Abstract
The protection of listed species through the Ecological Risk Assessment (ERA) process is encumbered by the number and diversity of species that need protection and the limited data available to inform assessments. Ecological communities within isolated ecosystems often contain a number of biologically diverse endemic, endangered, and threatened species, as well as providing numerous ecosystem services (ES). We propose an approach that develops community-level protection goals using isolated wetlands that includes both listed species and Service Providing Units (SPUs) that drive ES for ecological risk assessments (ERAs). Community-level protection goals are achieved by developing a protection community and weighing lines of evidence to determine a set of focal species within that community upon which to base the assessment. Lines of evidence include chemical mechanism of action, likely routes of exposure, and taxa susceptibility, as well as relationships among species, and other ecological factors. We demonstrate the process using case studies of chlorpyrifos in California vernal pools and coal ash effluent in Carolina bays. In the California vernal pool case study, listed species were the primary SPUs for the ES provided by the critical habitat. The weight of evidence demonstrated the honey bee as the focal species for the terrestrial environment and the vernal pool fairy shrimp as the focal species for the aquatic environment. The protection community within the Carolina bay case study was more taxonomically diverse than vernal pools for both listed species and SPUs, with amphibians identified as the focal species for which to target mitigation goals and hazard levels. The approach presented here will reduce the time and resource investment required for assessment of risk to listed species and adds an ES perspective to demonstrate value of assessments beyond listed species concerns.
Keywords: Ecological risk assessment, Listed species, Ecosystem services, Geographically isolated wetlands
1. Introduction
At first glance, protection goals for Ecological Risk Assessments (ERAs) that focus on threatened and endangered (listed) species and ecosystem services (ES) appear contradictory. Under the Endangered Species Act (ESA), private and federal actions may not jeopardize listed species pop-ulations, regardless of the benefit to humans. Conversely, by definition, ES benefit society through ecological processes that are valued and used by people. While the protection of listed species often is focused on the in-dividual and population-levels, protection of ES is enmeshed in community- or ecosystem-level dynamics. ERAs that target the protection of listed species do so on a species-by-species basis, whereas the current state of ES-based ERAs have been more focused on ES in general habitat types (e.g., urban, grassland, wetlands; Maltby et al., 2017) using broad and relatively large-scale endpoints (e.g., quantity and quality of food production; Munns Jr. et al., 2015). Here, we present an approach to ERA that aims to protect both listed species and ES in isolated wetlands using community-based protection goals. In doing so, we demonstrate the protection of listed species and ES are not contradictory, but can be leveraged together for broader environmental protection.
Isolated wetlands are surrounded by uplands, may or may not be ephemeral, and have hydrological input primarily through rainfall and runoff (Tiner, 2003). Ubiquitous throughout the United States, isolated wetlands vary across ecoregions with a unique combination of geological, climatic, and biotic features. They support a diverse array of species and communities, including many endemic species, listed species, and species of concern (e.g., plants, crustaceans, amphibians), and are critical habitat for several listed species (e.g., vernal pool fairy shrimp; USFWS, 2005). They also provide a variety of ES such as provisioning ES (e.g., genetic resources, fresh water) and regulatory ES (e.g., pollination, water regulation, water purification) (Tiner, 2003; Marton et al., 2015). Isolated wetlands face multiple threats including encroaching urbanization, agricultural conversion, hydrological changes, and other climatic stressors. In addition to their unique regional stressors, isolated wetlands are vulnerable to chemical contamination, which also may vary based on region (Tiner et al., 2002).
Under the ESA, federal and state agencies consult with the US Fish and Wildlife Service (USFWS) or the National Oceanic and Atmospheric Administration (NOAA) National Marine Fisheries Service (NMFS) (herein combined, the Services) to make certain that federal, federally-funded, or federally-permitted actions do not jeopardize the continued existence of listed species or result in the destruction or adverse modification of those species' critical habitat. Critical habitat of listed species is delineated by the Services and includes isolated wetlands for several species throughout the US. The ERA process is intended to determine what risks an action poses to listed species and their corresponding critical habitat. In assessing risks of pesticide exposure to listed species, the Services and EPA work in tandem in a three-step process to characterize the risk of an action to listed species' viability (NRC, 2013). The current process involves a species-by-species approach that often targets protection goals at the individual or population level, despite many habitats, such as isolated wetlands, containing multiple listed species spanning across taxa (e.g., plants, crustaceans, amphibians). Therefore, it is intuitive that ERAs focused on community-level protection as the endpoint provides a potential to protect multiple listed species across diverse taxa with one process.
The ERA process for conservation of ES is considerably less defined than that of listed species. The US EPA Risk Assessment Forum only recently added ES endpoints to a list of generic endpoints for ERA (USEPA, 2016a) with the goals of expanding the value of ERA in environmental decision making and improving the communication of risks to stakeholders (Munns Jr. et al., 2015). However, inclusion of ES endpoints is considerably less straight-forward, as additional analytical steps are required to link data typically available to risk assessors to ES. The classification of ES involves the designation of final ES that provide direct benefits to society and human well-being (e.g., the benefits of crop pollination realized by farmers), intermediate ES that are not used directly by beneficiaries but contribute to final ES (e.g., the environmental conditions necessary to support pollinator populations), and service providing units (SPUs), which are the ecological units from which ES originate (e.g., the pollinator populations). Ecological production functions are quantitative relationships between SPUs and stressors or management decisions that can alter SPUs. These production functions may not be linear or correlated with one another and can vary both spatially and temporally (Rodríguez et al., 2006). One of the largest challenges in using ES as endpoints in ERA is linking data that are commonly provided in support of ERAs with production functions, SPUs, or intermediate and final ES (Forbes et al., 2017). As such, frameworks and case studies are emerging to begin working through this challenge (e.g., Faber and van Wensem, 2012; Forbes et al., 2017; Galic et al., 2012; Nienstedt et al., 2012).
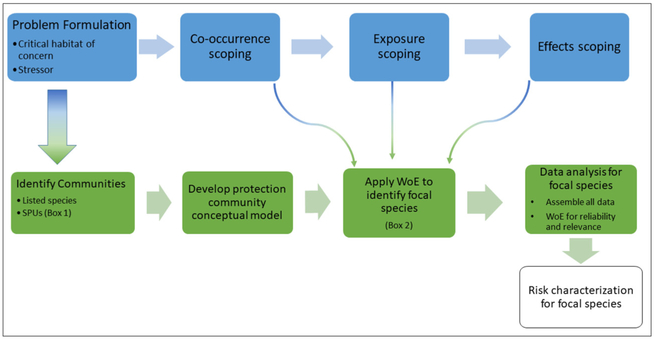
We propose a unified ERA process that evaluates the risks to all species which rely on a critical habitat and the ES services it provides using isolated wetlands as an example. Unique geographical and hydrological features are emblematic of isolated wetlands across all regions and we suggest that these characteristics can be used to standardize an approach to improve ERAs to be protective of the isolated ecosystems, including all listed species that rely on or occupy them. In our approach, a protection community (e.g., all listed species and SPUs occurring within the area of concern) is identified and co-occurrence, exposure, and effects information pertaining to these species is gathered in an initial scoping effort. Traits of the species within the protection community are considered along with community dynamics, co-occurrence, exposure, and effects information in a Weight of Evidence (WoE) to identify focal species within the protection community. The focal species are the species that can then be targeted for a definitive ERA that is protective of the entire community (Fig. 1). We demonstrate this approach using case studies of vernal pools in the California Central Valley and Carolina bays in the Southeast US.
Fig. 1.
Process for protecting communities containing listed species and Service Providing Units (SPUs). Blue steps are consistent with steps in traditional ERA processes. Green steps are demonstrated in the current study to identify the protection community and determine a set of focal species on which to base an assessment.
2. Community-level protection goals
Protection goals are determined in the problem formulation stage of ERAs by risk assessors and risk managers to define the objective of the assessment. For general ERAs that may be applied to non-specific locations, protection goals may be as broad as ensuring that chemicals will not pose any unreasonable risks to plants, wildlife, and the environment. Protection goals are met through the evaluation of assessment endpoints, which are measurable attributes used by decision makers to evaluate chemical hazards. Assessment endpoints are limited to available data or models and may be at the sub-organismal level (e.g., molecular pathways and adverse outcome pathways), the individual level (e.g., increased mortality, reduced reproduction, reduced growth), population level (e.g., population decline), or community level (e.g., taxa richness) (USEPA, 2016a).
The protection goal for an ERA involving a listed species is to avoid jeopardizing the continued existence of the species while avoiding excessive, adverse economic impact (NRC, 2013). Jeopardy occurs when an action directly or indirectly diminishes a species' abundance. Protection goals for listed species are straight forward and may be at the individual level for critically endangered species or at the population level where compensatory mechanisms may reduce the impacts of individual-level effects. Where listed species protection goals are at the population level, it must be demonstrated that impacts will not appreciably reduce the survival and recovery of the species. Assessment endpoints for listed species may include any evidence that the contaminant will result in harming an individual, including altering behavior that may affect foraging, sheltering, and breeding success. Protection goals for listed species extend to all locations of its critical habitat, even if individuals are not currently observed in a specific critical habitat. Since the goal of the ESA is to de-list the species, protecting its critical habitat even in the absence of individuals is essential to secure the potential for recovery. Under this protection goal, ERAs need to ensure that critical habitat maintains the biological integrity required for individuals of listed species to sustain or increase their populations or recolonize their critical habitat.
Protection goals for ES-based ERAs are to sustain the natural resources that provide ES. These goals are beneficiary-specific, habitat-specific, and require knowledge of the relevant stakeholder groups as well as an understanding of the ES provided and how they may be impacted by chemical stressors (Maltby et al., 2017). Nienstedt et al. (2012) provides a conceptual framework for identifying protection goals for ERAs using ES that involves: 1) listing ES of the habitat, 2) identifying which ES could be affected by chemical contaminants, 3) identifying the key drivers of those ES, and 4) developing protection goals for each key driver. There also have been calls for participatory stakeholder engagement to be part of the identification process (Munns Jr. et al., 2015). Generic ES assessment endpoints have been recommended by the US EPA Risk Assessment Forum and include quantitative metrics such as mass of wood produced, amount of food produced, volume of ground water recharged, and extent of crops pollinated, to name a few (Munns Jr. et al., 2015; USEPA, 2016a). These endpoints are more difficult to assess using data typically available to risk assessors (e.g., molecular initiating events, organism-level laboratory responses) and require models to translate available data into production functions. Such a process requires linking models across various levels of biological organization from molecular, to individuals, to populations, to SPUs, and finally to quantitate changes in the SPU to changes in the ES (Forbes and Galic, 2016; Galic et al., 2012). An ES approach to setting protection goals is one that is highly anthropocentric and utilitarian (Maltby et al., 2017). On its own this could put assessment and protection of ES in conflict with assessment and protection of listed species. However, the purpose of the approach outlined here is to identify, incorporate, and demonstrate ES thinking into a community-level ERA approach that will strengthen the protection goals while articulating benefits that might not otherwise be considered (Munns Jr. et al., 2015). Because of this, ES is a useful concept for facilitating communication between risk assessors, risk managers, and stakeholder groups and can be used to explore additional benefits that accrue alongside protection of listed species.
We propose that community-level protection goals can be developed that avoid jeopardizing listed species while maintaining the natural resources that support ES. These goals require the identification of a protection community (Section 3) along with an initial review of co-occurrence, exposure, and effects information (Section 4). Interactions among members of the protection community are depicted in conceptual diagrams to identify dependent relationships among community members. A WoE approach uses information obtained on co-occurrence, exposure, effects, and community dynamics to reduce the protection community down to one or two focal species for which more intensive data analysis can be performed to establish hazard levels or mitigation goals (Fig. 1).
3. Identifying the protection community
For California vernal pools and Carolina bays, we identified all listed species that have been documented to either use the habitat or rely on it as a critical habitat through review of literature (e.g., Tiner et al., 2002) and habitat conservation plans (USFWS, 2005). All species that were identified from this search as protected under federal or state law, as well as those identified as special concern, were confirmed using the USFWS and respective state conservation agency websites (Supplemental Information A). In a second approach to identify and verify listed species for each wetland type, state conservation websites were reviewed for species that were likely to occur in the system based on life history (e.g., amphibian life cycle) and habitat usage. For every species identified, a literature review was performed to determine if there was documented evidence of occurrence in the respective wetland. All federal and state listed species for which California vernal pools or Carolina bays is considered critical habitat were identified, as well as any whose geographic range obtained from FWS service websites over-lapped with the respective wetland distribution if the species could use the habitat for life support. For example, listed wading birds that may use the wetland for food but do not solely rely on it were included. Supplemental Information A provides the full list of protected species and species of concern for California vernal pools and Carolina bays.
The ES provided were identified for each wetland type. To properly identify the relevant ES for a community-based risk assessment approach, two aspects of ES were considered: the benefits or services that are being produced by the wetland and the stakeholder groups that are present or otherwise interacting with the wetland in order to receive those benefits. To address the first aspect, we started with the ES identified by Maltby et al. (2017) followed by a literature review for services by wetland type. In many cases, direct reference to an ES was completely lacking, in which case internet searches were performed to identify the potential for a service to be provided for each of the isolated wetlands. The Final Ecosystem Goods and Services Classification System (FEGS-CS) was then used to find likely environmental attributes of concern for each beneficiary type (Landers and Nahlik,2013). The combination of these attributes and the habitat's service potential was used to identify the SPUs to the closest taxonomic level possible. In some cases, ES and/or SPUs were based on best professional judgement. This process is presented in Box 1. The list of ES provided by California vernal pools and Carolina bays is presented in Table 1 and their SPUs and associated references are provided in Supplemental Information B. The list of ES in Table 1 should be considered a starting point and should be further refined with the scope of each assessment and regionally-specific knowledge and the stakeholder groups.
Box 1. Process for identifying Service Providing Units (SPUs).
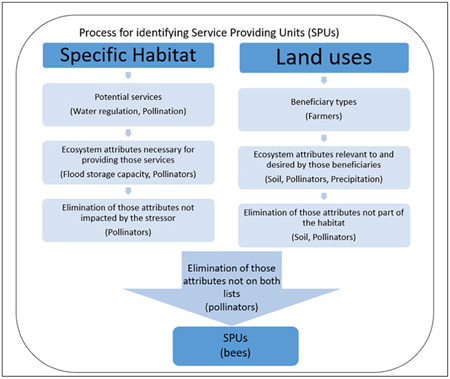
Table 1.
Summary of Ecosystem Services (ES) for Carolina Bays and California vernal pools.
| ES type | Ecosystem service | Carolina bays |
California vernal pools |
|---|---|---|---|
| Provisioning | Food | X | X |
| Fiber | X | ||
| Genetic resources | X | X | |
| Biochemical/natural medicines | X | ||
| Ornamental resources | X | ||
| Fresh water | |||
| Regulatory | Pollination | X | X |
| Pest and disease regulation | X | ||
| Climate regulation/carbon sequestration | X | ||
| Air quality regulation | |||
| Water regulation/flood water storage | X | X | |
| Water reservoir | X | ||
| Erosion regulation | |||
| Natural hazard regulation | |||
| Water purification/soil remediation/waste treatment | X | X | |
| Cultural | Spiritual and religious values | ||
| Education and inspiration | X | X | |
| Recreation and ecotourism | X | ||
| Cultural diversity and heritage | X | X | |
| Aesthetic values | X | X | |
| Supporting | Primary production | X | |
| Soil retention | X | ||
| Nutrient cycling | X | X |
For each case study, the interactions among members of the protection community are depicted in conceptual diagrams to identify dependent relationships among community members (Section 5). Visualization of the protection community dynamic is important to identify species or traits-based groups that provide critical ecosystem functions that will inform the lines of evidence described in Section 4.4. We do not prescribe a rigid structure for the conceptual diagrams, as noted by two different approaches used in the case studies in Section 5; however, diagrams should represent elements that are important to the habitats and aid in communication to stakeholders.
4. Data analysis
Data limitations are the principal source of uncertainty in all ERAs, particularly for listed species, and gaps between available metrics and their linkage to ES is a noted challenge (Forbes et al., 2017). The analysis phase of an ERA is typically separated into the exposure and effects analyses, which are integrated and evaluated during risk characterization as the co-occurrence of contaminant and receptor (USEPA, 1992). In our process, the potential for co-occurrence should be established prior to data-driven exposure and effects analyses to identify focal species, as described below in Section 5. Given the ephemeral nature and limited geospatial information on isolated wetlands, approaches for determining co-occurrence with chemical contaminants is discussed here as the first-tier analysis. This is followed by approaches for estimating exposure, modeling potential effects (including evaluation of chemical mode of action), and applying weight of evidence to achieve the protection goals of isolated wetlands exposed to various contaminants.
4.1. Co-occurrence of protection community and contaminant of concern
The co-occurrence of chemical contaminants and members of the protection community needs to be considered both spatially and temporally. For isolated wetlands, spatial co-occurrence is determined by the proximity of the wetland and sources of contaminants and must consider runoff and atmospheric drift potential. Spatial co-occurrence should be determined within the study area by first establishing as precise a study location as possible, then searching for GIS data on isolated wetlands in that area. The US Fish and Wildlife Service hosts the National Wetlands Inventory (NWI) dataset which provides wetland locations since the mid-1970s to the public and decision-makers, (https://www.fws.gov/wetlands); however, not all isolated wetlands are represented in the NWI. The NWI's minimum mapping unit varies among regions from 0.2 to 1.4 ha which excludes many small isolated wetlands. Photointerpretation of wetlands for the NWI is confounded by leaf cover in spring-summer photographs and is generally more difficult for forested wetland types (Tiner, 1997). Since 2010, the NWI Plus has improved functional assessment information for wetlands by incorporating descriptors such as water flow path and waterbody type to allow wetland position within the landscape and watershed to be better understood (Tiner, 2010). Isolated wetlands in certain areas of the US have been analyzed in greater depth using remote sensing techniques and GIS resources, resulting in higher estimates of wetlands compared to the NWI alone (Vance, 2009; Lane and D'Amico, 2016).
Temporal co-occurrence of contaminants and ecological receptors depends on the application, fate, and transport of the contaminant as well as the life history and behavior (e.g., migration) of the receptor. This may be evaluated prior to exposure modeling and used to prioritize exposure modeling scenarios. For example, the timing of pesticide exposure with bird nesting is critical in understanding long-term impacts to bird populations (Bennett and Etterson, 2009). Temporal co-occurrence is especially challenging with isolated wetlands because their seasonal changes in hydrology result in different ecological communities being present at different times of the year. For example, cycles of pooling and drying of ephemeral wetlands are based primarily on the precipitation regime of the region, though groundwater exchange as well as runoff can contribute to water levels in some areas (Brooks, 2005). As such, temporal co-occurrence of the habitat and chemical contaminants needs to consider the life cycle and migratory behavior of resident and non-resident species in the protection community as they correspond to various stages of inundation.
4.2. Estimating chemical exposure
Exposure pathways are dependent on the physiochemical properties of the contaminant as well as the environmental factors of the region within which the habitat is located. For isolated wetlands, the principal sources of hydrology are precipitation and runoff, although there can sometimes be groundwater contributions (Brooks, 2004; Zedler, 2003; Rains et al., 2006; Pyzoha et al., 2008). Runoff and erosion can redistribute contaminants deposited into isolated wetlands from other pathways, including near-term spray drift, longer-range atmospheric transport, and aquatic point sources. Receptors may be exposed to contaminants through relevant media (aqueous and sediment) and food web exposures. Estimated Exposure Concentrations (EECs) are derived via modeling and/or monitoring. Because understanding exposure in isolated wetlands is critical to identifying spatial and temporal co-occurrence, we discuss various approaches that may be used in this context below. Not all models presented below are cited in the following case studies.
Monitoring data are advantageous because they provide a direct measurement of the concentration in the media at a particular time and location, but may not consistently be available at many sites or may not have been collected during times of peak field concentrations. Monitoring data are limited and do not typically provide relevant temporal and spatial resolution that conform to robust statistical designs. However, databases such as the USEPA's Toxic Release Inventory (TRI; USEPA, 2018a; https://www.epa.gov/toxics-release-inventory-tri-program), USEPA's National Wetland Condition Assessment (NWCA; USEPA, 2018b; https://www.epa.gov/national-aquatic-resource-surveys/nwca), USEPA's Water Quality Data (WQX; https://www.epa.gov/waterdata/water-quality-data-wqx), USGS National Water Quality Assessment Program (NAWQA; https://cida.usgs.gov/nawqa_www/nawqa_data_redirect.html?p=nawqa), and the California Pesticide Use Reporting (PUR; https://www.cdpr.ca.gov/docs/pur/purmain.htm) may provide substantive information to assist in developing EECs. Sediment EECs are more static over time and are more likely to be directly estimated from monitoring data.
Models are often used to develop EECs for isolated wetlands regardless of whether field data are available due to their ability to estimate concentrations at times and places where field data are lacking. Ground-water linkages are key when considering exposure models for hydrologic transport of chemicals entering an isolated wetland. If there is little or no groundwater exchange, a model which includes runoff, sediment transport via erosion, and shallow subsurface and/or simplified groundwater fluxes is typically sufficient. Two popular semi-distributed watershed hydrologic and water quality models have been modified for use with isolated wetlands: The Soil and Water Assessment Tool (SWAT; Neitsch et al., 2005) and the Hydrological Simulation Program - FORTRAN (HSPF; Bicknell et al., 2001). In addition to hydrological models, more simplified aquatic fate and transport models can be applied to isolated wetlands when hydrologic data is scarce (e.g., Pesticide in Water Calculator [PWC]; Young, 2016). A groundwater flow model such as the US Geological Survey's MODFLOW (McDonald and Harbaugh, 1984) may also be applied where groundwater plays an important role in habitat hydrology. Groundwater models such as MODFLOW can be coupled with surface watershed models such as SWAT to simulate interactions between upland/surface flows and groundwater flow. However, coupled surface water-groundwater models are challenging to parameterize and typically require extensive field data, which may be a key limitation for use on isolated wetlands (Golden et al., 2014).
Pesticide drift processes for may occur over relatively short distances (e.g., 1000s of feet from application site) or as long-range transport via atmospheric drift. Shorter range drift can be modeled using AgDRIFT (Teske et al., 2002; Bird et al., 2002) and Agricultural DISPersal (AgDISP; Bilanin et al., 1989). Risk assessments that require longer-range deposition estimates suffer parameterization problems as they scale, and therefore typically rely on monitoring data for assessing long-range transport potential or relatively simple box models (Scheringer, 2015) approaches to capture transport. Models such as Globo-POP (Wania and Mackay, 1999), CliMoChem (Scheringer et al., 2004) and BETR-Global (MacLeod et al., 2005) have been developed for relating global organic chemical loadings to concentrations in relevant media and can be a point of departure for contaminant loading in isolated wetlands.
Food web models vary by habitat type and receptors of interest, and may be limited to the direct use of bioaccumulation factors to estimate the transfer of contaminants through components. There are many available models that predict EECs based on terrestrial (Armitage and Gobas, 2007; Kelly et al., 2007; Gobas et al., 2016) and aquatic (Mackay and Fraser, 2000; Arnot and Gobas, 2006) bioaccumulation principles. The primary consideration when tailoring these models for use in specific ecosystems is the selection of appropriate species. Aquatic bioaccumulation models are generally well-accepted and typically based on bioconcentration factors (BCFs), bioaccumulation factors (BAFs), and/or octanol-water partition coefficients (Kows), all of which are measures of partitioning between biota and water. The Comprehensive Aquatic Systems Model (CASM) (DeAngelis et al., 1989; Bartell et al., 1999) is a mass-balance aquatic food web modeling platform that has been used to identify the potential exposure for a variety of aquatic ecosystems. AQUATOX can predict the fate of organic chemicals and designate multiple trophic levels to simulate complex food webs. It simulates multiple environmental stressors in addition to chemicals (e.g., nutrients, temperature) and handles algal, macrophyte, invertebrate, and fish communities (USEPA, 2014; Yang et al., 2012). The Three-phase Ecological Food Web Analysis (TEFWA) (Preziosi and Pastorok, 2008) guides users through a process to construct a food web for risk assessment purposes, which may allow for a customized implementation that accounts for receptors and interactions within isolated wetlands. Attempts to transfer these aquatic-derived approaches to terrestrial models has been shown to be problematic as many chemicals have different bioaccumulative properties in aquatic versus air-breathing organisms.
4.3. Effects characterization
Limited effects data are confounding in ERAs, particularly for listed species and critical habitat assessments. The unique species composition of isolated wetlands requires the application of effects modeling and approaches beyond the use of surrogate species test data for protecting both listed species and ES. Common surrogate species (e.g., rainbow trout, fathead minnow, bobwhite quail, common rat) do not naturally occupy isolated wetlands, sufficiently relate to the benefits provided by ES, or adequately represent the species adapted to the unique features of these habitats that comprise their protection communities (Supplemental Information A and B). Additionally, there are significant gaps in the methods available to estimate effects from various exposure routes, such as dermal exposure for wading birds and other vertebrates that submerge in aquatic habitats. Regardless, effects data collection for community protection goals will follow the same processes used for other ERAs: 1) collection and evaluation of the most reliable available data for all possible ecological receptors (including incidence reports), 2) understanding the Adverse Outcome Pathway (AOP; Ankley et al., 2010) of the contaminant to the extent that it is available, 3) application of models to evaluate susceptibility and sensitivity of untested taxa (e.g., Lalone et al., 2013; Raimondo et al., 2010; USEPA, 2014; USEPA, 2017), and 4) development of Species Sensitivity Distributions (SSDs) from measured or extrapolated effects data.
In addition to these steps, our approach employs traits that influence susceptibility for each species in the protection community. Traits are characteristics that influence a population and may be at the individual (e.g., diet, body size), the population (e.g., life span, reproduction strategy), or landscape levels (e.g., dependency on hydroperiod) (van den Brink et al., 2013). Traits may be taxa-specific (e.g., bimodal life history of amphibians) or be shared across broad taxonomic groups (e.g., insectivorous diet). For their application here, we focus on traits that influence the vulnerability of each species relative to its potential co-occurrence with chemical stressors, inherent sensitivity, and potential routes of exposure. We use traits to aggregate species within the protection community into traits-based species groups that will be used in the weight of evidence approach described below. These traits will be specific to each ERA as they will be identified by relevant factors defined in the problem formulation stage of each assessment (e.g., type of contaminant, source of contaminant). The development of traits-based species groups is demonstrated in the case studies.
4.4. Weight of evidence
A well-designed WoE approach justifies decisions and clearly communicates findings to a wide range of audiences while increasing confidence in, and defensibility of, the ERA (Rhomberg et al., 2013; USEPA, 2016b). WoE approaches typically include three elements: 1) identifying and assembling relevant evidence as discussed in the previous exposure and effects sections, 2) evaluating the strength, reliability, and relevance of the available evidence through the most objective and repeatable process possible, and 3) integrating and interpreting that evidence (USEPA, 2016b). Here, a WoE approach is used to identify the species or group of species in the protection community that will be either most susceptible to impacts from contaminant exposure and/or otherwise serves as a critical species in the dynamic of the community and the contaminant (referred to here as focal species). The WoE pro-cess at this step will demonstrate, based on the evidence collected on exposure routes, species susceptibility, and community dynamics, that protecting the focal species will protect the remaining community members (Lambeck, 1997). The WoE approach is traditionally used in ERA to determine data acceptability and integration for risk characterization and has been described thoroughly elsewhere (USEPA, 2016b). Here, we will demonstration an additional application of WoE to identify the focal species of the protection community for which protection goals should be targeted.
WoE approaches are used to evaluate the support of a set of alternative causal hypotheses (USEPA, 2016b). In a typical ERA, the alternative hypotheses may be H0: the stressor is not likely to affect the species of concern and H1: the stressor is likely to affect the species of concern. When applying the WoE in this critical habitat approach, there is a much wider set of alternative hypotheses (e.g., H1: the stressor is likely to affect listed species A; H2: the stressor is likely to affect listed species B; H3: the stressor is likely to affect SPU species C; etc.) encompassing the stressor's potential to cause an effect on any species of concern and the null hypothesis would be correspondingly narrower, e.g., H0: the stressor is not likely to affect any of the species of concern in the habitat. Our approach allows regulators to avoid spending time on those hypotheses supported by weak or conflicting evidence and base their decisions on those hypotheses that are most strongly supported. In this process, the collective hypotheses pose the question: Which species in the protection community will preserve the biological integrity of the habitat such that no likely adverse effects will occur for the remaining species? To identify these species, we consider the lines of evidence listed in Box 2. These lines of evidence should be considered for both the parent contaminant as well as its metabolites and/or degradation products or separate toxic components (e.g., mixtures).
Box 2. Lines of evidence to identify focal species:
Temporally co-occurrences with the contaminant
Exposure pathway present for species uptake
Susceptible to the chemical mode of action
Demonstrated sensitivity of taxa to chemical
Traits that increase susceptibility
Traits that provide a critical ecosystem function
Provides habitat for other species in the protection community
Provides food for other species in the protection community
Indirect effects via food web
Vulnerable to additional non-chemical stressors (e.g., bacteria/viral prevalence; excluding loss of habitat).
5. Case studies
5.1. Chlorpyrifos in California vernal pools
This case study focuses on chlorpyrifos exposure to vernal pools located in the California Central Valley. Chlorpyrifos is an organophosphate insecticide applied in the Central Valley to a wide variety of food crops, forage fodder crops, tree nuts, field crops, and fruits. Chlorpyrifos is applied directly to sites as part of integrated pest management strategies and is relied on to control alfalfa weevil, ants, plant bugs, aphids, and whiteflies on alfalfa, almonds, citrus, and cotton (CDPR, 2014). The California Department of Pesticide Regulation (CDPR) reported over 40,000 lbs. of chlorpyrifos use in the Central Valley in 2016, with the peak application period occurring between May and August (CDPR, 2018). In 2017, the USEPA submitted a national-scale biological evaluation (BE) for chlorpyrifos and determined that most listed species and critical habitats, respectively, are likely to be adversely affected by its application based on the action area, its potential distribution, and toxicity (USEPA, 2016c). This case study builds off that BE to demonstrate the application of the community-level process described here for California vernal pools.
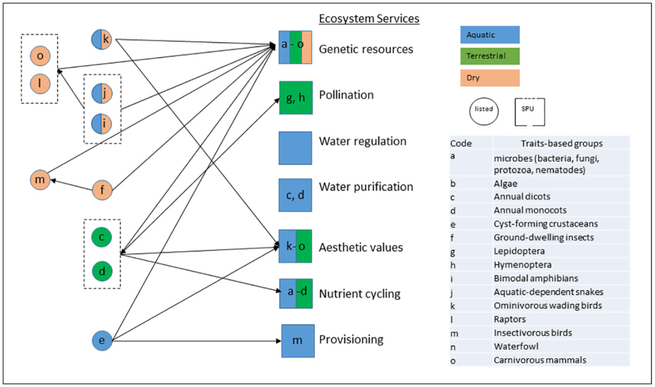
California vernal pools are characterized by a wet phase, when soils become saturated from rain and/or snowmelt and the pool becomes hydrated (November–April), a terrestrial or flowering phase, during which the pool begins drying and endemic, flowering plants emerge (April–May), and a dry phase, during which egg, cyst, spore, and seed life stages occur (May–November) (USFWS, 2005). The geomorphology of vernal pools makes them insular where they occur, with sharply defined boundaries marked by unique, endemic vegetation that distinctly separates them from surrounding areas (Holland and Jain, 1981). The protection community for these wetlands is depicted in Fig. 2, with species and taxa-specific information for listed species and SPUs provided in Supplemental Information A and B, respectively. As demonstrated in Fig. 2, the species or taxa within the protection community interact with each other, although many species are active only during one phase of the vernal pool. The protection goals used for this case study are to maintain the biological integrity of the system such that there are no likely adverse effects to listed species or SPUs.
Fig. 2.
Protection community of California vernal pools showing the three phases of the wetland. Traits-based groups are depicted for listed species and Service Providing Unites (SPU) for various ecosystem services. Arrows indicate where members of the traits-based groups influence other groups within the protection community.
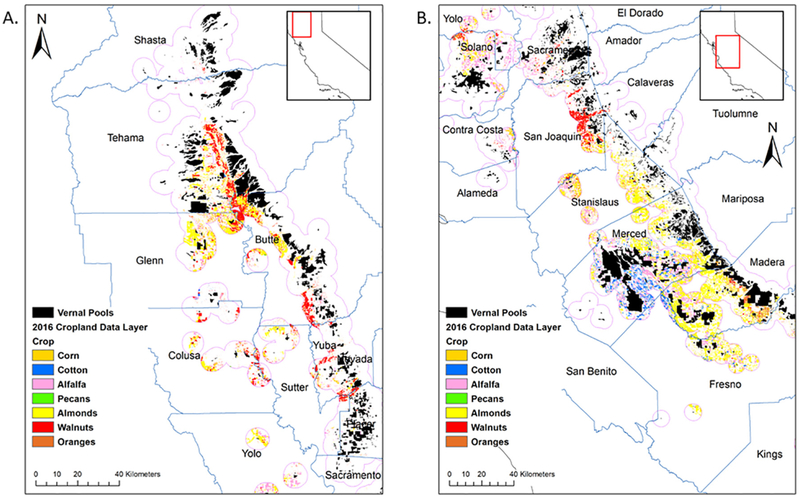
We used the Central Valley vernal pool geospatial data and California's Pesticide Usage Reporting (PUR) Database (CDPR, 2018) to determine the spatial co-occurrence of chlorpyrifos application near vernal pools. The vernal pool data was developed by a location shapefile (http://www.vernalpools.org, accessed March 13, 2017, Witham et al., 2014) that was buffered to 5 km in ArcMap v10.4. By employing a 5 km buffer around vernal pool habitats, this screening analysis demonstrates potential exposure of vernal pool species on a conservative spatial scale given long-range environmental transport of chlorpyrifos (Mackay et al., 2014). The 2016 PUR data for chlorpyrifos, which included application to agricultural lands and non-agricultural areas by commercial appliers, was summed by Section unit of COMTRS (County-Meridian-Township-Range-Section) and then joined by Section to Public Land Survey System COMTRS shapefile (https://www.cdpr.ca.gov/docs/emon/grndwtr/gis_shapefiles.htm, accessed November 18, 2016). The chlorpyrifos-by-COMTRS shapefile was extracted by the 5 km vernal pool buffer using Arcmap “Clip” tool. Almost 6000 pounds of chloropyrifos was applied to crops within 5 km of any vernal pool feature. The 2016 US Department of Agriculture's Cropland Data Layer or CDL (https://www.nass.usda.gov/Statistics_by_State/index.php, accessed July 17, 2018) was also extracted by the 5 km vernal pool buffer. When mapped with vernal pool networks, crops that are labeled for chlorpyrifos application are closest to pools in Tehama, Butte and Yuba counties in Northern California, and pools in San Joaquin, Stanislaus, Merced and Madera counties in central California (Fig. 3). This analysis did not account for errors in the CDL.
Fig. 3.
Buffer analysis demonstrating co-occurrence of vernal pools and crops for which chlorpyrifos is registered for application. A. Northern California. B. Central California.
Chlorpyrifos EECs were calculated to determine if there was potential for any estimated exposure of chlorpyrifos to vernal pools to evaluate spatial and temporal co-occurrence. The EECs were estimated using the Pesticide in Water Calculator (PWC) for vernal pools in Merced County, CA (Sinnathamby et al., In review). In brief, EECs were simulated by parameterizing PWC (version 1.59) for three agricultural vernal pool watersheds located in the San Joaquin River basin in the Central Valley of California. Among watersheds, simulated EECs varied with crop type, crop acreage, soil, application rate, application method etc. Simulated highest daily averages maximum EECs of chlorpyrifos were 13.06 and 6.79 ppb with levels that exceeded the USEPA benchmark for chlorpyrifos (0.04 ppb; USEPA, 2016c) in for 93 and 94% of days when applied to dormant almonds and grains (oats, winter wheat and barley), respectively. Maximum EECs were obtained when chlorpyrifos was applied during the dormant season (in almonds) or as a pre-planting soil treatment (in grains). The application methods during these crop scenarios were inferred using section-level application dates (CDPR, 2018) and are described in detail in Sinnathamby et al. (In review).
Chlorpyrifos effects were determined through evaluation of the AOP for acetylcholinesterase (AChE) inhibitors, evaluation of species susceptibility, and measured data. The molecular target of chlorpyrifos is AChE, which is present in vertebrate and invertebrate animal species. In the mechanism of action, ACh is blocked from binding to and being degraded by AChE, which results in unregulated excitation at neuromuscular junctions that cause neurotoxic symptoms (e.g., increased respiration, seizures, bradycardia) that can lead to mortality (Russom et al., 2014). Predictions of species susceptibility using Sequence Alignment to Predict Across Species Susceptibility (SeqAPASS) showed widespread susceptibility across the animal kingdom, while plants, fungi, and micro-organisms (e.g., protozoan) were less susceptible (Doering et al., 2018).
The species within the protection community were grouped into traits-based species groups based on broad taxonomic categorization, knowledge of the AOP and predicted species susceptibility, and potential routes of exposure. Listed species and SPUs may be included in the same traits-based group, as was done for plant groups and Lepidoptera in this example (Table 2). For the initial assessment of species susceptibility and sensitivity used in the WoE approach to identify focal species, we used the effects data obtained from the USEPA biological evaluation for chlorpyrifos and recent studies on adverse outcome pathways of AChE inhibitors (Doering et al., 2018; Russom et al., 2014; USEPA, 2016c).
Table 2.
Traits-based species groups for California vernal pools exposed to chlorpyrifos. Numbers correspond to lines of evidence listed in Box 2. The “+” symbols associated with each line of evidence indicate where there is strong0 (+++), moderate (++), or weak (+) association for each traits-based group. A “0” indicates where no information is available.
| |
Line of evidence |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Traits-based species groups | Number of listed species |
SPUs (yes/no) |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Various | microbes (bacteria, fungi, protozoa, nematodes) | 0 | Yes | ++ | +++ | + | 0 | 0 | +++ | 0 | +++ | 0 | 0 |
| Nonvascular plants | Algae | 0 | Yes | ++ | +++ | + | + | 0 | +++ | 0 | +++ | 0 | 0 |
| Vascular plants | Annual dicots | 5 | Yes | +++ | +++ | ++ | + | 0 | +++ | ++ | 0 | 0 | 0 |
| Annual monocots | 8 | Yes | +++ | +++ | ++ | + | 0 | +++ | ++ | 0 | 0 | 0 | |
| Crustacean | Cyst-forming crustaceans | 4 | Yes | +++ | +++ | +++ | +++ | 0 | 0 | 0 | +++ | 0 | 0 |
| Insects | Ground-dwelling insects | 1 | No | +++ | ++ | +++ | +++ | +++ | 0 | 0 | 0 | 0 | 0 |
| Lepidoptera | 0 | Yes | +++ | ++ | +++ | +++ | +++ | +++ | 0 | 0 | +++ | 0 | |
| Hymenoptera | 0 | Yes | +++ | ++ | +++ | +++ | +++ | +++ | 0 | 0 | 0 | +++ | |
| Amphibians | Bimodal amphibians | 1 | Yes | ++ | ++ | +++ | ++ | ++ | 0 | 0 | ++ | 0 | +++ |
| Reptiles | Aquatic-dependent snakes | 1 | No | ++ | + | +++ | ++ | + | 0 | 0 | + | 0 | 0 |
| Birds | Omnivorous wading birds | 1 | No | ++ | ++ | +++ | ++ | + | 0 | 0 | 0 | +++ | 0 |
| Raptors | 2 | No | +++ | 0 | +++ | 0 | + | 0 | 0 | 0 | + | 0 | |
| Insectivorous birds | 2 | No | +++ | ++ | +++ | +++ | + | 0 | 0 | 0 | +++ | 0 | |
| Waterfowl | 0 | Yes | ++ | + | +++ | ++ | + | 0 | 0 | 0 | + | 0 | |
| Mammals | Carnivorous mammals | 1 | No | +++ | 0 | +++ | ++ | + | 0 | 0 | 0 | + | 0 |
The weights for each line of evidence for each traits-based species group are presented in Table 2. Weights were assigned as high (+++), medium (++), low (+), or no evidence available (0). Since there is the potential for chlorpyrifos to be applied in the Central Valley year-round, all species had a medium to high weight for temporal co-occurrence, with a higher weight given to those species that are present during the peak application period (May–August). Higher weights were assigned for exposure pathways to species occupying the vernal pools full time (e.g., algae, cyst forming crustaceans). Since chlorpyrifos has low bioaccumulation potential, lower weights were applied to species that might encounter chlorpyrifos only via food web exposure routes and a weight of zero was applied to higher, opportunistic trophic groups that do not use vernal pools as habitat and do not rely on vernal pool species for food. Susceptibility to chemical mode of action were based on Doering et al. (2018). Demonstrated sensitivity was based on the availability and value of toxicity effects data available from USEPA (2016c), with higher weights given to relatively sensitive organisms in their respective habitats (aquatic, terrestrial) and no weight given to taxa where no toxicity data were available. Traits that increase susceptibility included permeable skin (e.g., amphibians) or behavior that increases potential for exposure (e.g., pollinators visiting multiple plants on which potential drift may occur). Traits that provide critical ecosystem function include primary production, nutrient cycling, and pollination. Lines of evidence for providing habitat and food for other members of the protection community were based on community dynamics of vernal pools (Zedler, 1987; USFWS, 2005) and do not include other species outside of the protection community. Indirect effects via food web consider whether a species food source, regardless of its inclusion in the protection community (e.g., insectivorous birds) would be impacted. Lastly, high weights were assigned to Hymenoptera (e.g., honey bees) and amphibians for the line of evidence for vulnerability to additional non-chemical stressors, due to their susceptibility to varroa mites and chytrid fungus, respectively.
Based on the weights assigned to the lines of evidence, Lepidoptera and Hymenoptera represented the focal taxa for the terrestrial and dry phase species; cyst-forming crustaceans were the focal species for aquatic phase species. Although bimodal amphibians could also be included as a focal species for the aquatic phase based on the WoE, we identified cyst-forming crustaceans as the focal species because available toxicity data demonstrate that they are more sensitive than amphibians (USEPA, 2016c), and a focus on this group will provide protection for amphibians as well. Following identification of the focal species, exposure and effects data would be integrated for these species to characterize risk to the community. As an example, USEPA (2016c) contained three acute mortality data points for cyst-forming crustaceans in the families Thamnocephalidae (Thamnocephalus platyurus; LC50 = 0.52 μg/L) and Streptocephalidae (3.48 and 8.25 μg/L). Because no data were available for any of the focal species families (Branchinectidae, Triopsidae; Supplemental Information A), the Web-based Interspecies Correlation Estimation (Web-ICE; https://www3.epa.gov/ceampubl/fchain/webice, accessed 7/27/2018; Raimondo et al., 2015) was used to estimate sensitivity to the family Branchinectidae (1.29 μg/L, confidence interval 0.227–7.40 μg/L) using the value obtained for Thamnocephalus platyurus reference above as a surrogate. Web-ICE contains log-linear regressions developed from existing data that predict an acute value of an untested species from a surrogate (Raimondo et al., 2010). A single data point was available for a butterfly in the family Nymphalidae (0.25 kg/ha) while several studies reported toxicity to bees at levels orders of magnitude lower than that reported for the butterfly (0.05 kg/ha; USEPA, 2016c). Since the measured effects endpoints for the focal species are less than the maximum EECs modeled, an additional WoE approach may be applied to the available data for bees and cyst-forming crustaceans to evaluate data reliability and robustness to complete the risk characterization of this case study. As this case study does not represent a final risk assessment of chlorpyrifos in vernal pool communities, final uncertainty analysis of exposure and effects information used here is not within the scope of this example.
5.2. Coal ash in Carolina bays
This case study focuses on coal ash deposited into Carolina bays in the southeast US (USEPA Region 4). Coal combustion is one of the principle processes used to produce energy in the US and produces approximately 110 million tons of residuals annually (Harkness et al., 2016; Rowe et al., 2002). Coal ash is the primary constituent of coal combustion residues; it is deposited into surface impoundments and landfills, is highly toxic to plants and animals, and leaches into ground water from disposal sites (Harkness et al., 2016; Rowe et al., 2002). The principle contaminants in coal ash are heavy metals, which readily bind to sediment and are toxic to both plants and animals (Lance et al., 2012; Rowe et al., 2002). A historical precedent has been established using isolated wetlands, including Carolina bays, as settling basins to remove contaminants from effluent (Lance et al., 2012). The use of isolated wetlands for coal ash water filtration and the associated environmental impacts and remediation is well documented for the Savannah River Site (SRS) in Aiken, SC (Rowe et al., 2002). This case study utilizes data assembled from SRS and represents a scenario in which the contaminant is introduced into the environment to obtain an ES (water purification via adsorption to sediment and uptake by biota) that is contrary to the protection of vulnerable populations. In scenarios such as these, assessments will inform management of effluent treatment to minimize impacts to focal species prior to establishment, as well as remediation of legacy sites that are no longer able to support diverse communities.
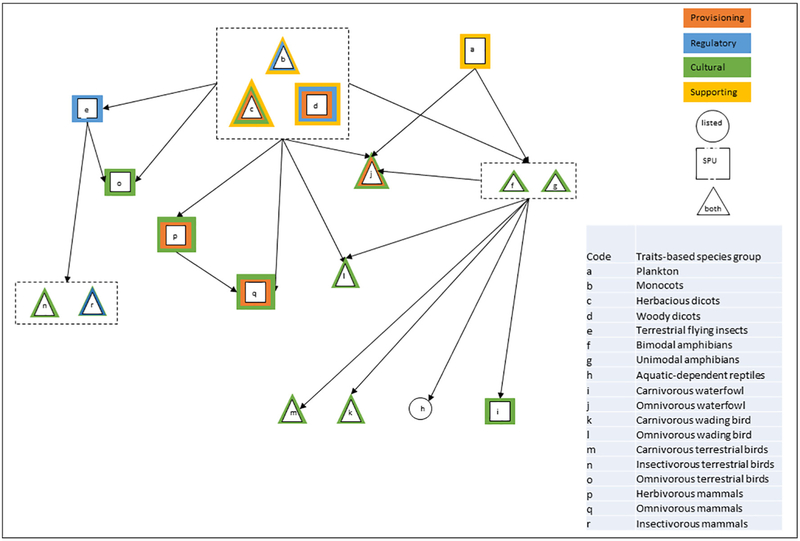
Carolina bays are elliptical depressions located from New Jersey to northern Florida with distinct geologic boundaries separating them from surrounding uplands. Many Carolina bays are ephemeral, with inundation and water levels dependent on rainfall and evapotranspiration rates, resulting in a community of diverse species that are well adapted to the habitat and its surrounding ecotome (Sharitz, 2003). Due to their size and diversity, Carolina bays support a large number of listed species and SPUs. The protection community and the interactions among species is depicted in Fig. 4, with species and taxa-specific information for listed species and SPUs provided in Supplemental Information A and B, respectively. In this case study, protection goals are to determine a threshold by which mitigation efforts may be targeted to increase the biological integrity of the system to support vulnerable populations.
Fig. 4.
Protection community for Carolina Bays depicting relationships between Service Providing Unites (SPU) for various ecosystem services and listed species of traits-based groups. Arrows indicate where members of the traits-based groups influence other groups within the protection community.
Since coal ash is a point-source pollutant, determining the spatial co-occurrence of contaminant and Carolina bays is more straight forward than it is for non-point source contaminants. For example, on the SRS, coal ash both passively flows into Carolina bay systems (e.g., Dunbarton Bay; Lance et al., 2012) and is actively pumped into a series of settling ponds and wetlands prior to entering the Savannah River watershed (Rowe et al., 2002). In these cases, spatial co-occurrence is simplified to physical overlap of the coal plume and the Carolina bays.
Heavy metals have been measured in the surface water, sediment, and biota of SRS impoundments (Rowe et al., 2002). Twenty-one metals were identified as constituents of concern, with arsenic (As), barium (Ba), cadmium (Cd), chromium (Cr), copper (Cu), selenium (Se), lead (Pb), and mercury (Hg) identified as the most common contaminants in Carolina bay surface water and sediment (Lance et al., 2012; Rowe et al., 2002; RTI, 2007). Rowe et al. (2002) compiled a review of the ecotoxicological implications of coal ash combustion and reported the following relative occurrence data for As, Cd, Cr, Cu, Pb, and Se measured on SRS basins: As was found in the highest concentration in settling basin surface water, followed by Se and Cu; As and Cu were found in the highest concentrations in sediment, followed by Se and Cr; all were reported in overlapping ranges in plant tissue; all were reported in animals, with the majority of studies reporting on Se, which was typically in the highest concentration in animal tissue when other metals were detected. Levels of As and Cu in biota were also reported in relatively high concentrations in some studies.
While metals were measured in plant tissues from SRS sites, little is documented on potential adverse effects. Conversely, plants are noted for their tolerance to heavy metals with multiple lines of defense engaging prior to the synthesis of stress-related proteins and signaling molecules (Manara, 2012). To assess the relative sensitivity of animal taxa to the three metals that were reported in the highest concentrations in water, sediment, and biota (As, Cu, Se), the Web-based Interspecies Correlation Estimation (Web-ICE; https://www3.epa.gov/ceampubl/fchain/webice, accessed 8/07/18) application was used to either obtain a compilation of measured acute sensitivity or to predict acute sensitivity across a diversity of taxa. Web-ICE uses log-linear least square regressions developed from a database of existing acute toxicity to estimate sensitivity to aquatic organisms (EC/LC50) and wildlife (LC50) from the measured toxicity of surrogate species (Raimondo et al., 2007,2010). Where Web-ICE did not provide sufficient sensitivity information for a taxonomic group, we referred to Rowe et al. (2002) to complete WoE process below. Assessment of relative species sensitivity was performed separately for aquatic and terrestrial species.
For aquatic species sensitivity to Cu, the Web-ICE database contained geometric mean acute values for 87 species (Supplemental Information C Table C-1). Tested species did not represent those found in Carolina Bays, so surrogate species were used to represent general sensitivity of traits-based groups. The Web-ICE database contained toxicity data for 10 aquatic species exposed to As, which were used as surrogate species to predict toxicity to 58 additional species using the Species Sensitivity Distribution (SSD) module ofWeb-ICE (Supplemental Information C Table C-2). The Web-ICE database contained toxicity values for 12 aquatic species exposed to Se, which were used as surrogates to predict sensitivity to 49 additional species (Supplemental Information C Table C-3). For terrestrial animals, the Web-ICE database contained geometric mean acute sensitivity values for 5 bird species exposed to As, which were used to predict to 17 birds and 1 mammal species (Supplemental Information C Table C-4). The Web-ICE database contained acute toxicity for two birds and one mammal exposed to Cu, which were used to predict sensitivity of 21 birds and 6 mammals (Supplemental Information C Table C-5). The use of Web-ICE in this case study was for screening level toxicity assessment. A final risk assessment of coal ash in Carolina Bays would include additional uncertainty analysis of the final data used for effects determination, which is not within the scope of this example.
The species within the protection community were grouped into traits-based species groups based on broad taxonomic group, diet, and habitat type (Fig. 4). Plankton included all phytoplankton and zooplankton. Plants were separated as monocots, herbaceous dicots, and woody dicots and all assumed to grow within the inundation boundaries of the wetland. Arthropods were only represented by terrestrial flying insects. Both bimodal amphibians as well as fully aquatic (unimodal) amphibians were within the protection community. Only aquatic-dependent reptiles were included in the protection community for that taxa. Birds were separated by diet and habitat. Bird diets included insectivorous, omnivorous, and carnivorous (including piscivorous species), which includes birds that eat both vertebrate and invertebrate prey. Their habitat types were lumped as swimmers (e.g., waterfowl), waders (e.g., egrets), and terrestrial (e.g., birds that hunt aquatic prey but spend the rest of their time in terrestrial habitats. Mammals were all primarily terrestrial insectivores or herbivores that were associated, but not dependent, on Carolina bays. Because Carolina bays contain a diversity of ES that will be important for stakeholder communication, inclusion of these services is depicted in the conceptual diagram of the protection community (Fig. 4).
The weights for each line of evidence of the traits-based species groups are presented in Table 3. Temporal co-occurrence was weighed high (+++) for groups that lived or hunted in the water as their primary habitat. Medium weights (++) were given for omnivorous groups who entered the wetland temporarily to obtain food or those whose diets included, but were not limited to, species within the wetland. Low weights (+) for temporal co-occurrence were assigned to those group that opportunistically use the wetland. Exposure pathway was weighted high for species that lived in wetland water or sediment or consumed organisms living in the wetland, with weights decreasing for groups that only opportunistically used the wetland. A weight of zero was assigned to terrestrial insects and insectivores that are unlikely to eat from or come in direct contact with the wetland water or sediment. Based on documented uptake of heavy metals in all taxa and their bioaccumulation potential, all groups were weighed high for susceptibility. Demonstrated sensitivity of metals was weighted lower for plants than animals based on their ability to assimilate high levels of metals without adverse effects (Manara, 2012). The SSDs developed for acute sensitivity of aquatic organisms exposed to Cu, As, and Se showed molluscs, aquatic arthropods (crustaceans and aquatic insects), and plankton as the most sensitive traits-based groups for all three metals (Supplemental Information C Fig. C-1). Of these, only plankton is listed in the protection community, so that group was weighted high for demonstrated sensitivity. Amphibians were in the middle to upper range of the SSDs, so were assigned a medium weight. Rowe et al. (2002) reported no mortality for aquatic-dependent reptiles collected from SRS sites, so this group was weighted with a low sensitivity. Sensitivity data to the selected metals were only available for omnivorous waterfowl, omnivorous terrestrial birds, herbivorous mammals, and omnivorous mammals. Based on their relative position in the SSDs, omnivorous mammals and omnivorous waterfowl were weighted with a medium sensitivity while omnivorous terrestrial birds and herbivorous mammals were weighted with a high sensitivity (Supplemental Information C Fig. C-2). Other terrestrial traits-based groups were assumed to have similar relative sensitivity as other birds and mammals, so were assigned a medium weight for this line of evidence. Traits that were considered to increase susceptibility included living in the wetland for all or part of its lifecycle (e.g., amphibians) or carnivorous diets that relied on food items supplied by the wetland. Plants were assigned a negative weight for traits increasing susceptibility based on their adaptations that provide multiple lines of physiological defense against metal toxicity (Manara, 2012). Traits that provided critical ecosystem functions included providing food, habitat, and pollination for other members of the protection community. Weights were assigned for a groups potential to provide habitat and food for other species within the protection community based on the relationships depicted in Fig. 4. Since the contaminant of this case study has high bioaccumulation potential, all non-herbivorous animals had high weights for indirect effects via the foodweb, with medium weights assigned to omnivorous animals. Of the species within the protection community, the only ones that were assigned high weights for vulnerability to additional non-chemical stressors were amphibians, which are susceptible to chytrid fungus.
Table 3.
Traits-based species groups for Carolina Bays exposed to coal ash. Terrestrial birds include songbirds, woodpeckers, and kingfishers; Waterfowl include ducks and geese; Wading birds include wading and shorebirds. Numbers correspond to lines of evidence listed in Box 2. The “+” symbols associated with each line of evidence indicate where there is strong0 (+++), moderate (++), or weak (+) association for each traits-based group. A “0” indicates where no information is available.
| Line of evidence |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | Traits-based species groups | Number of listed species | SPUs (yes/no) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Various | Plankton | 0 | Yes | +++ | +++ | +++ | +++ | + | +++ | 0 | +++ | 0 | 0 |
| Vascular plants | Monocots | 14 | Yes | +++ | +++ | +++ | + | 0 | +++ | + | +++ | 0 | 0 |
| Herbaceous dicots | 21 | Yes | +++ | +++ | +++ | + | 0 | +++ | + | +++ | 0 | 0 | |
| Woody dicots | 2 | Yes | +++ | +++ | +++ | + | 0 | +++ | ++ | ++ | 0 | 0 | |
| Insects | Terrestrial flying insects | 0 | Yes | + | 0 | +++ | 0 | 0 | +++ | 0 | ++ | 0 | 0 |
| Amphibians | Bimodal amphibians | 6 | Yes | +++ | +++ | +++ | ++ | +++ | +++ | 0 | +++ | ++ | +++ |
| Unimodal amphibians | 1 | Yes | +++ | +++ | +++ | ++ | +++ | +++ | 0 | +++ | ++ | +++ | |
| Reptiles | Aquatic-dependent reptiles | 4 | No | +++ | +++ | +++ | + | +++ | + | 0 | + | +++ | 0 |
| Birds | Carnivorous waterfowl | 0 | Yes | +++ | +++ | +++ | ++ | +++ | + | 0 | 0 | +++ | 0 |
| Omnivorous waterfowl | 0 | Yes | +++ | +++ | +++ | ++ | +++ | + | 0 | 0 | ++ | 0 | |
| Carnivorous wading bird | 0 | Yes | +++ | +++ | +++ | ++ | +++ | + | 0 | 0 | +++ | 0 | |
| Omnivorous wading bird | 1 | Yes | +++ | +++ | +++ | ++ | +++ | + | 0 | 0 | ++ | 0 | |
| Carnivorous terrestrial birds | 2 | Yes | ++ | +++ | +++ | ++ | +++ | + | 0 | 0 | +++ | 0 | |
| Insectivorous terrestrial birds | 1 | Yes | + | 0 | +++ | ++ | + | + | 0 | 0 | ++ | 0 | |
| Omnivorous terrestrial birds | 1 | Yes | ++ | + | +++ | +++ | + | + | 0 | 0 | ++ | 0 | |
| Mammals | Herbivorous mammals | 1 | Yes | + | ++ | +++ | +++ | + | + | 0 | 0 | ++ | 0 |
| Omnivorous mammals | 0 | Yes | + | ++ | +++ | ++ | + | + | 0 | 0 | ++ | 0 | |
| Insectivorous mammals | 1 | Yes | + | 0 | +++ | ++ | 0 | + | 0 | 0 | ++ | 0 | |
Based on the weights assigned to the lines of evidence, amphibians (both unimodal and bimodal) were identified as the focal species. Despite the higher, relative sensitivity of plankton in available laboratory measured studies, amphibians possess traits that increase their susceptibility to coal ash, are a critical component of the food web, and are vulnerable to pathogens and indirect effects via prey items not included in the protection community (Table 3). Managing mitigation goals to preserve and protect amphibian communities would involve reducing metal concentrations in Carolina bays to protect amphibians from sub-lethal effects, including those imposed by a potential reduction in prey, their ability to forage, and functional alterations that may impact long-term survival (Raimondo et al., 1998). Further analysis of amphibian effects data relative to exposure concentrations will provide a final risk characterization or mitigation goal that will be protective of the whole community.
6. Discussion
We demonstrate a community-level approach to protect critical habitat of isolated wetlands that also protects ES. Isolated wetlands are unique in that they often contain multiple species and taxa of concern in a single, spatially-explicit area. Many isolated wetlands, such as vernal pools and pond swamps, are subjects ofmulti-species recovery plans for conservation management (e.g., US Fish and Wildlife Service, 1998, 2005), supporting their protection under a multi-species risk assessment. The community-level approach demonstrated here targets critical habitat assessments and can reduce the time investment required of species-by-species assessments while adding an ES perspective to demonstrate the value of the habitat beyond listed species concerns; essentially moving towards doing more, quicker, with less. Its targeted application is for habitat types that provide refuge for communities of listed species, as demonstrated by isolated wetlands. As a critical habitat approach, it can identify potential impacts to the isolated ecosystem that could affect a listed species through impaired ecosystem integrity that may not be observed in a species-specific assessment. The approach demonstrated here can be used at various levels of governance and will be particularly effective at regional, state, and local scales where protection communities can be more accurately defined for specific sites and stakeholders are more actively engaged in site-specific assessments. While isolated wetlands are unique to their ecoregions (Tiner et al., 2002), they share characteristics that make the approach demonstrated here transferrable across different types of isolated wetlands.
Hydrologic isolation is a keystone characteristic of isolated wetlands, which creates systems of rare, endemic species and makes estimating exposure concentrations challenging. As discussed here, several exposure models are well suited to estimate the EEC in isolated wetlands from runoff, drift, industrial point source, and food-webs (e.g., Armitage and Gobas, 2007; Neitsch et al., 2005; Young, 2016), with potential exposure from groundwater re-introduction and atmospheric transport and deposition less understood. Although atmospheric and groundwater inputs of pesticides remain largely unknown, pesticides are one of the most common chemical threats to isolated wetlands across regions (Tiner et al., 2002), and the models described here remain widely applicable to estimate pesticide exposure concentration in all isolated wetland types. Similar to ERAs for pesticides, the disposal of wastewater (e.g., USEPA, 1993) or other point source pollution into isolated wetlands may also be well understood and modeled, allowing for the development of prospective ERAs or environmental impact statements. In cases of unintentional or accidental release of chemicals such as unpermitted discharges of metals, petroleum products and industrial pollutants as well as urban runoff, ERAs are most likely to occur retrospectively such that monitoring data may be used to determine concentration levels. All models will have advantages and disadvantages at different spatial and temporal scales and the use of any of the models discussed here should be considered on a case-by-case basis. The approach demonstrated here can be used to achieve mitigation goals by prioritizing effective mitigation efforts.
Although the species that occupy isolated wetlands vary across regions, the general taxonomic composition within them is somewhat consistent (Supporting Information A, B), as are the traits that allow species to persist in these highly variable environments. Traits such as desiccation-resistant dormancy (e.g., cysts, seeds), high levels of sensitivity to environmental cues, quick release from dormancy, and short maturation and reproduction periods are present in species that occupy ephemeral wetlands (Bliss and Zedler, 1997; Zedler, 1987; Zedler, 2003). These adaptations to the unique environmental conditions along with relative geographic and hydrologic isolation, have led to high rates of endemism in isolated wetland communities (Tiner, 2003; Zedler, 2003), making their populations particularly vulnerable to anthropogenic stressors. All isolated wetlands also are ecologically significant as important breeding sites for many amphibians, as the ephemeral nature of many precludes the establishment of predatory fish populations (Battaglin et al., 2009; Tiner, 2003; Zedler, 2003). In addition to inherent species sensitivity, traits can be used to evaluate a population's susceptibility to chemical contaminants. The application of traits-based risk assessment approaches is becoming more widespread as a means by which to compensate for species-specific data deficiencies (Baird and Van den Brink, 2007; van den Brink et al., 2013). As demonstrated by our case studies, traits-based species groups can be used to maximize the representativeness of available toxicity information.
Isolated wetlands tend to be consistent in the ES they provide (Supporting Information B). Maintaining some ES can enhance the protection of listed species, and in some cases, such as the one demonstrated here for vernal pools, listed species make up most of the SPUs that deliver the ES. Alternatively, some ES are contrary to the protection of listed species and SPUs of other ES, such as the one demonstrated here for Carolina bays. Management practices that use isolated wetlands (e.g., pond swamps, Carolina bays) to treat wastewater intentionally introduce contaminants into the habitat to utilize the water purification properties of the sediments and plants (USEPA, 1993). As demonstrated in the case study of Carolina bays, coal ash has been previously discarded into the wetlands to filter heavy metals and other industrial contaminants prior to recharging ground water (Rowe et al., 2002). This increases the exposure potential of contaminants, particularly for benthic organisms and those with increased potential for chemical absorption (i.e., amphibians), increasing risks to the unique communities within the Carolina bay ecosystems.
Characterization of chemical effects on ecological receptors is a source of significant uncertainty in ERAs stemming from limited data availability. This is particularly true for listed species, for which performing laboratory tests is either illegal, impractical, or requires permits that are time consuming to obtain. Understanding potential effects to ES is more complex as modeling impacts across multiple levels of biological organization is required (Forbes and Galic, 2016; Galic et al., 2012). Much of this uncertainty is due to the limited number of surrogate species to represent effects to diverse ecological communities. Even in the few cases of data rich chemicals, species diversity is poorly represented and extrapolation to targeted receptors is the only way to understand impacts to untested species. For the species that are tested, measured endpoints may be limited to acute estimates of lethal concentrations to 50% of tested organisms (e.g., LC50) or effects measured on survival, growth, or reproduction on longer term exposures, typically representing a single life cycle or less (i.e. partial life cycle tests). While models are used regularly to estimate exposure concentrations for ERA, effects models, including interspecies extrapolation and population models, are not regularly applied in ERA (Raimondo et al., 2018). Despite the recommendation to move towards effects modeling to reduce the uncertainty around the use of surrogate species, little has historically been done with surrogate species data in ERA beyond the application of safety factors (NRC, 2013). Our approach reduces the requirement on data collection for all species within the protection community by identifying the focal species for which models and extrapolation efforts may be targeted.
The WoE process is an integral component of ERA and we demonstrate an additional application of this approach to identify focal species within the protection community. Using a WoE approach as demonstrated here allows environmental resource managers to maximize resources in constrained environments and set explicit levels of uncertainty and provide unequivocal justification for the use of focal species. We provide 10 lines of evidence for focal species (Box 2) that may be applied or used as a guide for a specific assessment. Some lines are loosely defined here, e.g., vulnerable to additional non-chemical stressors, and may be more narrowly defined based on the protection community. For example, populations of many species that inhabit isolated wetlands operate as metapopulations, such that the subpopulation of a species that is present in one wetland is connected to a subpopulation in another through dispersal. Listed species metapopulation dynamics may be an important consideration when evaluating chemical risks, as more reliably-inundated pools may act as “sources” or genetic rescues to those that are less inundated (Zedler, 2003). Such dynamics should be considered in this approach if they are suspected to occur for one or more species within the protection community.
The approach described here is demonstrated using case studies of two different isolated wetland types (Section 5). Compared to Carolina bays and other wetlands, vernal pools are simple systems with a small protection community, allowing linkages among the species to be more clearly described. The distinct phases of vernal pools are critical components of these wetlands that should be considered in achieving protection goals. Thus, the conceptual diagram for this case study highlighted vernal pool phase (Fig. 2) and thus, focal species were identified for both aquatic and terrestrial phases. The Carolina bay case study is an example of an isolated wetland that provides a diversity of ES and contains a large, inter-connected protection community (Fig. 4). Due to their size, biological diversity, and irregular ephemeral nature, inundation of the wetland was not highlighted as a critical component in the conceptual diagram. Rather, we included ES provided by the SPUs in the conceptual diagram to aid in communicating the value of the focal species to stakeholders. Both case studies were intended to demonstrate the WoE process to identify focal species for which assessments can be targeted. Neither case study is intended to represent a final or complete risk assessment, which would include a full uncertainty analysis of final data used in risk determination.
We have highlighted several advantages associated with implementing community-level protections goals using traits-based WoE analysis and demonstrated a process that is ecologically comprehensive while considering limited data availability. The benefits of using a network or community-level approach for ERA includes a wider range of protection that is based on the integration of biotic and abiotic attributes (Rico et al., 2016). A larger, networked approach can also help explain some ambiguities and discrepancies in the evidence; different species and stressors found within a single critical habitat interact with one another and effects on one species may be the indirect cause of effects on another. These relationships are harder to discern when examining each species in isolation. While multispecies recovery plans have been found to be less effective than single species recovery plans (Clark and Harvey, 2002), chemical modes of action and adverse outcome pathways allow a more targeted evaluation of chemical impacts in complex communities (Russom et al., 2014). Therefore, this multi-species protection approach will establish comprehensive threshold values that not only protect individual listed species, but also conserve the ecological integrity that continues to provide ecosystem services for human benefit.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2019.01.153.
Supplementary Material
HIGHLIGHTS.
Isolated ecosystems provide habitat for rare species and ecosystem services.
Chemical contamination threatens these unique systems.
Community level protection goals assess risks to listed species and services.
The approach is demonstrated with geographically isolated wetlands.
The multi-species protection establishes comprehensive threshold values.
Acknowledgements
We thank Tanja Crk and Elizabeth George for critical review of a draft of this manuscript. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency (EPA). This document has been reviewed by the USEPA Office of Research and Development and approved for publication. Any mention of trade names, products, or services does not imply an endorsement by the US Government or the USEPA. This research was funded by the USEPA. The authors declare no conflicts of interest.
References
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL, 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicological research and risk assessment. Environ. Toxicol. Chem 29, 730–741. 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Armitage JM, Gobas FAPC, 2007. A terrestrial food-chain bioaccumulation model for POPs. Environ. Sci. Technol 41,4019–4025. 10.1021/es0700597. [DOI] [PubMed] [Google Scholar]
- Arnot JA, Gobas FAPC, 2006. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev 14 (4), 257–297. [Google Scholar]
- Baird DJ, Van den Brink PJ, 2007. Using biological traits to predict species sensitivity to toxic substances. Ecotoxicol. Environ. Saf 67, 296–301. 10.1016/j.ecoenv.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Bartell SM, Lefebvre G, Kaminski G, Carreau M, Campbell KR, 1999. An ecosystem model for assessing ecological risks in Quebec rivers, lakes, and reservoirs. Ecol. Model 124, 43–67. 10.1016/s0304-3800(99)00155-6. [DOI] [Google Scholar]
- Battaglin WA, Rice KC, Focazio MJ, Salmons S, Barry RX, 2009. The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005–2006. Environ. Monit. Assess 155, 281–307. 10.1007/s10661-008-0435-y. [DOI] [PubMed] [Google Scholar]
- Bennett RS, Etterson MA, 2009. Incorporating results of avian toxicity tests into a model of annual reproductive success. Integr. Environ. Assess. Manag 3,498–507. 10.1897/IEAM_2007-029.1. [DOI] [PubMed] [Google Scholar]
- Bicknell B, Imhoff J, Kittle J Jr., Jobes T, Donigian A Jr., 2001. Hydrological Simulation Program-Fortran (HSPF). User's Manual for Release 12 US EPA National Exposure Re-search Laboratory/in Cooperation with US Geological Survey. Water Resources Division, Athens, GA/Reston, VA [Google Scholar]
- Bilanin AJ, Teske ME, Barry JW, Ekblad RB, 1989. AGDISP: the aircraft spray dispersion model, code development and experimental validation. Trans. ASAE 32 (1), 0327–0334. 10.13031/2013.31005. [DOI] [Google Scholar]
- Bird SL, Perry SG, Ray SL, Teske ME, 2002. Evaluation of the AgDISP aerial spray algorithms in the AgDRIFT model. Environ. Toxicol. Chem 21,672–681. 10.1002/etc.5620210328. [DOI] [PubMed] [Google Scholar]
- Bliss SA, Zedler PH, 1997. The germination process in vernal pools: sensitivity to environmental conditions and effects on community structure. Oecologia 113, 67–73. 10.1007/s004420050354. [DOI] [PubMed] [Google Scholar]
- Brooks RT, 2004. Weather-related effects on woodland vernal pool hydrology and hydroperiod. Wetlands 24, 104–114. 10.1672/0277-5212(2004)024[0104:WEOWVP]2.0.CO;2. [DOI] [Google Scholar]
- Brooks RT, 2005. A review of basin morphology and pool hydrology of isolated ponded wetlands: implications for seasonal forest pools of the northeastern United States. Wetl. Ecol. Manag. 13, 335–348. 10.1007/s11273-004-7526-5. [DOI] [Google Scholar]
- California Department of Pesticide Regulation, 2014. Identifying and Managing Critical Uses of Chlorpyrifos Against key Pests of Alfalfa, Almonds, Citrus, and Cotton Califonia Department of Pesticide Regulation Agreement Number 13-C0054. University of California Agriculture and Natural Resources, Parlier, CA. [Google Scholar]
- California Department of Pesticide Regulation, 2018. Pesticide Use Reporting Data. https://www.cdpr.ca.gov/docs/pur/purmain.htm, 2016–2017. California Department of Pesticide Regulation, Environmental Monitoring and Pest Management Branch, Sacramento, CA: Accessed July 18, 2018. [Google Scholar]
- Clark JA, Harvey E, 2002. Assessing multi-species recovery plans under the endangered species act. Ecol. Appl 12, 655–662. 10.1890/1051-0761(2002)012[0655:AMSRPU]2.0.CO;2. [DOI] [Google Scholar]
- DeAngelis DL, Bartell SM, Brenkert AL, 1989. Effects of nutrient recycling and food-chain length on resilience. Am. Nat 134, 778–805. [Google Scholar]
- Doering JA, Lee S, Kristiansen K, Evenseth L, Barron MG, Sylte I, Lalone CA, 2018. In silico site-directed mutagenesis informs species-specific predictions of chemical susceptibility derived from the sequence alignment to predict across species susceptibility (SeqAPASS) tool. Toxicol. Sci. kfy 186 10.1093/toxsci/kfy186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber JH, van Wensem J, 2012. Elaborations on the use of the ecosystem services concept for application in ecological risk assessment for soils. Sci. Total Environ 415,3–8. 10.1016/j.scitotenv.2011.05.059. [DOI] [PubMed] [Google Scholar]
- Forbes VE, Galic N, 2016. Next-generation ecological risk assessment: predicting risk from molecular initiation to ecosystem service delivery. Environ. Int 91, 215–219. 10.1016/j.envint.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Forbes VE, Salice CJ, Birnir B, Bruins RJF, Calow P, Ducrot V, Galic N, Garber K, Harvey BC, Jager H, Kanarek A, Pastorok R, Railsback SF, Rebarber R, Thorbek P, 2017. A framework for predicting impacts on ecosystem services from (sub)organismal responses to chemicals. Environ. Toxicol. Chem 36, 845–859. 10.1002/etc.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic N, Schmolke A, Forbes V, Baveco H, van den Brink PJ, 2012. The role of ecological models in linking ecological risk assessment to ecosystem services in agroecosystems. Sci. Total Environ 415, 93–100. 10.1016/j.scitotenv.2011.05.065. [DOI] [PubMed] [Google Scholar]
- Gobas FAPC, Burkhard LP, Doucette WJ, Sappington KG, Verbruggen EMJ, Hope BK, Bonnell MA, Arnot JA, Tarazona JV, 2016. Review of existing terrestrial bioaccumulation models and terrestrial bioaccumulation modeling needs for organic chemicals. Integr. Environ. Assess. Manag 12,123–134. 10.1002/ieam.1690. [DOI] [PubMed] [Google Scholar]
- Golden HE, Lane CR, Amatya DM, Bandilla KW, Kiperwas HR, Knightes CD, Ssegane H, 2014. Hydrologic connectivity between isolated wetlands and surface water systems: a review of select modeling methods. Environ. Model. Softw 53, 190–206. 10.1016/j.envsoft.2013.12.004. [DOI] [Google Scholar]
- Harkness JS, Sulkin B, Vengosh A, 2016. Evidence for coal ash ponds leaking in the southeastern United States. Environ. Sci. Technol 50, 6583–6592. 10.1021/acs.est.6b01727. [DOI] [PubMed] [Google Scholar]
- Holland RF, Jain SK, 1981. Insular biogeography of vernal pools in the Central Valley of California. Am. Nat 117, 24–37. 10.1086/283684. [DOI] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC, 2007. Food web-specific biomagnification of persistent organic pollutants. Science 317, 236–239. 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
- Lalone CA, Villeneuve DL, Burgoon LD, Russom CL, Helgen HW, Berninger JP, Tietge JE, Severson MN, Cavallin JE, Ankley GT, 2013. Molecular target sequence similarity as a basis for species extrapolation to assess the ecological risk of chemicals with known modes of action. Aquat. Toxicol 144–145, 141–154. 10.1016/j.aquatox.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Lambeck RJ, 1997. Focal species: a multi-species umbrella for nature conservation. Conserv. Biol 11, 849–856. 10.1046/j.1523-1739.1997.96319.x. [DOI] [Google Scholar]
- Lance SL, Seaman JC, Scott DE, Bryan AL Jr., Singer JH, 2012. P-area wetland studies soils and biota. Final Report http://archive-srel.uga.edu/research/projects/docs/P-ash/P-AreaWetlands-FinalReport-2012.pdf(accessed 8/9/18).
- Landers DH, Nahlik AM, 2013. Final Ecosystem Goods and Services Classification System (FEGS-CS). EPA/600/R-13/ORD-004914. US Environmental Protection Agency, Corvallis, OR. [Google Scholar]
- Lane CR, D'Amico E, 2016. Identification of putative isolated wetlands of the conterminous United States. J. Amer. Wat. Res. Ass 52, 705–722. 10.1111/1752-1688.12421. [DOI] [Google Scholar]
- Mackay D, Fraser A, 2000. Bioaccumulation of persistent organic chemicals: mechanisms and models. Environ. Pollut 110, 375–391. 10.1016/s0269-7491(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Mackay D, Giesy JP, Solomon KR, 2014. Fate in the environment and long-range atmospheric transport of the organophosphorus insecticide, chlorpyrifos and its oxon In: Giesy J, Solomon K (Eds.), Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States. Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews) 231 Springer Cham. [DOI] [PubMed] [Google Scholar]
- MacLeod M, Riley WJ, Mckone TE, 2005. Assessing the influence of climate variability on atmospheric concentrations of polychlorinated biphenyls using a global-scale mass balance model (BETR-Global). Environ. Sci. Technol 39, 6749–6756. [DOI] [PubMed] [Google Scholar]
- Maltby L, Jackson M, Whale G, Brown AR, Hamer M, Solga A, Kabouw P, Woods R, Marshall S, 2017. Is an ecosystem services-based approach developed for setting specific protection goals for plant protection products applicable to other chemicals? Sci. Total Environ 580,1222–1236. 10.1016/j.scitotenv.2016.12.083. [DOI] [PubMed] [Google Scholar]
- Manara A, 2012. Plant responses to heavy metal toxicity In: Furini A (Ed.), Plants and Heavy Metals. Springer Briefs in Molecular Science. Springer, Dordrecht, pp. 27–53. [Google Scholar]
- Marton JM, Creed IF, Lewis DB, Lane CR, Basu NB, Cohen MJ, Craft CB, 2015. Isolated wetlands are important biogeochemical reactors on the landscape. Bioscience 65, 408–418. [Google Scholar]
- McDonald MG, Harbaugh AW, 1984. A modular three-dimensional finite-difference ground-water flow model. U.S. Geological Survey Open-File Report 83–875, p. 528. [Google Scholar]
- Munns WR Jr., Rea AW, Suter GW II, Martin L, Blake-Hedges L, Crk T, Davis C, Ferreira G, Jordan S, Mahoney M, Barron MG, 2015. Ecosystem services as assessment endpoints for ecological risk assessment. Integr. Environ. Assess. Manag 12 (3), 522–528. 10.1002/ieam.1707. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2013. Assessing Risks to Endangered and Threatened Species from Pesticides. The National Academies Press, Washington, DC: 10.17226/18344. [DOI] [Google Scholar]
- Neitsch SL, Arnold JG, Kiniry JR, Williams JR, 2005. Soil and water assessment tool: theoretical documentation, version 2005. Grassland, Soil and Water Research Center. Agricultural Research Service and Blackland Research Center, Texas Agricultural Experiment Station, Temple, Texas. [Google Scholar]
- Nienstedt KM, Brock TCM, van Wensem J, Montforts M, Hart A, Aagaard A, Alix A, Boesten J, Bopp SK, Brown C, Capri E, Forbes V, Kopp H, Liess M, Luttik R, Maltby L, Sousa JP, Streissl F, Hardy AR, 2012. Development of a framework based on an ecosystem services approach for deriving specific protection goals for environmental risk assessment of pesticides. Sci. Total Environ 415 10.1016/j.scitotenv.2011.05.057. (31–18) [DOI] [PubMed] [Google Scholar]
- Preziosi DV, Pastorok RA., 2008. Ecological food web analysis for chemical risk assessment. Sci. Total Environ 406, 491–502. 10.1016/j.scitotenv.2008.06.063. [DOI] [PubMed] [Google Scholar]
- Pyzoha JE, Callahan TJ, Sun G, Trettin CC, Miwa M, 2008. A conceptual hydrological model for a forested Carolina bay depressional wetland on the coastal plain of South Carolina, USA. Hydrol. Process 22, 2689–2698. [Google Scholar]
- Raimondo SM, Rowe CL, Congdon JD, 1998. Exposure to coal ash impacts swimming performance and predator avoidance in larval bullfrogs (Rana catesbeiana). J. Herpetol 32, 289–291. 10.2307/1565313. [DOI] [Google Scholar]
- Raimondo S, Mineau P, Barron MG, 2007. Estimation of chemical toxicity to wildlife species using interspecies correlation models. Environ. Sci. Technol 41,5888–5894. 10.1021/es070359o. [DOI] [PubMed] [Google Scholar]
- Raimondo S, Jackson CR, Barron MG, 2010. Influence of taxonomic relatedness and chemical mode of action in acute interspecies estimation models for aquatic species. Environ. Sci. Technol 44, 7711–7716. 10.1021/es101630b. [DOI] [PubMed] [Google Scholar]
- Raimondo S, Lilavois CR, Barron MG, 2015. Web-based Interspecies Correlation Estimation (Web-ICE) for Acute Toxicity: User Manual. Version 3.3, EPA/600/R-15/192. US Environmental Protection Agency, Office of Research and Development, Gulf Ecology Division, Gulf Breeze, FL. [Google Scholar]
- Raimondo S, Etterson M, Pollesch N, Garber K, Kanarek A, Lehmann W, Awkerman J, 2018. A framework for linking population model development with ecological risk assessment objectives. Integr. Environ. Assess. Manag 14, 369–380. 10.1002/ieam.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains MC, Fogg GE, Harter T, Dahlgren RA, Williamson RJ, 2006. The role of perched aquifers in hydrological connectivity and biogeochemical processes in vernal pool landscapes, Central Valley, California. Hydrol. Process 20, 1157–1175. [Google Scholar]
- Research Triangle International, 2007. Human and ecological risk assessment of coal combustion wastes. Prepared for the US Environmental Protection Agency; Research Triangle Park, NC: http://www.southeastcoalash.org/wp-content/uploads/2012/10/epa-coal-combustion-waste-risk-assessment.pdf, Accessed date: 2 August 2018. [Google Scholar]
- Rhomberg LR, Goodman JE, Bailey LA, Prueitt RL, Beck NB, Bevan C, Honeycutt M, Kaminski NE, Paoli G, Pottenger LH, Scherer RW, Wise KC, Beck RA, 2013. A survey of frameworks for best practices in weight-of-evidence analyses. Crit. Rev. Toxicol 43 (9), 753–784. 10.3109/10408444.2013.832727. [DOI] [PubMed] [Google Scholar]
- Rico A, van den Brink PJ, Gylstra R, Focks A, Brock TCM, 2016. Developing ecological scenarios for the prospective aquatic risk assessment of pesticides. Integr. Environ. Assess. Manag 12, 510–521. [DOI] [PubMed] [Google Scholar]
- Rodríguez JP, Beard TD Jr., Bennett EM, Cumming GS, Cork S, Agard J, Dobson AP, Peterson GD, 2006. Trade-offs across space, time, and ecosystem services. Ecol. Soc 11,28. [Google Scholar]
- Rowe CL, Hopkins WA, Congdon JD, 2002. Ecotoxicological implications of aquatic disposal of coal combustions residues in the United States: a review. Environ. Monit. Assess 80, 207–276. [DOI] [PubMed] [Google Scholar]
- Russom CL, LaLone CA, Villeneuve DL, Ankley GA, 2014. Development of an adverse outcome pathway for acetylcholinesterase inhibition leading to acute mortality. Environ. Toxicol. Chem 33, 2157–2169. 10.1002/etc.2662. [DOI] [PubMed] [Google Scholar]
- Scheringer M, 2015. Multimedia mass-balance models for chemicals in the environment: reliable tools or bold oversimplifications? Integr. Environ. Assess. Manag 11, 177–178. 10.1002/ieam.1591. [DOI] [PubMed] [Google Scholar]
- Scheringer M, Salzmann M, Stroebe M, Wegmann F, Fenner K, Hungerbühler K, 2004. Long-range transport and global fractionation of POPs: insights from multimedia modeling studies. Environ. Pollut 128, 177–188. 10.1016/j.envpol.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Sharitz RR, 2003. Carolina bays: unique habitats of the southeastern United States. Wetlands 23 (3), 550–562. 10.1672/0277-5212(2003)023[0550:cbwuho]2.0.co;2. [DOI] [Google Scholar]
- Sinnathamby S, Denton D, Hook J, Oliver L, Raimondo S, Pitchford A, Waits E, Yongping Y, Young D, Purucker ST, 2019. Sensitivity Analysis of Pesticide Concentrations in California Central Valley Vernal Pools In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske ME, Bird SL, Esterly DM, Curbishley TB, Ray SL, Perry SG, 2002. AgDrift®: a model for estimating near-field spray drift from aerial applications. Environ. Toxicol. Chem 21, 659–671. 10.1002/etc.5620210327. [DOI] [PubMed] [Google Scholar]
- Tiner RW, 1997. NWI maps: what they tell us. US Fish and Wildlife Service National Wetlands Newsletter, pp. 7–12 (Mar-Apr 1997). [Google Scholar]
- Tiner RW, 2003. Isolated wetlands of the United States. Wetlands 23 (3), 494–516. 10.1672/0277-5212(2003)023[0494:ISOLATEDWETLANDSOTU]2.0.CO;2. [DOI] [Google Scholar]
- Tiner RW, 2010. Wetlands of the Northeast: Results of the National Wetlands Inventory. U.S. Fish and Wildlife Service, Northeast Region, Hadley, MA: (71 pp.). [Google Scholar]
- Tiner RW, Berguist HC, DeAlessio GP, Starr MJ, 2002. Isolated Wetlands: a Preliminary Assessment of their Characteristics and Status in Selected Areas of the United States. US Department of the Interior, Fish and Wildlife Service, Northeast Region, Hadley, MA. [Google Scholar]
- US Environmental Protection Agency, 1992. Framework for Ecological Risk Assessment. EPA/630/R-92/001. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- US Environmental Protection Agency, 1993. Carolina Bays: a Natural Wastewater Treatment Program. EPA/832-R-93-005a. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- US Environmental Protection Agency, 2014. AQUATOX: Modeling Environmental Fate and Ecological Effects in Aquatic Ecosystems. Release 3.1 plus. EPA-820-R-14-005. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- US Environmental Protection Agency, 2016a. Generic Ecological Assessment Endpoints (GEAEs) for Ecological Risk Assessment: Second Edition with Generic Ecosystem Services Endpoints Added. EPA/100/F15/005. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- US Environmental Protection Agency, 2016b. Weight of Evidence in Ecological Assessment. EPA/100/R-16/001. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- US Environmental Protection Agency, 2016c. Biological Evaluation Chapters for chlorpyrifos ESA Assessment. https://www.epa.gov/endangered-species/biological-evaluation-chapters-chlorpyrifos-esa-assessment#executivesummary.
- US Environmental Protection Agency, 2017. Causal Analysis/Diagnosis Decision Information System (CADDIS). (Retrieved: August 13, 2018). US Environmental Protection Agency, Washington, DC: https://www.epa.gov/caddis. [Google Scholar]
- US Environmental Protection Agency, 2018a. Toxic Release Inventory 2018. https://www.epa.gov/toxics-release-inventory-tri-program.
- US Environmental Protection Agency, 2018b. National Wetland Condition Assessment 2018. https://www.epa.gov/national-aquatic-resource-surveys/nwca.
- US Fish and Wildlife Service, 1998. Multi-species Recovery Plan for the Threatened and Endangered Species of South Florida. vol. 2 US Fish and Wildlife Service, Atlanta, GA: https://fgcu.digital.flvc.org/islandora/object/fgcu%3A27171/datastream/OBJ/view/Multi-Species_Recovery_Plan_for_the_Threatened_and_Endangered_Species_of_South_Florida__Volume_II_of_II.pdf. [Google Scholar]
- US Fish and Wildlife Service, 2005. Recovery Plan for Vernal Pool Ecosystems of California and Southern Oregon. US Fish and Wildlife Service, Portland, Oregon: https://www.fws.gov/oregonfwo/documents/RecoveryPlans/Vernal_Pool_Fairy_Shrimp_RP.pdf. [Google Scholar]
- van den Brink PJ, Baird DJ, Baveco HJM, Focks A, 2013. The use of traits-based approaches and eco(toxico)logical models to advance the ecological risk assessment framework for chemicals. Integr. Environ. Assess. Manag 9, e47–e57. 10.1002/ieam.1443. [DOI] [PubMed] [Google Scholar]
- Vance LK, 2009. Isolated wetlands and intermittent/ephemeral streams in Montana: extent, distribution, and function Report to the Montana Department of Environmental Quality and the U.S. EPA. Montana Natural Heritage Program, Helena, MO. [Google Scholar]
- Wania F, Mackay D, 1999. The evolution of mass balance models of persistent organic pollutant fate in the environment. Environ. Pollut 100, 223–240. 10.1016/s0269-7491(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Witham CW, Holland RF, Vollmar JE, 2014. Changes in the distribution of great valley vernal pool habitats from 2005 to 2012 Sacramento, CA: Report Prepared for the U.S. Fish and Wildlife Service and Bureau of Reclamation CVPIA Habitat Restoration Program under Grant Agreement No. F11AP00169 with the USFWS. [Google Scholar]
- Yang Y, Chen H, Yang ZF, 2012. Integration of water quantity and quality in environmental flow assessment in wetlands. In: Yang Z, Chen B (Eds.), 18th Biennial ISEM Conference on Ecological Modelling for Global Change and Coupled Human and Natural Systems Procedia Environmental Sciences. Elsevier Science, Amsterdam, pp. 1535–1552 10.1016/j.proenv.2012.01.146. [DOI] [Google Scholar]
- Young DF, 2016. Pesticide in Water Calculator User Manual for Versions 1.50 and 1.52. U.S. Environmental Protection Agency, Office of Pesticide Programs, Washington, DC. [Google Scholar]
- Zedler PH, 1987. The ecology of southern California vernal pools: a community profile. US Fish and Wildlife Service. Biol. Reprod 85 (7.11) (136 pp.). [Google Scholar]
- Zedler PH, 2003. Vernal pools and the concept of “isolated wetlands”. Wetlands 23 (3), 297–607. 10.1672/0277-5212(2003)023[0597:VPATCO]2.0.CO;2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.