Occupational Applications
This paper provides an overview of a new consensus standards committee for exoskeletons, ASTM International F48, and describes the organization and current activities of this committee. Lack of product standards and certifications have been described as barriers to adoption of exoskeleton technologies in industry practice. While exoskeletons are not considered a traditional form of personal protective equipment (PPE) they are similarly wearable, and much of the interest in their application in the industrial/workplace domain is motivated by injury prevention. ASTM F48 believes that standards and certifications for exoskeletons in their manufacture, deployment, and use would enhance their adoption in the workplace.
Keywords: exoskeleton, exosuit, standards, wearable robotics
1. Introduction
This paper provides an overview of the formation and current structure of the new ASTM International Technical Committee on Exoskeletons and Exosuits (ASTM F48), its planned activities and liaisonships, and provides a perspective on their potential impact on workplace ergonomics programs and practices. For the purpose of this paper, an exoskeleton is defined as a wearable device that augments, enables, assists, or enhances motion, posture, or physical activity. This term will also be inclusive of exosuits, which are similar in function but are differentiated by predominantly soft and/or elastic structures. Exoskeletons have been described as being ‘active’, ‘passive’ or a combination of both active and passive devices and it is important to note that exoskeleton terminologies are still evolving as rapidly as their technologies. Typically, active devices have an electrical power source whereas passive devices tend to utilize levers, springs and other non-electrical means to assist the user. Standardization of technologies and their terminologies are an important part of the activities and scope of the F48 committee.
As of December 2016, the Exoskeleton Report (https://exoskeletonreport.com/, accessed 7/25/2018) identified 58 commercial and/or non-profit organizations that had developed, or were actively developing, exoskeletons or wearable robots and it is estimated that there are roughly 80 such commercial systems on the market as of August 2018. A thorough review of the many developments within military, healthcare, and academic research and development units is beyond the scope of this article. However, several publications exist that provide more detailed reviews on exoskeleton developments (e.g. Lee et al, 2012; de Looze et al, 2016). The emphasis of this paper is on the recent coordination and organization of communities of interest and stakeholder groups that are interested in developing consensus standards for these technologies.
2. Organization of Exoskeleton Interests and Stakeholders
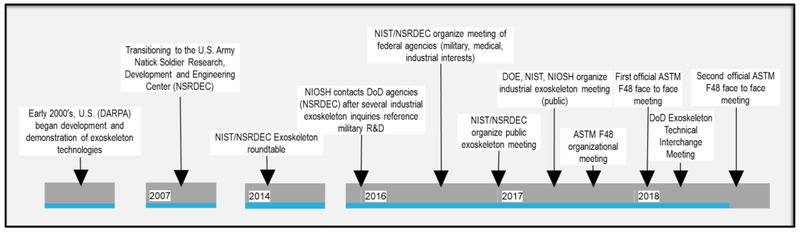
In the early 2000’s, the U.S. Defense Advanced Research Projects Agency (DARPA) began development and demonstration of “… critical technologies such as power, control, and actuation that will lead to a self-powered external structure to enable a Soldier to effortlessly carry over 100 pounds of additional weight.” (DARPA, 2006). The results of these efforts were transitioned to the U.S. Army Natick Soldier Research, Development, and Engineering Center (NSRDEC) in 2007. In 2014, NSRDEC invited representatives from the National Institute of Standards and Technology (NIST) to participate in an Augmentation Round Table to discuss challenges and future directions of this technology. In 2015, the National Institute for Occupational Safety and Health (NIOSH) contacted Department of Defense (DOD) agencies after receiving a series of inquiries from industry stakeholders and product developers about capabilities and efficacy of the technologies in industrial/occupational applications. These common interests and discussions led to the formation of an ad-hoc workgroup among these, and several other, U.S. federal and DOD agencies with interests in standards and/or best practices for the use of wearable human augmentation technologies (“exoskeletons”).
In August 2016, NIST hosted a preliminary meeting of federal (Department of Energy, Department of Homeland Security, Food and Drug Administration, NIOSH, Occupational Safety and Health Administration) and DOD (U.S. Army Research Lab, U.S. Army Research Institute of Environmental Medicine, U.S. Army Clinical and Rehabilitative Medicine Research Program, U.S. Army Communications-Electronics Command, U.S. Special Operations Command, U.S. Navy, U.S. Air Force) agencies to discuss the current state of standards within the exoskeleton and wearable robotics space and to identify gaps in standards for safety, performance, interoperability, ergonomics, and cybersecurity. In follow-up, an open public technical interchange meeting was held in January 2017, which was broadly inclusive of stakeholders across the military, medical, and industrial spaces and the academic community. The goals of the meeting were to: 1) identify gaps in current exoskeleton standards including terminology, test methods, and performance metrics in the industrial, military, and medical sectors; 2) facilitate the involvement of all interested parties in these developments as they progress; and 3) promote technological advances by building relationships among key stakeholders. Over 100 organizations, including 44 from industry, 35 from government, and 19 from academia, participated. There was strong agreement among the attendees that standard test methods to compare exoskeletons to the task were needed and more detailed future meetings were necessary to further coordination and efforts.
A subsequent meeting with focus specifically on industrial applications of exoskeletons and wearable robotics was hosted by the Department of Energy (DOE) in June 2017. This meeting was collaboratively organized by DOE, NIST, and NIOSH, with the purpose of engaging the robotics community on industrial applications of human-wearable and human-attachable, passive and active devices to enable and proliferate use among the various occupational groups. Other objectives were to: 1) identify opportunities to transfer knowledge and technologies from medical and military applications to industrial applications; 2) identify the key framework elements for a body of knowledge and a community of practice on human-wearable augmentation devices; 3) identify federal agencies and their respective interests, initiatives, projects, and deployments of human-wearable augmentation devices for industrial applications; and 4) discuss terminology and standards needs among industrial end-users, government industrial program leaders, insurance representatives, and the testing and standards community. A key outcome of this meeting was a vote to move forward with ASTM International as the standards development organization for exoskeletons.
NSRDEC hosted a Military Exoskeleton Technical Interchange meeting in April 2018 under the sponsorship of the Office of the Under Secretary of Defense for Research and Engineering. The meeting brought together experts from across the exoskeleton community to discuss the latest DOD exoskeleton needs, advancements, science and technology gaps, user applications, and lessons learned for the purpose of stimulating advances in exoskeleton technology. A timeline of the above described activities is shown in Figure 1.
Figure 1.
Timeline of selected activities of Department of Defense and other federal agencies leading to organization of ASTM F48. The emphasis is on industrial exoskeleton interest.
3. ASTM Committee F48
In September 2017, approximately 40 participants representing interests from various stakeholder groups, including military, federal agencies, exoskeleton manufacturers, component manufacturers, academia, user groups, and laboratories, convened at ASTM International’s headquarters for the purpose of organizing a new exoskeleton standards committee, now known as the ASTM International Committee F48 on Exoskeletons and Exosuits (https://www.astm.org/COMMITTEE/F48.htm).
F48 now broadly addresses active, passive, and quasi-active/passive exoskeletons and classifies their general use cases according to the following:
Medical: Amputee, injured, and/or physically disabled patients wearing exoskeletons can experience increased mobility and stability & enhanced physical therapy. This could accelerate return-to-work and recovery, benefiting patients, healthcare providers, and insurance companies.
Industrial: Employees working in logistics, warehouse, and factory settings could utilize exoskeletons for overhead, load carrying, tool-use, mobility, and squatting activities, allowing them to have a higher performance for a longer duration with less impact on the body, while also decreasing risk of injury. This could also increase operational productivity.
Military: Soldiers may benefit through use of exoskeletons that can allow them to march farther with less fatigue, move logistical loads more easily and safely, and carry more supplies or weaponry which may otherwise prove too heavy or burdensome on the body.
Public Safety: First responders may benefit from exoskeletons that can allow them to move larger objects when searching for victims within collapsed structures and to carry more equipment such as extra air bottles for firefighters and heavy bomb suits for explosive ordinance disposal technicians.
Consumer/Recreational: Consumers may benefit from exoskeletons for personal use, recreational sports (eg, skiing), home and yard work and other physically demanding tasks.
Subcommittees within Committee F48 were established under a framework that considers the exoskeleton product lifecycle rather than only based on use cases. The first official F48 meeting was held in February 2018 where subcommittee scopes were approved. As of August 2018, 18 task groups have been proposed within the subcommittees to draft specific standards, with a terminology standard that has passed ballot as ASTM F3233 and further terminology and product labelling work items currently in pre-ballot form.
All subcommittees will develop and maintain international standards that include, but are not limited to, standards for safety, quality, and efficiency. In addition, each subcommittee aims to develop standards for applications of exoskeleton technologies in the industrial, medical, military, consumer, and emergency response sectors. Furthermore, each subcommittee strives to understand, and make reference to, other existing and developing standards and works to coordinate with other ASTM International committees, as well as with other organizations having mutual interest. The F48 committee scope can be found at www.astm.org/COMMITTEE/F48.htm. In addition to an Executive Committee (F48.90), there are six other subcommittees within F48. The following is a brief outline of the current and future work of each of the subcommittees:
Design and Manufacturing (F48.01) - The F48.01 subcommittee is focused on exoskeleton design, validation, and manufacturing and is specifically targeting standardization of structural function, such as mechanical and electrical components, embedded components, energy systems, cooling and fluid power systems, software, and user experiences. In addition, F48.01 is working to provide guidelines on active and passive systems and to define system needs, especially for energy storage and transportation.
Human Factors and Ergonomics (F48.02) - Based on the disciplines of human factors, ergonomics, and safety, the F48.02 subcommittee is focused on user-centered design and selection of exoskeletons. More specifically, this subcommittee is working to develop physical-activity-based and anthropometric-based standards (excluding strength and mobility) for applications relevant to users from the consumer, industrial, medical, military, and emergency management sectors, while incorporating key human factors and ergonomic aspects such as: 1) design for population accommodation, 2) assessment of system usability, 3) assessment of system ergonomics, 4) assessment of system safety, and 5) assessment of system training. This subcommittee is also considering anthropometric variables
Task Performance and Environmental Considerations (F48.03) - The F48.03 subcommittee is targeting task performance and environmental considerations for consumers, public safety personnel, industrial/occupational users, and military personnel. This subcommittee is also working to generate a guide that maps applications to tasks, in order to provide a baseline on how the standards should be implemented. In addition, F48.03 is using F3218-17 (ASTM F3218—17, Standard Practice for Recording Environmental Effects for Utilization with A-UGV Test Methods) to understand possible analogous applications in recording and analyzing the effects of the environmental factors, particularly during exoskeleton utilization and testing.
Maintenance and Disposal (F48.04) - The F48.04 subcommittee is currently working to develop standards that consider the decontamination and/or disposal of exoskeleton systems following exposures in radioactive and chemical environments, as well as maintenance guidelines and general disposal procedures, including, exoskeleton system consumables and components.
Security and Information Technology (F48.05) - The F48.05 subcommittee is currently working to develop standards for the practice of security and privacy protocols to protect data associated with the exoskeleton system, including appropriate protocol testing methods.
Terminology (F48.91) - The F48.91 subcommittee maintains the F48 terminology standards in coordination with representatives from all F48 subcommittees. In this capacity, this subcommittee is working to create terms and definitions that are logically organized, readable, and understandable. The subcommittee also serves as an editorial resource for other subcommittees that need assistance to eliminate redundancies, harmonize variances, clarify meanings, and standardize formats of definitions.
The community of interests relevant to human augmentation, wearable robotics, and exoskeletons across medical, military, industrial, public safety, and consumer spaces is multidisciplinary and diverse. ASTM F48 has been establishing liaisons with relevant professional societies, technical committees, consortia, and stakeholder groups. Some of the key groups currently include: Human Factors and Ergonomics Society (HFES), Biomedical Engineering Society (BMES), IEEE Technical Committee on Wearable Robotics, American Society of Mechanical Engineers (ASME), ISO Technical Committee (TC) 299, Materials Handling Institute, NATO Committee HFM-266 (3D Scanning for Clothing Fit and Logistics), American Industrial Hygiene Association, International Society of Biomechanics, NextFlex Alliance on Flexible Hybrid Electronics (FHE), and European Cooperation in Science and Technology. There have also been interactions with newly forming specialized exoskeleton interest groups such as the North American AExG (Automotive Exoskeleton Group) and insurance providers focused on workers’ compensation and return-to-work.
4. Other Standards
An existing international standard, International Organization for Standardization (ISO) 13482:2014 (International Organization for Standardization, 2014) addresses safety requirements for personal care robots, some of which are considered exoskeletons. Portions of ISO 13482 are applicable to a subset of exoskeletons, but much of the exoskeleton space is outside of the scope of ISO 13482. ISO 13482 is not applicable to robots as medical devices, nor to military or public force application robots, both of which were intended to be within the scope of ASTM F48. ISO 13482:2014 is applicable to wearable physical assistant robot exoskeletons that may be used in the workplace. These technologies may enable increased work capacity or reduce biomechanical loads and/or worker fatigue and overexertion risk, among other use cases. ISO 13482 recognizes that, while physical assistant robots may augment certain human capabilities, their use introduces potential new hazards. Some of these hazards result from the fastening and direct contact of the robot device to the user while other hazards are similar to those of robots operating in the environment. The ISO standard emphasizes the need for risk assessment and hazard identification analysis for safe design and operation of these technologies. ISO Technical Committee (TC) 299 work groups are developing two test methods: 1) a skin stress test method for exoskeletons that looks at the possible maximum load on a user’s skin using a simulation device and 2) a test method for visible fracture, deformation, disengagement of parts, and functional damage of a robot, including those worn by a person, for durability assessment. (Bostelman and Hong, 2018). A second existing standard, Japanese Standards Association (JSA)-Japanese Industrial Standards (JIS) B 8456-1, Personal Care Robots - Part 1: Physical Assistant Robots for Lumbar Support (in Japanese), “…specifies performance requirements, safety requirements and indication requirements of wearable robots for lumbar support based on a consensus between manufacturers, consumers and other neutral bodies…” (Nabeshima et al, 2018; p. 1292).
5. Influence on Workplace Ergonomics, Safety and Health
Exoskeletons have demonstrated the potential to improve individual well-being and quality of life. Examples in the medical space are lower extremity exoskeletons improving standing, walking, and sitting ability for some multiple sclerosis patients (Kozlowski et al, 2017) and positive impact on ability to walk (Kozlowski et al 2015), quality of life, cardiovascular endurance, and motor neurological status in spinal cord injury patients (Raab et al, 2016). While the medical exoskeleton market share has, to date, well exceeded that of the industrial, a 2015 market research report (WinterGreen Research, 2015) projected the industrial market share to surpass the medical by 2021. The promise of industrial exoskeletons and exosuits, to the fully physically-abled, embodies both productivity to the organization and injury prevention for the individual user. The technologies are intended to augment a worker’s physiologic capabilities (stability, force and power production) in industrial task performance to increase work capacity, or reduce biomechanical loads and/or worker fatigue, thus mitigating overexertion risk.
Exoskeletons address a mismatch between workplace physical demands and human strength/endurance capabilities, consistent with any other ergonomic equipment intervention. However, by their wearable nature, exoskeletons have some characteristics of personal protective equipment (PPE). As such, in the technical interchange meetings described in Section 2, it was proposed that exoskeletons represent an emerging new class of PPE. A National Science Foundation program solicitation (NSF 18-518) under the National Robotics Initiative 2.0: Ubiquitous Collaborative Robots describes interest in “…wearable, prosthetic-like, exoskeletal, bionic, and other attachable human assistive robotic devices that can serve the workforce by functioning as (1) smart personal protective equipment (PPE) and/or (2) performance augmentation and amplification devices (PAADs).” Alternative to the PPE characterization, exoskeletons have been likened to other forms of work aid, material handling equipment, or tooling/equipment modifications that reduce the user/operator’s physical effort in executing a task, consistent with an engineering controls approach. However, the wearable nature of these devices that involve attachment, fitting/adjustment and donning/doffing may be more akin to PPE use with regards to acceptance by industrial workers. Traditional PPE (e.g. respirators, protective eyewear, hearing protection devices, head protection) is generally characterized by a physical barrier between the worker and the physical agent or hazard. PPE adoption is part of a safety management system, but is also persuaded by regulatory requirements and mandated use in environments where hazards cannot be eliminated, substituted, or mitigated with other engineering or process design approaches. Traditional PPE equipment have established certifications and test methods that communicate and/or certify protectiveness. For example, hearing protection devices have quantitative metrics for noise reduction to match their specifications to noise exposure levels and protective eyewear is certified to impact testing requirements.
Exoskeletons are protective of the user through the reduction of internal biomechanical loads across user’s joints, muscles and soft tissue, or reduction in their metabolic exertion. One challenge to standards development for exoskeletons will be to establish test methods and certifications to communicate this protectiveness in a manner analogous to PPE protectiveness while also recognizing the ability for exoskeletons to augment capabilities that traditional PPE does not. This will likely involve multiple test methods given the range of tasks and functions of these systems in industrial applications. Employers’ and workers’ (user’s) may be more confident in adopting these technologies if standards exist for certifying and communicating their protectiveness in a manner similar to PPE. A European automotive manufacturing study (Spada et al, 2017) recently pointed to the lack of standards as a barrier to introduction of “active exoskeleton-type solutions” in manufacturing industries.
Forthcoming exoskeleton standards that address device capabilities towards overexertion/musculoskeletal injury prevention in the workplace should be applied consistently with industrial hygiene principles and consensus standards. For example, in the Construction Industry, per ANSI/ASSE A10.40-2007, solutions for jobs/tasks with risk factors for musculoskeletal disorders should be based on the hierarchy of controls, prioritized as: 1) elimination, 2) substitution, 3) engineering controls, 4) administrative changes, 5) work practice changes, 6) training, 7) protective equipment, and 8) assessment of individual physical capabilities. Deployment of exoskeletons to protect employees from overexertion and/or cumulative trauma injury risks should consider the potential for overreliance on the technology. One way in which this has been addressed is with policies stating that exoskeleton deployment should not be an opportunity to increase throughput or work load that might negate a margin of safety afforded by the exoskeleton. In addition to standards for safe design and use, to minimize any new workplace risks introduced by exoskeletons, future work might emphasize test methods whereby exoskeletons can be evaluated in terms of the degree to which they mitigate user overexertion and musculoskeletal risk. The ability to standardize testing of an augmentation “multiplier”, that is, the extent to which the device increases an occupationally-relevant specific capability or tolerance to external loading, is needed to comparatively assess similarly purposed devices.
6. Recommendations for Future F48 Work
The primary challenge in this new technology market is the establishment of safety and trust. ASTM F48 is working to establish consensus standards so that the researchers, developers, and buyers of exoskeletons can trust that equipment safety and performance claims have been validated. Standard test methods will enable certification so that users have increased confidence in the exoskeletons they purchase and deploy. Additional recommended areas of future work are as follows:
Assessment techniques for powered and passive exoskeletons,
Design techniques around human anthropometry, shapes, and job functions,
System maintainability standards,
System training standards,
Device certification criteria,
Third party certification processes,
Adapting disruptive technologies to exoskeletons (e.g., augmented/extended reality, real-time health monitoring/risk assessment; access to big data systems; standards for Artificial Intelligence/Machine Learning safety/performance enhancements).
7. Acknowledgements
The authors are grateful for the support and guidance from Pat Picariello and Christine DeJong, ASTM.
Footnotes
Publisher's Disclaimer: Disclaimer: The opinions, findings, and conclusions in this report are those of the authors and do not necessarily reflect the policy or official position of the National Institute of Standards and Technology (NIST), or the National Institute for Occupational Safety and Health (NIOSH), Centers for Disease Control and Prevention. Mention of any company or product does not constitute a recommendation or endorsement by NIST or NIOSH, CDC.
Conflict of Interest: The authors have no conflict of interests.
Contributor Information
Brian D. Lowe, National Institute for Occupational Safety and Health.
William G. Billotte, National Institute of Standards and Technology.
Donald R. Peterson, College of Engineering and Engineering Technology, Northern Illinois University.
8. References
- ANSI/ASSE. A10.40-2007 (R2013). Reduction of musculoskeletal problems in construction American National Standard for Construction and Demolition Operations. Des Plaines, IL: American Society of Safety Engineers (ASSE). [Google Scholar]
- Bostelman R and Hong T (2018). Test methods for exoskeletons—lessons learned from industrial and response robotics In Wearable Exoskeleton Systems: Design, Control and Applications. S. Bai, G.S Virk, and T Sugar (Eds.) Institution of Engineering Technology, 335–361. [Google Scholar]
- Defense Advanced Research Projects Agency (2006). DARPA Director’s testimony to the Subcommittee on Terrorism, Unconventional Threats and Capabilities, House Armed Services Committee, United States House of Representatives, March 29, 2006. [Google Scholar]
- de Looze MP, Bosch T, Krause F, Stadler KS, and & O’Sullivan LW. (2016). Exoskeletons for industrial application and their potential effects on physical work load, Ergonomics, 59:5, 671–681. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization (2014). ISO 13482:2014: Robots and robotic devices -- Safety requirements for personal care robots.
- Kozlowski A, Bryce T, and Dijkers M (2015). Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Topics in Spinal Cord Injury Rehabilitation, 21 (2), 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowki AJ, Fabian M, Lad D, and Delgado AD (2017). Feasibility and safety of a powered exoskeleton for assisted walking for persons with multiple sclerosis: A single-group preliminary study. Archives of Physical Medicine and Rehabilitation, 98, 1300–1307. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim W, Han J, & Han C (2012). The technical trend of the exoskeleton robot system for human power assistance. International Journal of Precision Engineering and Manufacturing, 13(8), 1491–1497. [Google Scholar]
- Nabeshima C, Ayusawa K, Hochberg C, and Yoshida E (2018). Standard Performance Test of Wearable Robots for Lumbar Support. IEEE Robotics and Automation Letters, 3, 3, 2182–2189. [Google Scholar]
- Raab K, Krakow K, Tripp F, & Jung M (2016). Effects of training with the ReWalk exoskeleton on quality of life in incomplete spinal cord injury: a single case study. Spinal Cord Series and Cases, 2, 15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spada S, Ghibaudo L, Gilotta S, Gastaldi L, and Cavatorta MP, (2017). Investigation into the applicability of a passive upper-limb exoskeleton in automotive industry. Procedia Manufacturing 11: 1255–1262. [Google Scholar]
- Research WinterGreen (2015). Wearable Robots, Exoskeletons: Market Shares, Market Strategies, and Market Forecasts, 2015 to 2021. REPORT # SH26511914, Lexington, Massachusetts: WinterGreen Research, Inc. [Google Scholar]