Abstract
Background
The aim of this study was to explore the effect of dexmedetomidine (DEX)-mediated insulin-like growth factor 2 (IGF2) signal pathway on immune function and cancer cell invasion and migration in rats with ovarian cancer.
Material/Methods
Forty rats with ovarian cancer were divided into 4 groups: model group, and low dose (0.2 μg/kg/hour DEX), medium dose (1.0 μg/kg/hour DEX), and high dose (5.0 μg/kg/hour DEX) DEX groups. In addition, 10 Fischer344 rats were selected as a normal group. Human NUTU-19 poorly differentiated epithelial ovarian cancer cell line cells were divided into 4 groups: a blank group and low dose, medium dose, and high dose DEX NUTU-19 groups.
Results
Compared with the normal group, in the other groups the serum interleukin (IL)-2 and interferon gamma (INF-γ) levels, CD4+ and CD8+ percentages, CD4+/CD8+ ratio, and transformation rate of splenic lymphocytes were decreased, and the serum tumor necrosis factor alpha (TNF-α) level, IGF2, insulin-like growth factor 1 receptor (IGF1R), insulin receptor substrate 1 (IRS1) mRNA, and protein expressions in ovarian tissue were increased (all P<0.05). Results in the DEX groups compared with model group were the opposite of those in the other groups compared with normal group (all P<0.05). Compared with the blank group, in the other groups the proliferation, invasion, and migration of ovarian cancer cells were reduced significantly (all P<0.05). Compared with the low dose DEX NUTU-19 group, in the high dose DEX NUTU-19 group the invasion and migration of ovarian cancer cells weakened significantly (both P<0.05).
Conclusions
A certain dose of DEX can effectively inhibit IGF2 signal pathway activation to improve the immune function of rats with ovarian cancer, inhibiting the invasion and migration of ovarian cancer cells.
MeSH Keywords: Dexmedetomidine, Neoplasm Invasiveness, Ovarian Neoplasms
Background
Ovarian cancer is a common gynecological malignant tumor, with an incidence rate lower than uterine cancer and cervical cancer [1,2]. Because of the imperfection of early screening methods, most patients have been in an advanced stage when they are diagnosed with ovarian cancer, resulting in a high mortality rate [3]. Surgery and chemotherapy are the important means of clinical treatment for ovarian cancer at present [4]. Previous studies have confirmed that the immune system function in patients with ovarian cancer is significantly inhibited at the onset, and immune activity factors and cell function are significantly reduced, which further aggravates the growth and migration of tumor cells [5–7].
Dexmedetomidine (DEX) is currently a widely used drug for sedation in intensive care units and used in clinical anesthesia [8]. As a highly selective α2 adrenergic receptor agonist, DEX can combine with α2 receptors in the spinal cord and brainstem nucleus ceruleus to play the role of analgesia and anti-anxiety [9]. Compared with sedative drugs, such as propofol and fentanyl, the advantage of DEX is that it does not cause respiratory depression in patients [10]. At present, there are many studies on DEX in anesthesia, sedation, and organ protection, and few studies in tumor treatment.
The insulin-like growth factor 2 (IGF2) signal pathway is an important pathway in the course of cancer [11]. Lau et al. found that the IGF2 signal pathway and its key proteins play a major role in cisplatin-resistant ovarian cancer [12]. However, it is unclear whether the IGF2 signal pathway is affected by related drugs and whether it affects the biological characteristics of tumors.
Based on the molecular mechanism of pharmacotherapy, this study explored the effect of DEX-mediated IGF2 signal pathway on immune function and the invasion and migration of cancer cells in rats with ovarian cancer.
Material and Methods
Materials
Fifty adult female Fischer344 rats of clean grade (provided by the Beijing Vital River Laboratory Animal Technology Co., Ltd., China) with the weight of 140±10 g were used in this experiment after 1-week acclimating feeding. This study was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center, Guangzhou Medical University (protocol number 2016050213, approved May 2, 2016).
Establishment and grouping of ovarian cancer rat models
Ten Fischer344 rats were selected as the normal group, and the remaining 40 rats were used to construct the ovarian cancer rat model. The concentration of NUTU-19 poorly differentiated epithelial ovarian cancer cells in the logarithmic growth phase (purchased from the Shanghai Qiaodu Biotechnology Co., Ltd., China) was adjusted to 2×107 cells/mL, and 0.5 mL cell suspension was inoculated subcutaneously in the right armpit of each rat. The criterion of a successful model is that the rat has dry hair, significantly reduced weight and decreased physical activity. Rats were divided into 4 groups when the tumor in the right armpit grew to 0.4×0.4 cm2: a model group, a low dose DEX group (0.2 μg/kg/hour DEX), a medium dose DEX group (1.0 μg/kg/hour DEX), and a high dose DEX group (5.0 μg/kg/hour DEX). The rats were administrated DEX (purchased from the Cisen Pharmaceutical Co., Ltd., China) by intraperitoneal injection once a day for 15 days. Rats in the normal group and model group were injected with the same amount of normal saline [13].
Determination of levels of serum interleukin (IL)-2, interferon gamma (INF-γ), and tumor necrosis factor alpha (TNF-α) by enzyme-linked immunosorbent assay (ELISA)
The rats were anesthetized on the twenty-fifth day of this experiment and then extracted 5.0 mL aortic blood from the abdomen. The blood was placed in the centrifuge tube for 2 hours, and centrifuged with 3000 rpm at 4°C for 15 minutes. The serum was separated into 1.5 mL Eppendorf tubes and stored in a refrigerator at -20°C. The levels of interleukin (IL)-2, interferon gamma (INF-γ), and tumor necrosis factor alpha (TNF-α) in serum of rats were determined by ELISA kits (IL-2: ab223588, Abcam, UK; INF-γ: 1009251, Shanghai Westang Bio-Tech Co., Ltd., China; TNF-α: ab208348, Abcam, UK) according to the kit instruction.
Detection of distributions of serum T lymphocyte subsets (CD4+ and CD8+) by flow cytometry
The rats were anesthetized on the twenty-fifth day of this experiment and then we extracted 5 μL aortic blood from the rat abdomen. The blood was placed in flow tubes and then we added anticoagulant. Subsequently, 5 μL FITC-CD4 (ab218745, Abcam, UK) and PE-CD8 (ab37928, Abcam, UK) were added to the flow tubes. The blood was incubated for 15 minutes in a dark place. Then the blood was well mixed with 500 μL red blood cells lysis buffer and placed in a dark place for 15 minutes. Centrifugation was performed at 2000 rpm for 15 minutes. The supernatant was discarded. The sediment was washed twice with 500 μL phosphate-buffered saline (PBS). Finally, 500 μL PBS was added as a solvent to mix the sediment. The percentages of CD4+ and CD8+ were measured by flow cytometry (Cytomics FC 500, Beckman, Germany), and the CD4+/CD8+ ratio was calculated [14].
Detection of transformation rate of splenic lymphocytes by MTT assay
Rats were put to death by cervical dislocation on the twenty-fifth day of this experiment. Rats were soaked with 0.5% povidone iodine for 5 minutes. Then the spleens of the rats were removed and shredded under aseptic conditions and placed in sterile PBS. After grinding, the solution was filtered through a mesh size of 200 to prepare single cell suspensions. Centrifugation was performed at 1500 rpm for 10 minutes. The supernatant was discarded. Sediment was added to 10 mL NH4Cl and incubation in a water bath was performed at 37°C for 10 minutes. The sediment was then washed with 500 μL PBS, 3 times. Cells were suspended in 2 mL medium, and the concentration was adjusted to 2×106/mL. The prepared cell suspension was added to a 96-well culture plate at 100 μL per well, and the blank group was set up. The culture plate was placed in an incubator containing 5% CO2 at 37°C for 48 hours. The supernatant was discarded 4 hours before the end of the cell culture period. And 20 μL MTT solutions of 5 mg/mL were added to each well, and the mixture was further cultured for 4 hours. After the cell culture period, 100 μL dimethyl sulfoxide was added to each well, and the mixture was shaken, mixed, and placed in an incubator at 37°C for 30 minutes. Optical density (OD) value at a wavelength of 490 nm was measured by ELISA. The transformation rate of splenic lymphocytes in each group was reflected by stimulation index (SI=OD average value of experimental well/OD average value of control well) [15].
Detection of IGF2, IGF1R, and IRS1 mRNA expressions in ovarian tissue by quantitative real-time polymerase chain reaction (qRT-PCR)
Ovarian tissues from each group were stored at −80°C. After stirring with an electric homogenate machine, the tissues were placed in precooled lysis buffer (Beijing Aviva Systems Biology, China) for lysis on ice. Centrifugation was performed at 1200 rpm for 30 minutes. The supernatant was collected. Total RNA in the lysed tissues was extracted according to the instruction of TRIzol manufacturer (Invitrogen Corporation, USA), and the procedure was carried out according to the instruction of SYBRGreenRT-PCR reagent supplier. The primer sequences of IGF2, insulin-like growth factor 1 receptor (IGF1R), and insulin receptor substrate 1 (IRS1) are shown in Table 1. GAPDH was used as an internal reference. And 50 ng total RNA was synthesized from RNA to cDNA through reverse transcriptase. Then the PCR reaction was performed. The reaction system contained a total of 50 μL, including 25 μL SYBR® Premix Ex Taq™ II (2×), 2 μL forward primer of PCR, 2 μL reverse primer of PCR, l μL ROX Reference Dye (50×), 4 μL DNA templates, and 16 μL ddH2O. Real-time fluorescence quantitative PCR was performed through ABI PRISM 7300 system (Applied Biosystems, USA). Reaction conditions were as follows: pre-denaturation at 95°C for 30 seconds, denaturation at 95°C for 5 seconds, renaturation at 58°C for 30 seconds, and extension at 72°C for 15 seconds, with 40 cycles. The target gene expression level was calculated by 2−ΔΔCt.
Table 1.
Primer sequences of qRT-PCR.
| Item | Sequence |
|---|---|
| IGF2 | Forward primer: 5′-GTGGCATCGTTGAGGAGTG-3′ |
| Reverse primer: 5′-CACCTCCCTCTCGGACTTG-3′ | |
| IGF1R | Forward primer: 5′-ATGCTGACCTCTGTTACCTCT-3′ |
| Reverse primer: 5′-CGCTTATTCCCCACAATGTAGTT-3′ | |
| IRS1 | Forward primer: 5′-TATGCCAGCATCAGTTTCCA 3′ |
| Reverse primer: 5′-GGATTTGCTGAGGTCATTAGG-3′ | |
| GAPDH | Forward primer: 5′-TCTCTACTGGGCATTAGGG -3′ |
| Reverse primer: 5′-GTGTCCGAGGAAGATACTTG-3′ |
IGF2 – insulin-like growth factor 2; IGF1R – insulin-like growth factor 1 receptor; IRS1 – insulin receptor substrate 1.
Detection of IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein expressions in ovarian tissue by western blot
Ovarian tissues from each group were washed twice with PBS for 3 minutes. The tissues were ground in liquid nitrogen, and then were ground into homogenate with the addition of lysis buffer. Centrifugation was performed at 12 000 rpm at 4°C for 30 minutes. The supernatant was collected. Total protein concentration was determined by BCA kit (Shanghai Beyotime Biotechnology, China). Then 50 μg protein were added to 2×SDS loading buffer and boiled at 100°C for 5 minutes. Gel electrophoresis was performed by 10% SDS-PAGE. The protein was transferred to a PVDF membrane by wetting transfer. Then 5% nonfat dried milk was added and then membrane was sealed at room temperature for 1 hour, and incubated with the addition of rabbit anti-mouse primary antibodies IGF2 (1: 500, ab170304, Abcam, UK), IGF1R (1: 500, ab39675, Abcam, UK), p-IGF1R (1: 500, ab39398, Abcam, UK), IRS1 (1: 500, ab52167, Abcam, UK), p-IRS1 (1: 250, ab1194, Abcam, UK), GAPDH (1: 2500, ab9485, Abcam, UK). After rinsing 3 times with TBST, goat anti-rabbit second antibody (1: 2,000, ab6721, Abcam, UK) was added to incubate for 1 hour. Then rinsing with TBST, color development with ECL, x-ray exposure, and photographing were performed. The OD of each band was analyzed by a gel imaging analysis system. The relative content of sample protein=average OD of sample/average OD of the corresponding internal reference. The experiment was repeated 3 times.
Ovarian cancer cell culture
NUTU-19 poorly differentiated epithelial ovarian cancer cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and placed in an incubator containing 5% CO2 and 100% humidity at 37°C. The medium was replaced every 24 hours. A cell passage was performed every 2 days depending on cell growth.
Detection of invasion of ovarian cancer cells by Transwell
A total of 200 μL Matrigel were added to 200 μL serum-free medium (YB356234, Shanghai Yubo Biological Technology Co., Ltd., China) at 4°C and mixed well. Then 50 μL Matrigel was added to Transwell chamber and incubated in an incubator for 2 to 3 hours. After cells were digested and counted, the cell suspension was prepared with 2% serum-containing medium. Then 200 μL cell suspension was added to the Transwell chamber of each well. DEX was added to the culture plates with the final concentration of 1 ng/mL, 10 ng/mL, and 100 ng/mL respectively, in the low dose DEX group, the medium dose DEX group, and the high dose DEX group. And 800 μL conditioned medium containing 20% FBS was added to the culture plate for the blank group. The Transwell plates were incubated at 37°C for 24 hours, then rinsed with PBS for 3 minutes, immersed in formaldehyde for 10 minutes, and washed 3 times with water for 3 minutes each time. Then 0.1% crystal violet staining was performed. After placing at room temperature for 1 hour, the Transwell plates were rinsed with PBS twice, and the cells were wiped off with cotton balls. Transwell plates were air-dried and observed under an inverted microscope (Leica, Germany). Five fields were randomly selected, and the average was calculated [16].
Detection of migration of ovarian cancer cells by scratch adhesion test
A horizontal line was drawn every 0.5 cm behind the 12-well plate by a marker to ensure that at least 5 lines crossed each well. About 5×105 cells were added to ensure that the plate was overspread overnight. The next day, the scratch adhesin test was performed with the assistance of a ruler to ensure to the greatest extent that the pipette tips were perpendicular to the back of the plate to scratch test, avoiding inclining the plate. Rinsing with PBS was performed 3 times, for 1 minute each time. The crossed cells were removed. DEX was added with the final concentration of 1 ng/mL, 10 ng/mL, and 100 ng/mL respectively, for the low dose DEX group, the medium dose DEX group, and the high dose DEX group. Normal saline was added in the blank group. Cells in each group were cultured in an incubator containing 5% CO2 at 37°C. Samples were taken at 0 hours and 48 hours. The scratch width was photographed and analyzed to assess cell migration.
Statistical analysis
SPSS 21.0 statistical software was used to analyze data. All data were expressed as mean ± standard deviation. The comparison between 2 groups in accordance with normal distribution was analyzed by t-test, and the comparison among groups was analyzed by one-way analysis of variance (ANOVA). There was a significant difference at P<0.05.
Results
Levels of serum IL-2, INF-γ, and TNF-α
Levels of serum IL-2, INF-γ, and TNF-α of rats in each group was detected by ELISA (Figure 1). Compared with the normal group, in the other groups the levels of serum IL-2 and INF-γ were significantly decreased, and the TNF-α level was significantly increased (all P<0.05). Compared with the model group, in the low dose DEX group, the medium dose DEX group and the high dose DEX group the levels of serum IL-2 and INF-γ were significantly increased, and the TNF-α level was significantly decreased (all P<0.05). There was no significant difference between the low dose DEX group and the medium dose DEX group (P>0.05). Compared with the low dose DEX group, in the high dose DEX group the levels of serum IL-2 and INF-γ were significantly increased, and the TNF-α level was significantly decreased (P<0.05).
Figure 1.
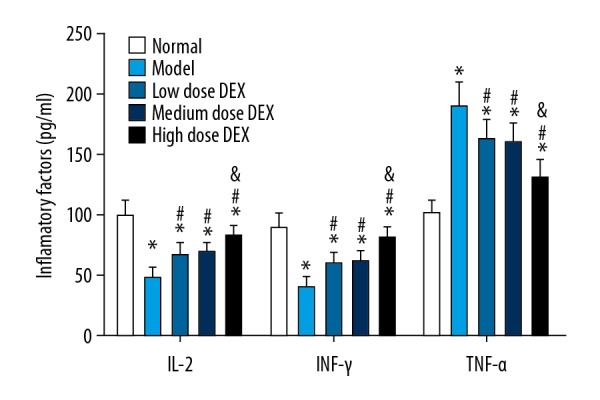
Levels of serum IL-2, INF-γ and TNF-α in each group. Compared with the normal group, * P<0.05; compared with the model group, # P<0.05 compared with the low dose DEX group, & P<0.05. DEX – dexmedetomidine; IL – interleukin; INF – interferon gamma; TNF – tumor necrosis factor.
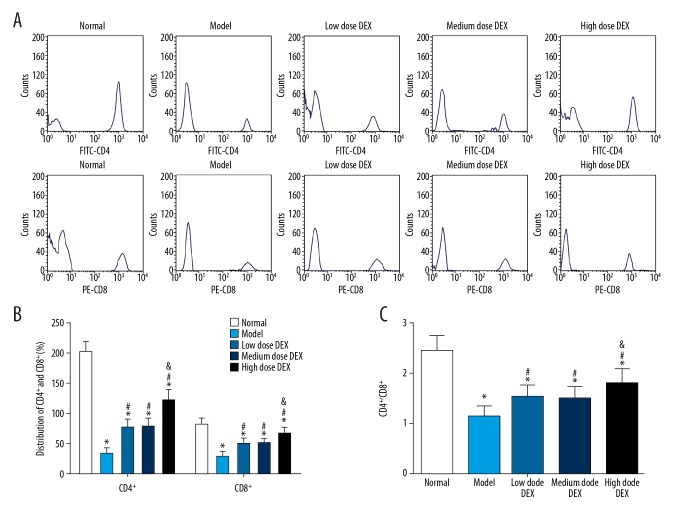
Distributions of serum T lymphocyte subsets (CD4+ and CD8+)
Serum T lymphocyte subsets (CD4+ and CD8+) in each group were detected by flow cytometry (Figure 2). Compared with the normal group, in the other groups the percentages of CD4+ and CD8+ and the CD4+/CD8+ ratios were significantly decreased (all P<0.05). Compared with the model group, in the low dose DEX group, the medium dose DEX group, and the high dose DEX group, the percentages of CD4+ and CD8+ and the CD4+/CD8+ ratios were significantly increased (all P<0.05). There was no significant difference between the low dose DEX group and the medium dose DEX group (all P>0.05). Compared with the low dose DEX group, in the high dose DEX group the percentages of CD4+ and CD8+ and the CD4+/CD8+ ratios were significantly increased (all P<0.05).
Figure 2.
Distributions of serum T lymphocyte subsets (CD4+ and CD8+) in each group: (A) flow diagram of CD4+ and CD8+ in blood of rats in each group; (B) histogram of the percentages of CD4+ and CD8+ in each group; (C) histogram of CD4+/CD8+ ratio in each group. Compared with the normal group, * P<0.05; compared with the model group, # P<0.05; compared with the low dose DEX group, & P<0.05. DEX – dexmedetomidine.
Transformation rate of splenic lymphocytes
Transformation rate of splenic lymphocytes in each group was detected by MTT assay (Figure 3). Compared with the normal group, in the other groups the transformation rates of splenic lymphocytes were significantly decreased (all P<0.05). Compared with the model group, in the low dose DEX group, the medium dose DEX group, and the high dose DEX group the transformation rate of splenic lymphocytes was significantly increased (all P<0.05). There was no significant difference between the low dose DEX group and the medium dose DEX group (all P>0.05). Compared with the low dose DEX group, in the high dose DEX group the transformation rate of splenic lymphocytes was significantly increased (all P<0.05).
Figure 3.
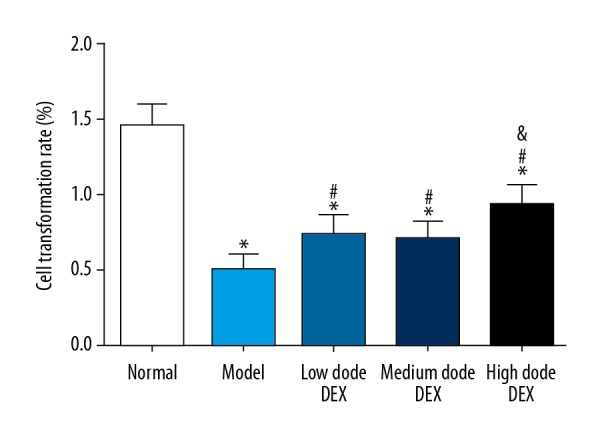
Transformation rate of splenic lymphocytes in each group. Compared with the normal group, * P<0.05; compared with the model group, # P<0.05; compared with the low dose DEX group, & P<0.05. DEX – dexmedetomidine.
Expressions of IGF2, IGF1R, and IRS1 mRNA in ovarian tissues
The results of qRT-PCR are shown in Figure 4. Compared with the normal group, in the other groups the IGF2, IGF1R, and IRS1 mRNA expressions in ovarian tissues were significantly increased (all P<0.05). Compared with the model group, in the low dose DEX group, the medium dose DEX group, and the high dose DEX group, expression levels of IGF2, IGF1R, and IRS1 mRNA in ovarian tissues were significantly decreased (all P<0.05). There was no significant difference between the low dose DEX group and the medium dose DEX group (all P>0.05). Compared with the low dose DEX group, in the high dose DEX group, the expression levels of IGF2, IGF1R, and IRS1 mRNA in ovarian tissues were significantly decreased (all P<0.05).
Figure 4.
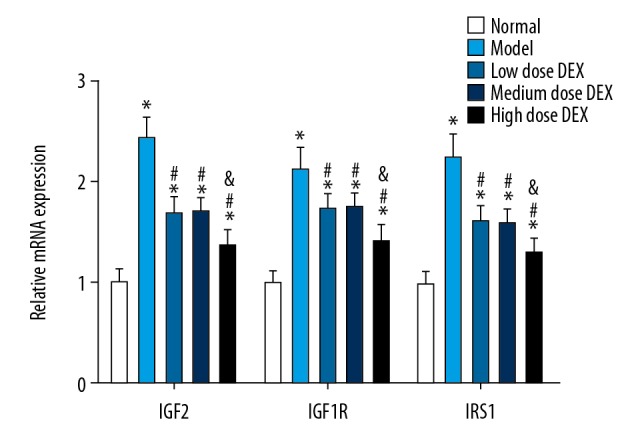
Expressions of IGF2, IGF1R and IRS1 mRNA in ovarian tissues. Compared with the normal group, * P<0.05; compared with the model group, # P<0.05; compared with the low dose DEX group, & P<0.05. IGF2 – insulin-like growth factor 2; IGF1R – insulin-like growth factor 1 receptor; IRS1 – insulin receptor substrate 1; DEX – dexmedetomidine.
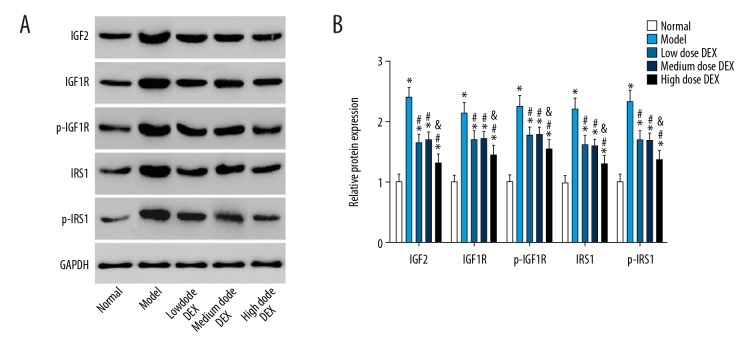
IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein expressions in ovarian tissues
The results of western blot are shown in Figure 5. Compared with the normal group, in the other groups IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein expressions in ovarian tissues were significantly increased (all P<0.05). Compared with the model group, in the low dose DEX group, the medium dose DEX group, and the high dose DEX group, the expression level of IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 proteins in ovarian tissues were significantly decreased (all P<0.05). There was no significant difference between the low dose DEX group and the medium dose DEX group (all P>0.05). Compared with the low dose DEX group, in the high dose DEX group IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein expressions in ovarian tissues were significantly decreased (all P<0.05).
Figure 5.
IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein expressions in ovarian tissues: (A) electrophoretogram of IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein in ovarian tissues in each group; (B) histogram of IGF2, IGF1R, p-IGF1R, IRS1, and p-IRS1 protein expressions in ovarian tissues in each group. Compared with the normal group, (* P<0.05; compared with the model group, # P<0.05; compared with the low dose DEX group, & P<0.05. IGF2 – insulin-like growth factor 2; IGF1R – insulin-like growth factor 1 receptor; IRS1 – insulin receptor substrate 1; DEX – dexmedetomidine.
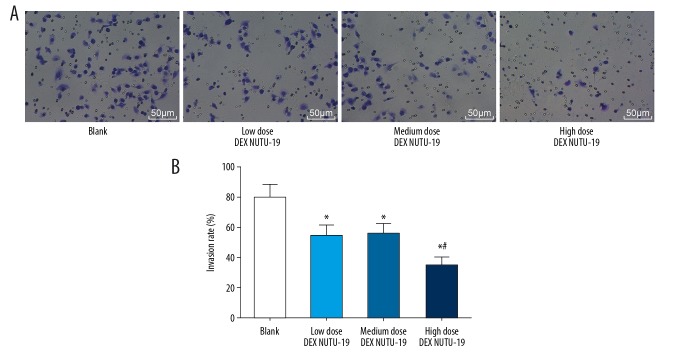
Invasion of ovarian cancer cells
Invasion of ovarian cancer cells in each group was detected by Transwell assay (Figure 6). Compared with the blank group, in the other groups the invasion of ovarian cancer cells was reduced significantly (all P<0.05). There was no significant difference between the low dose DEX NUTU-19 group and the medium dose DEX NUTU-19 group (P>0.05). Compared with the low dose DEX NUTU-19 group, in the high dose DEX NUTU-19 group the invasion of ovarian cancer cells was weakened significantly (P<0.05).
Figure 6.
Transwell chamber micrograph and invasion rate in each group: (A) Transwell chamber micrograph (200×); (B) invasion rate of ovarian cancer cells in each group. Compared with the blank group, * P<0.05; compared with the low dose DEX NUTU-19 group, # P<0.05. DEX – dexmedetomidine.
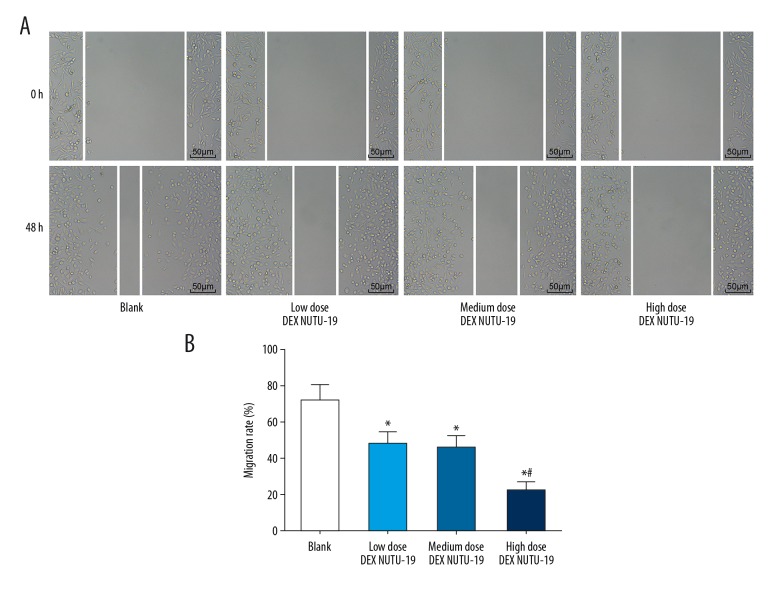
Migration of ovarian cancer cells
Migration of ovarian cancer cells in each group was detected by scratch adhesion test (Figure 7). Compared with the blank group, in the other groups the migration of ovarian cancer cells was reduced significantly (all P<0.05). There was no significant difference between the low dose DEX NUTU-19 group and the medium dose DEX NUTU-19 group (P>0.05). Compared with the low dose DEX NUTU-19 group, in the high dose DEX NUTU-19 group the migration of ovarian cancer cells was weakened significantly (P<0.05).
Figure 7.
Migration distance at 0 hours and 48 hours and migration rate by scratch adhesion test: (A) migration micrograph (200×) by scratch adhesion test; (B) migration rate of ovarian cancer cells. Compared with the blank group, * P<0.05; compared with the low dose DEX NUTU-19 group, # P<0.05. DEX – dexmedetomidine.
Discussion
The incidence of ovarian cancer ranks second in gynecological malignant tumors, but the mortality rate is the highest [17]. Most patients are in the middle or advanced stage at the time of initial diagnosis and are difficult to treat by surgical resection. Therefore, they can only be treated by neoadjuvant chemotherapy or platinum-based chemotherapy [18]. However, nearly 30% of patients will develop drug resistance when receiving chemotherapy, which is very likely to cause tumor recurrence [19].
DEX is currently widely used in intensive care units and in surgical treatments [20]. Nagamine et al. confirmed in their study that DEX was beneficial for the promotion of the reduction of serum IL-6, IL-8, and TNF-α levels in patients with thyroid cancer, thereby reducing the inflammatory injury in the central nervous system, which was of great significance to the recovery of postoperative cognitive function [21]. The clinical study of Lykke et al. showed that DEX helped patients with rectal cancer resection to restore gastrointestinal function and improve gastrointestinal motility [22]. Our study found that DEX had a regulating effect on the improvement of immune function in rats with ovarian cancer, and that an appropriate amount of DEX was beneficial to reduce the invasion and migration of cancer cells of patients with ovarian cancer. Compared with the model group, in the low dose DEX group, the medium dose DEX group and the high dose DEX group levels of serum IL-2 and INF-γ were significantly increased, and serum TNF-α level was decreased. The results of flow cytometry showed that compared with the model group, in the low dose DEX group, the medium dose DEX group, and the high dose DEX group the percentages of serum T lymphocyte subsets CD4+ and CD8+ were significantly increased. IL-2 is a common cytokine in human body. CD4+ and CD8+ and T cells are important components of IL-2 and important factors in the regulation of immune function [23]. IL-2 can stimulate T cells to produce INF-γ, activating multiple immune cells [24]. A previous study confirmed that a low level of TNF-α plays a protective effect on human immune function, and TNF-α level exceeding a certain range will cause damages to human tissues and organs [25]. Our study further confirmed that DEX was of great importance to improve the immune function of rats with ovarian cancer. The transformation rate of splenic lymphocytes has been shown to be an important factor in maintaining lymphocyte and immune function. Zhang et al. found in their study that norepinephrine aggravated the toxicity of splenic lymphocyte through β-AR-cAMP signal pathway, reducing the transformation rate of splenic lymphocytes and causing immune dysfunction [26]. Our study confirmed the positive effects of DEX on the transformation rate of splenic lymphocytes in rats with ovarian cancer.
A previous study confirmed that IGF2 signal pathway plays an important role in the adhesion process of tumor cells [27]. The upregulation of IGF2 further activates the expressions of IGF1R and INSR [28]. A study on prostate cancer found that the inhibition of IGF1R and INSR expressions can significantly increase the chemosensitivity in patients with prostate cancer [29]. In our study, the results of qRT-PCR and western blot showed that IGF2 signal pathway in ovarian tissue was significantly activated and IGF1R and INSR expressions were significantly increased in rats with ovarian cancer. After the application of DEX, the indicators were significantly downregulated, and the signal pathway was inhibited. Cell experiments further confirmed the positivity of DEX in reducing the invasion and migration of cancer cells in rats with ovarian cancer. One previous study confirmed that mifepristone and propofol have certain effects in anti-tumor therapy [30]. IN addition, Check et al. found that propofol drugs could affect the biological behavior of breast cancer cells, and the right amount of propofol could reduce the invasion and migration of cancer cells [31]. However, there is currently no research on the effect of DEX on cancer treatment. For the first time, in our study, we found the important value of DEX in the treatment of ovarian cancer. However,, further research and clinic studies are needed to confirm and improve our reported study results.
Conclusions
DEX can enhance the immune function of rats with ovarian cancer and reduce the invasion and migration of ovarian cancer cells by inhibiting the activation of IGF2 signal pathway, showing a positive significance in the treatment of ovarian cancer.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Jänicke F, Hölscher M, Kuhn W, et al. Radical surgical procedure improves survival time in patients with recurrent ovarian cancer. Cancer. 2015;70:2129–36. doi: 10.1002/1097-0142(19921015)70:8<2129::aid-cncr2820700820>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Jones MB, Krutzsch H, Shu H, et al. Proteomic analysis and identification of new biomarkers and therapeutic targets for invasive ovarian cancer. Proteomics. 2015;2:76–84. [PubMed] [Google Scholar]
- 3.Piver MS, Jishi MF, Tsukada Y, Nava G. Primary peritoneal carcinoma after prophylactic oophorectomy in women with a family history of ovarian cancer. A report of the Gilda Radner Familial Ovarian Cancer Registry. Cancer. 2015;71:2751–55. doi: 10.1002/1097-0142(19930501)71:9<2751::aid-cncr2820710911>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 4.Turner TB, Buchsbaum DJ, Straughn JM, Jr, et al. Ovarian cancer and the immune system – The role of targeted therapies. Gynecol Oncol. 2016;142:349–56. doi: 10.1016/j.ygyno.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Urh A, Romano N, Han A, et al. Determination of HE4-mediated roles in tumor immune system modulation in epithelial ovarian cancer (EOC) Gynecol Oncol. 2015;137:174. [Google Scholar]
- 6.Yoshida M, Kawana K, Taguchi A, et al. Oncogenes (K-ras and c-Myc) modulate tumor immune system and enhance peritoneal carcinomatosis in the ovarian cancer. J Reprod Immunol. 2015;112:130. [Google Scholar]
- 7.Knutson KL, Maurer MJ, Preston CC, et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol Immunother. 2015;64:1495–504. doi: 10.1007/s00262-015-1753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong X, Xing Q, Li Y, et al. Dexmedetomidine protects against ischemia-reperfusion injury in rat skeletal muscle. J Surg Res. 2014;186:240–45. doi: 10.1016/j.jss.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Anttila M, Penttilä J, Helminen A, et al. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2015;56:691–93. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueki M, Kawasaki T, Habe K, et al. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69:693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Huang J, Ma L, et al. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016;371:171–81. doi: 10.1016/j.canlet.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Lau MT, Leung PCK. The PI3K/Akt/mTOR signaling pathway mediates insulin-like growth factor 1-induced E-cadherin down-regulation and cell proliferation in ovarian cancer cells. Cancer Lett. 2012;326:191–98. doi: 10.1016/j.canlet.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Wei W, Zhang T, Zafarnia S, et al. Establishment of a rat model: Associating liver partition with portal vein ligation for staged hepatectomy. Surgery. 2016;159:1299–307. doi: 10.1016/j.surg.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Keersmaecker BD, Fostier K, Corthals J, et al. Immunomodulatory drugs improve the immune environment for dendritic cell-based immunotherapy in multiple myeloma patients after autologous stem cell transplantation. Cancer Immunol Immunother. 2014;63:1023–36. doi: 10.1007/s00262-014-1571-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pei X, Duan Z, Ma M, et al. Role of Ca/CaN/NFAT signaling in IL-4 expression by splenic lymphocytes exposed to phthalate (2-ethylhexyl) ester in spleen lymphocytes. Mol Biol Rep. 2014;41:2129–42. doi: 10.1007/s11033-014-3062-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Zhao F, Wang ZN, et al. Altered expression of miR-152 and miR-148a in ovarian cancer is related, to cell proliferation. Oncol Rep. 2012;27:447–54. doi: 10.3892/or.2011.1482. [DOI] [PubMed] [Google Scholar]
- 17.Beltran PJ, Calzone FJ, Mitchell P, et al. Ganitumab (AMG 479) inhibits IGF-II-dependent ovarian cancer growth and potentiates platinum-based chemotherapy. Clin Cancer Res. 2014;20:2947–58. doi: 10.1158/1078-0432.CCR-13-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y, Li J, Han F, et al. High IGF2 expression is associated with poor clinical outcome in human ovarian cancer. Oncol Rep. 2015;34:936–42. doi: 10.3892/or.2015.4048. [DOI] [PubMed] [Google Scholar]
- 19.Brouwer-Visser J, Horwitz SB, Huang GS. Abstract A33: in ovarian cancer cell lines resistant to microtubule-stabilizing agents, expression of IGF2 and ABCB1 is increased and IGF1R inhibition restores drug sensitivity. Clin Cancer Res. 2010;16:A33. [Google Scholar]
- 20.Freeman J, Buggy DJ. Modelling the effects of perioperative interventions on cancer outcome: lessons from dexmedetomidine. Br J Anaesth. 2018;120:15–17. doi: 10.1016/j.bja.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Nagamine T, Komasawa N, Fujiwara S, et al. [Successful airway management using the air-q intubating laryngeal airway under dexmedetomidine and laryngeus superior nerve block for severe tracheal stenosis due to thyroid cancer invasion]. Masui. 2015;64:543–45. [in Japanese] [PubMed] [Google Scholar]
- 22.Lykke J, Jess P, Roikjaer O Danish Colorectal Cancer Group. Increased lymph node yield is associated with improved survival in rectal cancer irrespective of neoadjuvant treatment: results from a national cohort study. Dis Colon Rectum. 2015;58:823–30. doi: 10.1097/DCR.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 23.Vanhanen R, Tuulasvaara A, Mattila J, et al. Common gamma chain cytokines promote regulatory T cell development and survival at the CD4+ CD8+ stage in the human thymus. Scand J Immunol. 2018 doi: 10.1111/sji.12681. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Rafiq K, Kasran A, Peng X, et al. Cyclosporin A increases IFN-gamma production by T cells when co-stimulated through CD28. Eur J Immunol. 2015;28:1481–91. doi: 10.1002/(SICI)1521-4141(199805)28:05<1481::AID-IMMU1481>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Yeom JS, Koh YG, et al. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J Orthop Res. 2014;32:551–56. doi: 10.1002/jor.22532. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JH, Hu CW, Zhu YZ, et al. Effects of norepinephrine on immune functions of cultured splenic lymphocytes exposed to aluminum trichloride. Biol Trace Elem Res. 2013;154:275–80. doi: 10.1007/s12011-013-9729-1. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Ma J, Ma Q, et al. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem. 2013;20:4185–94. doi: 10.2174/09298673113209990251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidal SJ, Rodriguez-Bravo V, Quinn SA, et al. A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell. 2015;27:223–39. doi: 10.1016/j.ccell.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ofer P, Heidegger I, Eder IE, et al. Both IGF1R and INSR knockdown exert antitumorigenic effects in prostate cancer in vitro and in vivo. Mol Endocrinol. 2015;29:1694–707. doi: 10.1210/me.2015-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu B, Liang Y, Dong Z, et al. Glucocorticoid receptor mediated the propofol self-administration by dopamine D1 receptor in nucleus accumbens. Neuroscience. 2016;328:184–93. doi: 10.1016/j.neuroscience.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 31.Check JH, Check D, Aly J, Wilson C. Abstract 4876: Long-term high-quality survival with single agent mifepristone treatment despite advanced lung cancer and advanced renal cell carcinoma – 2 case reports. Cancer Res. 2016;76:4876. doi: 10.21873/anticanres.11251. [DOI] [PubMed] [Google Scholar]