Abstract
Background
Neurosteroids mediate stress signaling and have been implicated in the pathogenesis of post-traumatic stress disorder (PTSD) in both preclinical and clinical studies. Compared to controls, subjects with PTSD exhibit altered neurosteroid levels in peripheral blood and cerebrospinal fluid as well as hypoactivity in the medial orbital frontal cortex (mOFC). Therefore, the aim of this study was to compare neurosteroid levels in the mOFC of subjects with PTSD (n = 18) and controls (n = 35).
Methods
Gray matter was dissected from fresh-frozen mOFC, and levels of the neurosteroids pregnenolone, allopregnanolone, pregnanolone, epiallopregnanolone, epipregnanolone, tetrahydrodeoxycorticosterone, and androsterone were determined by gas chromatography-tandem mass spectrometry.
Results
Analyses of unadjusted levels revealed that males with PTSD had significantly decreased levels of allopregnanolone (p = 0.03) compared to control males, and females with PTSD had significantly increased levels of pregnenolone (p = 0.03) relative to control females. After controlling for age, postmortem interval, and smoking status, results showed that males with PTSD had significantly decreased levels of androsterone (t46 = 2.37, p = 0.02) compared to control males and females with PTSD had significantly increased levels of pregnanolone (t46 = −2.25, p = 0.03) relative to control females.
Conclusions
To our knowledge, this is the first report of neurosteroid levels in postmortem brain tissue of subjects with PTSD. Although replication is required in other brain regions and a larger cohort of subjects, the results suggest a dysregulation of allopregnanolone and androsterone in males with PTSD and pregnanolone in females with PTSD in the mOFC.
Keywords: neurosteroids, stress, sex differences, post-traumatic stress disorder, frontal cortex, human postmortem brain
Introduction
Neurosteroids are naturally occurring molecules synthesized de novo in the brain from cholesterol.1 Pregnenolone is synthesized from cholesterol and serves as a precursor for other neurosteroids, including the pregnane neurosteroids, such as allopregnanolone, pregnanolone, epiallopregnanolone, epipregnanolone, and tetrahydrodeoxycorticosterone (THDOC), as well as androstane neurosteroids, such as androsterone.2 In addition to synthesis in the brain, steroids are also synthesized in the periphery, and these neurosteroids appear to cross the blood–brain barrier and mediate stress-signaling pathways.3,4 Neurosteroids impact pathways that modulate gene expression and rapidly alter excitatory and inhibitory neurotransmission through ligand-gated ion channels, including gamma-aminobutyric acid (GABA) type A5,6 and N-methyl-D-aspartate receptors,7 as well as other cell surface receptors.8 For example, allopregnanolone, pregnanolone, THDOC, and androsterone are positive allosteric modulators of GABAA receptors, while epiallopregnanolone and epipregnanolone are negative allosteric modulators of GABAA receptors.9,10 In addition, neurosteroids affect multiple cellular processes, such as myelination, apoptosis, neuritic outgrowth, and neurogenesis,4,6,8 as well as neuronal systems, including those underlying cognition, emotion, and motivation.11
Neurosteroids have been implicated in stress response regulation6 and the pathophysiology underlying several mental disorders, especially stress and stress-related psychiatric disorders.12–17 Multiple studies have shown that the endogenous production of neurosteroids is affected by stress16 and that patients with various neuropsychiatric conditions, including bipolar disorder, schizophrenia, major depression, traumatic brain injury, and post-traumatic stress disorder (PTSD), exhibit disruptions of their hypothalamic-pituitary-adrenocortical axis and altered levels, as well as reactivity, of neurosteroids in peripheral blood, cerebrospinal fluid, and postmortem brain tissue.2,8,12,18 Both clinical and preclinical studies have shown that the levels of neurosteroids, particularly those that impact GABAergic neurotransmission, are decreased in subjects with PTSD2,19 and differ by sex.2,19
A number of animal models have shown that neurosteroids are involved in the development and treatment of PTSD-related behaviors.2,20 The administration of an allopregnanolone stimulant alleviates fear extinction deficits in a rodent model of PTSD that exhibits decreased allopregnanolone levels in corticolimbic regions.21,22 The biosynthesis of allopregnanolone is also decreased in patients with PTSD.15,23 In addition, androsterone, an inhibitory androstane neurosteroid, and its precursor, dehydroepiandrosterone, have been shown to be neuroprotective and associated with stress resilience.15,24 Both the serum and brain levels of androsterone increase in response to acute stress.25
Accumulating evidence collected from postmortem and neuroimaging studies of PTSD suggests that brain structure and function, especially in subregions of frontal cortex, amygdala, and hippocampus, are altered in patients with PTSD.26,27 The hippocampal and anterior cingulate cortical volumes are decreased in patients with PTSD.28 Functional imaging studies of patients with PTSD have reported hypoactivity of the ventromedial prefrontal cortex, including the medial orbital frontal cortex (mOFC).29–33 Few postmortem studies of subjects with PTSD have examined the mOFC. Interestingly, studies involving human postmortem mOFC have reported stress-induced differences in dendritic spine density34 and reductions in spine density in subjects with PTSD.35 The mOFC is involved in the extinction of conditioned fear and reward processing,36,37 processes that are abnormal in subjects with PTSD.38,39 Therefore, in this study, neurosteroid levels were quantified in the mOFC of postmortem subjects with PTSD in order to gain a greater understanding of how the mOFC may contribute to dysregulation of these pathways.
The pathophysiological processes underlying the observed alterations of neurosteroid levels in patients with PTSD remain unclear. In addition, to our knowledge, no studies to date have measured neurosteroid levels in postmortem brain tissue from individuals with PTSD. Therefore, guided by prior research, the aim of this preliminary study was to compare neurosteroid levels in the mOFC of postmortem subjects with PTSD and controls.
Methods
Fresh-frozen tissue was obtained from the brains of subjects with PTSD (n = 18) and controls (n = 35) from the Duke Neuropsychiatric Brain Bank. Informed consent for brain donation was obtained from the next of kin (NOK) of each subject. All aspects of this work were approved by the Duke University School of Medicine institutional review board.
Information about the donor’s medical history was obtained from interviews with the NOK that also included a structured interview using the Mini-International Neuropsychiatric Interview.40 Psychiatric diagnoses were determined by a consensus review process conducted by at least two psychiatrists who reviewed the NOK interviews and medical records of each donor. In any cases involving disagreement, a third psychiatrist was consulted to determine the diagnosis. The criteria in the Diagnostic and Statistical Manual of Mental Disorders, Version IV,41 were utilized for determining postmortem psychiatric diagnosis. Subjects were categorized as controls if their medical records and NOK interviews confirmed they had no known history of psychiatric symptoms or substance abuse.
Subject Demographics
A total of 53 subjects were included in this study (PTSD: n = 18, controls: n = 35; see Table 1). The groups did not differ significantly for age or sex. However, the groups differed significantly for postmortem interval (PMI; t51 = 2.57; p = 0.013), ethnicity (Fisher’s exact test, p = 0.001), and smoking (Fisher’s exact test, p = 0.001). Compared to the control group, the subjects with PTSD had a shorter PMI (26.6 ± 10 h vs. 35.5 ± 12.8 h), a greater racial proportion more likely to be white (72.2% vs. 28.6%), and a history of smoking (94.4% vs. 20.0%) (see Table 1).
Table 1.
Subject demographic information.
| PTSD n = 18 | Control n = 35 | p value | |
|---|---|---|---|
| Sex (% male) | 66.7% | 54.3% | NS |
| Age (years) | 42.4 ± 13.9 | 45.9 ± 13.4 | NS |
| PMI (hours) | 26.6 ± 10.0 | 35.5 ± 12.8 | 0.013 |
| Ethnicity (% white) | 72.2% | 28.6% | ≤0.001a |
| Comorbid MDD (%) | 50% | 0% | ≤0.001a |
| Smoking (% yes) | 94.4% | 20.0% | ≤0.001a |
PTSD: post-traumatic stress disorder; NS: not significant; PMI: postmortem interval; MDD: major depressive disorder.
Fisher’s exact test.
Postmortem Brain Samples
All specimens were free of confounding gross or microscopic neuropathology. Fresh-frozen tissue samples from the mOFC (Brodmann area 11) were sampled from each subject, and approximately 80 mg of tissue was collected from cryosections. The collected tissue from each sample was stored in individually washed and salinized vials at −80℃ until analysis.
Quantification of Neurosteroids
The levels of neurosteroids, including pregnenolone, allopregnanolone, pregnanolone, epiallopregnanolone, epipregnanolone, THDOC, and androsterone were determined in each subject using gas chromatography-tandem mass spectrometry (GC/MS/MS), preceded by high-performance liquid chromatography (HPLC). This technique has a limit of quantification of 2 pg, as described previously,17,42,43 and was modified to use electron impact ionization mode.
The tissue was initially subjected to triplicate liquid-liquid extractions using ethyl acetate. Combined extracts were further purified by collecting the fractions of interest from an Agilent 1100 HPLC system equipped with a 25 cm × 4.6 mm × 5 µm LiChrosorb® Diol-5 HPLC column under normal-phase conditions. Purified samples were subsequently derivatized with heptafluoroacetic anhydride and injected onto an Agilent 7013A gas chromatography/triple quadrupole mass spectrometer (GC/MS/MS) with chromatographic separations performed on the interfaced Agilent 7890B GC. A 2 -µl splitless injection was used on a 30.0 m × 0.25 mm × 0.25 µm installed HP-5MS GC column.
Twenty percent of the injections were performed in duplicate. The mean intra-assay coefficients of variation for the subset of samples (20% of the entire sample set) run in duplicate were 4.8% for pregnenolone, 2.6% for allopregnanolone, 12.1% for pregnanolone, 4.7% for epiallopregnanolone, 7.9% for epipregnanolone, 7.2% for THDOC, and 6.5% for androsterone. Multiple reactions monitoring transition states were optimized for precursor and product ions using the two most intense precursor-to-product ion transitions for each analyte. The more intense transition was used for target ion quantitation, and the second one was used as the reference ion for structural confirmation (qualifier ion) and added specificity. All neurosteroid measurements were conducted blind to group condition.
Statistical Analysis
The descriptive data are presented as means, standard deviations, minimums, lower quartiles (Q1), medians, upper quartiles (Q3), and maximums separately for males and females. Differences in the levels of neurosteroids between the subjects with PTSD and controls were assessed in R (version 3.4.4, 2018-03-15).44 The initial analyses stratified the data by gender and compared the two groups using Wilcoxon rank-sum tests. Subsequently, linear regression analyses controlling for smoking status, PMI, and age, which have been shown to affect postmortem and/or neurosteroid measures,17,45,46 were used to examine the data for each neurosteroid in all of the subjects. Because neurosteroid levels (and possibly also the underlying pathophysiology of PTSD) differ according to gender,46,47 between-group differences were next examined within each gender using the estimated marginal means that were derived from the regression. Prior to the linear regression analyses, the data for each neurosteroid were transformed to normality using a Box–Cox transformation48 via the following formula: T(Y) = (Yλ − 1)/λ, where Y is the neurosteroid level, and λ is the transformation parameter. For λ = 0, the formula ln (Y + 1) was used in place of the above formula. λ values that ranged from −2 to 2 with 0.1 increments were used to transform each neurosteroid level, and the appropriate λ was selected based on the highest Shapiro–Wilk test statistic and the significance value closest to 1.0 (indicating that the transformed values were normally distributed). p values < 0.05 were considered statistically significant, and p values > 0.05 and < 0.10 were reported as trends. Effect sizes for significant comparisons were calculated based on p values and number of subjects.49
Results
Neurosteroid Levels in the mOFC
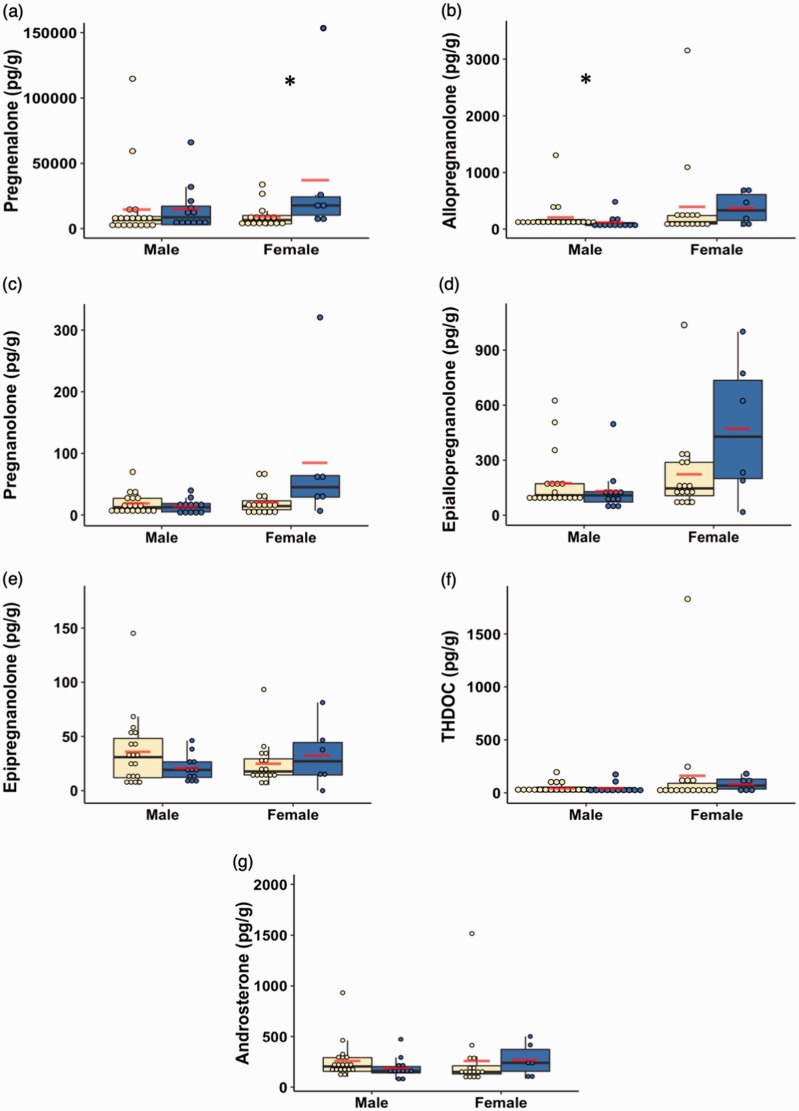
The neurosteroids pregnenolone, allopregnanolone, pregnanolone, epiallopregnanolone, epipregnanolone, THDOC, and androsterone were all detected at physiologically relevant concentrations in the mOFC of the subjects with PTSD and controls (see Table 2 and Figure 1).42
Table 2.
Neurosteroid concentrations (pg/g) in the medial orbitofrontal cortex.
| N | Mean | SD | Min | Q1 | Median | Q3 | Max | |
|---|---|---|---|---|---|---|---|---|
| Pregnenolone | 53 | 15,752.2 | 26,502.4 | 912.4 | 3,751.9 | 7,026.4 | 14,521.9 | 146,702.8 |
| Allopregnanolone | 53 | 262.7 | 473.2 | 37.2 | 91.8 | 112.6 | 223.2 | 3,152.3 |
| Pregnanolone | 53 | 26.1 | 44.9 | 0.0 | 8.2 | 13.4 | 29.0 | 320.6 |
| Epiallopregnanolone | 53 | 213.9 | 226.9 | 16.8 | 92.2 | 121.2 | 188.6 | 1,044.9 |
| Epipregnanolone | 53 | 28.9 | 25.4 | 0.0 | 12.8 | 20.6 | 37.7 | 145.2 |
| THDOC | 53 | 83.6 | 251.3 | 0.0 | 12.0 | 28.3 | 83.7 | 1,831.6 |
| Androsterone | 53 | 244.0 | 228.1 | 70.7 | 141.4 | 185.6 | 267.7 | 1,515.7 |
SD: standard deviation; Min: minimum; Q1: quartile 1; Q3: quartile 3; Max: maximum; THDOC: tetrahydrodeoxycorticosterone.
Figure 1.
Box and dot plots of the neurosteroid concentrations in units of (pg/g) (a. pregnenolone, b. allopregnanolone, c. pregnanolone, d. epiallopregnanolone, e. epipregnanolone, f. THDOC, g. androsterone) stratified by sex and group. Yellow indicates control subjects, and blue indicates subjects with post-traumatic stress disorder. The lines indicate median values, while the lower and upper box boundaries indicate first and third quartiles, respectively. The red lines indicate group mean values. *p < 0.05.
THDOC: tetrahydrodeoxycorticosterone.
Group Differences in Neurosteroids Stratified by Sex
Primary Analysis
In the primary analysis (see Tables 3 and 4, Figure 1), the raw data for each neurosteroid were stratified by gender and compared between the two groups. The results revealed that, compared to the control subjects, males with PTSD had significantly decreased levels of allopregnanolone (p = 0.03, effect size = 0.8307) and a trend for decreased levels of androsterone (p = 0.08, effect size = 0.6666; Table 3). Females with PTSD had significantly increased levels of pregnenolone (p = 0.03, effect size = 1.0964) and a trend for increased levels of pregnanolone (p = 0.07, effect size = 0.9129; Table 4).
Table 3.
Unadjusted neurosteroid concentrations (pg/g) in the medial orbitofrontal cortex among males.
| N | Mean | SD | Min | Q1 | Median | Q3 | Max | p valuea | |
|---|---|---|---|---|---|---|---|---|---|
| Pregnenolone | |||||||||
| Control | 19 | 14,824.0 | 27,356.3 | 912.4 | 3,650.3 | 6,678.0 | 9,305.8 | 114,766.7 | |
| PTSD | 12 | 15,246.3 | 18,440.3 | 2,520.2 | 2,957.8 | 8,714.4 | 17,235.6 | 66,197.5 | |
| Overall | 31 | 14,987.4 | 23,953.0 | 912.4 | 3,271.7 | 7,026.4 | 13,777.4 | 114,766.7 | 0.516 |
| Allopregnanolone | |||||||||
| Control | 19 | 208.1 | 280.2 | 72.0 | 99.0 | 105.5 | 166.6 | 1,302.3 | |
| PTSD | 12 | 125.2 | 120.0 | 37.6 | 63.8 | 90.2 | 113.4 | 479.9 | |
| Overall | 31 | 176.0 | 232.6 | 37.6 | 90.9 | 101.4 | 161.8 | 1,302.3 | 0.032 * |
| Pregnanolone | |||||||||
| Control | 19 | 19.3 | 16.1 | 1.9 | 10.1 | 12.6 | 27.1 | 69.8 | |
| PTSD | 12 | 14.3 | 11.4 | 0.0 | 5.1 | 12.7 | 18.6 | 39.7 | |
| Overall | 31 | 17.4 | 14.5 | 0.0 | 7.8 | 12.6 | 23.0 | 69.8 | 0.465 |
| Epiallopregnanolone | |||||||||
| Control | 19 | 175.8 | 152.5 | 79.8 | 92.9 | 108.5 | 171.2 | 624.9 | |
| PTSD | 12 | 133.0 | 121.9 | 37.4 | 71.6 | 107.8 | 128.0 | 496.7 | |
| Overall | 31 | 159.3 | 140.9 | 37.4 | 91.8 | 108.5 | 165.2 | 624.9 | 0.44 |
| Epipregnanolone | |||||||||
| Control | 19 | 35.9 | 32.8 | 5.5 | 12.0 | 30.9 | 48.2 | 145.2 | |
| PTSD | 12 | 20.9 | 11.9 | 7.3 | 12.3 | 19.2 | 26.5 | 46.2 | |
| Overall | 31 | 30.1 | 27.4 | 5.5 | 12.0 | 23.0 | 40.2 | 145.2 | 0.23 |
| THDOC | |||||||||
| Control | 19 | 43.1 | 50.5 | 2.9 | 8.8 | 20.8 | 53.8 | 195.3 | |
| PTSD | 12 | 41.8 | 51.2 | 0.0 | 10.7 | 22.7 | 48.3 | 173.7 | |
| Overall | 31 | 42.6 | 49.9 | 0.0 | 8.8 | 20.8 | 50.7 | 195.3 | 0.84 |
| Androsterone | |||||||||
| Control | 19 | 258.6 | 183.5 | 100.4 | 156.1 | 203.5 | 290.3 | 930.5 | |
| PTSD | 12 | 189.4 | 106.9 | 70.7 | 140.2 | 158.5 | 202.8 | 472.0 | |
| Overall | 31 | 231.8 | 159.9 | 70.7 | 149.9 | 191.5 | 259.2 | 930.5 | 0.081 |
SD: standard deviation; Min: minimum; Q1: quartile 1; Q3: quartile 3; Max: maximum; PTSD: post-traumatic stress disorder; THDOC: tetrahydrodeoxycorticosterone.
Statistical test based on Wilcoxon rank-sum test.
p < 0.05. Significant p-values are bolded.
Table 4.
Unadjusted neurosteroid levels (pg/g) in the medial orbitofrontal cortex among females.
| N | Mean | SD | Min | Q1 | Median | Q3 | Max | p valuea | |
|---|---|---|---|---|---|---|---|---|---|
| Pregnenolone | |||||||||
| Control | 16 | 9,189.1 | 8,933.3 | 1,702.6 | 3,636.6 | 6,651.8 | 10,077.9 | 33,828.7 | |
| PTSD | 6 | 37,205.4 | 54,121.1 | 6,543.5 | 10,307.8 | 17,739.2 | 24,442.4 | 146,702.8 | |
| Overall | 22 | 16,829.9 | 30,290.3 | 1,702.6 | 4,698.8 | 7,301.0 | 15,273.4 | 146,702.8 | 0.033 * |
| Allopregnanolone | |||||||||
| Control | 16 | 391.3 | 776.6 | 55.3 | 89.2 | 120.7 | 237.8 | 3,152.3 | |
| PTSD | 6 | 368.1 | 286.1 | 37.2 | 152.2 | 328.5 | 611.2 | 715.6 | |
| Overall | 22 | 385.0 | 67.1 | 37.2 | 94.1 | 163.8 | 263.1 | 3,152.3 | 0.376 |
| Pregnanolone | |||||||||
| Control | 16 | 20.9 | 19.9 | 0.0 | 8.6 | 14.5 | 23.0 | 71.3 | |
| PTSD | 6 | 85.2 | 117.3 | 6.7 | 29.1 | 45.1 | 63.9 | 320.6 | |
| Overall | 22 | 38.4 | 66.5 | 0.0 | 9.3 | 20.0 | 32.1 | 320.6 | 0.071 |
| Epiallopregnanolone | |||||||||
| Control | 16 | 222.6 | 237.8 | 54.7 | 106.1 | 145.9 | 287.9 | 1,044.9 | |
| PTSD | 6 | 472.5 | 384.2 | 16.8 | 199.6 | 427.9 | 735.4 | 1,001.0 | |
| Overall | 22 | 290.8 | 297.5 | 16.8 | 112.5 | 164.6 | 317.2 | 1,044.9 | 0.160 |
| Epipregnanolone | |||||||||
| Control | 16 | 25.1 | 20.7 | 5.2 | 14.5 | 17.7 | 29.3 | 93.3 | |
| PTSD | 6 | 32.7 | 29.2 | 0.0 | 14.5 | 27.1 | 44.4 | 81.3 | |
| Overall | 22 | 27.1 | 22.8 | 0.0 | 14.1 | 17.8 | 35.0 | 93.3 | 0.560 |
| THDOC | |||||||||
| Control | 16 | 163.8 | 449.4 | 8.6 | 14.7 | 23.4 | 89.7 | 1,831.6 | |
| PTSD | 6 | 81.7 | 68.9 | 0.0 | 35.7 | 68.1 | 128.9 | 180.2 | |
| Overall | 22 | 141.4 | 383.1 | 0.0 | 15.3 | 29.3 | 92.1 | 1,831.6 | 0.420 |
| Androsterone | |||||||||
| Control | 16 | 259.0 | 346.1 | 80.5 | 129.9 | 149.2 | 210.0 | 1,515.7 | |
| PTSD | 6 | 267.5 | 161.9 | 82.7 | 156.1 | 238.6 | 371.5 | 500.6 | |
| Overall | 22 | 261.3 | 303.1 | 80.5 | 130.1 | 161.2 | 260.9 | 1,515.7 | 0.417 |
SD: standard deviation; Min: minimum; Q1: quartile 1; Q3: quartile 3; Max: maximum; PTSD: post-traumatic stress disorder; THDOC: tetrahydrodeoxycorticosterone.
Statistical test based on Wilcoxon rank-sum test.
p < 0.05. Significant p-values are bolded.
Linear Regression Models Adjusting for Potential Confounds
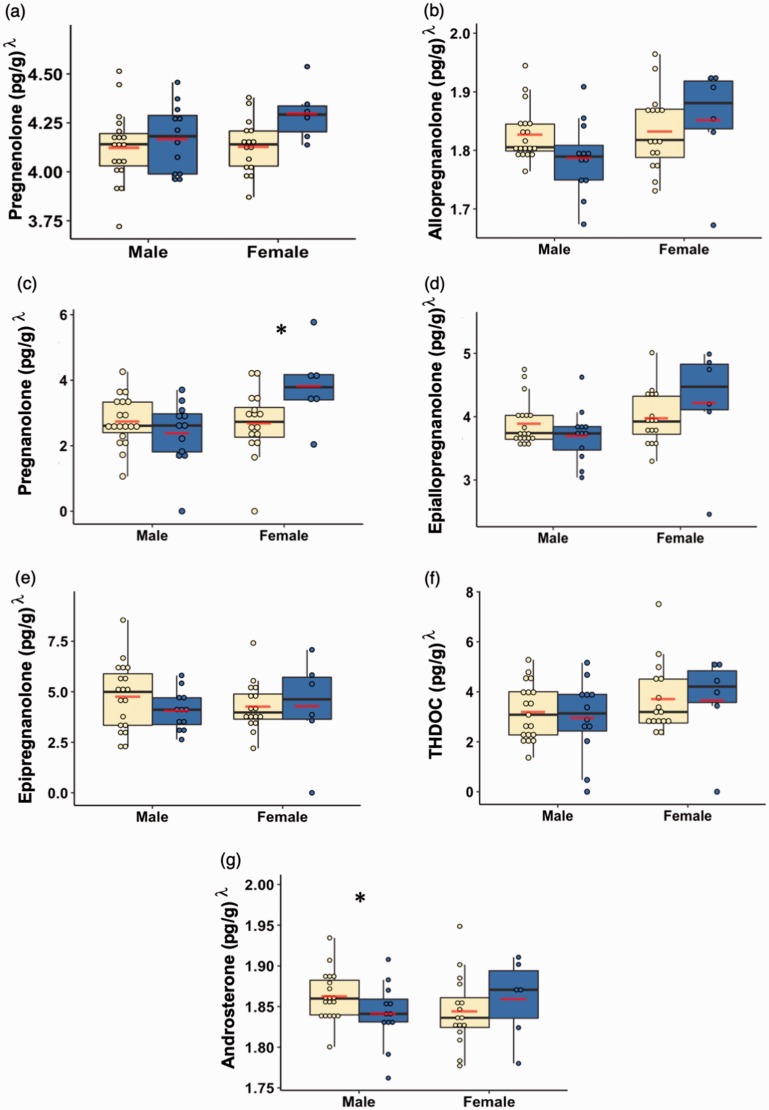
We further analyzed the data for each neurosteroid by first transforming the data to normality using a Box–Cox transformation and then using a linear regression model that controlled for age, PMI, and smoking status. Between-group differences were then examined within each gender using the estimated marginal means derived from the regression. As shown in Figure 2 (Box–Cox transformed data with adjusted p values shown), compared to the control subjects, males with PTSD exhibited significantly decreased levels of androsterone (t46 = 2.37, p = 0.02, effect size = 0.6788) and a trend for decreased levels of allopregnanolone (t46 = 1.82, p = 0.08, effect size = 0.8932), while females with PTSD only had significantly increased levels of pregnanolone (t46 = −2.25, p = 0.03, effect size = 1.1215).
Figure 2.
Box and dot plots of the neurosteroid concentrations in units of (pg/g)λ (a. pregnenolone, b. allopregnanolone, c. pregnanolone, d. epiallopregnanolone, e. epipregnanolone, f. THDOC, g. androsterone) stratified by gender and group after Box–Cox transformation and controlling for age, postmortem interval, and smoking status. Yellow indicates control subjects, and blue indicates subjects with post-traumatic stress disorder. The lines indicate median values, while the lower and upper box boundaries indicate first and third quartiles, respectively. The red lines indicate group mean values. *p < 0.05.
THDOC: tetrahydrodeoxycorticosterone.
Discussion
To our knowledge, this study is the first to quantify neurosteroids in postmortem brain tissue of subjects with PTSD. The results of this preliminary study in the mOFC showed that the levels of androsterone (and to a lesser extent allopregnanolone) were decreased in males with PTSD compared with control males. Conversely, levels of pregnanolone were significantly increased in females with PTSD compared with control females. These results suggest that the levels of some neurosteroids may be altered in the mOFC of subjects with PTSD and that these alterations may differ by sex.
Previous reports have suggested that neurosteroids are decreased in subjects with PTSD.16,19,23 For example, we previously reported that the serum levels of pregnenolone, allopregnanolone, and androsterone were significantly decreased in male patients with PTSD.50 Similarly, a recent study has shown that the levels of allopregnanolone and pregnanolone in cerebrospinal fluid are negatively correlated with total PTSD symptoms in males with PTSD.19 The present results in human postmortem brain tissue showing a significant reduction of androsterone and allopregnanolone in males with PTSD are consistent with these prior observations in peripheral samples. Although preliminary, the present results potentially converge to suggest a dysregulation of androsterone in males with PTSD in at least one brain region previously linked with PTSD. It is unclear why we found a significant increase in the levels of pregnanolone in females with PTSD. However, multiple studies have suggested that steroid levels exhibit sex differences51 and that the pathophysiological mechanisms underlying PTSD in females may differ from those in males.52 Additionally, as discussed in the limitations below, there are numerous potential confounds that impact the interpretations of male versus female findings in PTSD.
A number of neurosteroids, including allopregnanolone, pregnanolone, and androsterone act as positive allosteric modulators of the GABAA receptor.6 Changes in this receptor and its modulators are thought to reflect the pathophysiological mechanisms that underlie the anxiety observed in patients with PTSD.13 The results of multiple studies have suggested that the levels of neurosteroids, including allopregnanolone, decrease in response to chronic stress.21 Thus, our observed changes in the levels of neurosteroids in subjects with PTSD may reflect evidence that subjects with PTSD have impaired GABAergic neurotransmission, as reviewed elsewhere.6,15,16
This preliminary study had a number of limitations. First, there was no information regarding menstrual status or whether subjects were taking supplemental hormones, all of which may have an effect on neurosteroid levels. For example, neurosteroid levels differ according to a female’s menstrual, menopausal, and oral contraceptive use status,2,17,23 which might have uniquely impacted the female PTSD group.27 In addition, selective serotonin reuptake inhibitors, which are used to treat patients with PTSD, have been shown to induce allopregnanolone synthesis.53,54 Second, the females with PTSD were slightly younger (age range: 35–51 years) than the female controls (age range: 44–57 years). Such an age difference could contribute to our finding of significantly higher levels of pregnanolone in the female subjects with PTSD since neurosteroids have been shown to decrease with age.55 Third, smoking status differed significantly between the groups. In the control group, 20% of the subjects smoked, while 94.4% of the subjects with PTSD smoked. Subjects with PTSD have a higher incidence of smoking compared with that of the general population,56 and smoking status needs to be accounted for in studies of PTSD.15 However, previous findings that smoking is positively associated with allopregnanolone levels57 suggest that our findings of decreased levels of allopregnanolone in male subjects with PTSD were not due to smoking and might actually underestimate the true levels in these subjects.
Because of these limitations and the relatively small number of samples included, the current results should be viewed as preliminary as they await replication in a larger sample of subjects carefully matched for age, smoking status, and PMI. Furthermore, neurosteroid levels should be measured in other brain regions, such as the prefrontal cortex, amygdala, and hippocampus, regions that have been directly implicated in the pathophysiology of PTSD.27
Future directions include expanding the number of samples in these cohorts and examining neurosteroid expression levels in subjects with other diagnoses, such as major depression, as well as measuring the activity of enzymes involved in the production of neurosteroids, in order to gain better insight into the sex differences of neurosteroid levels in subjects with PTSD.
Conclusion
Consistent with prior and emerging literature, these preliminary results suggest that males with PTSD exhibit differences in the levels of some neurosteroids, particularly androsterone and allopregnanolone, in the mOFC. More extensive investigations of neurosteroids in larger samples and additional brain regions are needed.
Acknowledgments
The authors would like to acknowledge the valuable input in the planning and design of this project by the members of the Department of Veterans Affairs National Center for PTSD Brain Bank.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Marx is a coapplicant on pending U.S. patent applications focusing on the use of neurosteroids for the treatment of central nervous system disorders; no patents issued; no licensing in place; VA 208 waiver in place.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided in part by 5I01CX001141 from the U.S. Department of Veterans Affairs (Williamson), VA Merit Review 1101RX000571-01 (PI Marx), VA Merit Review 1I01CX000794-01 (PI Marx), and VA Mid-Atlantic Mental Illness Research, Education, and Clinical Center/MIRECC (PI Fairbank).
References
- 1.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990; 37: 395–403. [DOI] [PubMed] [Google Scholar]
- 2.Rasmusson AM, Marx CE, Pineles SL, et al. Neuroactive steroids and PTSD treatment. Neurosci Lett 2017; 649: 156–163. [DOI] [PubMed] [Google Scholar]
- 3.Paul SM, Purdy RH. Neuroactive steroids. FASEB J 1992; 6: 2311–2322. [PubMed] [Google Scholar]
- 4.Diotel N, Charlier TD, Lefebvre d’Hellencourt C, et al. Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Front Neurosci 2018; 12: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen RW. GABAA receptor: positive and negative allosteric modulators. Neuropharmacology 2018; 136: 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol 2015; 36: 28–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnell ES, Irvine M, Fang G, Sapkota K, Jane DE, Monaghan DT. Positive and negative allosteric modulators of N-methyl-D-aspartate (NMDA) receptors: structure–activity relationships and mechanisms of action. J Med Chem 2019; 62: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schverer M, Lanfumey L, Baulieu E-E, Froger N, Villey I. Neurosteroids: non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacol Ther 2018; 191: 190–206. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs TT, Farb DH. Direct modulation of amino acid receptors by neuroactive steroids: physiological and pharmacological implications. In: Smith SS. (ed). Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor, Boca Raton, FL: CRC Press LLC, 2004, pp. 339–358. [Google Scholar]
- 10.Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA A receptor function and seizure susceptibility. J Neurosci 2002; 22: 3795–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossetti MF, Cambiasso MJ, Holschbach MA, Cabrera R. Oestrogens and progestagens: synthesis and action in the brain. J Neuroendocrinol 2016; 28. [DOI] [PubMed] [Google Scholar]
- 12.Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol 2014; 113: 79–87. [DOI] [PubMed] [Google Scholar]
- 13.Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat 2015; 11: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pineles SL, Nillni YI, Pinna G, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 2018; 93: 133–141. [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson AM, Pineles SL. Neurotransmitter, peptide, and steroid hormone abnormalities in PTSD: biological endophenotypes relevant to treatment. Curr Psychiatry Rep 2018; 20: 52. [DOI] [PubMed] [Google Scholar]
- 16.Locci A, Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol 2017; 174: 3226–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marx CE, Stevens RD, Shampine LJ, et al. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology 2006; 31: 1249–1263. [DOI] [PubMed] [Google Scholar]
- 18.Marx CE, Naylor JC, Kilts JD, et al. Neurosteroids and traumatic brain injury: translating biomarkers to therapeutics; overview and pilot investigations in Iraq and Afghanistan era veterans. In: Laskowitz D, Grant G. (eds). Translational Research in Traumatic Brain Injury, Boca Raton, FL: CRC Press/Taylor and Francis Group, 2016. [PubMed] [Google Scholar]
- 19.Rasmusson AM, King MW, Valovski I, et al. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology 2019; 102: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology 2013; 38: 1895–1911. [DOI] [PubMed] [Google Scholar]
- 21.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A 2008; 105: 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinna G. In a mouse model relevant for post-traumatic stress disorder, selective brain steroidogenic stimulants (SBSS) improve behavioral deficits by normalizing allopregnanolone biosynthesis. Behav Pharmacol 2010; 21: 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmusson AM, Pinna G, Paliwal P, et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 2006; 60: 704–713. [DOI] [PubMed] [Google Scholar]
- 24.Cho I, Cho YJ, Kim HW, Heo K, Lee BI, Kim WJ. Effect of androsterone after pilocarpine-induced status epilepticus in mice. J Epilepsy Res 2014; 4: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Servatius RJ, Marx CE, Sinha S, et al. Brain and serum androsterone is elevated in response to stress in rats with mild traumatic brain injury. Front Neurosci 2016; 10: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duman RS, Girgenti MJ. Molecular and cellular studies of PTSD: postmortem transcriptome analysis and novel therapeutic targets. J Neurosci Res 2019; 97: 292–299. [DOI] [PubMed] [Google Scholar]
- 27.Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci 2018; 19: 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logue MW, van Rooij SJH, Dennis EL, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry 2018; 83: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety 2007; 24: 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhe X, Liu K, Mu YF, et al. Decreased regional cerebral perfusion at resting state in acute posttraumatic stress disorder resulting from a single, prolonged stress event. Acad Radiol 2016; 23: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 31.Jackowski AP, Araújo Filho GM, Almeida AG, et al. The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings. Braz J Psychiatry 2012; 34: 207–212. [DOI] [PubMed] [Google Scholar]
- 32.Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 2004; 61: 168–176. [DOI] [PubMed] [Google Scholar]
- 33.Lindauer RJ, Booij J, Habraken JB, et al. Cerebral blood flow changes during script-driven imagery in police officers with posttraumatic stress disorder. Biol Psychiatry 2004; 56: 853–861. [DOI] [PubMed] [Google Scholar]
- 34.Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 2012; 216: 103–109. [DOI] [PubMed] [Google Scholar]
- 35.Young KA, Thompson PM, Cruz DA, Williamson DE, Selemon LD. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol Stress 2015; 2: 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 2006; 60: 376–382. [DOI] [PubMed] [Google Scholar]
- 37.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex 2000; 10: 284–294. [DOI] [PubMed] [Google Scholar]
- 38.Jovanovic T, Norrholm SD, Fennell JE, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res 2009; 167: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, Olff M. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev 2015; 51: 189–204. [DOI] [PubMed] [Google Scholar]
- 40.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59: 22–33. [PubMed] [Google Scholar]
- 41.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, Washington, DC: Author, 1994. [Google Scholar]
- 42.Naylor JC, Hulette CM, Steffens DC, et al. Cerebrospinal fluid dehydroepiandrosterone levels are correlated with brain dehydroepiandrosterone levels, elevated in Alzheimer’s disease, and related to neuropathological disease stage. J Clin Endocrinol Metab 2008; 93: 3173–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marx CE, Lee J, Subramaniam M, et al. Proof-of-concept randomized controlled trial of pregnenolone in schizophrenia. Psychopharmacology (Berl) 2014; 231: 3647–3662. [DOI] [PubMed] [Google Scholar]
- 44.Team RC. R: a language and environment for statistical computing. http://www.r-project.org. Accessed March 4, 2019.
- 45.Nagy C, Maheu M, Lopez JP, et al. Effects of postmortem interval on biomolecule integrity in the brain. J Neuropathol Exp Neurol 2015; 74: 459–469. [DOI] [PubMed] [Google Scholar]
- 46.Del Río JP, Alliende MI, Molina N, Serrano FG, Molina S, Vigil P. Steroid hormones and their action in women’s brains: the importance of hormonal balance. Front Public Heal 2018; 6: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolea-Alamanac B, Bailey SJ, Lovick TA, Scheele D, Valentino R. Female psychopharmacology matters! Towards a sex-specific psychopharmacology. J Psychopharmacol 2018; 32: 125–133. [DOI] [PubMed] [Google Scholar]
- 48.Box GEP, Cox DR. An analysis of transformations. J R Stat Soc Ser B 1964; 26: 211–252. [Google Scholar]
- 49.Lipsey MW, Wilson DB. Practical meta-analysis, Thousand Oaks, CA: Sage Publications, 2001. [Google Scholar]
- 50.Marx C, Naylor J, Kilts J, et al. Neurosteroids and inflammatory markers in PTSD and TBI. Biol Psychiatry 2017; 81: S304. [Google Scholar]
- 51.Giatti S, Garcia-Segura LM, Barreto GE, Melcangi RC. Neuroactive steroids, neurosteroidogenesis and sex. Prog Neurobiol 2018, pp. pii: S0301–0082(18)30008–X. [DOI] [PubMed] [Google Scholar]
- 52.Kimerling R, Allen MC, Duncan LE. Chromosomes to social contexts: sex and gender differences in PTSD. Curr Psychiatry Rep 2018; 20: 114. [DOI] [PubMed] [Google Scholar]
- 53.Marx CE, Shampine LJ, Khisti RT, et al. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav 2006; 84: 609–617. [DOI] [PubMed] [Google Scholar]
- 54.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A 1996; 93: 12599–12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morley JE, Kaiser F, Raum WJ, et al. Potentially predictive and manipulable blood serum correlates of aging in the healthy human male: progressive decreases in bioavailable testosterone, dehydroepiandrosterone sulfate, and the ratio of insulin-like growth factor 1 to growth hormone. Proc Natl Acad Sci U S A 1997; 94: 7537–7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001; 70: 561–570. [DOI] [PubMed] [Google Scholar]
- 57.Marx CE, Trost WT, Shampine L, et al. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006; 186: 462–472. [DOI] [PubMed] [Google Scholar]