Abstract
Magnetic particle imaging (MPI) is a new medical imaging technique that enables three-dimensional real-time imaging of a magnetic tracer material. Although it is not yet in clinical use, it is highly promising, especially for vascular and interventional imaging. The advantages of MPI are that no ionizing radiation is necessary, its high sensitivity enables the detection of very small amounts of the tracer material, and its high temporal resolution enables real-time imaging, which makes MPI suitable as an interventional imaging technique. As MPI is a tracer-based imaging technique, functional imaging is possible by attaching specific molecules to the tracer material. In the first part of this article, the basic principle of MPI will be explained and a short overview of the principles of the generation and spatial encoding of the tracer signal will be given. After this, the used tracer materials as well as their behavior in MPI will be introduced. A subsequent presentation of selected scanner topologies will show the current state of research and the limitations researchers are facing on the way from preclinical toward human-sized scanners. Furthermore, it will be briefly shown how to reconstruct an image from the tracer materials’ signal. In the last part, a variety of possible future clinical applications will be presented with an emphasis on vascular imaging, such as the use of MPI during cardiovascular interventions by visualizing the instruments. Investigations will be discussed, which show the feasibility to quantify the degree of stenosis and diagnose strokes and traumatic brain injuries as well as cerebral or gastrointestinal bleeding with MPI. As MPI is not only suitable for vascular medicine but also offers a broad range of other possible applications, a selection of those will be briefly presented at the end of the article.
Keywords: cardiovascular intervention, functional imaging, image reconstruction, magnetic nanoparticles, medical imaging, MPI scanner, quantitative imaging, real-time imaging
Introduction
Several medical imaging techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET), are used in clinical routine today. The continuous development of all these techniques has taken in vivo diagnostics a decisive step forward. CT utilizes the attenuation of X-rays depending on the material density to image anatomical information. MRI measures the tissue-dependent proton-spin relaxation times. External magnetic fields are applied, which excite the nuclear spin of hydrogen atoms in the tissue. The relaxation time of the spins after such an excitation is tissue dependent, such that high-contrast images can be obtained from soft tissue. Further, MRI can provide information about flow and diffusion. The physical principles of both CT and MRI are based on a direct interaction with tissue material. In contrast, PET is a tracer-based imaging modality. As radioactive markers are attached to functional molecules, PET is a suitable imaging technique for the investigation of metabolic processes.
CT is widely used for imaging of all parts of the body, i.e. head and neck, chest abdomen and musculoskeletal system. To visualize the lumen of blood vessels, digital subtraction angiography (DSA) plays an important role in clinical routine for vascular interventions. An X-ray image is taken before and after the administration of a contrast agent into the blood vessel. The images are being subtracted in order to remove disturbing structures and to only visualize the blood vessels. The drawback of these techniques are that ionizing radiation is needed and the iodine-based contrast agent material can be nephrotoxic, which is a severe issue especially for older patients as well as for patients suffering from hyperthyroidism.
Magnetic particle imaging (MPI) is a novel imaging technique that is not yet in clinical use but is highly promising for several medical applications, and especially for applications in diagnostic vascular in vivo imaging and imaging-guided vascular interventions. MPI was invented by Bernhard Gleich and Jürgen Weizenecker at the Philips Research Laboratories Hamburg and was first published in 2005 [1]. No harmful radiation, such as X-rays in CT and DSA or gamma rays caused by radioactive tracers in PET, is needed. Similar to PET and DSA, MPI images the spatial and temporal distribution of tracers. However, instead of using a radioactive material or iodine, MPI uses magnetic nanoparticles (MNPs), which are biocompatible, well tolerated, and offer the potential to be quantitatively imaged. Promising experiments have shown the potential of a spatial resolution in the submillimeter range and that a high sensitivity of MNP detection can be reached. Three-dimensional (3D) images can be acquired in a short acquisition time, enabling real-time and thus interventional imaging.
Basic imaging principles
The basic imaging principles of MPI rely on the interaction of the MNPs with specific magnetic fields that are externally applied. Because of this interaction, a characteristic particle signal can be detected and later reconstructed to an image representation of the particle suspension.
Signal generation and encoding
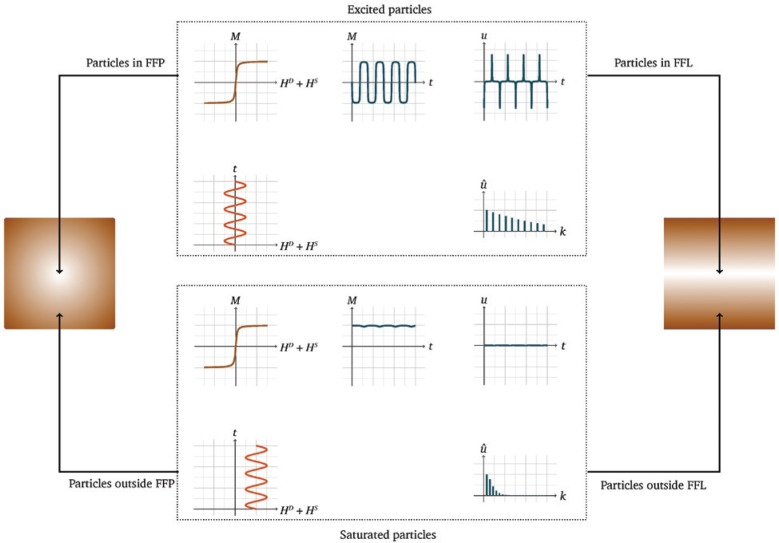
To explain the basic physical principle of MPI, we consider an MNP having a magnetic moment imaginable as a compass needle. An externally applied alternating magnetic field, called drive field HD(t), causes the magnetic moment to flip, such as a compass needle that aligns with an external magnetic field. Because the particles are excited by the drive field, this field is referred to as the excitation field. The frequency of the drive field is often chosen around 25 kHz [1], [2], [3] and is described as excitation frequency due to its effect on the particles. The magnetic moment of the MNP then flips with the same frequency. However, when the magnetic moments of the particles are completely aligned with the externally applied magnetic field, the particles’ magnetization is saturated, i.e. the particles have reached their saturation magnetization. This can be imagined as the point when a compass needle has reached its maximum deflection and thus is completely aligned with the external magnetic field. A further increase of the applied magnetic field strength therefore has no impact on the magnetization. This behavior results in a characteristic non-linear magnetization curve as a function of the magnetic field strength. Hence, the temporal evolution of the magnetization has a plateau at its minimum and maximum. That means that the magnetic moments of the MNPs flip with the same frequency as the drive field, but the temporal evolution of the magnetization is modulated. The change of the magnetic moments induces a characteristic voltage u(t) in a receiving electromagnetic coil. This voltage is a detectable, characteristic particle signal and the key to MPI (see Figure 1). The induced particle signal, which is the derivation of the magnetization, not only consists of the excitation frequency but also of higher harmonics due to the non-linear magnetization behavior of the MNPs. This can be used to determine the spatial and temporal distribution of MNP concentrations and can be seen as the fingerprint of the MNPs.
Figure 1:
The signal encoding principle in MPI is based on the excitation of MNPs by an oscillating magnetic field called drive field HD(t).
Based on a modulation of the drive field by the particles’ non-linear magnetization curve, a characteristic receive signal u(t) can be induced. As shown by the frequency spectrum û(t) of such a voltage signal, the modulation of HD(t) causes the generation of higher harmonics of the applied frequency. The principle for spatial encoding in MPI is based on the application of a selection field HS(i). All MNPs outside the area of the FFP or FFL or rather in a close vicinity to it remain in saturation, and thus do not contribute to the particle signal.
Spatial encoding
In order to spatially encode the particle signal, only MNPs in a defined area should be allowed to contribute to the signal at a given time. This can be realized by using an additional magnetic field featuring a high magnetic field strength and spatial inhomogeneity, i.e. a strong magnetic gradient field. Such a field, referred to as selection field HS(i) (with i∈{x, y, z} being the spatial direction), results in a saturation of all MNPs except those in a specific area (see Figure 1). The selection field generates a specific area in the form of a field-free point (FFP) [1] or a field-free line (FFL) [4] to spatially encode the particle signal. In both settings, the magnetic field strength of the selection field HS(i) is zero in the FFP or the FFL, respectively. Outside the FFP or FFL, the magnetization of the MNPs by the selection field HS(i) is high enough to inhibit the effect of the temporally variable drive field HD(t). Compared to the FFP approach, the FFL allows for a spatial encoding along a line, which yields in addition to an increased sensitivity also an increased signal-to-noise ratio [4], [5]. The use of an FFL can also be seen in analogy to the imaging principles of CT. This is particularly interesting when it comes to the reconstruction of the received particle signals (see “Image reconstruction” section) and the possibility of applying sophisticated reconstruction algorithms as used in CT [6], [7]. The signal and spatial encoding principle in MPI based on an FFP or FFL is illustrated for a 1D scenario in Figure 1.
As it is of interest to not only cover one position but an entire area or volume given by a defined field of view (FOV), it is necessary to change the position of the FFP or the FFL relative to the imaged object containing the MNPs. A sufficiently large amplitude of the drive field leads to a significant movement of the FFP or FFL along a line. A drive field applied in two or three dimensions enables a movement of the FFP or FFL in a plane or volume, respectively. The established trajectories for FFP imaging are the Cartesian trajectory and the Lissajous trajectory [8] that differ in their complexity regarding technical realization and the later image reconstruction procedures. The latter enables 3D real-time imaging [9]. An illustration is given in Figure 2. In case of an FFL, the trajectory consists of a translation as well as a rotation [6].
Figure 2:
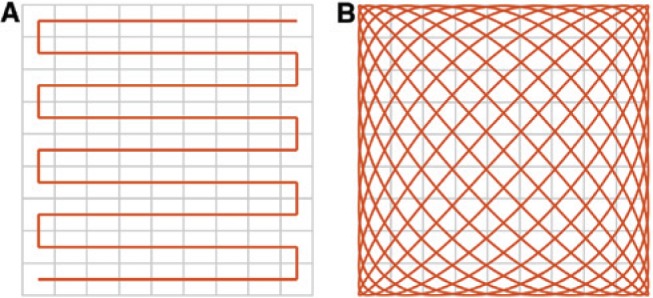
By use of a continuous data acquisition, different trajectories can be applied to cover the FOV.
For a 2D or even 3D imaging approach, a sequential movement (A) or a Lissajous trajectory (B) can be used.
Towards clinical use: enlargement of the imaging region and safety considerations
The sizes of the FOV covered by a 3D Lissajous trajectory of today’s available scanners are about a few centimeters in all directions. A full scan of this volume can be taken within several 10 ms, which enables real-time imaging. Aiming at the clinical use of MPI, the upscaling of the FOV is of high interest. As a possibility to further enlarge the potential imaging region, additional magnetic fields, known as focus fields, can be used, which allow the volume covered by the Lissajous trajectory to be shifted [10], [11]. Consecutively, it becomes possible to merge these volumes to one large volume, enabling the visualization of larger organs or vascular branches. However, field imperfections might cause artifacts at edges of the imaging area.
MPI is a non-hazardous imaging technique, as no ionizing radiation is needed, which makes MPI promising as an interventional imaging method. The biocompatibility of the tracer material can be achieved by a biocompatible coating of the MNPs (see “MNPs” section). However, for use in clinical routine, the tracer material needs to be officially approved. Further, it needs to be mentioned that certain combinations of drive field frequencies and amplitudes can lead to nerve stimulation and tissue heating. Considering this, parameters need to be carefully chosen and safety limits should be investigated thoroughly in order to avoid those negative side effects by the applied magnetic fields [12], [13], [14], [15]. As human-sized MPI scanners would need large field amplitudes, this is a challenging task; however, their feasibility has already been demonstrated (see “MPI scanner” section).
MNPs
Nanoparticles are usually defined as objects ranging in size from 1 to 100 nm. Nowadays, they can be found in different materials of everyday use, such as colors, solar cells, cosmetics, sunscreen, or clothes. Moreover, they are being developed as contrast agents or drug carriers for future medical applications. Nanosized iron oxide particles are used as a tracer material in MPI. The particles are so small that only a single magnetic domain forms throughout a particle. This allows the particles to behave in a very specific way, called superparamagnetic, i.e. the non-linear magnetization curve looks like the one of a paramagnetic material with a high saturation magnetization (see “Signal generation and encoding” section). Therefore, the particles are often called superparamagnetic iron oxide nanoparticles (SPIONs). In general, the MNPs consist of a superparamagnetic core and a biocompatible coating. In contrast to iodine-based tracer material used for DSA, MNPs are not nephrotoxic and uncritical for patients with hyperthyroidism. In contrast to tracer materials used in PET, MNPs are not radioactive; therefore, neither the patient nor the clinician are being exposed to radiation and the tracer material is easier to handle. In the last decade, Resovist (Bayer Schering Pharma AG, Berlin, Germany) has been established as a gold standard for experimental and preclinical MPI tracers, because it provides good performance for MPI. Resovist is made of MNPs covered with carboxydextrane in a water-based isotonic suspension and was an approved contrast agent for application of MRI in liver evaluations. It has been used in MPI, even though only a few percent of the nanoparticles in the suspension actually contribute to the MPI signal, which is why the development of tailored nanoparticles for MPI is part of ongoing research [16], [17]. In the future, optimized tracer materials need to be evaluated and approved for clinical applications.
The combination of the magnetic iron core with its magnetic characteristics and the magnetically neutral coating makes MNPs a suitable MPI tracer material.
In a simplified model, the non-linear magnetization behavior of MNPs can be described by the Langevin theory of paramagnetism as long as anisotropy, a particle’s magnetic directional dependence, particle interactions, and hysteresis effects are neglected.
When particles are exposed to an external magnetic field, the magnetic moments align in the direction of the magnetic field and change their magnetization direction. This magnetization process can be described as a function of the magnetic field. For ideal particles, the magnetization shows a very characteristic behavior. With an increasing field strength, the magnetization rises to a saturation magnetization and decreases back to zero, when the field strength decreases. The saturation effect of the particle’s magnetization can mathematically be described by the Langevin function.
The steepness of the magnetization curve determines the achievable spatial resolution in MPI and is mostly dependent on the size of the iron core [3], [18], [19]. In Figure 1, the magnetization behavior of MNPs according to the Langevin theory can be seen.
MPI scanner
Today, a variety of MPI scanner prototypes have been developed by various research groups [2]. The main challenges are the achievement of a high spatial and/or temporal resolution, an efficient field generation, patient safety, and an upscaling to larger scanners. Typically, the design of the developed prototypes and preclinical scanners is focused either on the improvement of sensitivity or acquisition time.
The first MPI scanner was developed at the Philips Research Laboratories Hamburg in 2005 [1]. The scanner consists of two permanent magnets to generate an FFP selection field with a magnetic field gradient of 3.4 T/m and three opposing pairs of drive field coils for each direction in space with an amplitude of 10 mT. The drive field coils generate a homogeneous field inside the scanning volume and excite the particles. These homogenous fields are also used to move the FFP. Additionally, the object can be mechanically moved by using a robot to enlarge the FOV. Two receive coils are used to record the induced signal. First experiments using a phantom that consists of holes with a diameter of 0.5 mm filled with Resovist were performed at 52×52 data points, within a scanning volume of 9.4×9.4 mm2. A schematic drawing of an FFP scanner with closed bore geometry can be seen in Figure 3 on the left side.
Figure 3:
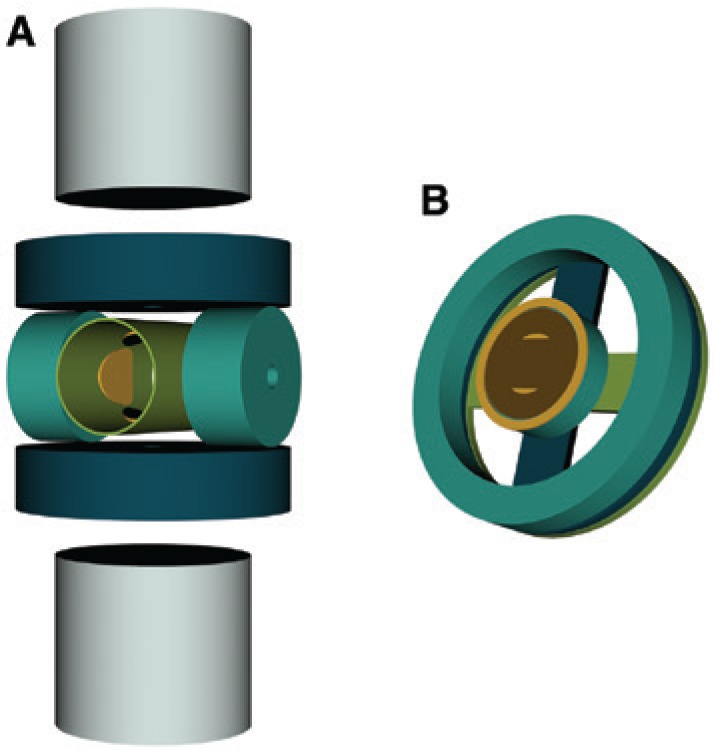
Schematic drawings of MPI scanners in bore and single sided geometry.
(A) Schematics of the principal scanner setup developed by Philips (according to Ref. [1]). (B) The principle coil geometry of a single-sided MPI scanner. It consists of two concentrically aligned transmit coils and a dedicated receive coil. For 3D imaging, additional D-shaped transmit and receive coils need to be added (according to Refs. [20], [21]).
Up until now, several MPI scanner devices with bore geometry were developed aiming either at 3D real-time imaging [9], extending the FOV [10], or improving the sensitivity [22].
Compared to the closed bore geometry, which only allows the imaging of objects as large as the bore, a single-sided geometry does not limit the sample size. A schematic drawing of an MPI scanner in single sided geometry can be seen in Figure 3 on the right side. However, due to the arrangement of the coils, the penetration depth of the FFP is limited. This means that the FOV can only cover those parts of an object that are close to the scanner surface and that the resolution decreases with increasing distance to the coils. The first single-sided scanner was presented in Ref. [20] and was able to acquire image data up to 15 mm in depth. Later, first 2D images were presented in Ref. [21], in which the FFP trajectory covered an area of 30×30 mm2 and images of a phantom in 10 mm depth were shown.
As described in “Spatial encoding” section, the spatial encoding of the received particle signal can also be performed by use of an FFL instead of an FFP. It yields a better signal-to-noise ratio and therefore an increased sensitivity by a factor of 10 [4], as more particles contribute to the signal. The first experimental setup was presented by Knopp et al. [23]. A static FFL is generated by two orthogonal Maxwell coil pairs. The currents of each of the opposing coils flow in opposite directions. Thus, each of the coil pairs generates an FFP. The superposition of both magnetic fields leads to an FFL along the bore axis of the scanner. This first FFL scanner obtained a FOV of 28×28 mm2. Instead of Maxwell coils, permanent magnets can be used as well, resulting in higher magnetic field gradients. By use of such a static FFL, the object or the whole scanner setup needs to be moved in order to scan the object [6], [22]. Usually, the full FOV is scanned electronically by a translation and rotation of the FFL, which can be realized much faster than moving the sample or the scanner [6], [24].
The first clinical demonstrator was built at the Philips Research Laboratories with a bore diameter of 34 cm in vertical direction and 45 cm in horizontal direction to fit a human and a FOV of 20 cm in diameter [2], [25]. For scaling up MPI, two problems are being particularly faced. First, the power consumption of a whole-body scanner will be much larger as for preclinical scanners and, second, peripheral nerve stimulation and tissue heating become severe issues, because larger field strengths are needed for sufficient image resolution (see also “Towards clinical use: enlargement of the imaging region and safety considerations” section) [26].
A preclinical MPI scanner, allowing 3D real-time imaging performing at an acquisition rate of 46 frames per second, is provided by Bruker BioSpin GmbH (Ettlingen, Germany). An open bore of 12 cm in diameter enables imaging of small animals. When applying maximal drive field strength (12 mT) and maximal gradient (2.5 T/m), a FOV of 19.2×19.2×9.6 mm2 can be achieved; however, the scanning volume can be further enlarged by use of focus fields as discussed in “Towards clinical use: enlargement of the imaging region and safety considerations” section [27].
Another preclinical MPI scanner, which applies one excitation frequency, is offered by Magnetic Insight Inc. (Alameda, CA, USA). This scanner lays the focus on sensitivity, leading to a drawback in temporal resolution [28].
Image reconstruction
In MPI, the presence of MNPs within the FOV is detected by applying magnetic fields and measuring the resulting voltage induced in dedicated receive coils (see “Basic imaging principles” section). It can be concluded from the resulting signal if particles are present; however, a spatial correlation has to be established by a reconstruction of the actual position of the particles.
Currently, there are two established ways to solve the reconstruction problem: a system-matrix-based reconstruction and an x-space reconstruction [29].
The system matrix in MPI calibrates the imaging system with respect to the used imaging sequence and the used tracer. Any particle signal can be expressed as the linear combination of the independent particle distribution signals. Thus, if the signal of every individual position in the FOV is known, any other combination can be calculated from the known signals. Therefore, a series of measurements with the MPI system is performed that calibrates and stores the signal of individual positions in a system matrix. Figure 4 illustrates this concept.
Figure 4:
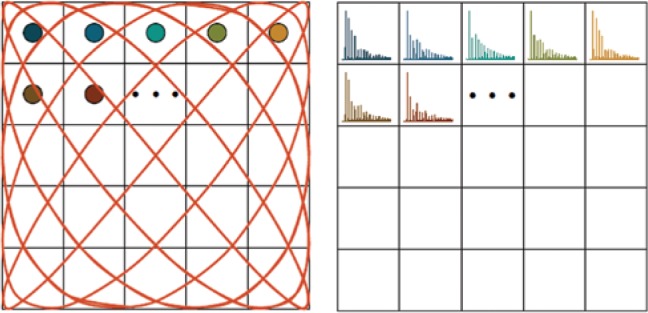
Principle concept of the system matrix acquisition: a tracer sample with known volume and tracer concentration is placed sequentially at every position in the FOV.
For every sample position, the corresponding receive signal is acquired and stored in frequency space as one entry in the system matrix.
The acquisition of the system matrix was originally proposed by placing a defined sample at every voxel position (e.g. by using a robot to shift the sample accurately) within the FOV and measuring the response when a sequence is applied [9]. This method is often named measurement-based or robot-based system matrix acquisition, and includes all possible influences on the signal generation. However, due to the movement of the sample, this kind of calibration is very time consuming. Alternatively, it is possible to calculate the system matrix via physical models for the applied magnetic fields and the particle behavior [30]; however, a reasonable computation time is only possible with simple methods that result in less accurate image reconstruction results [29]. Recently, a combination of modeling the magnetic fields and measuring the particle response has become a valuable alternative [31]. The idea of this hybrid approach is to omit the movement of the tracer sample, which is the reason for most of the calibration time. This is achieved by varying a magnetic offset field to emulate the spatial correspondence between trajectory and particle sample [29]. This method can be performed with the actual imaging system [32] or with a magnetic particle spectrometer [31], [33]. The acquisition of the system matrix is time consuming. However, when thinking of a routine use of MPI, this only has to be done once for one scanner and kind of tracer material. With the system matrix S, the signal u of any combination of particle positions can be calculated via
with c being the desired particle concentration in the FOV. To solve this, different mathematical concepts can be used [34]. However, this image reconstruction concept typically needs a reconstruction time of several seconds to minutes [29] and an acceleration is only possible with loss of image quality [35].
MPI has one of its strengths in the fast signal encoding, allowing the examination of fast processes such as blood flow quantification [36]. Consequentially, there is desire for a real-time image reconstruction to visualize the images instantaneously to use them as a direct feedback, e.g. in vascular interventions [37]. One possibility for a fast image reconstruction is the direct mapping of the measurement signal to the corresponding spatial position. This method is referred to as x-space reconstruction [38], because a mapping of the time signal u(t) is performed to the spatial domain x. This is possible because the trajectory of the FFP is known by design (see “Spatial encoding” section). One drawback of this image reconstruction concept is that it currently can be only used for data acquired with a Cartesian trajectory.
Applications of MPI
MNPs can be bound to a huge variety of different molecules and materials. Therefore, the application of MPI as a molecular imaging tool is an interesting research topic. Further, due to the real-time capability of MPI, its application scenarios in vascular and cardiac imaging seem to be promising. Furthermore, the high sensitivity and quantitative nature of MPI offer applications that are not possible with today’s medical imaging technologies. In the following, a selection of experimental and preclinical investigations is presented, which was intended to pave the way to clinical application.
MPI in vascular medicine
Typically, MNPs are administered into a blood vessel and allow for visualization of the blood flow, as shown with the first in vivo images of the blood flow in a beating mouse heart (see Figure 5) [9]. As most MPI scanners have no depth limitation (see “MPI scanner” section) and the tissue of the patient does not attenuate or shield the received MPI signal, it is possible to image the MNPs everywhere in the patient.
Figure 5:
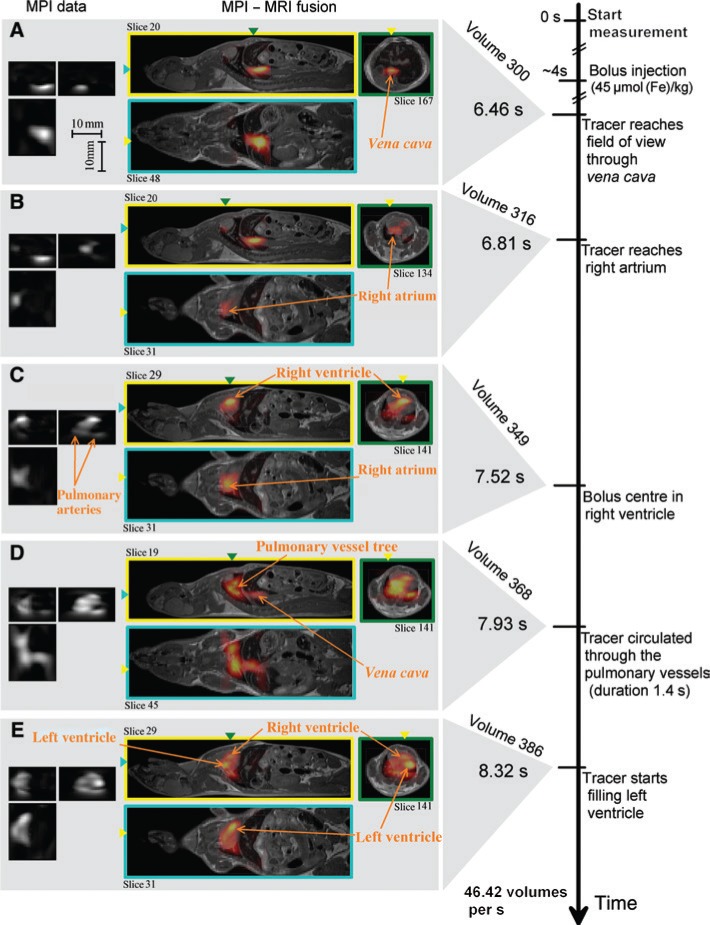
In vivo measurements of a beating mouse heart.
Resovist was injected into the tail vein of the mouse. The scanner achieves a FOV of 20.4×12×16.8 mm3 and a temporal resolution of 21.5 ms. The MPI images are fused with static MRI images (with permission taken from Ref. [9]). (A) Bolus reaches field of view through vena cava. (B) Tracer reaches right artrium. (C) Bolus centre in right ventricle. (D) Tracer circulated through the pulmonary vessel. (E) Tracer starts filling left ventricle.
As MNPs can be easily administered into the vascular system, there is a wide range of medical applications for vascular interventions and imaging. In the future, it might even be possible that it will replace today’s procedures such as DSA, which utilizes ionizing radiation. One key advantage of MPI is that it simultaneously features a high spatial and a high temporal resolution, why it is especially interesting for cardiovascular applications. This was already demonstrated in different proof-of-concept studies, where images of beating mouse hearts were acquired [9], [39].
When MNPs are administered into the blood flow, there is typically just a small time window of several minutes to image the particles during their circulation before they accumulate in the liver and spleen [40], [41]. By the administration of Resovist to New Zealand rabbits, it was found that after 10 min, the MPI signal decreased to about 20% compared to the signal directly after the administration. Within 30 min, the signal was not recordable anymore [42]. However, for many applications, it is necessary to have longer circulation times. It is possible to adapt the chemical properties of an MNP to increase the circulation time, which could be shown for MPI applications [43]. Another possibility to achieve an increased blood circulation time is to incorporate the MNPs in red blood cells [44], which results in a detectable MPI signal even hours after the injection [36].
Visualization of instruments for cardiovascular interventions
As MPI visualizes only MNP distributions, it is necessary to adapt the instruments potentially used for interventions with MPI such that a physician receives information about the positioning of the instrument. For example, in order to visualize a balloon catheter, it is possible to either directly label the catheter with MNPs or administer an MNP bolus into the blood vessel. Both methods were shown to be feasible by use of a vessel phantom [37]. Figure 6 shows that the catheter is clearly visible with a high temporal (21.54 ms per image) and spatial (<3 mm) resolution for both cases. A very interesting possibility in this context is to also use color coding in the reconstructed images by discriminating different particle properties [45], which, for example allows the discrimination between a catheter and a filled lumen by the use of different MNPs (see Figure 7) [46]. In further studies, it was demonstrated that diagnostic catheters as well as guide wires can be coated with a special varnish containing MNPs. It has been shown that the coating does not interfere with the handling of the catheters or the guide wires. The instruments were visualized in 3D with a high temporal resolution, paving the way for interventional imaging scenarios [47]. In addition to this, it is feasible to guide these catheters magnetically as the magnetic fields of an MPI scanner can apply a magnetic force on a magnetic material [48]. Altogether, this makes MPI a very promising application for precise image-guided minimally invasive interventions.
Figure 6:
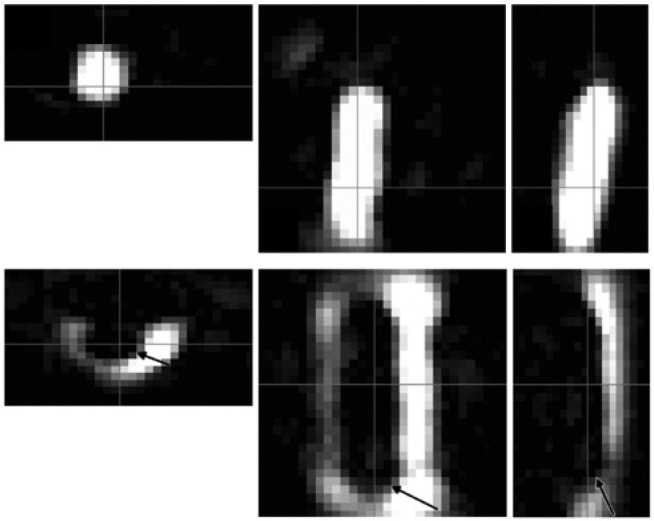
The axial, sagittal, and coronal images (left to right) show a balloon catheter labeled with MNPs (top) and a non-labeled balloon catheter in a vessel phantom filled with MNPs (bottom).
The FOV was 20×36×36 mm3 (with permission taken from Ref. [37]).
Figure 7:
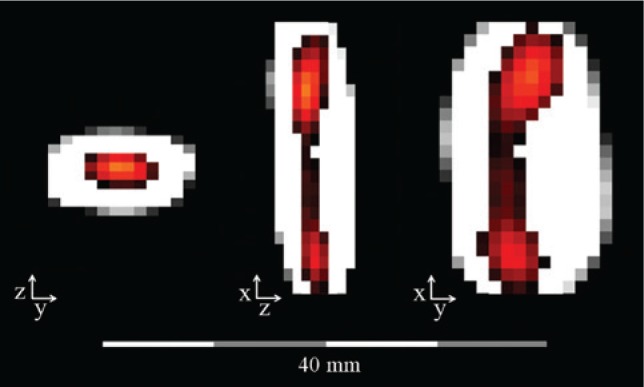
Color coding can be used to differentiate between a varnished catheter (colored) and a filled lumen (gray scale).
This is possible because the physical properties of MNPs in varnish are different from the properties of MNPs in a fluid (with permission taken from Ref. [46]).
For safety reasons, the heating of the instruments needs to be considered, which can occur due to the high frequency of the drive fields and the resulting eddy currents within the instruments’ material. This may pose a limitation for the use of instruments for vascular interventions as guide wire temperatures of up to 85°C have been observed due to their material and shape [49]. However, many devices did not show any heating behavior.
Further research investigated the safe use of endovascular stents of different sizes and materials [50]. It could be shown that with few exceptions, the safe use of endovascular stents in MPI is possible. This would enable the visualization of the inner stent volume without artifacts such that a reocclusion would be possible to diagnose.
Imaging of vascular stenosis
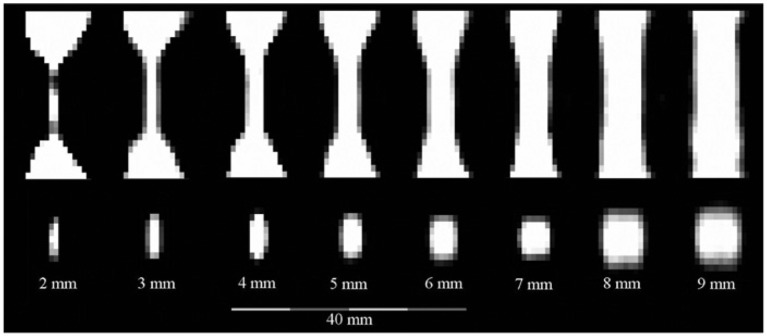
As MPI features a high spatial resolution, it is interesting to be used for investigating the degree of stenosis [51]. By use of a stenosis phantom, which has a normal lumen of 10 mm diameter and allows a narrowing from 9 to 1 mm, referring to a stenosis degree of 19%–99%, the visualization of the stenosis degree with MPI was possible. It was shown that with an MNP concentration suitable for clinical use, a stenosis down to 2 mm could be imaged (see Figure 8) [52].
Figure 8:
Sagittal (top) and axial (bottom) slices extracted from reconstructed 3D image data of the stenosis phantoms.
The lumen of the 1 mm stenosis is not discernible (with permission taken from Ref. [52]).
An in vitro angioplasty was performed by Salamon et al. [53]. A balloon catheter was inflated in a stenotic vessel phantom. Real-time MPI images were acquired to visualize the placement of the balloon catheter at the stenosis and the following inflation of the balloon with MNPs. In a second scenario, the catheter balloon was inflated with saline after filling the vessel phantom with MNPs. In both scenarios, the balloon catheter was clearly distinguishable from the surrounding vessel phantom. In addition, bimodal fiducial markers were used to enable the merging of MPI and MRI images (for multimodality approaches in MPI, see “Multimodality with MPI” section).
Imaging of brain injuries
For diagnosing a traumatic brain injury, usually the degree of awareness is subjectively quantified according to the Glasgow Coma Scale or further analyzed with imaging modalities such as CT or MRI. However, with these techniques, it is difficult to diagnose mild degrees of traumatic brain injuries [54]. It has been demonstrated in mouse experiments that with MPI, the affected regions could be clearly seen with a half-life of the particle signal in the skull of 4 days. The clearance of MNPs in the affected region as well as in the lymph nodes was significantly higher than the clearance of these regions observed for healthy mice [54]. Furthermore, the diagnosis of stroke by use of MPI is of high potential as the cerebral perfusion can be visualized very fast. Real-time MPI images of mice suffering an ischemic stroke proved the suitability in vivo [55].
Imaging of blood flow with MPI
In an in vitro experiment, the suitability of MPI for the assessment of aneurysms was shown by the characterization of the hemodynamics in a 3D aneurysm model. The results obtained were similar to MRI and DSA procedures used in clinical practice today [56]. First results revealed the possibility to depict lung perfusion in rats [57]. MNPs were bound to macroaggregated albumin-forming larger clusters of several 10 μm, which are trapped in the lung capillaries after 10 min of injection into the tail vein of rats.
It is also possible to image gastrointestinal bleeding with MPI in order to get a fast and precise localization of the bleeding, which has been demonstrated in vivo [58]. Also, a quantitative diagnosis of bleed rates is possible with MPI.
Furthermore, a successful in vivo tracking of a tracer bolus, which was injected into the tail vein of mice, was performed. A 3D volume scan within about 20 ms enabled real-time imaging. By this, the assessment of the blood flow velocity has been demonstrated [59], [60].
It is not only possible to distinguish different kinds of particles, but also different properties, such as the viscosity of the particle matrix [61]. This would allow for real-time monitoring of biological processes in terms of viscosity changes, which can be used, e.g. to monitor blood or tissue coagulation [62].
Further applications of MPI
Apart from vascular imaging and vascular interventions, multiple other applications are feasible with MPI. The advantages of MPI – high temporal and spatial resolution, as well as no radioactive- or iodine-based tracer material – are promising for a broad range of medical applications.
Diagnostics and interventions with MPI
MPI can visualize MNPs at any location in the FOV. Thus, if the particles can be administered or transported to the location of interest, MPI can be used to examine the medical condition in situ. It had already been shown that MPI allows imaging of tumors [63] or specific organs; a first in vivo liver visualization was shown in 2017 [64]. MNPs can be modified with respect to their coating or by adding ligands. These ligands can be very different and are used for an active targeting of the MNPs, e.g. they can be bound to cancerous tissue.
Another possible application for MPI is breast cancer staging. Here, the MNPs are injected in the close vicinity of the cancerous tissue inside the breast. They are transported via the lymphatic system and accumulate in the sentinel lymph node, which is the first lymph node behind the tumor. The sentinel lymph node has to be removed in order to examine whether metastases are present outside the primary tumor region. Using a single-sided coil arrangement (see “MPI scanner” section), the sentinel lymph node can be precisely localized and then removed with minimal invasive surgery [20], [65], [66]. This technique allows the resection of as much tissue as necessary and as less tissue as possible. MPI would be an improvement in breast cancer treatment as the current state-of-the-art methods use either radioactive material or blue dye, which has a very low resolution and therefore leads to the removal of healthy tissue and large surgical wounds.
Multimodality with MPI
MPI suffers from the lack of direct anatomical information of the subjects. Hence, it is desirable to combine MPI with other imaging technologies to gain the missing anatomical information. An obvious choice would be the combination with MRI, because both MPI and MRI use magnetic fields. It is possible to perform the imaging in different devices and combine the information in a post-processing step [53], [67]. As the object needs to be transferred between two different scanners, the spatial confidence is a severe issue and in vivo real-time imaging is not feasible. A device combining both imaging modalities in a single system was introduced by Vogel et al. [68], in which a traveling wave MPI scanner was inserted to a low field MRI system, showing promising 2D phantom measurement results. In Ref. [69], a fully integrated MPI-MRI system for static 3D imaging was presented. MPI and MRI need very different magnetic field topologies, and the magnetic field strength needed for MRI is so large that the SPIONs get into saturation. Therefore, simultaneous imaging is hard to implement and a sequential data acquisition was realized. As both modalities share the same FOV, high spatial registration accuracy can be achieved. To correlate the information of both modalities, fiducial markers can be used [70].
Another possible combination of MPI is with ultrasound (US). In addition to morphological evaluation, US can be used for flow imaging and even thermal therapies. A design study of an MPI-compatible US transducer has already been published [71].
Recently, an approach for bimodal intravascular optical coherence tomography (IVOCT) MPI was investigated [72]. IVOCT provides a very high spatial and temporal resolution but lacks penetration depth. Thus, an accurate 3D positioning is not possible. In Ref. [72], MPI was used to accurately place the IVOCT device within several vessel phantoms.
Additionally, it might be useful to develop tracer materials that are not just visible with MPI but in combination with other imaging modalities as well. In Ref. [73], such tracer materials, being suitable for MPI and several other imaging techniques, have been investigated.
Cellular imaging with MPI
In Ref. [74], the biocompatibility of MNPs has been evaluated as well as their impact on biological properties and their cellular uptake using head and neck squamous cancer cells and mouse fibroblast cells, respectively. In Ref. [44], an approach for the use of erythrocytes as particle carriers has been developed. The tracking of neural cells and stem cells in vivo is a very promising application for MPI and is in the focus of investigations [75], [76], [77], [78], [79]. The feasibility of molecular imaging with MPI has been proven in 2015 [80], when glioma cells with lactoferrin-conjugated MNPs were detected. It was also possible to label cells with MNPs ex vivo and monitor their behavior in vivo using MPI after reinjection [81].
The sensitivity of an MPI scanner defines the possible medical applications. In order to detect a single stem cell loaded with tracer material, an MPI scanner would need to detect about 10–40 pg of iron [76], [82]. In Ref. [82], it was shown that 250 cells were imaged by MPI after implantation, corresponding to an amount of 7.8 ng of iron. Taking the newly reached sensitivity limits of 1 ng [83] and 192 pg [84] into account, this number could be surpassed now.
Thermo-therapy with MPI
Fast oscillating magnetic fields cause two reactions of the MNPs: a change of the magnetization direction and a movement of the particles, referred to as Néel and Brown relaxation. The energy needed to change the magnetization direction is converted into heat and the friction in a viscous medium caused by a mechanical movement of the particles also leads to heating of the particles. Using specific frequencies and amplitudes, it is possible to use this effect for a controlled heating of the particles. Such a heating might, for instance, be used to destroy cancerous tissue. Thus, MPI allows for an image-guided magnetic hyperthermia therapy [85]. However, as imaging and heating of particles impose different particle requirements, it is necessary to tailor the particles to the application at hand [86]. As shown in Ref. [87], it is possible to monitor the heating of nanoparticles, as particles are also distinguishable by their temperature.
Magnetic manipulation and drug targeting with MPI
The magnetic fields that are used in MPI for the imaging of MNPs can also be used to manipulate magnetic material. In a first study, it could be shown that the fields applied in MPI can be used to move and hold a magnetic object while imaging its location [88]. With this technique, it is possible to manipulate catheters to support physicians in an intervention [48]. In Ref. [25], it was shown that even a spatially selective magnetic manipulation is possible for screws and screw-like objects.
Not only macroscopic devices but also MNPs can be manipulated by applying magnetic forces. Magnetic drug targeting is a very promising approach to deliver therapeutic agents more efficiently. Drugs such as chemotherapeutics are bound to MNPs. The MNPs can be directed by external magnetic fields toward a targeted volume, e.g. a tumor or an inflammation. This allows lower dosages of drugs, and healthy tissue is less affected. First human trials, in which a strong permanent magnet holds the chemotherapeutic agent bound to MNPs in a shallow tumor, were successfully performed [89]. For in vivo measurements, a tomographic imaging technique is needed to monitor and control the movement of the particles. Here, MPI has great potential to offer this missing information. As a first step, in Ref. [90], an accumulation of particles at a certain position could be imaged with MPI. First setups are being investigated in which an MPI scanner is integrated into a coil setup for steering the MNPs, where steering and imaging is alternately operated [91]. The combination of magnetic drug targeting and MPI is an ongoing and very promising field of research.
Summary and conclusion
MPI is a new medical imaging technique that enables a variety of applications with the great advantage that no ionizing radiation or radioactive tracers are required. It is especially promising for vascular imaging, as it provides 3D real-time imaging with a high sensitivity and submillimeter resolution. In comparison to the usual procedures for vascular imaging, such as DSA, no nephrotoxic tracers such as iodine are required. The principle of MPI is based on the excitation of MNPs by externally applied magnetic fields. Those MNPs are biocompatible and can be administered as a bolus or in a functionalized form. As nowadays, only preclinical MPI scanners are available, featuring a FOV of several centimeters, ongoing research is aiming at upscaling and verifying different scanner topologies, such as the FFL approach or the single-sided scanner geometry.
Various experiments demonstrated the high potential of MPI for vascular imaging, which was shown in 2009 with a beating mouse heart. This was followed by the visualization of instruments, which would enable MPI image guidance of vascular interventions. Further application scenarios in the field of vascular imaging are quantifying the degree of stenosis, diagnosing brain injuries more precisely, and measuring the blood flow velocity. However, MPI is not only promising for vascular imaging, but also has a broad potential for many other applications. It has impressively demonstrated its potential for imaging in diagnostics and therapy.
Supporting Information
Supplementary Material
The article (iss-2018-2026) offers reviewer assessments as supplementary material.
Author Statement
Research funding: Funding by the German Research Foundation (grant nos. BU 1436/7-1, BU 1436/9-1, and BU 1436/10-1), the Federal Ministry of Education and Research (grant nos. 01DL17010A, 13GW0071D, and 13GW0069A), and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604448 is gratefully acknowledged. Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animal use.
Author Contributions
Anna C. Bakenecker: writing – original draft. Mandy Ahlborg: writing – review & editing. Christina Debbeler: writing – review & editing. Christian Kaethner: writing – review & editing. Thorsten M. Buzug: writing – review & editing. Kerstin Lüdtke-Buzug: writing – review & editing.
References
- [1].Gleich B, Weizenecker J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005;435:1214–7. [DOI] [PubMed]; Gleich B, Weizenecker J. Tomographic imaging using the nonlinear response of magnetic particles. Nature. 2005;435:1214–7. doi: 10.1038/nature03808. [DOI] [PubMed] [Google Scholar]
- [2].Panagiotopoulos N, Duschka RL, Ahlborg M, Bringout G, Debbeler C, Graeser M, et al. Magnetic particle imaging: current developments and future directions. Int J Nanomed 2015;10:3097–114. [DOI] [PMC free article] [PubMed]; Panagiotopoulos N, Duschka RL, Ahlborg M, Bringout G, Debbeler C, Graeser M. et al. Magnetic particle imaging: current developments and future directions. Int J Nanomed. 2015;10:3097–114. doi: 10.2147/IJN.S70488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Knopp T, Buzug TM. Magnetic Particle Imaging: an Introduction to Imaging Principles and Scanner Instrumentation. Berlin: Springer; 2012. DOI: 10.1007/978-3-642-04199-0.; Knopp T, Buzug TM. Magnetic Particle Imaging: an Introduction to Imaging Principles and Scanner Instrumentation. Berlin: Springer; 2012. [DOI] [Google Scholar]
- [4].Weizenecker J, Gleich B, Borgert J. Magnetic particle imaging using a field free line. J Phys D Appl Phys 2008;41:105009.; Weizenecker J, Gleich B, Borgert J. Magnetic particle imaging using a field free line. J Phys D Appl Phys. 2008;41:105009. [Google Scholar]
- [5].Erbe M, Knopp T, Sattel TF, Biederer S, Buzug TM. Experimental generation of an arbitrary rotated field-free line for use in magnetic particle imaging. Med Phys 2011;38:5200–7. [DOI] [PubMed]; Erbe M, Knopp T, Sattel TF, Biederer S, Buzug TM. Experimental generation of an arbitrary rotated field-free line for use in magnetic particle imaging. Med Phys. 2011;38:5200–7. doi: 10.1118/1.3626481. [DOI] [PubMed] [Google Scholar]
- [6].Knopp T, Erbe M, Sattel TF, Biederer S, Buzug TM. A Fourier slice theorem for magnetic particle imaging using a field-free line. Inverse Probl 2011;27:095004.; Knopp T, Erbe M, Sattel TF, Biederer S, Buzug TM. A Fourier slice theorem for magnetic particle imaging using a field-free line. Inverse Probl. 2011;27:095004. [Google Scholar]
- [7].Konkle JJ, Goodwill PW, Carrasco-Zevallos O, Conolly SM. Projection reconstruction magnetic particle imaging. IEEE Trans Med Imaging 2013;32:338–47. [DOI] [PMC free article] [PubMed]; Konkle JJ, Goodwill PW, Carrasco-Zevallos O, Conolly SM. Projection reconstruction magnetic particle imaging. IEEE Trans Med Imaging. 2013;32:338–47. doi: 10.1109/TMI.2012.2227121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Werner F, Gdaniec N, Knopp T. First experimental comparison between the Cartesian and the Lissajous trajectory for magnetic particle imaging. Phys Med Biol 2017;62:3407–21. [DOI] [PubMed]; Werner F, Gdaniec N, Knopp T. First experimental comparison between the Cartesian and the Lissajous trajectory for magnetic particle imaging. Phys Med Biol. 2017;62:3407–21. doi: 10.1088/1361-6560/aa6177. [DOI] [PubMed] [Google Scholar]
- [9].Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. Three-dimensional real-time in vivo magnetic particle imaging. Phys Med Biol 2009;54:L1–10. [DOI] [PubMed]; Weizenecker J, Gleich B, Rahmer J, Dahnke H, Borgert J. Three-dimensional real-time in vivo magnetic particle imaging. Phys Med Biol. 2009;54:L1–10. doi: 10.1088/0031-9155/54/5/L01. [DOI] [PubMed] [Google Scholar]
- [10].Schmale I, Rahmer J, Gleich B, Kanzenbach J, Schmidt JD, Bontus C, et al. First phantom and in vivo MPI images with an extended field of view. SPIE Med Imaging Biomed Appl Mol Struct Funct Imaging 2011:7965.; Schmale I, Rahmer J, Gleich B, Kanzenbach J, Schmidt JD, Bontus C. et al. First phantom and in vivo MPI images with an extended field of view. SPIE Med Imaging Biomed Appl Mol Struct Funct Imaging. 2011;7965 [Google Scholar]
- [11].Kaethner C, Ahlborg M, Bringout G, Weber M, Buzug TM. Axially elongated field-free point data acquisition in magnetic particle imaging. IEEE Trans Med Imaging 2015;34:381–7. [DOI] [PubMed]; Kaethner C, Ahlborg M, Bringout G, Weber M, Buzug TM. Axially elongated field-free point data acquisition in magnetic particle imaging. IEEE Trans Med Imaging. 2015;34:381–7. doi: 10.1109/TMI.2014.2357077. [DOI] [PubMed] [Google Scholar]
- [12].Doessel O, Bohnert J. Safety considerations for magnetic fields of 10 mT to 100 mT amplitude in the frequency range of 10 kHz to 100 kHz for magnetic particle imaging. Biomed Tech/Biomed Eng 2013;58:611–21. [DOI] [PubMed]; Doessel O, Bohnert J. Safety considerations for magnetic fields of 10 mT to 100 mT amplitude in the frequency range of 10 kHz to 100 kHz for magnetic particle imaging. Biomed Tech/Biomed Eng. 2013;58:611–21. doi: 10.1515/bmt-2013-0065. [DOI] [PubMed] [Google Scholar]
- [13].Schmale I, Gleich B, Rahmer J, Bontus C, Schmidt J, Borgert J. MPI safety in the view of MRI safety standards. IEEE Trans Magn 2015;51:6502604.; Schmale I, Gleich B, Rahmer J, Bontus C, Schmidt J, Borgert J. MPI safety in the view of MRI safety standards. IEEE Trans Magn. 2015;51:6502604. [Google Scholar]
- [14].Saritas EU, Goodwill PW, Zhang GZ, Yu W, Conolly SM. Safety limits for human-size magnetic particle imaging systems. Springer Proc Phys 2012;140:325–30.; Saritas EU, Goodwill PW, Zhang GZ, Yu W, Conolly SM. Safety limits for human-size magnetic particle imaging systems. Springer Proc Phys. 2012;140:325–30. [Google Scholar]
- [15].Saritas EU, Goodwill PW, Zhang GZ, Conolly SM. Magnetostimulation limits in magnetic particle imaging. IEEE Trans Med Imaging 2013;32:1600–10. [DOI] [PubMed]; Saritas EU, Goodwill PW, Zhang GZ, Conolly SM. Magnetostimulation limits in magnetic particle imaging. IEEE Trans Med Imaging. 2013;32:1600–10. doi: 10.1109/TMI.2013.2260764. [DOI] [PubMed] [Google Scholar]
- [16].Bauer LM, Situ SF, Griswold MA, Samia ACS. Magnetic particle imaging tracers: state-of-the-art and future directions. J Phys Chem Lett 2016;6:2509–17. [DOI] [PubMed]; Bauer LM, Situ SF, Griswold MA, Samia ACS. Magnetic particle imaging tracers: state-of-the-art and future directions. J Phys Chem Lett. 2016;6:2509–17. doi: 10.1021/acs.jpclett.5b00610. [DOI] [PubMed] [Google Scholar]
- [17].Ferguson RM, Khandar AP, Kemp SJ, Arami H, Saritas EU, Croft LR, et al. Magnetic particle imaging with tailored iron oxide nanoparticle tracers. IEEE Trans Med Imaging 2015;34:1077–84. [DOI] [PMC free article] [PubMed]; Ferguson RM, Khandar AP, Kemp SJ, Arami H, Saritas EU, Croft LR. et al. Magnetic particle imaging with tailored iron oxide nanoparticle tracers. IEEE Trans Med Imaging. 2015;34:1077–84. doi: 10.1109/TMI.2014.2375065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weizenecker J, Borgert J, Gleich B. A simulation study on the resolution and sensitivity of magnetic particle imaging. Phys Med Biol 2007;52:6363–74. [DOI] [PubMed]; Weizenecker J, Borgert J, Gleich B. A simulation study on the resolution and sensitivity of magnetic particle imaging. Phys Med Biol. 2007;52:6363–74. doi: 10.1088/0031-9155/52/21/001. [DOI] [PubMed] [Google Scholar]
- [19].Graeser M, Bente K, Buzug TM. Dynamic single-domain particle model for magnetite particles with combined crystalline and shape anisotropy. J Phys D Appl Phys 2015;48:275001.; Graeser M, Bente K, Buzug TM. Dynamic single-domain particle model for magnetite particles with combined crystalline and shape anisotropy. J Phys D Appl Phys. 2015;48:275001. [Google Scholar]
- [20].Sattel TF, Knopp T, Biederer S, Gleich B, Weizenecker J, Borgert J, et al. Single-sided device for magnetic particle imaging. J Phys D Appl Phys 2009;42:022001. [DOI] [PubMed]; Sattel TF, Knopp T, Biederer S, Gleich B, Weizenecker J, Borgert J. et al. Single-sided device for magnetic particle imaging. J Phys D Appl Phys. 2009;42:022001. doi: 10.1088/0031-9155/54/2/014. [DOI] [PubMed] [Google Scholar]
- [21].Gräfe K, von Gladiss A, Bringout G, Ahlborg M, Buzug TM. 2D images recorded with a single-sided magnetic particle imaging scanner. IEEE Trans Med Imaging 2016;35: 1056–65. [DOI] [PubMed]; Gräfe K, von Gladiss A, Bringout G, Ahlborg M, Buzug TM. 2D images recorded with a single-sided magnetic particle imaging scanner. IEEE Trans Med Imaging. 2016;35:1056–65. doi: 10.1109/TMI.2015.2507187. [DOI] [PubMed] [Google Scholar]
- [22].Goodwill PW, Lu K, Zheng B, Conolly SM. An x-space magnetic particle imaging scanner. Rev Sci Instrum 2012;83:033708. [DOI] [PMC free article] [PubMed]; Goodwill PW, Lu K, Zheng B, Conolly SM. An x-space magnetic particle imaging scanner. Rev Sci Instrum. 2012;83:033708. doi: 10.1063/1.3694534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Knopp T, Erbe M, Sattel TF, Biederer S, Buzug TM. Generation of a static magnetic field-free line using two Maxwell coil pairs. Appl Phys Lett 2010;97:092505.; Knopp T, Erbe M, Sattel TF, Biederer S, Buzug TM. Generation of a static magnetic field-free line using two Maxwell coil pairs. Appl Phys Lett. 2010;97:092505. [Google Scholar]
- [24].Bente K, Weber M, Graeser M, Sattel TF, Erbe M, Buzug TM. Electronic field free line rotation and relaxation deconvolution in magnetic particle imaging. IEEE Trans Med Imaging 2015;34:644–51. [DOI] [PubMed]; Bente K, Weber M, Graeser M, Sattel TF, Erbe M, Buzug TM. Electronic field free line rotation and relaxation deconvolution in magnetic particle imaging. IEEE Trans Med Imaging. 2015;34:644–51. doi: 10.1109/TMI.2014.2364891. [DOI] [PubMed] [Google Scholar]
- [25].Rahmer J, Stehning C, Gleich B. Spatially selective remote magnetic actuation of identical helical micromachines. Sci Robot 2017;2:2845. [DOI] [PubMed]; Rahmer J, Stehning C, Gleich B. Spatially selective remote magnetic actuation of identical helical micromachines. Sci Robot. 2017;2:2845. doi: 10.1126/scirobotics.aal2845. [DOI] [PubMed] [Google Scholar]
- [26].Borgert J, Schmidt JD, Schmale I, Bontus C, Gleich B, David B, et al. Perspectives on clinical magnetic particle imaging. Biomed Tech/Biomed Eng 2013;58:551–6. [DOI] [PubMed]; Borgert J, Schmidt JD, Schmale I, Bontus C, Gleich B, David B. et al. Perspectives on clinical magnetic particle imaging. Biomed Tech/Biomed Eng. 2013;58:551–6. doi: 10.1515/bmt-2012-0064. [DOI] [PubMed] [Google Scholar]
- [27].Bruker Biospin GmbH. Available from: https://www.bruker.com/nc/news-records/single-view/article/bruker-announces-first-customer-installation-of-its-preclinical-magnetic-particle-imaging-mpi-scan.html. Accessed 29 September 2018.; Bruker Biospin GmbH. Available from: https://www.bruker.com/nc/news-records/single-view/article/bruker-announces-first-customer-installation-of-its-preclinical-magnetic-particle-imaging-mpi-scan.html. Accessed 29 September 2018. [Google Scholar]
- [28].Magnetic Insight Inc. Available from: http://www.magneticinsight.com/momentum-imager. Accessed 29 September 2018.; Magnetic Insight Inc. Available from: http://www.magneticinsight.com/momentum-imager. Accessed 29 September 2018. [Google Scholar]
- [29].Grüttner M, Knopp T, Franke J, Heidenreich M, Rahmer J, Halkola A, et al. On the formulation of the image reconstruction problem in magnetic particle imaging. Biomed Tech/Biomed Eng 2013;58:583–91. [DOI] [PubMed]; Grüttner M, Knopp T, Franke J, Heidenreich M, Rahmer J, Halkola A. et al. On the formulation of the image reconstruction problem in magnetic particle imaging. Biomed Tech/Biomed Eng. 2013;58:583–91. doi: 10.1515/bmt-2012-0063. [DOI] [PubMed] [Google Scholar]
- [30].Knopp T, Biederer S, Sattel TF, Rahmer J, Weizenecker J, Gleich B, et al. 2D model-based reconstruction for magnetic particle imaging. Med Phys 2010;37:485–91. [DOI] [PubMed]; Knopp T, Biederer S, Sattel TF, Rahmer J, Weizenecker J, Gleich B. et al. 2D model-based reconstruction for magnetic particle imaging. Med Phys. 2010;37:485–91. doi: 10.1118/1.3271258. [DOI] [PubMed] [Google Scholar]
- [31].von Gladiss A, Graeser M, Szwargulski P, Knopp T, Buzug TM. Hybrid system calibration for multidimensional magnetic particle imaging. Phys Med Biol 2017;62:3392–406. [DOI] [PubMed]; von Gladiss A, Graeser M, Szwargulski P, Knopp T, Buzug TM. Hybrid system calibration for multidimensional magnetic particle imaging. Phys Med Biol. 2017;62:3392–406. doi: 10.1088/1361-6560/aa5340. [DOI] [PubMed] [Google Scholar]
- [32].Halkola A, Rahmer J, Gleich B, Borgert J, Buzug TM. System calibration unit for magnetic particle imaging: system matrix. In: International Workshop on Magnetic Particle Imaging, Berkeley, CA, USA: IEEE Xplore Digital Library; 2013. DOI: 10.1109/IWMPI.2013.6528344.; Halkola A, Rahmer J, Gleich B, Borgert J, Buzug TM. International Workshop on Magnetic Particle Imaging. Berkeley, CA, USA: IEEE Xplore Digital Library; 2013. System calibration unit for magnetic particle imaging: system matrix. [DOI] [Google Scholar]
- [33].Grüttner M, Gräser M, Biederer S, Sattel TF, Wojtczyk H, Tenner W, et al. 1D-image reconstruction for magnetic particle imaging using a hybrid system function. IEEE Nucl Sci Symp Med Imaging Conf 2011:2545–8. Available from: https://ieeexplore.ieee.org/document/6152687.; Grüttner M, Gräser M, Biederer S, Sattel TF, Wojtczyk H, Tenner W. et al. 1D-image reconstruction for magnetic particle imaging using a hybrid system function. IEEE Nucl Sci Symp Med Imaging Conf. 2011:2545–8. Available from: https://ieeexplore.ieee.org/document/6152687. [Google Scholar]
- [34].Knopp T, Rahmer J, Sattel TF, Biederer S, Weizenecker J, Gleich B, et al. Weighted iterative reconstruction for magnetic particle imaging. Phys Med Biol 2010;55:1577–89. [DOI] [PubMed]; Knopp T, Rahmer J, Sattel TF, Biederer S, Weizenecker J, Gleich B. et al. Weighted iterative reconstruction for magnetic particle imaging. Phys Med Biol. 2010;55:1577–89. doi: 10.1088/0031-9155/55/6/003. [DOI] [PubMed] [Google Scholar]
- [35].Knopp T, Hofmann M. Online reconstruction of 3D magnetic particle imaging data. Phys Med Biol 2016;61:N257–67. [DOI] [PubMed]; Knopp T, Hofmann M. Online reconstruction of 3D magnetic particle imaging data. Phys Med Biol. 2016;61:N257–67. doi: 10.1088/0031-9155/61/11/N257. [DOI] [PubMed] [Google Scholar]
- [36].Rahmer J, Antonelli A, Sfara C, Tiemann B, Gleich B, Magnani M, et al. Nanoparticle encapsulation in red blood cells enables blood-pool magnetic particle imaging hours after injection. Phys Med Biol 2013;58:3965–77. [DOI] [PubMed]; Rahmer J, Antonelli A, Sfara C, Tiemann B, Gleich B, Magnani M. et al. Nanoparticle encapsulation in red blood cells enables blood-pool magnetic particle imaging hours after injection. Phys Med Biol. 2013;58:3965–77. doi: 10.1088/0031-9155/58/12/3965. [DOI] [PubMed] [Google Scholar]
- [37].Haegele J, Rahmer J, Gleich B, Borgert J, Wojtczyk H, Panagiotopoulos N, et al. Magnetic particle imaging: visualization of instruments for cardiovascular intervention. Radiology 2012;265:933–8. [DOI] [PubMed]; Haegele J, Rahmer J, Gleich B, Borgert J, Wojtczyk H, Panagiotopoulos N. et al. Magnetic particle imaging: visualization of instruments for cardiovascular intervention. Radiology. 2012;265:933–8. doi: 10.1148/radiol.12120424. [DOI] [PubMed] [Google Scholar]
- [38].Goodwill PW, Conolly SM. Multi-dimensional X-space magnetic particle imaging. IEEE Trans Med Imaging 2011;30:1581–90. [DOI] [PMC free article] [PubMed]; Goodwill PW, Conolly SM. Multi-dimensional X-space magnetic particle imaging. IEEE Trans Med Imaging. 2011;30:1581–90. doi: 10.1109/TMI.2011.2125982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vogel P, Rückert MA, Klauer P, Kullmann WH, Jakob PM, Behr VC. First in vivo traveling wave magnetic particle imaging of a beating mouse heart. Phys Med Biol 2016;61:6620–34. [DOI] [PubMed]; Vogel P, Rückert MA, Klauer P, Kullmann WH, Jakob PM, Behr VC. First in vivo traveling wave magnetic particle imaging of a beating mouse heart. Phys Med Biol. 2016;61:6620–34. doi: 10.1088/0031-9155/61/18/6620. [DOI] [PubMed] [Google Scholar]
- [40].Khandhar AP, Ferguson RM, Arami H, Krishnan KM. Monodisperse magnetite nanoparticle tracers for in vivo magnetic particle imaging. Biomaterials 2013;34:3837–45. [DOI] [PMC free article] [PubMed]; Khandhar AP, Ferguson RM, Arami H, Krishnan KM. Monodisperse magnetite nanoparticle tracers for in vivo magnetic particle imaging. Biomaterials. 2013;34:3837–45. doi: 10.1016/j.biomaterials.2013.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thanh NTK. Clinical Applications of Magnetic Nanoparticles. Boca Raton, FL: CRC Press; 2018. ISBN: 9781138051553.; Thanh NTK. Clinical Applications of Magnetic Nanoparticles. Boca Raton, FL: CRC Press; 2018. ISBN: 9781138051553. [Google Scholar]
- [42].Haegele J, Duschka RL, Graeser M, Schaecke C, Panagiotopoulos N, Lüdtke-Buzug K, et al. Magnetic particle imaging: kinetics of the intravascular signal in vivo. Int J Nanomed 2014;9:4203–9. [DOI] [PMC free article] [PubMed]; Haegele J, Duschka RL, Graeser M, Schaecke C, Panagiotopoulos N, Lüdtke-Buzug K. et al. Magnetic particle imaging: kinetics of the intravascular signal in vivo. Int J Nanomed. 2014;9:4203–9. doi: 10.2147/IJN.S49976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Keselman P, Yu EY, Zhou XY, Goodwill PW, Chandrasekharan P, Ferguson RM, et al. Tracking short-term biodistribution and long-term clearance of SPIO tracers in magnetic particle imaging. Phys Med Biol 2017;62:3440. [DOI] [PMC free article] [PubMed]; Keselman P, Yu EY, Zhou XY, Goodwill PW, Chandrasekharan P, Ferguson RM. et al. Tracking short-term biodistribution and long-term clearance of SPIO tracers in magnetic particle imaging. Phys Med Biol. 2017;62:3440. doi: 10.1088/1361-6560/aa5f48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Antonelli A, Sfara C, Rahmer J, Gleich B, Borgert J, Magnani M. Red blood cells as carriers in magnetic particle imaging. Biomed Tech/Biomed Eng 2013;58:517–25. [DOI] [PubMed]; Antonelli A, Sfara C, Rahmer J, Gleich B, Borgert J, Magnani M. Red blood cells as carriers in magnetic particle imaging. Biomed Tech/Biomed Eng. 2013;58:517–25. doi: 10.1515/bmt-2012-0065. [DOI] [PubMed] [Google Scholar]
- [45].Rahmer J, Halkola A, Gleich B, Schmale I, Borgert J. First experimental evidence of the feasibility of multi-color magnetic particle imaging. Phys Med Biol 2015;60:1775–91. [DOI] [PubMed]; Rahmer J, Halkola A, Gleich B, Schmale I, Borgert J. First experimental evidence of the feasibility of multi-color magnetic particle imaging. Phys Med Biol. 2015;60:1775–91. doi: 10.1088/0031-9155/60/5/1775. [DOI] [PubMed] [Google Scholar]
- [46].Haegele J, Vaalma S, Panagiotopoulos N, Barkhausen J, Vogt FM, Borgert J, et al. Multi-color magnetic particle imaging for cardiovascular interventions. Phys Med Biol 2016;61:N415–26. [DOI] [PubMed]; Haegele J, Vaalma S, Panagiotopoulos N, Barkhausen J, Vogt FM, Borgert J. et al. Multi-color magnetic particle imaging for cardiovascular interventions. Phys Med Biol. 2016;61:N415–26. doi: 10.1088/0031-9155/61/16/N415. [DOI] [PubMed] [Google Scholar]
- [47].Haegele J, Cremers S, Rahmer J, Franke J, Vaalma S, Heidenreich M, et al. Magnetic particle imaging: a Resovist based marking technology for guide wires and catheters for vascular interventions. IEEE Trans Med Imaging 2016;35:2312–8. [DOI] [PubMed]; Haegele J, Cremers S, Rahmer J, Franke J, Vaalma S, Heidenreich M. et al. Magnetic particle imaging: a Resovist based marking technology for guide wires and catheters for vascular interventions. IEEE Trans Med Imaging. 2016;35:2312–8. doi: 10.1109/TMI.2016.2559538. [DOI] [PubMed] [Google Scholar]
- [48].Rahmer J, Wirtz D, Bontus C, Borgert J, Gleich B. Interactive magnetic catheter steering with 3D real-time feedback using multi-color magnetic particle imaging. IEEE Trans Med Imaging 2017;36:1449–56. [DOI] [PubMed]; Rahmer J, Wirtz D, Bontus C, Borgert J, Gleich B. Interactive magnetic catheter steering with 3D real-time feedback using multi-color magnetic particle imaging. IEEE Trans Med Imaging. 2017;36:1449–56. doi: 10.1109/TMI.2017.2679099. [DOI] [PubMed] [Google Scholar]
- [49].Duschka R, Wojtczyk H, Panagiotopoulos N, Haegele J, Bringout G, Buzug TM, et al. Safety measurements for heating of instruments for cardiovascular interventions in magnetic particle imaging (MPI) – first experiences. J Healthcare Eng 2014;5:79–94. [DOI] [PubMed]; Duschka R, Wojtczyk H, Panagiotopoulos N, Haegele J, Bringout G, Buzug TM. et al. Safety measurements for heating of instruments for cardiovascular interventions in magnetic particle imaging (MPI) – first experiences. J Healthcare Eng. 2014;5:79–94. doi: 10.1260/2040-2295.5.1.79. [DOI] [PubMed] [Google Scholar]
- [50].Wegner F, Friedrich T, Panagiotopoulos N, Vaalma S, Goltz JP, Vogt FM, et al. First heating measurements of endovascular stents in magnetic particle imaging. Phys Med Biol 2018;63:045005. [DOI] [PubMed]; Wegner F, Friedrich T, Panagiotopoulos N, Vaalma S, Goltz JP, Vogt FM. et al. First heating measurements of endovascular stents in magnetic particle imaging. Phys Med Biol. 2018;63:045005. doi: 10.1088/1361-6560/aaa79c. [DOI] [PubMed] [Google Scholar]
- [51].Herz S, Vogel P, Kampf T, Ruckert MA, Veldhoen S, Behr VC, et al. Magnetic particle imaging for quantification of vascular stenoses: a phantom study. IEEE Trans Med Imaging 2017;37:61–7. [DOI] [PubMed]; Herz S, Vogel P, Kampf T, Ruckert MA, Veldhoen S, Behr VC. et al. Magnetic particle imaging for quantification of vascular stenoses: a phantom study. IEEE Trans Med Imaging. 2017;37:61–7. doi: 10.1109/TMI.2017.2717958. [DOI] [PubMed] [Google Scholar]
- [52].Vaalma S, Rahmer J, Panagiotopoulos N, Duschka RL, Borgert J, Barkhausen J, et al. Magnetic particle imaging (MPI): experimental quantification of vascular stenosis using stationary stenosis phantoms. PLoS One 2017;12:e0168902. [DOI] [PMC free article] [PubMed]; Vaalma S, Rahmer J, Panagiotopoulos N, Duschka RL, Borgert J, Barkhausen J. et al. Magnetic particle imaging (MPI): experimental quantification of vascular stenosis using stationary stenosis phantoms. PLoS One. 2017;12:e0168902. doi: 10.1371/journal.pone.0168902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Salamon J, Hofmann M, Jung C, Kaul MG, Werner F, Them K, et al. Magnetic particle/magnetic resonance imaging: in-vitro MPI-guided real time catheter tracking and 4D angioplasty using a road map and blood pool tracer approach. PLoS One 2016;11:e0156899. [DOI] [PMC free article] [PubMed]; Salamon J, Hofmann M, Jung C, Kaul MG, Werner F, Them K. et al. Magnetic particle/magnetic resonance imaging: in-vitro MPI-guided real time catheter tracking and 4D angioplasty using a road map and blood pool tracer approach. PLoS One. 2016;11:e0156899. doi: 10.1371/journal.pone.0156899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Orendorff R, Peck AJ, Zheng B, Shirazi SN, Ferguson RM, Khandhar AP, et al. First in vivo traumatic brain injury imaging via magnetic particle imaging. Phys Med Biol 2017;62: 3501–9. [DOI] [PMC free article] [PubMed]; Orendorff R, Peck AJ, Zheng B, Shirazi SN, Ferguson RM, Khandhar AP. et al. First in vivo traumatic brain injury imaging via magnetic particle imaging. Phys Med Biol. 2017;62:3501–9. doi: 10.1088/1361-6560/aa52ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ludewig P, Gdaniec N, Sedlacik J, Forkert ND, Szwargulski P, Graeser M, et al. Magnetic particle imaging for real-time perfusion imaging in acute stroke. ACS Nano 2017;11:10480–8. [DOI] [PubMed]; Ludewig P, Gdaniec N, Sedlacik J, Forkert ND, Szwargulski P, Graeser M. et al. Magnetic particle imaging for real-time perfusion imaging in acute stroke. ACS Nano. 2017;11:10480–8. doi: 10.1021/acsnano.7b05784. [DOI] [PubMed] [Google Scholar]
- [56].Sedlacik J, Frölich A, Spallek J, Forkert ND, Faizy TD, Werner F, et al. Magnetic particle imaging for high temporal resolution assessment of aneurysm hemodynamics. PLoS One 2016;11:e0160097. [DOI] [PMC free article] [PubMed]; Sedlacik J, Frölich A, Spallek J, Forkert ND, Faizy TD, Werner F. et al. Magnetic particle imaging for high temporal resolution assessment of aneurysm hemodynamics. PLoS One. 2016;11:e0160097. doi: 10.1371/journal.pone.0160097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhou XY, Jeffris KE, Yu EY, Zheng B, Goodwill PW, Nahid P, et al. First in vivo magnetic particle imaging of lung perfusion in rats. Phys Med Biol 2017;62:3510–22. [DOI] [PMC free article] [PubMed]; Zhou XY, Jeffris KE, Yu EY, Zheng B, Goodwill PW, Nahid P. et al. First in vivo magnetic particle imaging of lung perfusion in rats. Phys Med Biol. 2017;62:3510–22. doi: 10.1088/1361-6560/aa616c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yu EY, Chandrasekharan P, Berzon R, Tay ZW, Zhou XY, Khandhar AP, et al. Magnetic particle imaging for highly sensitive, quantitative, and safe in vivo gut bleed detection in a murine model. ACS Nano 2017;11:12067–76. [DOI] [PMC free article] [PubMed]; Yu EY, Chandrasekharan P, Berzon R, Tay ZW, Zhou XY, Khandhar AP. et al. Magnetic particle imaging for highly sensitive, quantitative, and safe in vivo gut bleed detection in a murine model. ACS Nano. 2017;11:12067–76. doi: 10.1021/acsnano.7b04844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Jung C, Salamon J, Hofmann M, Kaul MG, Adam G, Ittrich H, et al. MPI as high temporal resolution imaging technique for in vivo bolus tracking of Ferucarbotran in mouse model. SPIE Med Imag 2016:9788.; Jung C, Salamon J, Hofmann M, Kaul MG, Adam G, Ittrich H. et al. MPI as high temporal resolution imaging technique for in vivo bolus tracking of Ferucarbotran in mouse model. SPIE Med Imag. 2016:9788. [Google Scholar]
- [60].Kaul MG, Salamon J, Knopp T, Ittrich H, Adam G, Weller H, et al. Magnetic particle imaging for in vivo blood flow velocity measurements in mice. Phys Med Biol 2018;63:064001. [DOI] [PubMed]; Kaul MG, Salamon J, Knopp T, Ittrich H, Adam G, Weller H. et al. Magnetic particle imaging for in vivo blood flow velocity measurements in mice. Phys Med Biol. 2018;63:064001. doi: 10.1088/1361-6560/aab136. [DOI] [PubMed] [Google Scholar]
- [61].Rauwerdink AM, Weaver JB. Viscous effects on nanoparticle magnetization harmonics. J Magn Magn Mater 2010;322: 609–13.; Rauwerdink AM, Weaver JB. Viscous effects on nanoparticle magnetization harmonics. J Magn Magn Mater. 2010;322:609–13. [Google Scholar]
- [62].Murase K, Song R, Hiratsuka S. Magnetic particle imaging of blood coagulation. Appl Phys Lett 2014;104:252409.; Murase K, Song R, Hiratsuka S. Magnetic particle imaging of blood coagulation. Appl Phys Lett. 2014;104:252409. [Google Scholar]
- [63].Yu EY, Bishop M, Zheng B, Ferguson RM, Khandhar AP, Kemp SJ, et al. Magnetic particle imaging: a novel in vivo imaging platform for cancer detection. Nano Lett 2017;17:1648–54. [DOI] [PMC free article] [PubMed]; Yu EY, Bishop M, Zheng B, Ferguson RM, Khandhar AP, Kemp SJ. et al. Magnetic particle imaging: a novel in vivo imaging platform for cancer detection. Nano Lett. 2017;17:1648–54. doi: 10.1021/acs.nanolett.6b04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Dieckhoff J, Kaul MG, Mummert T, Jung C, Salamon J, Adam G, et al. In vivo liver visualizations with magnetic particle imaging based on the calibration measurement approach. Physics Med Biol 2017;62:3470–82. [DOI] [PubMed]; Dieckhoff J, Kaul MG, Mummert T, Jung C, Salamon J, Adam G. et al. In vivo liver visualizations with magnetic particle imaging based on the calibration measurement approach. Physics Med Biol. 2017;62:3470–82. doi: 10.1088/1361-6560/aa562d. [DOI] [PubMed] [Google Scholar]
- [65].Ruhland B, Baumann K, Knopp T, Sattel T, Biederer S, Lüdtke-Buzug K, et al. Magnetic particle imaging with superparamagnetic nanoparticles for sentinel lymph node detection in breast cancer. Geburtsh Frauenheilk 2009;69:758.; Ruhland B, Baumann K, Knopp T, Sattel T, Biederer S, Lüdtke-Buzug K. et al. Magnetic particle imaging with superparamagnetic nanoparticles for sentinel lymph node detection in breast cancer. Geburtsh Frauenheilk. 2009;69:758. [Google Scholar]
- [66].Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG multicentre trial. Ann Surg Oncol 2014;21:1237–45. [DOI] [PubMed]; Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D. et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG multicentre trial. Ann Surg Oncol. 2014;21:1237–45. doi: 10.1245/s10434-013-3379-6. [DOI] [PubMed] [Google Scholar]
- [67].Kaul MG, Weber O, Heinen U, Reitmeier A, Mummert T, Jung C, et al. Combined preclinical magnetic particle imaging and magnetic resonance imaging: initial results in mice. Rofo 2015;187:347–52. [DOI] [PubMed]; Kaul MG, Weber O, Heinen U, Reitmeier A, Mummert T, Jung C. et al. Combined preclinical magnetic particle imaging and magnetic resonance imaging: initial results in mice. Rofo. 2015;187:347–52. doi: 10.1055/s-0034-1399344. [DOI] [PubMed] [Google Scholar]
- [68].Vogel P, Lother S, Rückert MA, Kullmann WH, Jakob PM, Fidler F, et al. MRI meets MPI: a bimodal MPI-MRI tomograph. IEEE Trans Med Imaging 2014;33:1954–9. [DOI] [PubMed]; Vogel P, Lother S, Rückert MA, Kullmann WH, Jakob PM, Fidler F. et al. MRI meets MPI: a bimodal MPI-MRI tomograph. IEEE Trans Med Imaging. 2014;33:1954–9. doi: 10.1109/TMI.2014.2327515. [DOI] [PubMed] [Google Scholar]
- [69].Franke J, Heinen U, Lehr H, Weber A, Jaspard F, Ruhm W, et al. System characterization of a highly integrated preclinical hybrid MPI-MRI scanner. IEEE Trans Med Imaging 2016;35:1993–2004. [DOI] [PubMed]; Franke J, Heinen U, Lehr H, Weber A, Jaspard F, Ruhm W. et al. System characterization of a highly integrated preclinical hybrid MPI-MRI scanner. IEEE Trans Med Imaging. 2016;35:1993–2004. doi: 10.1109/TMI.2016.2542041. [DOI] [PubMed] [Google Scholar]
- [70].Werner F, Jung C, Hofmann M, Werner R, Salamon J, Säring D, et al. Geometry planning and image registration in magnetic particle imaging using bimodal fiducial markers. Med Phys 2016;43:2884. [DOI] [PubMed]; Werner F, Jung C, Hofmann M, Werner R, Salamon J, Säring D. et al. Geometry planning and image registration in magnetic particle imaging using bimodal fiducial markers. Med Phys. 2016;43:2884. doi: 10.1118/1.4948998. [DOI] [PubMed] [Google Scholar]
- [71].Kranemann TC, Ersepke T, Schmitz G. Towards the integration of an MPI compatible ultrasound transducer. Int J Magn Particle Imaging 2017;3:1703016.; Kranemann TC, Ersepke T, Schmitz G. Towards the integration of an MPI compatible ultrasound transducer. Int J Magn Particle Imaging. 2017;3:1703016. [Google Scholar]
- [72].Latus S, Griese F, Gräser M, Möddel M, Schlüter M, Otte C, et al. Towards bimodal intravascular OCT MPI volumetric imaging. Proc SPIE Med Imaging 2018:105732E. [DOI] [PubMed]; Latus S, Griese F, Gräser M, Möddel M, Schlüter M, Otte C. et al. Towards bimodal intravascular OCT MPI volumetric imaging. Proc SPIE Med Imaging. 2018:105732E. doi: 10.1002/mp.13388. [DOI] [PubMed] [Google Scholar]
- [73].Arami H, Khandhar A, Tomitaka A, Yu E, Goodwill P, Conolly S, et al. In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents. Biomaterials 2015;52:251–61. [DOI] [PMC free article] [PubMed]; Arami H, Khandhar A, Tomitaka A, Yu E, Goodwill P, Conolly S. et al. In vivo multimodal magnetic particle imaging (MPI) with tailored magneto/optical contrast agents. Biomaterials. 2015;52:251–61. doi: 10.1016/j.biomaterials.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lindemann A, Lüdtke-Buzug K, Fräderich BM, Gräfe K, Pries R, Wollenberg B. Biological impact of superparamagnetic iron oxide nanoparticles for magnetic particle imaging of head and neck cancer cells. Int J Nanomed 2014;9:5025–40. [DOI] [PMC free article] [PubMed]; Lindemann A, Lüdtke-Buzug K, Fräderich BM, Gräfe K, Pries R, Wollenberg B. Biological impact of superparamagnetic iron oxide nanoparticles for magnetic particle imaging of head and neck cancer cells. Int J Nanomed. 2014;9:5025–40. doi: 10.2147/IJN.S63873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bulte JWM, Walczak P, Gleich B, Weizenecker J, Markov DE, Aerts HCJ, et al. MPI cell tracking: what can we learn from MRI? SPIE Med Imaging Biomed Appl Mol Struct Funct Imaging 2011;7965:79605z. [DOI] [PMC free article] [PubMed]; Bulte JWM, Walczak P, Gleich B, Weizenecker J, Markov DE, Aerts HCJ. et al. MPI cell tracking: what can we learn from MRI? SPIE Med Imaging Biomed Appl Mol Struct Funct Imaging. 2011;7965:79605z. doi: 10.1117/12.879844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zheng B, Vazin T, Goodwill PW, Conway A, Verma A, Saritas EU, et al. Magnetic particle imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Sci Rep 2015;5:14055. [DOI] [PMC free article] [PubMed]; Zheng B, Vazin T, Goodwill PW, Conway A, Verma A, Saritas EU. et al. Magnetic particle imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Sci Rep. 2015;5:14055. doi: 10.1038/srep14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zheng B, von See MP, Yu E, Gunel B, Lu K, Vazin T, et al. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics 2016;6:291–301. [DOI] [PMC free article] [PubMed]; Zheng B, von See MP, Yu E, Gunel B, Lu K, Vazin T. et al. Quantitative magnetic particle imaging monitors the transplantation, biodistribution, and clearance of stem cells in vivo. Theranostics. 2016;6:291–301. doi: 10.7150/thno.13728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bulte JWM, Walczak P, Janowski M, Krishnan KM, Arami H, Halkola A, et al. Quantitative “hot-spot” imaging of transplanted stem cells using superparamagnetic tracers and magnetic particle imaging. Tomography 2015;1:91–7. [DOI] [PMC free article] [PubMed]; Bulte JWM, Walczak P, Janowski M, Krishnan KM, Arami H, Halkola A. et al. Quantitative “hot-spot” imaging of transplanted stem cells using superparamagnetic tracers and magnetic particle imaging. Tomography. 2015;1:91–7. doi: 10.18383/j.tom.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Them K, Salamon J, Szwargulski P, Sequeira S, Kaul MG, Lange C, et al. Increasing the sensitivity for stem cell monitoring in system-function based magnetic particle imaging. Phys Med Biol 2016;61:3279–90. [DOI] [PubMed]; Them K, Salamon J, Szwargulski P, Sequeira S, Kaul MG, Lange C. et al. Increasing the sensitivity for stem cell monitoring in system-function based magnetic particle imaging. Phys Med Biol. 2016;61:3279–90. doi: 10.1088/0031-9155/61/9/3279. [DOI] [PubMed] [Google Scholar]
- [80].Tomitaka A, Arami H, Gandhi S, Krishnan KM. Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic particle imaging. Nanoscale 2015;7:16890–8. [DOI] [PMC free article] [PubMed]; Tomitaka A, Arami H, Gandhi S, Krishnan KM. Lactoferrin conjugated iron oxide nanoparticles for targeting brain glioma cells in magnetic particle imaging. Nanoscale. 2015;7:16890–8. doi: 10.1039/c5nr02831k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ittrich H, Peldschus K, Raabe N, Kaul M, Adam G. Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy. Rofo 2013;185:1149–66. [DOI] [PubMed]; Ittrich H, Peldschus K, Raabe N, Kaul M, Adam G. Superparamagnetic iron oxide nanoparticles in biomedicine: applications and developments in diagnostics and therapy. Rofo. 2013;185:1149–66. doi: 10.1055/s-0033-1335438. [DOI] [PubMed] [Google Scholar]
- [82].Song G, Chen M, Zhang Y, Cui L, Qu H, Zheng X, et al. Janus iron oxides @ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett 2017;18:182–9. [DOI] [PMC free article] [PubMed]; Song G, Chen M, Zhang Y, Cui L, Qu H, Zheng X. et al. Janus iron oxides @ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett. 2017;18:182–9. doi: 10.1021/acs.nanolett.7b03829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Arami H, Teeman E, Troska A, Bradshaw H, Saatchi K, Tomitaka A, et al. Tomographic magnetic particle imaging of cancer targeted nanoparticles. Nanoscale 2017;9:18723–30. [DOI] [PMC free article] [PubMed]; Arami H, Teeman E, Troska A, Bradshaw H, Saatchi K, Tomitaka A. et al. Tomographic magnetic particle imaging of cancer targeted nanoparticles. Nanoscale. 2017;9:18723–30. doi: 10.1039/c7nr05502a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Graeser M, Knopp T, Szwargulski P, Friedrich T, Gladiss Av, Kaul M, et al. Towards picogram detection of superparamagnetic iron-oxide particles using a gradiometric receive coil. Nat Sci Rep 2017;7:6872. [DOI] [PMC free article] [PubMed]; Graeser M, Knopp T, Szwargulski P, Friedrich T, Gladiss Av, Kaul M. et al. Towards picogram detection of superparamagnetic iron-oxide particles using a gradiometric receive coil. Nat Sci Rep. 2017;7:6872. doi: 10.1038/s41598-017-06992-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tay ZW, Chandrasekharan P, Chiu-Lam A, Hensley DW, Dhavalikar R, Zhou XY, et al. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano 2018;12:3699–713. [DOI] [PMC free article] [PubMed]; Tay ZW, Chandrasekharan P, Chiu-Lam A, Hensley DW, Dhavalikar R, Zhou XY. et al. Magnetic particle imaging-guided heating in vivo using gradient fields for arbitrary localization of magnetic hyperthermia therapy. ACS Nano. 2018;12:3699–713. doi: 10.1021/acsnano.8b00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Bauer LM, Situ SF, Griswold MA, Samia AC. High-performance iron oxide nanoparticles for magnetic particle imaging-guided hyperthermia (hMPI). Nanoscale 2016;8:12162–9. [DOI] [PubMed]; Bauer LM, Situ SF, Griswold MA, Samia AC. High-performance iron oxide nanoparticles for magnetic particle imaging-guided hyperthermia (hMPI) Nanoscale. 2016;8:12162–9. doi: 10.1039/c6nr01877g. [DOI] [PubMed] [Google Scholar]
- [87].Stehning C, Gleich B, Rahmer J. Simultaneous magnetic particle imaging (MPI) and temperature mapping using multi-color MPI. Int J Magn Part Imaging 2016;2:1612001.; Stehning C, Gleich B, Rahmer J. Simultaneous magnetic particle imaging (MPI) and temperature mapping using multi-color MPI. Int J Magn Part Imaging. 2016;2:1612001. [Google Scholar]
- [88].Nothnagel N, Rahmer J, Gleich B, Halkola A, Buzug TM, Borgert J. Steering of magnetic devices with a magnetic particle imaging system. IEEE Trans Biomed Eng 2016;63:2286–93. [DOI] [PubMed]; Nothnagel N, Rahmer J, Gleich B, Halkola A, Buzug TM, Borgert J. Steering of magnetic devices with a magnetic particle imaging system. IEEE Trans Biomed Eng. 2016;63:2286–93. doi: 10.1109/TBME.2016.2524070. [DOI] [PubMed] [Google Scholar]
- [89].Lübbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K, et al. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res 1996;56:4686–93. [PubMed]; Lübbe AS, Bergemann C, Riess H, Schriever F, Reichardt P, Possinger K. et al. Clinical experiences with magnetic drug targeting: a phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996;56:4686–93. [PubMed] [Google Scholar]
- [90].Kuboyabu T, Ohki A, Banura N, Murase K. Usefulness of magnetic particle imaging for monitoring the effect of magnetic targeting. Open J Med Imaging 2016;6:33–41.; Kuboyabu T, Ohki A, Banura N, Murase K. Usefulness of magnetic particle imaging for monitoring the effect of magnetic targeting. Open J Med Imaging. 2016;6:33–41. [Google Scholar]
- [91].Mahmood A, Dadkhah M, Ok Kim M, Yoon J. A novel design of an MPI-based guidance system for simultaneous actuation and monitoring of magnetic nanoparticles. IEEE Trans Magn 2015:51:1–5.; Mahmood A, Dadkhah M, Ok Kim M, Yoon J. A novel design of an MPI-based guidance system for simultaneous actuation and monitoring of magnetic nanoparticles. IEEE Trans Magn. 2015;51:1–5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.