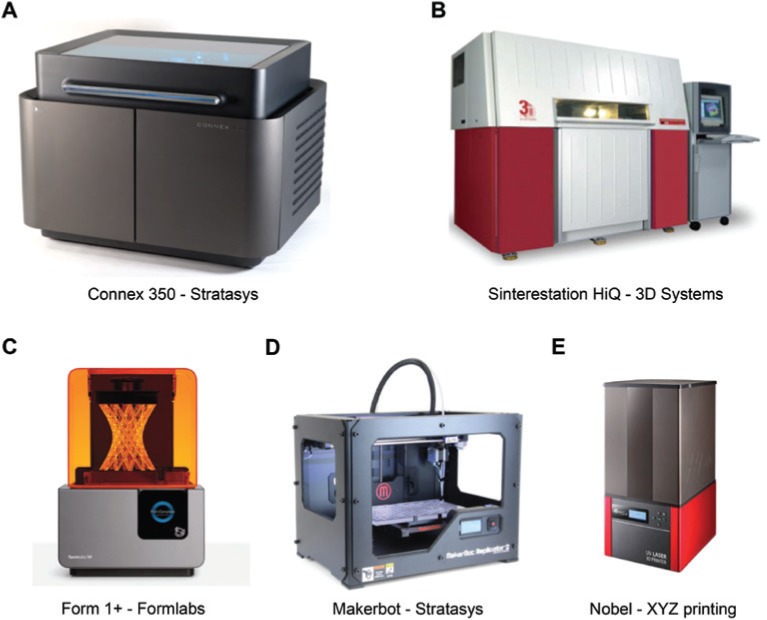
Recent advances in 3D printing technology have led to the development of new resources, making the use of a wide variety of materials from plastic to metals possible. It is possible to directly 3D print hollow flexible models, which is a one-step process; therefore, it is faster and more accurate than the commonly used lost wax technique [27]. The choice of the 3D printer depends on the application. Figure 3 shows some 3D printers available. Some of the following characteristics should be considered: cost, accuracy, speed and materials available [12].
Figure 3:
Examples of 3D printers available for the production of vascular models.
A and B are industrial machines. C, D and E are desktop machines. The pictures were collected from the websites (www.stratsys.com, www.3dsystems.com, www.formlabs.com and www.xyzprinting.com). The companies agreed to the use of the pictures.
Industrial machines, such as Connex from Stratasys [33], are versatile (have a great number of printing materials available and allow the combination of different materials in the same object), accurate (has a layer resolution of 16 μm) and have a big printing platform (up to 490×390×200 mm). Nevertheless, the cost of the 3D printer and the resins is high (one 3D printer costs around US$270,000.00, and the cost to 3D print an aneurysm is US$1670.00). Desktop machines, in general, have smaller printing platforms, smaller accuracy and limited materials available. Therefore, they cost less (Form1+, US$3000.00; MakerBot, US$2118.00; Nobel, US$2214.00), which makes their use outside study protocols feasible [19]. The companies Stratasys, Formlabs and MakerBot informed the cost of the 3D printers through invoices. The cost of the materials was calculated by two different 3D printing companies, based on the mean quantity of the material necessary for one aneurysm.
The characteristics of some 3D printers previously tested for producing models for endovascular training are shown in Table 2.
Table 2:
3D printers tested for the production of endovascular training models.
| 3D printer | Connex 350 | Form 1+ | Nobel | MakerBot | Sinterstation HiQ |
|---|---|---|---|---|---|
| Technology | Polyjet | Stereolithography | Stereolithography | Fused deposition modelling | Selective laser sintering |
| Industrial machine | Desktop machine | Desktop machine | Desktop machine | Industrial machine | |
| Printing platform (mm) | 340×340×200 | 125×125×165 | 128×128×200 | 295×195×165 | 381×330×457 |
| Layer resolution (μm) | 16 | 25–100 | 25 | 100 | 70 |
| Advantages | High-resolution, large printing platform, possibility of material combination | Low cost, high resolution | Low cost | Large printing platform, good printing speed | |
| Disadvantages | High cost, materials with insufficient transparency and/or resistance | Small printing platform | No flexible or translucent material available | No flexible or translucent material available | |
| Materials available | Transparent, biocompatible, rigid opaque, rubber like, polypropylene like | Photopolymer resins: transparent, white, resistant, flexible, castable dental SG | Photopolymer resins: standard, castable, flexible, rigid, tough | PLA filament, ABS filament, absorbable filament | DuraForm PA, DuraForm GF, DuraForm EX, DuraForm Flex, DuraForm AF, LaserForm A6 and CastForm materials |
| Webpage | http://www.stratasys.com/3d-printers/production-series/connex3-systems | https://formlabs.com/3d-printers/form-1-plus/ | https://www.xyzprinting.com/en-US/product/nobel-1-0 | https://www.makerbot.com/replicator/ | http://ntk-mt.ru/pdf/ds_sinterstation_hiq_rev.pdf |
ABS, acrylonitrile butadiene styrene; PLA, polilatic acid; SG, surgical guides.
The 3D printing materials are specific to each 3D printer and are not interchangeable. In general, the characteristics desired for endovascular training are transparency (for training with no need for radiation), good resistance (to avoid leakages and ruptures during the training sessions) and good navigability of the endovascular material (for precision during the deployment of the stent graft and the use of the endovascular material).
It is possible to produce a simulator for training in endovascular aneurysm repair (EVAR) using Connex (Stratasys), Form1+ (Formlabs), MakerBot and Nobel (XYZ Printing) 3D printers. The properties of the materials are shown in Table 3.
Table 3:
Materials available for producing transparent vascular models for endovascular training.
| Material | Typical properties of the material |
Major limitation during training sessionf | ||
|---|---|---|---|---|
| Shore | Elongation at break (%) | Tensile strength (MPa) | ||
| TangoPlusa | A 26–68 | 170–220 | 0.8–1.5 | Transparency and resistance |
| Vero Clearb | D 83–86 | 10–25 | 50–65 | Transparency and navigability |
| TangoPlus and Vero Clearc | A 57–63 | 75–85 | 2.5–4.0 | Transparency |
| Flexible Resin Formlabsd | A 80–90 | 90 | 5.95–6.5 | Resistance |
| Flexible Resin XYZ Printing | – | – | – | Transparency and Resistance |
| Siliconee | A 30 | 470 | 5 | Navigability |
aPolyjet Material Rubber FLX930. bPolyjet Material Standard Plastic RGD810. cPolyjet Digital Material Tango Plus + Vero-Clear Shore 60. dFormlabs Flexible Photopolymer Resin for Form1+. eDow Corning Silastic [34]. fAccording to our previous study [19], analysing transparency, resistance and navigability.
Unfortunately, no 3D printing material has all the properties required for endovascular training: insufficient transparency or resistance are the main problems. Most flexible resins available (e.g. Tango Plus from Stratasys, the flexible resin from Formlabs and the flexible resin from XYZ Printing) allow a single training session to be performed, due to the poor resistance of the material, which makes ruptures and leakages frequent. It is possible to reproduce the vascular model in silicone to overcome this issue. For this reproduction, a solid model is made (using a low-cost fused deposition modelling printer such as MakerBot or Sinterstation HiQ printer) and reproduced in silicone. Nevertheless, the process is not simple; knowledge of the material, skills and a good infrastructure are necessary. However, in a test of different materials (shown in Table 2) in a simulator for EVAR training, silicone was the only flexible material capable of resisting the training sessions with no leakages or ruptures [19].
Printed model post-processing
After 3D printing, the object needs to be post-processed. Each material requires different work [19].
If the Polyjet technology is used (e.g. Connex 3D printer from Stratasys), the support material has to be removed with a water jet. Ruptures may occur during the cleaning process and have to be repaired (using the resin itself or glue). The vascular models should be exposed to ultraviolet light for 24–48 h to improve transparency. Figure 4 shows the process.
Figure 4:
Post-processing of the aneurysms in Material 1.
(A) 3D-printed aneurysm with the support material. (B) 3D-printed aneurysm after removing the support material. (C) Areas reinforced with silicone. (D) 3D-printed aneurysm connected to the simulator.
If STL technology is used (e.g. Form1+ from Formlabs, Nobel from XYZ Printing, Projet from 3D Systems), pillars are produced to sustain the vascular model during the printing process (shown in Figure 5). These pillars have to be removed. In general, the printing area is small and the vascular models have to be produced in two or three parts, which can be assembled together using the resin and a proper laser. For curing purposes, the vascular models should be exposed to ultraviolet light for 24–48 h.
Figure 5:

Pillars produced during the printing process of the Form1+ 3D printer.
Test with an opaque material and an aneurysm produced in two parts.
If a solid model is 3D printed, it can be reproduced in silicone in several different ways: the lost wax technique can be used, or silicone can be directly applied on the surface of the solid model, cured under rotation and heat, and thereafter cut, removed from the solid model and restored. A soluble resin can be used to produce a solid vascular model, and then silicone is applied on the surface of the soluble model and cured, and afterwards the soluble resin is removed by submerging it in a solution produced with caustic soda and water.
Production of a patient-specific simulator for training in endovascular procedures
After post-processing, the vascular models should be connected to a pulsatile flow system to improve the navigability of the endovascular material and to allow the procedure to be performed under antegrade flow. There are commercially available pumps and generic silicone vascular models (e.g. http://www.elastrat.ch/models/assets/files/Products%20PDF/Pump-Tank%203 lt.pdf). A 3D-printed model can be incorporated in a generic silicone model, as shown in Figure 4. Although no 3D printing materials show all the properties required for training, the simulators produced using 3D-printed models allowed efficient patient-specific training prior to endovascular procedures [19].
Applications of 3D-printed vascular models
Surgical training
Simulation-based training is not a substitute for clinical practice; however, it offers a consistent method of instruction that allows skill development and assessment. It is more efficient than simple demonstrations (because the resident can truly practice the procedure), more accurate (a patient-specific condition can be reproduced) and less expensive than training in animals. Simulations avoid the exposure of patients to unnecessary risks, and avoid ethical and legal issues related to teaching and learning processes inside a hospital [34]. University hospitals can use simulators to assess the surgical skills of residents during their training, identifying their difficulties in order to improve the program.
Senior residents in the United States reported limited experience and low self-confidence in performing complex endovascular procedures. Residents who performed simulation-based training reported an increase in self-confidence; nevertheless, only 25% of residents in the United States had access to this kind of training [10]. Among educators, there is a concern that graduated residents are not ready to independently perform all vascular surgery procedures in their field [10].
Training in patient-specific simulators, produced with 3D-printed vascular models, improves the residents’ surgical performance (reducing fluoroscopy time, total procedure time and amount of contrast used) and increases their self-confidence [19].
Surgical planning
Preoperative planning is a crucial part of endovascular procedures [21], [35], [36], [37]. The choice of stents and grafts is based on CT images; however, in complex cases, there can still be considerable uncertainty [7], [15], [26]. 3D-printed vascular models can be created from CT data and used to test the device selection to assist in the preoperative planning [25], [26], [27], [31].
Tam et al. [38] conducted a pilot study to investigate the role of 3D-printed models in surgical planning and clinical decision making for cases with aortic aneurysms with challenging anatomical features (e.g. short, angulated or conical necks). They analysed 28 endovascular operators who planned six different cases. After planning the procedure based on the patients’ angioCT, the surgeons were presented with the equivalent 3D-printed models and asked to review their decisions. The plan changed in 20% of the cases, and the level of confidence increased in 43% [38].
3D aortic models are also a valuable tool to test a custom-made stent graft before implantation, and may avoid adverse events associated with misaligned fenestrations and unconnected aortic branches. Taher et al. [26] analysed 60 patients who underwent fenestrated endovascular aortic repair and observed that 21.7% of the stent grafts were modified after the surgeon tested the stent graft in a 3D-printed aortic model.
Similarly, Koleilat et al. [25] assessed the accuracy of the measurements obtained with automated 3D centreline reconstruction from imaging data compared with 3D-printed aortic models. They found substantial differences in inter-observer measurements compared with 3D-printed aortic models and concluded that vessel angles are not accurately measured. This may lead to an eclipsing phenomenon, which may contribute to branched or fenestrated vessel failure and re-intervention.
Itagaki [13] manufactured a vascular model to assist surgical planning prior to endovascular treatment of a patient with multiple splenic artery aneurysms. He used free software and low-cost printing services, and concluded that the models produced are useful in preoperative planning and intraoperative guidance.
In addition, 3D-printed vascular models significantly improve the ability of trainees to properly plan for complex endovascular procedures such as EVAR [21]. Therefore, a simulator produced using 3D-printed vascular models is a good tool to teach procedural planning. Surgeons under training have the opportunity to plan the procedure on the patients’ CT and test their tactics on the simulator, which allows for a better understanding of endovascular material behaviour.
Discussion
Increased emphasis on patient outcome and quality improvement has led to the use of 3D printing technology in the medical field, especially in school hospitals, where surgeries are performed by surgeons under training [39].
The effect of residents’ involvement in perioperative outcomes is a topic of debate and has been evaluated across different surgeries [39], [40]. Iannuzzi et al., analysing lower-extremity amputation, concluded that resident involvement increased the odds of major morbidity, operative time and the risk of intraoperative transfusions [41]. Scarborough et al. noticed that resident involvement in lower-extremity bypass was an independent risk factor for graft failure [42]. DiDato et al. [39] analysed the effect of resident involvement in EVAR, and concluded that it was not associated with major adverse perioperative outcomes. However, it was associated with an increased operative time and length of stay, and therefore may lead to increased resource utilisation and cost [39].
The increased operative time related to residents’ involvement in EVAR was reversed with patient-specific training in a school hospital in Brazil. During 1 year, the residents trained for all steps of the surgery using a simulator produced with 3D printing technology, which led to an improvement in residents’ surgical performance (total procedure time was reduced by 29%, fluoroscopy time by 31% and time for contralateral limb gate cannulation by 54%) and increased their self-confidence [19]. These findings agree with a systematic review published by See et al. in 2016 [43], which reported a reduction in surgical metrics (procedure time and fluoroscopy time) after the technical training of the residents because the residents became more familiar with the procedure. The reduction in fluoroscopy time and total procedure time is an important issue in a procedure that exposes the patient and the surgical team to ionizing radiation [44], [45].
A multicentre, prospective, randomised trial in Europe showed that patient-specific rehearsal prior to EVAR reduced perioperative errors and the number of angiograms required to deploy the stent graft. Therefore, it may improve patient safety and procedural efficiency [5].
The CT scan measurements for programming endovascular procedures are based on a centreline of flow. However, even modern workstations cannot accurately predict the treatment lengths in patients with severe aortoiliac tortuosity [46]. These patients tend to show a substantial shorten due to a combination of remodelling of the native aorta, stent-graft conformability, and stiffness of guidewires and delivery systems used in endovascular surgeries. The arterial deformations caused by the endovascular equipment depend on multiple factors, such as the morphology of the arteries, the state and degree of calcification of the arterial wall, and the type of device used. Today, their prediction relies mainly on the surgeon’s experience [47].
3D printing can be a valuable tool to predict the deformations of the arteries due to the insertion of the endovascular material, which can help improve surgical plans, avoiding complications and use of unnecessary material [26], [47], especially prior to complex surgeries such as implantation of fenestrated aortic grafts. Planning and construction of fenestrated stent grafts for complex aortic anatomies are challenging: the exact fit and positioning of the graft are paramount to allow cannulation of the aortic branches [26].
A retrospective study at Stanford University Medical Center showed a 30% cost increase in EVAR when the use of stent graft extensions was necessary, compared to cases where the standard number of pieces was used (mean device-related cost US$13,220 vs. US$17,107, p<0.01); the authors concluded that appropriate preoperative planning and device selection can minimize the cost [48]. Improvement in surgical planning and surgical efficiency can make the use of simulators cost-effective. The cost to produce 3D-printed models (Itagaki, US$50.34–232.03; Torres and De Luccia, US$200.00–1200.00) seems reasonable compared to the cost of the endovascular material [13], [19]. Nevertheless, a study designed to analyse cost reduction after simulations is necessary to confirm this conclusion.
An important limitation of training using 3D-printed models is the need for endovascular material for training purposes, which is wasteful and adds to the cost of training [11]. However, endovascular materials that are close to expiration are generally collected and incinerated. This material may be donated to hospitals where training protocols are implemented. In addition, some endovascular material can be recapped and deployed several times. Moreover, in complex cases, the company may produce a non-sterile prototype for training purposes, which is already commercially available for Terumo Anaconda fenestrated grafts, for example [26].
Currently, there are virtual-reality simulators that overcome the need for endovascular material for training. Numerous studies have shown good results with endovascular training using virtual-reality simulators [4], [5], [8], [11], [49], [50], [51], [52]. However, virtual-reality simulators are expensive devices [11], [50], [53] that are prone to technical failure and require regular calibration and maintenance [11].
Virtual-reality simulators and 3D-printed models are both interesting and promising technologies. Virtual-reality simulators have proven useful in choosing the C-arm angle and offer quantitative data for performance analysis [4]. 3D-printed models may help in understanding the behaviour of the endovascular material in three dimensions, inside a specific anatomy. In addition, 3D-printed models can be directly manipulated and inspected, which can help identify some details that were not noticed on the CT scan. Both technologies have shown good results for training and surgical planning [4], [5], [19]; the choice depends on the institution infrastructure, budget and personal preferences.
The traditional apprenticeship model training was stated by Halsted in 1904 and basically consists of supervised training with progressive exposure of the residents to the procedures [11]. Although patient safety is assured by the presence of a senior surgeon [39], this teaching method may not be valid in the modern practice of vascular surgery [54], [55], [56]. Simulation-based training offers a consistent method of instruction that allows skill development and assessment. Simulations avoid the exposure of patients to unnecessary risks, and avoid ethics and legal issues [34]. 3D-printed vascular models allow the production of simulators for endovascular training, which can improve procedural efficiency and patient safety.
Supporting Information
Acknowledgments
This manuscript was edited for proper English language by American Journal Experts: certification verification key FE66-DAD5-33A9-ED42-D5B1.
Supplementary Material
The article (iss-2018-0020) offers reviewer assessments as supplementary material.
Author Statement
Research funding: Authors state no funding involved. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animals use.
Author Contributions
Inez Torres: performed the research, wrote the manuscript; Nelson De Luccia: reviewed the manuscript.
References
- [1].Mills JL Sr. Vascular surgery training in the United States: a half-century of evolution. J Vasc Surg 2008;48:90S–7S; discussion 97S. [DOI] [PubMed]; Mills JL Sr. Vascular surgery training in the United States: a half-century of evolution. J Vasc Surg. 2008;48:90S–7S. doi: 10.1016/j.jvs.2008.07.090. discussion 97S. [DOI] [PubMed] [Google Scholar]
- [2].Moreira RC. Critical issues in vascular surgery: education in Brazil. J Vasc Surg 2008;48:87S–9S; discussion 89S. [DOI] [PubMed]; Moreira RC. Critical issues in vascular surgery: education in Brazil. J Vasc Surg. 2008;48:87S–9S. doi: 10.1016/j.jvs.2008.08.095. discussion 89S. [DOI] [PubMed] [Google Scholar]
- [3].Kim AH, Kendrick DE, Moorehead PA, Nagavalli A, Miller CP, Liu NT, et al. Endovascular aneurysm repair simulation can lead to decreased fluoroscopy time and accurately delineate the proximal seal zone. J Vasc Surg 2016;64:251–8. [DOI] [PubMed]; Kim AH, Kendrick DE, Moorehead PA, Nagavalli A, Miller CP, Liu NT. et al. Endovascular aneurysm repair simulation can lead to decreased fluoroscopy time and accurately delineate the proximal seal zone. J Vasc Surg. 2016;64:251–8. doi: 10.1016/j.jvs.2016.01.050. [DOI] [PubMed] [Google Scholar]
- [4].Desender L, Rancic Z, Aggarwal R, Duchateau J, Glenck M, Lachat M, et al. Patient-specific rehearsal prior to EVAR: a pilot study. Eur J Vasc Endovasc Surg 2013;45:639–47. [DOI] [PubMed]; Desender L, Rancic Z, Aggarwal R, Duchateau J, Glenck M, Lachat M. et al. Patient-specific rehearsal prior to EVAR: a pilot study. Eur J Vasc Endovasc Surg. 2013;45:639–47. doi: 10.1016/j.ejvs.2013.03.006. [DOI] [PubMed] [Google Scholar]
- [5].Desender LM, Van Herzeele I, Lachat ML, Rancic Z, Duchateau J, Rudarakanchana N, et al. Patient-specific rehearsal before EVAR: influence on technical and nontechnical operative performance. A randomized controlled trial. Ann Surg 2016;264:703–9. [DOI] [PubMed]; Desender LM, Van Herzeele I, Lachat ML, Rancic Z, Duchateau J, Rudarakanchana N. et al. Patient-specific rehearsal before EVAR: influence on technical and nontechnical operative performance. A randomized controlled trial. Ann Surg. 2016;264:703–9. doi: 10.1097/SLA.0000000000001871. [DOI] [PubMed] [Google Scholar]
- [6].Grantcharov TP, Kristiansen VB, Bendix J, Bardram L, Rosenberg J, Funch-Jensen P. Randomized clinical trial of virtual reality simulation for laparoscopic skills training. Br J Surg 2004;91:146–50. [DOI] [PubMed]; Grantcharov TP, Kristiansen VB, Bendix J, Bardram L, Rosenberg J, Funch-Jensen P. Randomized clinical trial of virtual reality simulation for laparoscopic skills training. Br J Surg. 2004;91:146–50. doi: 10.1002/bjs.4407. [DOI] [PubMed] [Google Scholar]
- [7].Tam MD, Laycock SD, Brown JR, Jakeways M. 3D printing of an aortic aneurysm to facilitate decision making and device selection for endovascular aneurysm repair in complex neck anatomy. J Endovasc Ther 2013;20:863–7. [DOI] [PubMed]; Tam MD, Laycock SD, Brown JR, Jakeways M. 3D printing of an aortic aneurysm to facilitate decision making and device selection for endovascular aneurysm repair in complex neck anatomy. J Endovasc Ther. 2013;20:863–7. doi: 10.1583/13-4450MR.1. [DOI] [PubMed] [Google Scholar]
- [8].Willaert WI, Aggarwal R, Van Herzeele I, O’Donoghue K, Gaines PA, Darzi AW, et al. Patient-specific endovascular simulation influences interventionalists performing carotid artery stenting procedures. Eur J Vasc Endovasc Surg 2011;41:492–500. [DOI] [PubMed]; Willaert WI, Aggarwal R, Van Herzeele I, O’Donoghue K, Gaines PA, Darzi AW. et al. Patient-specific endovascular simulation influences interventionalists performing carotid artery stenting procedures. Eur J Vasc Endovasc Surg. 2011;41:492–500. doi: 10.1016/j.ejvs.2010.12.013. [DOI] [PubMed] [Google Scholar]
- [9].Seymour NE, Gallagher AG, Roman SA, O’Brien MK, Bansal VK, Andersen DK, et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg 2002;236:458–63; discussion 463–4. [DOI] [PMC free article] [PubMed]; Seymour NE, Gallagher AG, Roman SA, O’Brien MK, Bansal VK, Andersen DK. et al. Virtual reality training improves operating room performance: results of a randomized, double-blinded study. Ann Surg. 2002;236:458–63. doi: 10.1097/00000658-200210000-00008. discussion 463–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg 2013;58:524–8. [DOI] [PubMed]; Duran C, Bismuth J, Mitchell E. A nationwide survey of vascular surgery trainees reveals trends in operative experience, confidence, and attitudes about simulation. J Vasc Surg. 2013;58:524–8. doi: 10.1016/j.jvs.2012.12.072. [DOI] [PubMed] [Google Scholar]
- [11].Neequaye SK, Aggarwal R, Van Herzeele I, Darzi A, Cheshire NJ. Endovascular skills training and assessment. J Vasc Surg 2007;46:1055–64. [DOI] [PubMed]; Neequaye SK, Aggarwal R, Van Herzeele I, Darzi A, Cheshire NJ. Endovascular skills training and assessment. J Vasc Surg. 2007;46:1055–64. doi: 10.1016/j.jvs.2007.05.041. [DOI] [PubMed] [Google Scholar]
- [12].Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol 2016;45:2–9. [DOI] [PubMed]; Marro A, Bandukwala T, Mak W. Three-dimensional printing and medical imaging: a review of the methods and applications. Curr Probl Diagn Radiol. 2016;45:2–9. doi: 10.1067/j.cpradiol.2015.07.009. [DOI] [PubMed] [Google Scholar]
- [13].Itagaki MW. Using 3D printed models for planning and guidance during endovascular intervention: a technical advance. Diagn Interv Radiol 2015;21:338–41. [DOI] [PMC free article] [PubMed]; Itagaki MW. Using 3D printed models for planning and guidance during endovascular intervention: a technical advance. Diagn Interv Radiol. 2015;21:338–41. doi: 10.5152/dir.2015.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sheth R, Balesh ER, Zhang YS, Hirsch JA, Khademhosseini A, Oklu R. Three-dimensional printing: an enabling technology for IR. J Vasc Interv Radiol 2016;27:859–65. [DOI] [PubMed]; Sheth R, Balesh ER, Zhang YS, Hirsch JA, Khademhosseini A, Oklu R. Three-dimensional printing: an enabling technology for IR. J Vasc Interv Radiol. 2016;27:859–65. doi: 10.1016/j.jvir.2016.02.029. [DOI] [PubMed] [Google Scholar]
- [15].Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg 2010;5:335–41. [DOI] [PubMed]; Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU. et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010;5:335–41. doi: 10.1007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- [16].Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK, et al. Medical 3D printing for the radiologist. Radiographics 2015;35:1965–88. [DOI] [PMC free article] [PubMed]; Mitsouras D, Liacouras P, Imanzadeh A, Giannopoulos AA, Cai T, Kumamaru KK. et al. Medical 3D printing for the radiologist. Radiographics. 2015;35:1965–88. doi: 10.1148/rg.2015140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Biglino G, Verschueren P, Zegels R, Taylor AM, Schievano S. Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing. J Cardiovasc Magn Reson 2013;15:2. [DOI] [PMC free article] [PubMed]; Biglino G, Verschueren P, Zegels R, Taylor AM, Schievano S. Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing. J Cardiovasc Magn Reson. 2013;15:2. doi: 10.1186/1532-429X-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mafeld S, Nesbitt C, McCaslin J, Bagnall A, Davey P, Bose P, et al. Three-dimensional (3D) printed endovascular simulation models: a feasibility study. Ann Transl Med 2017;5:42. [DOI] [PMC free article] [PubMed]; Mafeld S, Nesbitt C, McCaslin J, Bagnall A, Davey P, Bose P. et al. Three-dimensional (3D) printed endovascular simulation models: a feasibility study. Ann Transl Med. 2017;5:42. doi: 10.21037/atm.2017.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Torres IO, De Luccia N. A simulator for training in endovascular aneurysm repair: the use of three dimensional printers. Eur J Vasc Endovasc Surg 2017;54:247–53. [DOI] [PubMed]; Torres IO, De Luccia N. A simulator for training in endovascular aneurysm repair: the use of three dimensional printers. Eur J Vasc Endovasc Surg. 2017;54:247–53. doi: 10.1016/j.ejvs.2017.05.011. [DOI] [PubMed] [Google Scholar]
- [20].Biglino G, Verschueren P, Zegels R, Taylor AM, Schievano S. Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing. J Cardiovasc Magn Reson 2013;15:7. [DOI] [PMC free article] [PubMed]; Biglino G, Verschueren P, Zegels R, Taylor AM, Schievano S. Rapid prototyping compliant arterial phantoms for in-vitro studies and device testing. J Cardiovasc Magn Reson. 2013;15:7. doi: 10.1186/1532-429X-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wilasrusmee C, Suvikrom J, Suthakorn J, Lertsithichai P, Sitthiseriprapip K, Proprom N, et al. Three-dimensional aortic aneurysm model and endovascular repair: an educational tool for surgical trainees. Int J Angiol 2008;17:129–33. [DOI] [PMC free article] [PubMed]; Wilasrusmee C, Suvikrom J, Suthakorn J, Lertsithichai P, Sitthiseriprapip K, Proprom N. et al. Three-dimensional aortic aneurysm model and endovascular repair: an educational tool for surgical trainees. Int J Angiol. 2008;17:129–33. doi: 10.1055/s-0031-1278295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Håkansson A, Rantatalo M, Hansen T, Wanhainen A. Patient specific biomodel of the whole aorta – the importance of calcified plaque removal. Vasa 2011;40:453–9. [DOI] [PubMed]; Håkansson A, Rantatalo M, Hansen T, Wanhainen A. Patient specific biomodel of the whole aorta – the importance of calcified plaque removal. Vasa. 2011;40:453–9. doi: 10.1024/0301-1526/a000148. [DOI] [PubMed] [Google Scholar]
- [23].Yuan D, Luo H, Yang H, Huang B, Zhu J, Zhao J. Precise treatment of aortic aneurysm by three-dimensional printing and simulation before endovascular intervention. Sci Rep 2017;7:795. [DOI] [PMC free article] [PubMed]; Yuan D, Luo H, Yang H, Huang B, Zhu J, Zhao J. Precise treatment of aortic aneurysm by three-dimensional printing and simulation before endovascular intervention. Sci Rep. 2017;7:795. doi: 10.1038/s41598-017-00644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dong M, Chen G, Li J, Qin K, Ding X, Peng C, et al. Three-dimensional brain arteriovenous malformation models for clinical use and resident training. Medicine (Baltimore) 2018;97:e9516. [DOI] [PMC free article] [PubMed]; Dong M, Chen G, Li J, Qin K, Ding X, Peng C. et al. Three-dimensional brain arteriovenous malformation models for clinical use and resident training. Medicine (Baltimore) 2018;97:e9516. doi: 10.1097/MD.0000000000009516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koleilat I, Jaeggli M, Ewing JA, Androes M, Simionescu DT, Eidt J. Interobserver variability in physician-modified endograft planning by comparison with a three-dimensional printed aortic model. J Vasc Surg 2016;64:1789–96. [DOI] [PubMed]; Koleilat I, Jaeggli M, Ewing JA, Androes M, Simionescu DT, Eidt J. Interobserver variability in physician-modified endograft planning by comparison with a three-dimensional printed aortic model. J Vasc Surg. 2016;64:1789–96. doi: 10.1016/j.jvs.2015.09.044. [DOI] [PubMed] [Google Scholar]
- [26].Taher F, Falkensammer J, McCarte J, Strassegger J, Uhlmann M, Schuch P, et al. The influence of prototype testing in three-dimensional aortic models on fenestrated endograft design. J Vasc Surg 2017;65:1591–7. [DOI] [PubMed]; Taher F, Falkensammer J, McCarte J, Strassegger J, Uhlmann M, Schuch P. et al. The influence of prototype testing in three-dimensional aortic models on fenestrated endograft design. J Vasc Surg. 2017;65:1591–7. doi: 10.1016/j.jvs.2016.10.108. [DOI] [PubMed] [Google Scholar]
- [27].Tam MD, Latham T, Brown JRI, Jakeways M. Use of a 3D printed hollow aortic model to assist EVAR planning in a case with complex neck anatomy: potential of 3D printing to improve patient outcome. J Endovasc Ther 2014;21:760–2. [DOI] [PubMed]; Tam MD, Latham T, Brown JRI, Jakeways M. Use of a 3D printed hollow aortic model to assist EVAR planning in a case with complex neck anatomy: potential of 3D printing to improve patient outcome. J Endovasc Ther. 2014;21:760–2. doi: 10.1583/14-4810L.1. [DOI] [PubMed] [Google Scholar]
- [28].Takao H, Amemiya S, Shibata E, Ohtomo K. 3D printing of preoperative simulation models of a splenic artery aneurysm: precision and accuracy. Acad Radiol 2017;24:650–3. [DOI] [PubMed]; Takao H, Amemiya S, Shibata E, Ohtomo K. 3D printing of preoperative simulation models of a splenic artery aneurysm: precision and accuracy. Acad Radiol. 2017;24:650–3. doi: 10.1016/j.acra.2016.12.015. [DOI] [PubMed] [Google Scholar]
- [29].O’Hara RP, Chand A, Vidiyala S, Arechavala SM, Mitsouras D, Rudin S, et al. Advanced 3D mesh manipulation in stereolithographic files and post-print processing for the manufacturing of patient-specific vascular flow phantoms. Proc SPIE Int Soc Opt Eng 2016;9789. [DOI] [PMC free article] [PubMed]; O’Hara RP, Chand A, Vidiyala S, Arechavala SM, Mitsouras D, Rudin S. et al. Advanced 3D mesh manipulation in stereolithographic files and post-print processing for the manufacturing of patient-specific vascular flow phantoms. Proc SPIE Int Soc Opt Eng. 2016:9789. doi: 10.1117/12.2217036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Russ M, O’Hara R, Setlur Nagesh SV, Mokin M, Jimenez C, Siddiqui A, et al. Treatment planning for image-guided neuro-vascular interventions using patient-specific 3D printed phantoms. Proc SPIE Int Soc Opt Eng 2015;9417. [DOI] [PMC free article] [PubMed]; Russ M, O’Hara R, Setlur Nagesh SV, Mokin M, Jimenez C, Siddiqui A. et al. Treatment planning for image-guided neuro-vascular interventions using patient-specific 3D printed phantoms. Proc SPIE Int Soc Opt Eng. 2015:9417. doi: 10.1117/12.2081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Meess KM, Izzo RL, Dryjski ML, Curl RE, Harris LM, Springer M, et al. 3D printed abdominal aortic aneurysm phantom for image guided surgical planning with a patient specific fenestrated endovascular graft system. Proc SPIE Int Soc Opt Eng 2017;10138. [DOI] [PMC free article] [PubMed]; Meess KM, Izzo RL, Dryjski ML, Curl RE, Harris LM, Springer M. et al. 3D printed abdominal aortic aneurysm phantom for image guided surgical planning with a patient specific fenestrated endovascular graft system. Proc SPIE Int Soc Opt Eng. 2017:10138. doi: 10.1117/12.2253902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Govsa F, Yagdi T, Ozer MA, Eraslan C, Alagoz AK. Building 3D anatomical model of coiling of the internal carotid artery derived from CT angiographic data. Eur Arch Otorhinolaryngol 2017;274:1097–102. [DOI] [PubMed]; Govsa F, Yagdi T, Ozer MA, Eraslan C, Alagoz AK. Building 3D anatomical model of coiling of the internal carotid artery derived from CT angiographic data. Eur Arch Otorhinolaryngol. 2017;274:1097–102. doi: 10.1007/s00405-016-4355-0. [DOI] [PubMed] [Google Scholar]
- [33].Stratasys Ltd. Connex 3 Objet 500 and Objet 350; 2018.; Stratasys Ltd. Connex 3 Objet 500 and Objet 350; 2018. [Google Scholar]
- [34].Dawson DL, Meyer J, Lee ES, Pevec WC. Training with simulation improves residents’ endovascular procedure skills. J Vasc Surg 2007;45:149–54. [DOI] [PubMed]; Dawson DL, Meyer J, Lee ES, Pevec WC. Training with simulation improves residents’ endovascular procedure skills. J Vasc Surg. 2007;45:149–54. doi: 10.1016/j.jvs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [35].Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004;351:1607–18. [DOI] [PubMed]; Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R. et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351:1607–18. doi: 10.1056/NEJMoa042002. [DOI] [PubMed] [Google Scholar]
- [36].United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863–71. [DOI] [PubMed]; United Kingdom EVAR Trial Investigators; Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D. et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–71. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- [37].Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet 2004;364:843–8. [DOI] [PubMed]; Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364:843–8. doi: 10.1016/S0140-6736(04)16979-1. [DOI] [PubMed] [Google Scholar]
- [38].Tam MD, Latham TR, Lewis M, Khanna K, Zaman A, Parker M, et al. A pilot study assessing the impact of 3-D printed models of aortic aneurysms on management decisions in EVAR planning. Vasc Endovasc Surg 2016;50:4–9. [DOI] [PubMed]; Tam MD, Latham TR, Lewis M, Khanna K, Zaman A, Parker M. et al. A pilot study assessing the impact of 3-D printed models of aortic aneurysms on management decisions in EVAR planning. Vasc Endovasc Surg. 2016;50:4–9. doi: 10.1177/1538574415623651. [DOI] [PubMed] [Google Scholar]
- [39].DiDato S, Farber A, Rybin D, Kalish JA, Eslami MH, Moreira CC, et al. The effect of trainee involvement on perioperative outcomes of abdominal aortic aneurysm repair. J Vasc Surg 2016;63:16–22. [DOI] [PubMed]; DiDato S, Farber A, Rybin D, Kalish JA, Eslami MH, Moreira CC. et al. The effect of trainee involvement on perioperative outcomes of abdominal aortic aneurysm repair. J Vasc Surg. 2016;63:16–22. doi: 10.1016/j.jvs.2015.07.071. [DOI] [PubMed] [Google Scholar]
- [40].Meguid RA, Brooke BS, Perler BA, Freischlag JA. Impact of hospital teaching status on survival from ruptured abdominal aortic aneurysm repair. J Vasc Surg 2009;50:243–50. [DOI] [PMC free article] [PubMed]; Meguid RA, Brooke BS, Perler BA, Freischlag JA. Impact of hospital teaching status on survival from ruptured abdominal aortic aneurysm repair. J Vasc Surg. 2009;50:243–50. doi: 10.1016/j.jvs.2009.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Iannuzzi JC, Chandra A, Rickles AS, Kumar NG, Kelly KN, Gillespie DL, et al. Resident involvement is associated with worse outcomes after major lower extremity amputation. J Vasc Surg 2013;58:827–31; e821. [DOI] [PubMed]; Iannuzzi JC, Chandra A, Rickles AS, Kumar NG, Kelly KN, Gillespie DL. et al. Resident involvement is associated with worse outcomes after major lower extremity amputation. J Vasc Surg. 2013;58:827–31. doi: 10.1016/j.jvs.2013.04.046. e821. [DOI] [PubMed] [Google Scholar]
- [42].Scarborough JE, Pappas TN, Cox MW, Bennett KM, Shortell CK. Surgical trainee participation during infrainguinal bypass grafting procedures is associated with increased early postoperative graft failure. J Vasc Surg 2012;55:715–20. [DOI] [PubMed]; Scarborough JE, Pappas TN, Cox MW, Bennett KM, Shortell CK. Surgical trainee participation during infrainguinal bypass grafting procedures is associated with increased early postoperative graft failure. J Vasc Surg. 2012;55:715–20. doi: 10.1016/j.jvs.2011.06.122. [DOI] [PubMed] [Google Scholar]
- [43].See KW, Chui KH, Chan WH, Wong KC, Chan YC. Evidence for endovascular simulation training: a systematic review. Eur J Vasc Endovasc Surg 2016;51:441–51. [DOI] [PubMed]; See KW, Chui KH, Chan WH, Wong KC, Chan YC. Evidence for endovascular simulation training: a systematic review. Eur J Vasc Endovasc Surg. 2016;51:441–51. doi: 10.1016/j.ejvs.2015.10.011. [DOI] [PubMed] [Google Scholar]
- [44].Attigah N, Oikonomou K, Hinz U, Knoch T, Demirel S, Verhoeven E, et al. Radiation exposure to eye lens and operator hands during endovascular procedures in hybrid operating rooms. J Vasc Surg 2016;63:198–203. [DOI] [PubMed]; Attigah N, Oikonomou K, Hinz U, Knoch T, Demirel S, Verhoeven E. et al. Radiation exposure to eye lens and operator hands during endovascular procedures in hybrid operating rooms. J Vasc Surg. 2016;63:198–203. doi: 10.1016/j.jvs.2015.08.051. [DOI] [PubMed] [Google Scholar]
- [45].Lipsitz EC, Veith FJ, Ohki T, Heller S, Wain RA, Suggs WD, et al. Does the endovascular repair of aortoiliac aneurysms pose a radiation safety hazard to vascular surgeons? J Vasc Surg 2000;32:704–10. [DOI] [PubMed]; Lipsitz EC, Veith FJ, Ohki T, Heller S, Wain RA, Suggs WD. et al. Does the endovascular repair of aortoiliac aneurysms pose a radiation safety hazard to vascular surgeons? J Vasc Surg. 2000;32:704–10. doi: 10.1067/mva.2000.110053. [DOI] [PubMed] [Google Scholar]
- [46].Lee K, Leci E, Forbes T, Dubois L, DeRose G, Power A. Endograft conformability and aortoiliac tortuosity in endovascular abdominal aortic aneurysm repair. J Endovasc Ther 2014;21:728–34. [DOI] [PubMed]; Lee K, Leci E, Forbes T, Dubois L, DeRose G, Power A. Endograft conformability and aortoiliac tortuosity in endovascular abdominal aortic aneurysm repair. J Endovasc Ther. 2014;21:728–34. doi: 10.1583/14-4663MR.1. [DOI] [PubMed] [Google Scholar]
- [47].Gindre J, Bel-Brunon A, Kaladji A, Duménil A, Rochette M, Lucas A, et al. Finite element simulation of the insertion of guidewires during an EVAR procedure: example of a complex patient case, a first step toward patient-specific parameterized models. Int J Numer Method Biomed Eng 2015;31:e02716. [DOI] [PubMed]; Gindre J, Bel-Brunon A, Kaladji A, Duménil A, Rochette M, Lucas A. et al. Finite element simulation of the insertion of guidewires during an EVAR procedure: example of a complex patient case, a first step toward patient-specific parameterized models. Int J Numer Method Biomed Eng. 2015;31:e02716. doi: 10.1002/cnm.2716. [DOI] [PubMed] [Google Scholar]
- [48].Chandra V, Greenberg JI, Al-Khatib WK, Harris EJ, Dalman RL, Lee JT. Cost impact of extension cuff utilization during endovascular aneurysm repair. Ann Vasc Surg 2012;26:86–92. [DOI] [PubMed]; Chandra V, Greenberg JI, Al-Khatib WK, Harris EJ, Dalman RL, Lee JT. Cost impact of extension cuff utilization during endovascular aneurysm repair. Ann Vasc Surg. 2012;26:86–92. doi: 10.1016/j.avsg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- [49].Neequaye SK, Aggarwal R, Brightwell R, Van Herzeele I, Darzi A, Cheshire NJ. Identification of skills common to renal and iliac endovascular procedures performed on a virtual reality simulator. Eur J Vasc Endovasc Surg 2007;33:525–32. [DOI] [PubMed]; Neequaye SK, Aggarwal R, Brightwell R, Van Herzeele I, Darzi A, Cheshire NJ. Identification of skills common to renal and iliac endovascular procedures performed on a virtual reality simulator. Eur J Vasc Endovasc Surg. 2007;33:525–32. doi: 10.1016/j.ejvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- [50].Davis GR, Illig KA, Yang G, Nguyen TH, Shames ML. An approach to EVAR simulation using patient specific modeling. Ann Vasc Surg 2014;28:1769–74. [DOI] [PubMed]; Davis GR, Illig KA, Yang G, Nguyen TH, Shames ML. An approach to EVAR simulation using patient specific modeling. Ann Vasc Surg. 2014;28:1769–74. doi: 10.1016/j.avsg.2014.05.007. [DOI] [PubMed] [Google Scholar]
- [51].Saratzis A, Calderbank T, Sidloff D, Bown MJ, Davies RS. Role of simulation in endovascular aneurysm repair (EVAR) training: a preliminary study. Eur J Vasc Endovasc Surg 2017;53:193–8. [DOI] [PubMed]; Saratzis A, Calderbank T, Sidloff D, Bown MJ, Davies RS. Role of simulation in endovascular aneurysm repair (EVAR) training: a preliminary study. Eur J Vasc Endovasc Surg. 2017;53:193–8. doi: 10.1016/j.ejvs.2016.11.016. [DOI] [PubMed] [Google Scholar]
- [52].Rudarakanchana N, Van Herzeele I, Desender L, Cheshire NJ. Virtual reality simulation for the optimization of endovascular procedures: current perspectives. Vasc Health Risk Manag 2015;11:195–202. [DOI] [PMC free article] [PubMed]; Rudarakanchana N, Van Herzeele I, Desender L, Cheshire NJ. Virtual reality simulation for the optimization of endovascular procedures: current perspectives. Vasc Health Risk Manag. 2015;11:195–202. doi: 10.2147/VHRM.S46194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Starosolski ZA, Kan JH, Rosenfeld SD, Krishnamurthy R, Annapragada A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr Radiol 2014;44:216–21. [DOI] [PubMed]; Starosolski ZA, Kan JH, Rosenfeld SD, Krishnamurthy R, Annapragada A. Application of 3-D printing (rapid prototyping) for creating physical models of pediatric orthopedic disorders. Pediatr Radiol. 2014;44:216–21. doi: 10.1007/s00247-013-2788-9. [DOI] [PubMed] [Google Scholar]
- [54].Chaer RA, Derubertis BG, Lin SC, Bush HL, Karwowski JK, Birk D, et al. Simulation improves resident performance in catheter-based intervention: results of a randomized, controlled study. Ann Surg 2006;244:343–52. [DOI] [PMC free article] [PubMed]; Chaer RA, Derubertis BG, Lin SC, Bush HL, Karwowski JK, Birk D. et al. Simulation improves resident performance in catheter-based intervention: results of a randomized, controlled study. Ann Surg. 2006;244:343–52. doi: 10.1097/01.sla.0000234932.88487.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Willems MC, van der Vliet JA, Williams V, Kool LJ, Bergqvist D, Blankensteijn JD. Assessing endovascular skills using the Simulator for Testing and Rating Endovascular Skills (STRESS) machine. Eur J Vasc Endovasc Surg 2009;37:431–6. [DOI] [PubMed]; Willems MC, van der Vliet JA, Williams V, Kool LJ, Bergqvist D, Blankensteijn JD. Assessing endovascular skills using the Simulator for Testing and Rating Endovascular Skills (STRESS) machine. Eur J Vasc Endovasc Surg. 2009;37:431–6. doi: 10.1016/j.ejvs.2008.12.021. [DOI] [PubMed] [Google Scholar]
- [56].Bismuth J, Donovan MA, O’Malley MK, El Sayed HF, Naoum JJ, Peden EK, et al. Incorporating simulation in vascular surgery education. J Vasc Surg 2010;52:1072–80. [DOI] [PubMed]; Bismuth J, Donovan MA, O’Malley MK, El Sayed HF, Naoum JJ, Peden EK. et al. Incorporating simulation in vascular surgery education. J Vasc Surg. 2010;52:1072–80. doi: 10.1016/j.jvs.2010.05.093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.