Abstract
Background
Radiofrequency ablation (RFA) represents a treatment option for non-resectable liver malignancies. Larger ablations can be achieved with a temporary hepatic inflow occlusion (Pringle maneuver – PM). However, a PM can induce dehydration and carbonization of the target tissue. The objective of this study was to evaluate the impact of an intermittent PM on the ablation size.
Methods
Twenty-five multipolar RFAs were performed in porcine livers ex vivo. A perfused glass tube was used to simulate a natural vessel. The following five test series (each n=5) were conducted: (1) continuous PM, (2–4) intermittent PM, and (5) no PM. Ablations were cut into half. Ablation area, minimal radius, and maximal radius were compared.
Results
No change in complete ablation size could be measured between the test series (p>0.05). A small rim of native liver tissue was observed around the glass tube in the test series without PM. A significant increase of ablation area could be measured on the margin of the ablations with an intermittent PM, starting without hepatic inflow occlusion (p<0.05).
Conclusion
An intermittent PM did not lead to smaller ablations compared to a continuous or no PM ex vivo. Furthermore, an intermittent PM can increase the ablation area when initial hepatic inflow is succeeded by a PM.
Keywords: cooling effect, hepatic, liver, multipolar, radiofrequency ablation
Introduction
Radiofrequency ablation (RFA) is an important therapy option for the treatment of non-resectable malignant liver tumors [1], [2]. Electrical current administered through RFA applicators induces thermal heating of the target tissue around the applicator and therefore causes tumor necrosis [3]. The vascular cooling effect (“heat sink”) of adjacent liver vessels restricts the ablation size. A temporary hepatic inflow occlusion of both the hepatic artery and portal vein (“Pringle maneuver”) can reduce vascular cooling effects in RFA [4]. Higher local temperatures are observed when a Pringle maneuver is performed. Therefore, larger ablations can be achieved [5]. However, temperatures >100°C result in dehydration and carbonization of the target tissue. Carbonization leads to an increase in electrical resistance, which results in an electrical isolation of the tissue and a reduction of energy transmission. RFA is then limiting itself in regard to the ablation size [6].
Internal cooling of radiofrequency applicators is used in some RFA devices to reduce ablation temperatures around the applicators and consequently prevent carbonization of the target tissue adjacent to the applicators [6], [7]. A similar effect may exist around hepatic vessels. Although a positive effect on ablation size could be demonstrated with a complete Pringle maneuver, no study has investigated the effect of an intermittent Pringle maneuver so far [5]. An intermittent Pringle maneuver may lead to a more even distribution of thermal energy within the target tissue as carbonization of the tissue is reduced. Larger ablation volumes may result.
The objective of this study was to compare the impact of an intermittent hepatic inflow occlusion to that of a complete inflow occlusion and a no inflow occlusion in RFA ex vivo. Total ablation area and vascular cooling effects were evaluated.
Materials and methods
Experimental setup
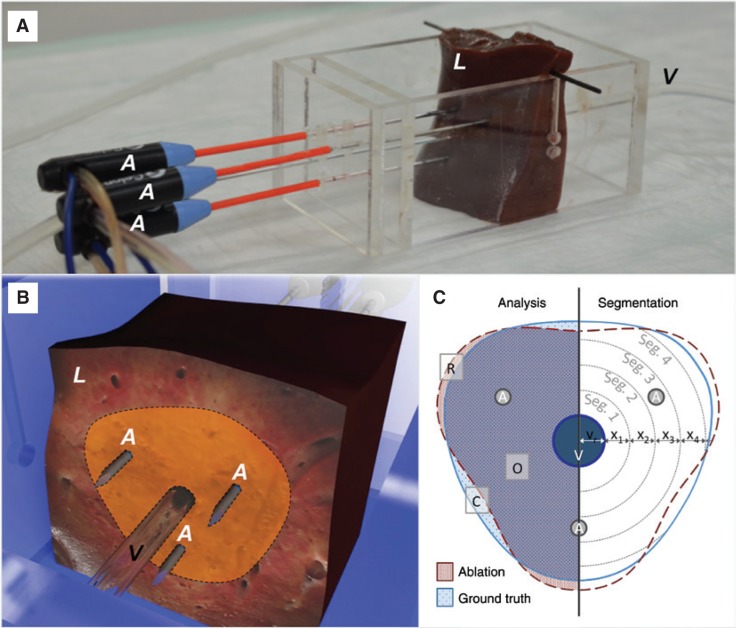
Multipolar RFA was performed in porcine liver ex vivo. A multipolar radiofrequency generator (CelonLab POWER System; Olympus Surgical Technologies Europe, Hamburg, Germany) was used with three internally cooled bipolar applicators (CelonProSurge T-20, Olympus Surgical Technologies Europe). A triple peristaltic pump (CelonAquaflowIII, Olympus Surgical Technologies Europe) ensured the internal cooling of each applicator. Applicators were set parallel to each other at a distance of 20 mm (Figure 1A). A preinstalled resistance controlled automatic power mode regulated the energy delivery into the liver tissue. The starting power was set to 60 W (according to manufacturer specifications) [8]. Ablations were stopped at an energy input of 40 kJ [9]. A glass tube (hereafter referred to as “vessel”) was used to simulate a natural liver vessel (inner/outer diameter: 3.4/5.0 mm). The vessel was set parallel to the applicators in the ablation center point. The vessel was perfused with water at room temperature with a peristaltic pump and a continuous flow rate of 100 mL/min (physiological parameters of a human with 70 kg: blood flow in the hepatic artery is 300 mL/min, blood flow in the portal vein is 1150 mL/min [10]). In previous experiments, we could demonstrate that a cooling effect already occurs at a flow rate of 100 mL/min [9]. Stopping the peristaltic pump simulated a Pringle maneuver. The test settings, which are specified above, corresponded to parameters that were established within our research group [9], [11], [12].
Figure 1:
Experimental implementation of the ex vivo test series.
(A) Three internally cooled bipolar radiofrequency applicators (A) were used in porcine livers (L) ex vivo.
A perfused glass tube (V) was used to simulate a natural liver vessel. The glass tube was situated in the center of the ablations, running parallel to the applicators. (B) Ablations were cut into half, orthogonally to the applicators on the height of the isolators (located at the tip of the applicator). Ablation areas were measured (dotted line). (C) An annular segmental model (Seg 1–4, …) with an adjustable segment width of “x” mm was used to compare ablations areas to an averaged mask (consisting of ablations with continuous Pringle maneuver reffered to as “ground truth”). Three areas have to be distinguished in analysis: (1) ablations are congruent (“O”); (2) an increase of ablation area compared to ground truth exists (“R”); and (3) the ablation is smaller than ground truth (cooling effects: “C”).
The following abbreviations are used to visualize the different test settings (Figure 2):
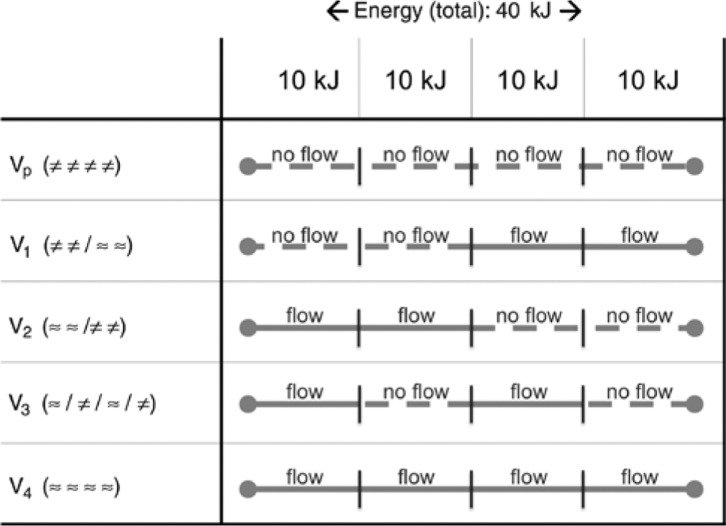
Figure 2:
Test series with corresponding energy input.
Five test series (n=5) were planned: (1) a continuous Pringle maneuver (Vp); (2–4) intermittent Pringle maneuvers (V1–V3); and (5) no Pringle maneuver (V4). Total energy input was set to 40 kJ (“≈”: perfusion; “≠”: Pringle maneuver).
“≈”: vascular flow,
“≠”: no vascular flow (Pringle maneuver),
“/”: alteration in perfusion (“flow→no flow”; respectively “no flow→flow”).
Five experimental settings were planned with five ablations each (Figure 2). For each ablation, energy input was set to 40 kJ (respectively 4 equal units of 10 kJ or 2 equal units of 20 kJ):
Vp: ≠≠≠≠ (continuous Pringle maneuver),
V1: ≠≠/≈≈ (intermittent Pringle maneuver: 20 kJ no flow/20 kJ flow),
V2: ≈≈/≠≠ (intermittent Pringle maneuver: 20 kJ flow/20 kJ no flow),
V3: ≈/≠/≈/≠ (intermittent Pringle maneuver: 10 kJ flow/10 kJ no flow/10 kJ flow/10 kJ no flow),
V4: ≈≈≈≈ (no Pringle maneuver).
Liver tissue was obtained from a slaughterhouse. Ablations were performed within 6 h after euthanasia of the animals at room temperature to minimize the effects of autolysis [11]. The livers were randomly assigned to the experimental settings.
Analysis
Ablations were cut in half after ablation along a plane defined by the three isolators situated between the two electrodes, located at the tip of the applicators (Figure 1B). Deformations caused by the cutting process of the soft liver tissue were subsequently adjusted using a thin spline landmark registration [13], [14]. The ablation area as well as minimal and maximal ablation radii were measured within this plane [15], [16]. Ablations were compared to a geometrically averaged mask (“ground truth”), comprising the ablations without perfusion, where no cooling effects are expected. The impact of a (temporary) blood flow occlusion was analyzed with circular segments defined by the vessel as center point (Figure 1C). Segment width was set to 2.5 mm (equivalent to the vessel radius). Ablation areas were compared within each circular segment to the averaged ground truth mask [9], [14]. Three different cases can occur in this analysis:
Statistical analysis
Analyses were conducted with a statistical software (SPSS version 20; SPSS Inc., Chicago, IL, USA). Data are expressed as median (minimum – maximum). The Kruskal-Wallis test was used for comparisons between more than two independent groups; the Mann-Whitney U-test was used for two independent groups. The level of significance was 0.05 (two sided) for each statistical testing.
Results
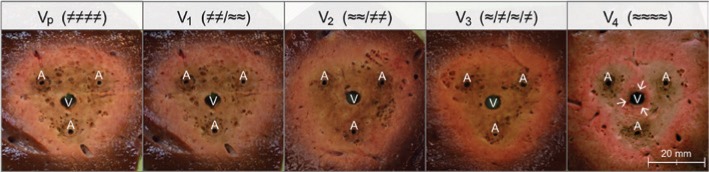
A total of 25 ablations were performed in 10 porcine livers. Figure 3 exemplarily shows cross sections of each experimental setting. All ablations led to rounded and homogeneous lesions. No difference in ablation area could macroscopically be observed between ablations with continuous and intermittent Pringle maneuver. However, a macroscopic small rim of native liver tissue could be detected immediately around the artificial vessel in all ablations of the test series without Pringle maneuver (Figure 3; V4 “→”). This “native” rim did not occur in ablations with continuous or intermittent Pringle maneuver (Vp, V1–V3).
Figure 3:
Exemplary cross-sectional areas of all five test series.
Homogeneous ablation areas could be observed in ablations in which a (temporary) Pringle maneuver was performed (Vp, V1–V3). A vascular cooling effect is seen around the vessel in the test series with continuous perfusion (V4) (V, vessel; A, applicator).
The characteristics of the test series are presented in Table 1. Five ablations without perfusion (Vp ≠≠≠≠) were carried out (cf. Table 1 and Figure 3). The geometrical averaged area of this experimental setting without any perfusion was 936 mm2. No difference in ablation area and ablation radius (minimum and maximum) could be observed between the five experimental settings (p>0.05).
Table 1:
Mean values of ablation area and ablation radii (min–max) for each test setting.
| Vp (≠≠≠≠) | V1 (≠≠/≈≈) | V2 (≈≈/≠≠) | V3 (≈/≠/≈/≠) | V4 (≈≈≈≈) | p-Value | |
|---|---|---|---|---|---|---|
| Area, mm2 | 936 (812–1186) | 1136 (878–1351) | 953 (885–1141) | 1058 (863–1194) | 897 (795–935) | >0.05 |
| Rmin, mm | 14 (14–17) | 15 (15–16) | 17 (14–19) | 16 (15–17) | 13 (13–17) | >0.05 |
| Rmax, mm | 20 (19–23) | 20 (19–22) | 22 (19–22) | 22 (20–22) | 20 (17–21) | >0.05 |
No differences in ablation area and ablation radius were observed between the five test series.
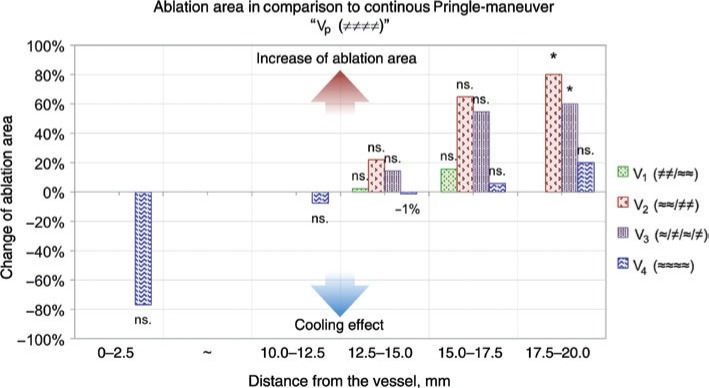
Additionally, ablation areas were measured within segments of 2.5 mm width to analyze the impact of vascular cooling effects with higher precision (Figure 4). A vascular cooling effect of almost 80% could be measured within 2.5 mm around the vessel in the test setting with continuous perfusion (V4 ≈≈≈≈). This cooling effect was not significant (p>0.05). No significant cooling effects could be observed in the test settings with intermittent Pringle maneuver. Additionally, no change of complete ablation area could be discovered between a continuous Pringle maneuver and the test settings without perfusion in the beginning (V1 ≠≠/≈≈). However, an increase of ablation area was measured in the periphery of the ablations in the test settings with intermittent Pringle maneuver, starting without blood flow occlusion (V2 ≈≈/≠≠, V3 ≈/≠/≈/≠).
Figure 4:
A vascular cooling effect could be observed around the vessel in all ablations of the test series without Pringle maneuver (V4).
However, this cooling effect was not significant (p>0.05). An increase of ablation area could be observed on the margin of the ablations in the test series with intermittent Pringle maneuver, starting without hepatic inflow occlusion (V1–V3). In between 2.5 and 10.0 mm (“~”), no change in ablation area could be observed between the five test series (*, significant; ns., not significant; p>0.05).
Discussion
In the actual study, we could demonstrate that an intermittent Pringle maneuver did not lead to smaller ablations compared to a continuous or even no Pringle maneuver ex vivo. Furthermore, an intermittent Pringle maneuver can increase the ablation area when initial hepatic inflow is succeeded by a Pringle maneuver.
No change of ablation area, minimal radius, and maximal radius could be observed between test series with continuous (Vp), intermittent (V1–V3), or without (V4) Pringle maneuver in our ex vivo study. A small rim of native liver tissue was observed macroscopically around the vessel in the test series without Pringle maneuver (V4). This “cooling effect” was not significant, because its size was too small/inhomogeneous in comparison to the total ablation area. However, an increased risk for local tumor recurrence after RFA appears to be likely around the vessel in these cases. Therefore, a Pringle maneuver seems to be a reasonable method for ablations close to major hepatic vessels. No cooling effect was observed in the test series with intermittent Pringle maneuver. Although the study was performed ex vivo, it demonstrated that intermittent vascular inflow occlusion is not inferior to continuous inflow occlusion. Moreover, we could observe that an intermittent Pringle maneuver increases the ablation area in the periphery of the ablations. This occurs especially when an initial hepatic inflow is succeeded by a Pringle maneuver during the course of the ablation (V2, V3). High temperatures >100°C within the ablation zone limit the efficiency of RFA due to dehydration and carbonization of the target tissue. An early carbonization of the tissue in the ablation center may be prevented by a persisting hepatic inflow in the beginning of an ablation. Residual tumor/liver tissue around major hepatic vessels may be finally destructed by a Pringle maneuver performed in the further course of an ablation. In summary, this so-called intermittent Pringle maneuver seems to ensure a more uniform distribution of thermal energy within the target tissue, resulting in larger ablation sizes. To our knowledge, no study examining the effect of an intermittent Pringle maneuver on hepatic RFA has been performed so far.
The effect of a Pringle maneuver in surgical hepatic resection has been controversially discussed in the literature. A Pringle maneuver is often used in patients with poor parenchymal conditions, larger tumors, or longer surgeries. A Pringle maneuver may have protective effects in surgical hepatic resection for high-risk cases by minimizing perioperative blood loss [17]. In several clinical studies, no difference in perioperative complications, postoperative liver function, tumor recurrence, or overall survival could be shown between hepatic resection and a Pringle maneuver/an intermittent Pringle maneuver [17], [18], [19], [20], [21]. However, an aggravation of gut barrier dysfunction with more aggressive translocation of endotoxins and intestinal bacteria has been described while performing a Pringle maneuver during RFA [22]. In an in vivo study by Kim et al., a Pringle maneuver resulted in severe pathologic changes in the portal vein, bile ducts, and liver parenchyma surrounding the ablation zone. Therefore, a Pringle maneuver should be performed with caution to avoid unintended thermal injuries [23]. An increased risk of ischemia-reperfusion injury was reported after RFA when performing a continuous Pringle maneuver in vivo [24]. However, a prolonged Pringle maneuver may lead to undesired thermal injuries. Therefore, an intermittent Pringle maneuver can represent an opportunity to combine the advantages of a Pringle maneuver with the disadvantages of a prolonged hepatic inflow occlusion.
The limitations of our study are the lack of a tumor model and the simulation of a natural liver vessel with a glass tube. The evaluation of vascular cooling effects in vivo is challenging due to the complexity of the existing natural vascular hepatic blood supply [25]. The cooling effect of a single hepatic vessel can hardly be estimated in vivo. Therefore, the study was performed with a glass tube that simulated a liver vessel to achieve a standardized setup. The isolating properties of the glass tube can be neglected, as it was situated outside of the electrical field of the applicators [9], [11], [12], [26]. Energy transmission took place at the position of the glass tube by direct temperature transfer. Glass has thermal properties that are similar to those of liver tissue and does not interfere with heat conduction [11], [26]. The thermal properties of the vascular wall itself were disregarded within this study. A standardized and reproducible test setting was used in our study. The perfusion of the glass tube was the only variable that was altered. Therefore, the impact of an intermittent Pringle maneuver could be exactly evaluated in this ex vivo model. The experiments were conducted at room temperature. Previous tests have demonstrated that a hepatic heat sink effect with similar extent to the heat sink effect at body temperature can occur at room temperature [12]. Ablations were performed in native porcine liver due to a lack of an adequate porcine tumor model. Porcine liver tissue has physiological properties similar to those of human liver tissue [27].
Our study suggests that an intermittent Pringle maneuver has a positive effect on RFA. In comparison to a continuous Pringle maneuver and to uninterrupted blood flow, an intermittent approach has favorable results regarding the ablation size. Functional disorders of the remnant liver may be reduced when performing an intermittent Pringle maneuver. In vivo experiments will be necessary to confirm the results of this ex vivo study.
Supporting Information
Supplementary Material
The article (iss-2018-0008) offers reviewer assessments as supplementary material.
Author Statement
Research funding: This study was supported by a grant from the “Deutsche Forschungsgemeinschaft” (ref. no. RI1131/3-3). Conflict of interest: Authors state no conflict of interest. Informed consent: Informed consent is not applicable. Ethical approval: The conducted research is not related to either human or animal use.
Author Contributions
Franz G.M. Poch: conceptualization; formal analysis; investigation; methodology; project administration; software; supervision; writing – original draft. Christina A. Neizert: data curation; formal analysis; investigation; methodology; project administration; writing – original draft. Ole Gemeinhardt: conceptualization; formal analysis; writing – review and editing. Beatrice Geyer: formal analysis; investigation; methodology; project administration; supervision; writing – review and editing. Katharina Eminger: supervision; writing – review and editing. Christian Rieder: software; validation; visualization; writing – review and editing. Stefan M. Niehues: conceptualization; writing – review and editing. Janis Vahldiek: conceptualization; writing – review and editing. Stefan F. Thieme: writing – review and editing. Kai S. Lehmann: conceptualization; funding acquisition; methodology; project administration; supervision; writing – review and editing.
Publication Funding
The German Society of Surgery funded the article processing charges of this article.
References
- [1].Nicholl MB, Bilchik AJ. Thermal ablation of hepatic malignancy: useful but still not optimal. Eur J Surg Oncol 2008;34:318–23. [DOI] [PubMed]; Nicholl MB, Bilchik AJ. Thermal ablation of hepatic malignancy: useful but still not optimal. Eur J Surg Oncol. 2008;34:318–23. doi: 10.1016/j.ejso.2007.07.203. [DOI] [PubMed] [Google Scholar]
- [2].Cai H, Kong W, Zhou T, Qiu Y. Radiofrequency ablation versus reresection in treating recurrent hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2014;93:e122. [DOI] [PMC free article] [PubMed]; Cai H, Kong W, Zhou T, Qiu Y. Radiofrequency ablation versus reresection in treating recurrent hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2014;93:e122. doi: 10.1097/MD.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990;25:267–70. [DOI] [PubMed]; McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990;25:267–70. doi: 10.1097/00004424-199003000-00011. [DOI] [PubMed] [Google Scholar]
- [4].Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541–9. [DOI] [PMC free article] [PubMed]; Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:541–9. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chinn SB, Lee FT Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC 3rd, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol 2001;176:789–95. [DOI] [PubMed]; Chinn SB, Lee FT Jr, Kennedy GD, Chinn C, Johnson CD, Winter TC 3rd. et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789–95. doi: 10.2214/ajr.176.3.1760789. [DOI] [PubMed] [Google Scholar]
- [6].Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol 1996;3:636–44. [DOI] [PubMed]; Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol. 1996;3:636–44. doi: 10.1016/s1076-6332(96)80188-7. [DOI] [PubMed] [Google Scholar]
- [7].Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol 1996;3:556–63. [DOI] [PubMed]; Lorentzen T. A cooled needle electrode for radiofrequency tissue ablation: thermodynamic aspects of improved performance compared with conventional needle design. Acad Radiol. 1996;3:556–63. doi: 10.1016/s1076-6332(96)80219-4. [DOI] [PubMed] [Google Scholar]
- [8].Frericks BB, Ritz JP, Roggan A, Wolf K-J, Albrecht T. Multipolar radiofrequency ablation of hepatic tumors: initial experience. Radiology 2005;237:1056–62. [DOI] [PubMed]; Frericks BB, Ritz JP, Roggan A, Wolf K-J, Albrecht T. Multipolar radiofrequency ablation of hepatic tumors: initial experience. Radiology. 2005;237:1056–62. doi: 10.1148/radiol.2373041104. [DOI] [PubMed] [Google Scholar]
- [9].Poch FG, Rieder C, Ballhausen H, Knappe V, Ritz JP, Gemeinhardt O, et al. The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int J Hyperthermia 2016;32:749–56. [DOI] [PubMed]; Poch FG, Rieder C, Ballhausen H, Knappe V, Ritz JP, Gemeinhardt O. et al. The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int J Hyperthermia. 2016;32:749–56. doi: 10.1080/02656736.2016.1196395. [DOI] [PubMed] [Google Scholar]
- [10].Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993;10:1093–5. [DOI] [PubMed]; Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- [11].Lehmann KS, Ritz JP, Valdeig S, Knappe V, Schenk A, Weihusen A, et al. Ex situ quantification of the cooling effect of liver vessels on radiofrequency ablation. Langenbecks Arch Surg 2009;394:475–81. [DOI] [PubMed]; Lehmann KS, Ritz JP, Valdeig S, Knappe V, Schenk A, Weihusen A. et al. Ex situ quantification of the cooling effect of liver vessels on radiofrequency ablation. Langenbecks Arch Surg. 2009;394:475–81. doi: 10.1007/s00423-009-0480-1. [DOI] [PubMed] [Google Scholar]
- [12].Lehmann KS, Poch FG, Rieder C, Schenk A, Stroux A, Frericks BB, et al. Minimal vascular flows cause strong heat sink effects in hepatic radiofrequency ablation ex vivo. J Hepatobiliary Pancreat Sci 2016;23:508–16. [DOI] [PubMed]; Lehmann KS, Poch FG, Rieder C, Schenk A, Stroux A, Frericks BB. et al. Minimal vascular flows cause strong heat sink effects in hepatic radiofrequency ablation ex vivo. J Hepatobiliary Pancreat Sci. 2016;23:508–16. doi: 10.1002/jhbp.370. [DOI] [PubMed] [Google Scholar]
- [13].Rohr K, Stiehl HS, Sprengel R, Buzug TM, Weese J, Kuhn MH. Landmark-based elastic registration using approximating thin-plate splines. IEEE Trans Med Imaging 2001;20:526–34. [DOI] [PubMed]; Rohr K, Stiehl HS, Sprengel R, Buzug TM, Weese J, Kuhn MH. Landmark-based elastic registration using approximating thin-plate splines. IEEE Trans Med Imaging. 2001;20:526–34. doi: 10.1109/42.929618. [DOI] [PubMed] [Google Scholar]
- [14].Rieder C, Poch F, Tiesler H, Lehmann K, Preusser T. Software tool for the analysis of the coagulation zone from multipolar radiofrequency ablation. In: Conference Paper: Computer- und Roboterassistierte Chirurgie (CURAC), Düsseldorf; 2012.; Rieder C, Poch F, Tiesler H, Lehmann K, Preusser T. Software tool for the analysis of the coagulation zone from multipolar radiofrequency ablation; Conference Paper: Computer- und Roboterassistierte Chirurgie (CURAC); Düsseldorf. 2012. [Google Scholar]
- [15].Ng KK, Lam CM, Poon RT, Shek TW, Yu WC, To JY, et al. Porcine liver: morphologic characteristics and cell viability at experimental radiofrequency ablation with internally cooled electrodes. Radiology 2005;235:478–86. [DOI] [PubMed]; Ng KK, Lam CM, Poon RT, Shek TW, Yu WC, To JY. et al. Porcine liver: morphologic characteristics and cell viability at experimental radiofrequency ablation with internally cooled electrodes. Radiology. 2005;235:478–86. doi: 10.1148/radiol.2352040425. [DOI] [PubMed] [Google Scholar]
- [16].Gemeinhardt O, Poch FG, Hiebl B, Kunz-Zurbuchen U, Corte GM, Thieme SF, et al. Comparison of bipolar radiofrequency ablation zones in an in vivo porcine model: correlation of histology and gross pathological findings. Clin Hemorheol Microcirc 2016;64:491–9. [DOI] [PubMed]; Gemeinhardt O, Poch FG, Hiebl B, Kunz-Zurbuchen U, Corte GM, Thieme SF. et al. Comparison of bipolar radiofrequency ablation zones in an in vivo porcine model: correlation of histology and gross pathological findings. Clin Hemorheol Microcirc. 2016;64:491–9. doi: 10.3233/CH-168123. [DOI] [PubMed] [Google Scholar]
- [17].Famularo S, Giani A, Di Sandro S, Sandini M, Giacomoni A, Pinotti E, et al. Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? A Western series of 441 patients. J Surg Oncol 2018;117:198–206. [DOI] [PubMed]; Famularo S, Giani A, Di Sandro S, Sandini M, Giacomoni A, Pinotti E. et al. Does the Pringle maneuver affect survival and recurrence following surgical resection for hepatocellular carcinoma? A Western series of 441 patients. J Surg Oncol. 2018;117:198–206. doi: 10.1002/jso.24819. [DOI] [PubMed] [Google Scholar]
- [18].Sanjay P, Ong I, Bartlett A, Powell JJ, Wigmore SJ. Meta-analysis of intermittent Pringle manoeuvre versus no Pringle manoeuvre in elective liver surgery. ANZ J Surg 2013;83:719–23. [DOI] [PubMed]; Sanjay P, Ong I, Bartlett A, Powell JJ, Wigmore SJ. Meta-analysis of intermittent Pringle manoeuvre versus no Pringle manoeuvre in elective liver surgery. ANZ J Surg. 2013;83:719–23. doi: 10.1111/ans.12312. [DOI] [PubMed] [Google Scholar]
- [19].Weiss MJ, Ito H, Araujo RL, Zabor EC, Gonen M, D’Angelica MI, et al. Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: no impact on survival or hepatic recurrence. Ann Surg Oncol 2013;20:285–94. [DOI] [PubMed]; Weiss MJ, Ito H, Araujo RL, Zabor EC, Gonen M, D’Angelica MI. et al. Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: no impact on survival or hepatic recurrence. Ann Surg Oncol. 2013;20:285–94. doi: 10.1245/s10434-012-2583-0. [DOI] [PubMed] [Google Scholar]
- [20].Huang J, Tang W, Hernandez-Alejandro R, Bertens KA, Wu H, Liao M, et al. Intermittent hepatic inflow occlusion during partial hepatectomy for hepatocellular carcinoma does not shorten overall survival or increase the likelihood of tumor recurrence. Medicine (Baltimore) 2014;93:e288. [DOI] [PMC free article] [PubMed]; Huang J, Tang W, Hernandez-Alejandro R, Bertens KA, Wu H, Liao M. et al. Intermittent hepatic inflow occlusion during partial hepatectomy for hepatocellular carcinoma does not shorten overall survival or increase the likelihood of tumor recurrence. Medicine (Baltimore) 2014;93:e288. doi: 10.1097/MD.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Xu W, Xu H, Yang H, Liao W, Ge P, Ren J, et al. Continuous Pringle maneuver does not affect outcomes of patients with hepatocellular carcinoma after curative resection. Asia Pac J Clin Oncol 2017;13:e321–30. [DOI] [PubMed]; Xu W, Xu H, Yang H, Liao W, Ge P, Ren J. et al. Continuous Pringle maneuver does not affect outcomes of patients with hepatocellular carcinoma after curative resection. Asia Pac J Clin Oncol. 2017;13:e321–30. doi: 10.1111/ajco.12585. [DOI] [PubMed] [Google Scholar]
- [22].Ypsilantis P, Lambropoulou M, Grapsa A, Tentes I, Tsigalou C, Panopoulou M, et al. Pringle maneuver deteriorates gut barrier dysfunction induced by extended-liver radiofrequency ablation. Dig Dis Sci 2011;56:1548–56. [DOI] [PubMed]; Ypsilantis P, Lambropoulou M, Grapsa A, Tentes I, Tsigalou C, Panopoulou M. et al. Pringle maneuver deteriorates gut barrier dysfunction induced by extended-liver radiofrequency ablation. Dig Dis Sci. 2011;56:1548–56. doi: 10.1007/s10620-010-1462-4. [DOI] [PubMed] [Google Scholar]
- [23].Kim SK, Lim HK, Ryu J, Choi D, Lee WJ, Lee JY, et al. Radiofrequency ablation of rabbit liver in vivo: effect of the Pringle maneuver on pathologic changes in liver surrounding the ablation zone. Korean J Radiol 2004;5:240–9. [DOI] [PMC free article] [PubMed]; Kim SK, Lim HK, Ryu J, Choi D, Lee WJ, Lee JY. et al. Radiofrequency ablation of rabbit liver in vivo: effect of the Pringle maneuver on pathologic changes in liver surrounding the ablation zone. Korean J Radiol. 2004;5:240–9. doi: 10.3348/kjr.2004.5.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ypsilantis P, Lambropoulou M, Anagnostopoulos C, Tsigalou C, Vasiliadis C, Kortsaris A, et al. Pringle maneuver exacerbates systemic inflammatory response and multiple-organ injury induced by extended liver radiofrequency ablation. Hum Exp Toxicol 2011;30:1855–64. [DOI] [PubMed]; Ypsilantis P, Lambropoulou M, Anagnostopoulos C, Tsigalou C, Vasiliadis C, Kortsaris A. et al. Pringle maneuver exacerbates systemic inflammatory response and multiple-organ injury induced by extended liver radiofrequency ablation. Hum Exp Toxicol. 2011;30:1855–64. doi: 10.1177/0960327111401438. [DOI] [PubMed] [Google Scholar]
- [25].Kröger T, Pätz T, Altrogge I, Schenk A, Lehmann KS, Frericks BB, et al. Fast estimation of the vascular cooling in RFA based on numerical simulation. Open Biomed Eng J 2010;4:16–26. [DOI] [PMC free article] [PubMed]; Kröger T, Pätz T, Altrogge I, Schenk A, Lehmann KS, Frericks BB. et al. Fast estimation of the vascular cooling in RFA based on numerical simulation. Open Biomed Eng J. 2010;4:16–26. doi: 10.2174/1874120701004020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Welp C, Siebers S, Ermert H, Werner J. Investigation of the influence of blood flow rate on large vessel cooling in hepatic radiofrequency ablation. Biomed Tech (Berl) 2006;51:337–46. [DOI] [PubMed]; Welp C, Siebers S, Ermert H, Werner J. Investigation of the influence of blood flow rate on large vessel cooling in hepatic radiofrequency ablation. Biomed Tech (Berl) 2006;51:337–46. doi: 10.1515/BMT.2006.067. [DOI] [PubMed] [Google Scholar]
- [27].Hiebl B, Müller C, Hünigen H, Gemeinhardt O, Plendl J, Jung F, et al. Gross anatomical variants of the vasculature of the GöttingenTM minipig. Appl Cardiopulm Pathophysiol 2010;14:236–43.; Hiebl B, Müller C, Hünigen H, Gemeinhardt O, Plendl J, Jung F. et al. Gross anatomical variants of the vasculature of the GöttingenTM minipig. Appl Cardiopulm Pathophysiol. 2010;14:236–43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.