Abstract
Friedel–Crafts (FC) acylation reactions were exploited in the preparation of ketone-functionalized aromatics. Environmentally more friendly, solvent-free mechanochemical reaction conditions of this industrially important reaction were developed. Reaction parameters such as FC catalyst, time, ratio of reagents and milling support were studied to establish the optimal reaction conditions. The scope of the reaction was explored by employment of different aromatic hydrocarbons in conjunction with anhydrides and acylation reagents. It was shown that certain FC-reactive aromatics could be effectively functionalized by FC acylations carried out under ball-milling conditions without the presence of a solvent. The reaction mechanism was studied by in situ Raman and ex situ IR spectroscopy.
Keywords: ball milling, Friedel–Crafts reaction, mechanochemistry
Introduction
The Friedel–Crafts reaction (FCR) is a very powerful tool in organic chemistry for the synthesis of aromatic ketones. It is of great industrial importance and widely used in fine chemicals production [1–2]. In recent years, public awareness of the negative impact of chemical processes on the environment instigates chemists to improve processes by the reduction of waste material, energy consumption and reagents (materials). In this respect, carrying out FCR at room temperature without the use of solvents, which are usually highly toxic (halogenated hydrocarbons) will improve the eco-friendliness of the process. Until now, FCRs have been rarely applied to organic functionalizations which are carried out in solid state by mortar and pestle [3–5]. We are aware of only a few examples of FCRs employing manual grinding: reserpine acylation with AlCl3 [6] and acylation reaction of aromatics [7]. One of the reasons for this scarcity is the hygroscopic nature of the aluminum trichloride catalyst [8–12] when exposed to air humidity. This problem could be easily avoided by conducting the reaction in a closed vessel, by the aid of automated ball milling, which became a very effective synthetic method in recent time [13–18]. The first account on mechanochemical FC alkylation by Borchardt [19] demonstrates the utility of the mechanochemical method in the synthesis of covalent triazine frameworks. Herein, we report related results on solvent-free FC acylation reactions conducted in a ball mill, which is the continuation of our program in organic mechanosynthesis [20–24].
Results and Discussion
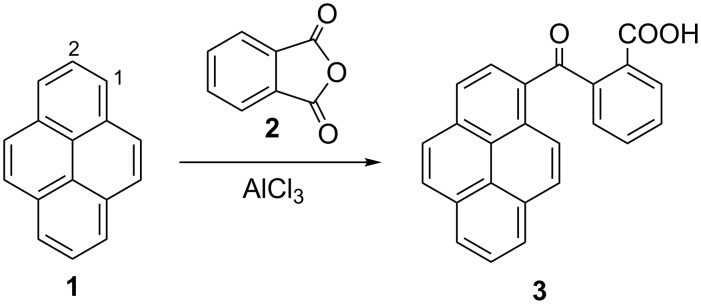
Mechanochemical FCR of pyrene (1) and phthalic anhydride (2) producing 1-(o-carboxybenzoyl)pyrene (3) was selected for the optimization of reaction conditions since all reagents and catalyst are solids (Scheme 1, Table 1) [25]. In solution, this reaction is facile and the product could be obtained in quantitative yield (Table 1, entry 15). The results on optimization of reaction conditions in the ball mill indicate that FC acylation could be effectively carried out mechanochemically. The best mechanochemical reaction conditions (Table 1, entry 4): 2 h, equimolar amount of phthalic anhydride and 2.5 equivalents of AlCl3, afforded product 3 in high yield (79%). Identical yields were obtained by the change of reaction time to 1 h and alternative work-up procedures (Table 1, entries 1 and 4). When the catalyst amount was decreased to one equivalent, a significant decrease of yield was attained (Table 1, entry 2). Addition of various grinding additives to improve mass transfer and prevent pasting of the reaction mixture [26–28] (Table 1, entries 5–8) was detrimental to reaction yields. The addition of a small amount of solvents which was reported to facilitate several ball milling reactions (liquid assisted grinding, LAG) [29–32], also decreased yields (Table 1, entries 9 and 10). The reaction carried out in a planetary mill (Table 1, entry 11) afforded yields comparable to the MM400 vibrational mill. We have also performed screening of efficacy of various Lewis acid catalysts [33–38] (Table 1, entries 18–23), which did not lead to formation of products.
Scheme 1.
FCR of pyrene and phthalic anhydride.
Table 1.
Reaction of pyrene with phthalic anhydridea.
| Entry | Conditions | Work-upb | Yield (%)c |
| 1 | 1 h | B | 78 |
| 2 | 1 h, ratio 1:1:1 | A | 44 |
| 3 | 1 h | A | 76 |
| 4 | 2 h | A | 79 |
| 5 | 1 h, silicagel 1 g | A | n.r. |
| 6 | 1 h, dry silicagel 0.5 g | A | 42 |
| 7 | 1 h, dry NaCl 0.5 g | A | 37 |
| 8 | 1 h, dry Na2SO4 0.5 g | A | 43 |
| 9 | 1 h, LAG dry DCM | A | 51 |
| 10 | 1 h, LAG dry THF | A | 16 |
| 11 | 1 h, planetary milld | A | 79 |
| 12 | 1 h, teflon jar | A | 71 |
| 13 | 3 h, reflux, dry DCM | B | 94 [39] |
| 14 | 1 h, reflux, dry DCM | B | 98 |
| 15 | 1 h, reflux, dry DCM | A | 83 |
| 16 | 1 h, rt, dry DCM | A | 99 |
| 17 | 10 min, melt, 180 °C, dry NaCl | C [40] | n.r.e |
| 18 | 1 h, FeCl3 | A | n.r. |
| 19 | 1 h, ZnCl2 | A | n.r. |
| 20 | 1 h, ZnI2 | A | n.r. |
| 21 | 1 h, ZnBr2 | A | n.r. |
| 22 | 1 h, CuBr2 | A | n.r. |
| 23 | 1 h, CuCl2 | A | n.r. |
| 24 | 3 h, scale-up | A | 73f |
aRetsch MM400 ball mill, 16 mL stainless steel vial, 1 × 12 mm stainless steel ball, 30 Hz, substrate/anhydride/AlCl3 ratio 1:1:2.5; bWork-up A: mixture suspended in H2O, pH adjusted with conc. HCl, chromatography; work-up B: identical to work-up A, but recrystallisation from AcOH instead of chromatography; work-up C: suspended in aq oxalic acid, extracted with DCM, chromatography; cisolated yields; dRetsch planetary ball mill PM-200, 500 rpm, 25 mL stainless steel vial, 30 × 3 mm steel balls; emelted in open flask; fscaled up to 500 mg of pyrene.
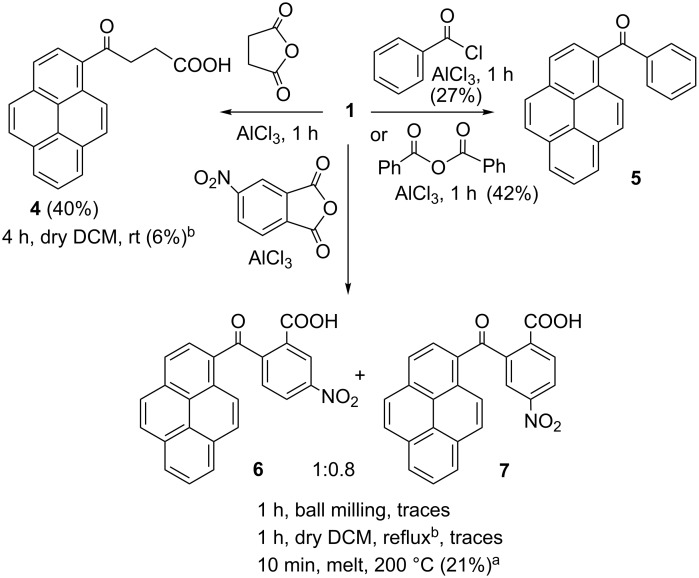
Experiments collected in Table 1 demonstrate that a FC acylation reaction could be effectively carried out under ball-milling conditions at room temperature without the use of solvent. This reaction could be easily scaled up from 94 to 500 mg of pyrene without the decrease in yield (Table 1, entry 24) [41–42]. To investigate the scope of the reaction, several acylation reagents were employed in conjunction with pyrene (Scheme 2) and a variety of aromatic substrates was subjected to FC acylation (Scheme 3).
Scheme 2.
Scope of acylation reagents in FCR under mechanochemical activation conditions and comparison with other reaction conditions (isolated yields); aconversion from NMR analysis; bsolution reaction in flask, substrate/acylation reagent/AlCl3 ratio is 1:1:2.5; ball-milling details are given in Table 1.
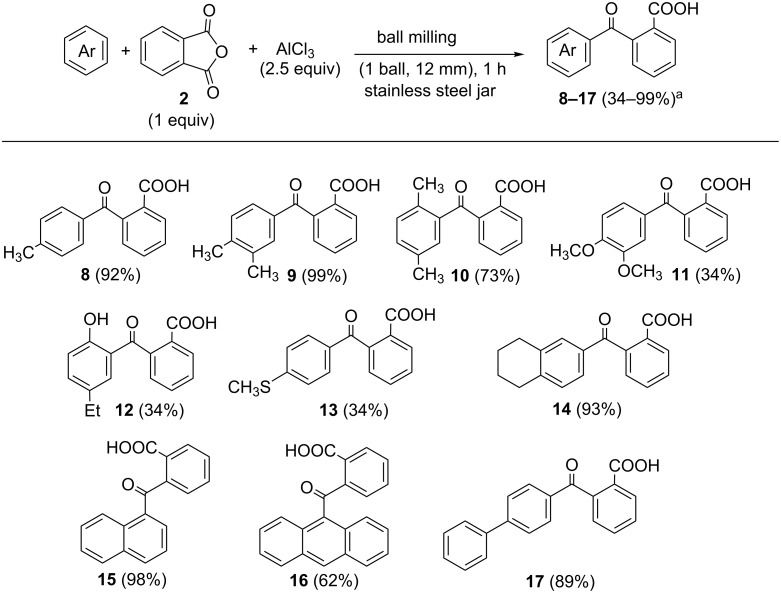
Scheme 3.
Scope of aromatic substrates in FCR under mechanochemical activation conditions. aIsolated yields.
Acylation reagents shown in Scheme 2 were less reactive in comparison to phthalic anhydride. Benzoic anhydride was used as a substitute for benzoyl chloride and the reaction proceeded in better yield. The observed disparity in reactivity might be associated with the difference in the physical state of the reagents. Furthermore, succinic anhydride poorly reacted with pyrene, but the reaction proceeds well with the more reactive biphenyl (69%, see Supporting Information File 1). Di-tert-butyl dicarbonate and 4-nitrobenzoyl chloride were unreactive under ball-milling conditions. Similary unreactive was 4-nitrophthalic anhydride, which only in forced conditions (by melting at 200 °C) reacted sluggishly with pyrene affording mixture of regioisomeric products 6 and 7. The advantage of the employment of mechanochemical conditions is evidenced by solid state milling of pyrene with succinic anhydride which showed remarkably better performance than the reaction carried out in solution (40% vs 6% yield).
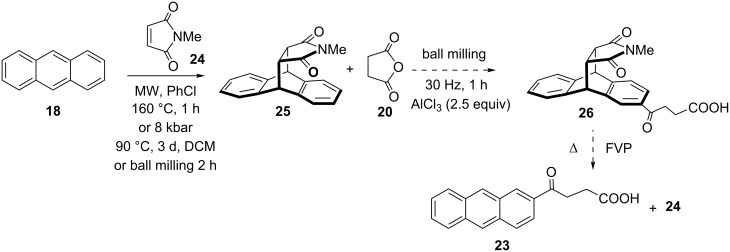
The screening of substrates showed disparate reactivities, ranging from quantitative to low (Scheme 3). Most rewarding are reactions of toluene, o-xylene, naphthalene and tetralin. Interestingly, ball milling of 4-ethylanisole provided phenol 12, in which acylation was accompanied with the cleavage of the methoxy group [43–45]. A striking advantage of the automated ball milling over manual grinding [46] is evident in the reaction of anthracene with phthalic anhydride which gave no product by manual grinding and the yield of the toluene reaction is increased from 68% to 92%.
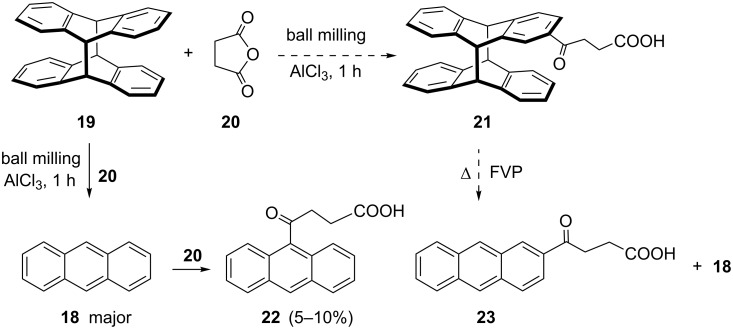
When anthracene was subjected to a milling reaction with succinic anhydride, 9-substituted product 22 was obtained in low yield, and accompanied with a small amount of 2-acylated product 23 (Scheme 4), with same regioselectivity to that reported in the literature [47–48]. FC acylation at the 2-position of anthracene was achieved by Levy by the employment of 9,10-dihydroanthracene and subsequent oxidation to anthracene. To direct the acylation towards the 2-position, we devised the use of anthracene photodimer 19 [49] for the protection of 9,10-positions. The photodimer would act as 9,10-dihydroanthracene, and 2-acylated product should be regioselectively formed, which could be converted by thermal retrocyclization via flash vacuum pyrolysis (FVP) [50–51] to 23. However, ball milling of 19 with 20 provided 95% conversion of 19 to anthracene, with a small amount (<5%) of 22. This result indicates that rapid [4π – 4π] cycloreversion of 19 takes place, even in solid state ball-milling conditions at room temperature. Produced anthracene then subsequently participates in FCR. In control reaction of milling of photodimer 19 itself for 1 h was converted to anthracene in 95% yield. This [4π + 4π] cycloreversion in mechanochemical conditions is analogous to previously described dissociation of labile anthracene/C60 cycloadduct [52]. When the reaction of 19 with 20 was carried out in solution (DCM, overnight), 60% of dimer was converted to anthracene, and traces of FC product 22 were observed. Further attempts were made to lower the reaction temperature by cryomilling [53] (reaction vessel was cooled down by dipping into liquid nitrogen every 3–5 min, and ball milled for 30 min in total). This procedure partially suppressed cycloreversion and led to the mixture of 19 and 18 (3:2 ratio), accompanied with a small amount of 22.
Scheme 4.
Mechanochemical regiodirected FCR of anthracene dimer and succinic anhydride.
As a substitute for dianthracene 19, thermally more stable substrate, anthracene-N-methyl maleimide adduct 25 [54] was prepared by Diels–Alder reaction under high pressure conditions as well as by microwave-assisted reaction and mechanochemically (Scheme 5). In this molecule, N-methylmaleimide could be used as protection of the 9,10-positions of anthracene and then removed by FVP. We thought that the maleimide moiety will not be affected in the FC acylation, since the precedencies exist in the literature on imide moiety withstanding the FC reaction [55–56]. However, mechanochemical reaction of 25 with succinic anhydride and 2.5 equiv of AlCl3 showed no reaction and the increase of the excess of catalyst to 5 equiv gave a very complex mixture.
Scheme 5.
Regioselectivity direction by protection of 9,10-anthracene ring positions.
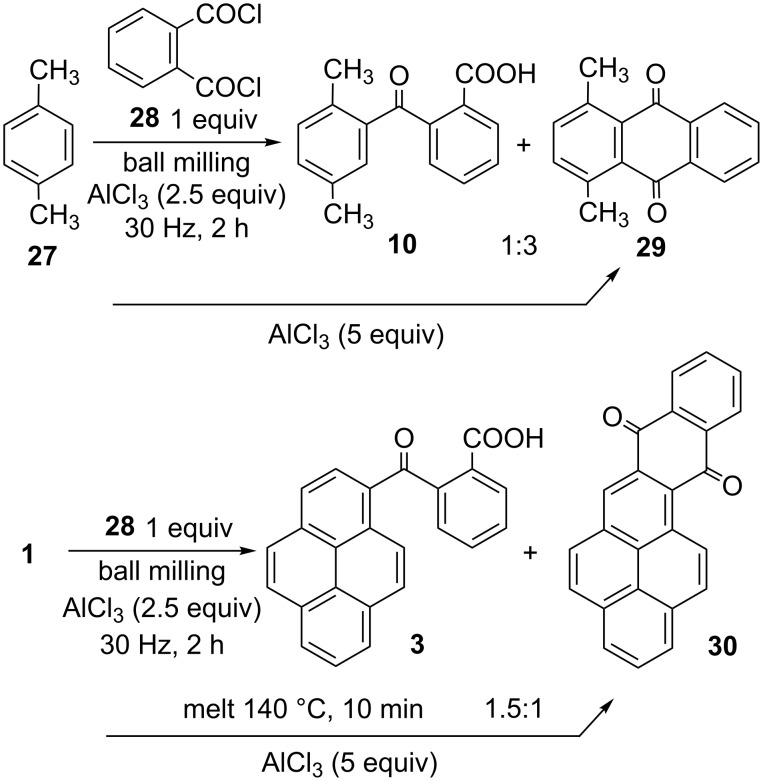
Phthaloyl chloride was applied in mechanochemical FCR with the goal of obtaining a double reaction leading to the anthraquinone core in a single reaction pot in solid state [57–58]. Indeed, milling of p-xylene, AlCl3 and phthaloyl chloride led to the formation of a mixture of 10 and intramolecular FC product 29 [59] in a 1:3 ratio (Scheme 6). The ratio of 1,4-dimethylanthraquinone (29) did not increase in the presence of 5 equivalents of AlCl3. Formation and ratio of these two products could be conveniently established by 1H NMR analysis, due to a difference in the symmetry of products: there are two methyl signals for 10 and a single methyl line at δ 2.81 ppm in the case of 29. Pyrene and naphthalene were less reactive under the same ball milling conditions and reactions stopped at the stage of formation of product 3 and 15. One-step preparation of quinone 30 [39], was achieved by melting reactants at 140 °C for 10 min. Under these conditions, a mixture of adducts 3 and 30 (1.5:1 ratio) was obtained. The product ratio was established by 1H NMR analysis of the characteristic H-10 proton signal of product 3 (peak resonance doublet at δ 9.2 ppm), which is shifted towards lower magnetic field in quinone 30 (δ 10.0 ppm), and concurrent appearance of singlet for H-3 at δ 9.1 ppm. These experiments demonstrate that quinones could be prepared by simple one-pot FC protocols in the case of reactive aromatics.
Scheme 6.
Double FCR of phthaloyl chloride and aromatics.
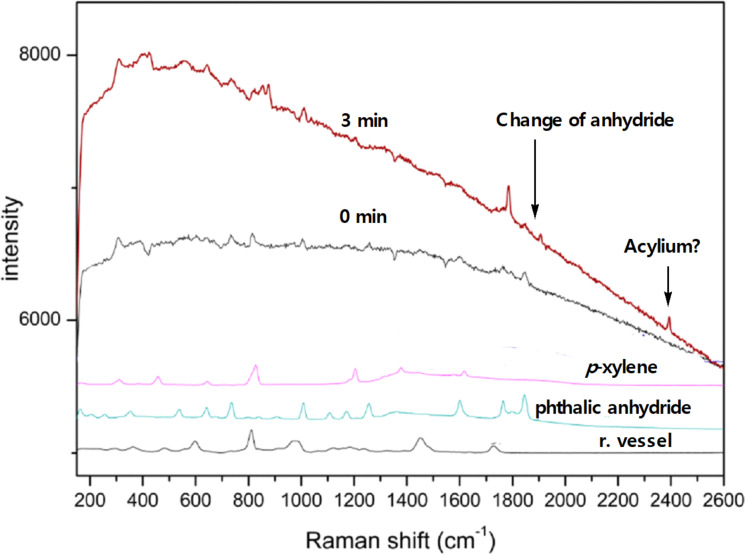
In situ Raman spectroscopy [60] was applied to study mechanistic aspects of the solid state reaction of phthalic anhydride with p-xylene. Raman spectra were simulated and positions of signals for transient reactive intermediates were predicted by density functional theory method B3LYP/6-31G* (Supporting Information File 1) [61]. The stretching of the +C≡O bond of the acylium ion was predicted to be at about 2300 cm−1. Raman spectroscopy revealed that the complexation of phthalic anhydride with AlCl3 is rapid, and within 3 minutes of milling all anhydride is consumed (Figure 1). After 3 minutes of milling, high fluorescence prevents further following of the reaction progress. These spectra indicate that rapid complexation of anhydride with AlCl3 takes place, whereas the formation of the acylium ion intermediate could not be unequivocally verified.
Figure 1.
In situ Raman monitoring of reaction of phthalic anhydride with p-xylene.
Similar conclusions could be drawn from ex situ IR spectroscopy [62] which indicates rapid complexation and disappearance of phthalic anhydride (Supporting Information File 1, Figures S43 and S44). A further study was carried on complexation of phthalic anhydride with AlCl3 (Supporting Information File 1, Figure S45). Although there are weak signals at 2300 and 3050 cm−1 which could be associated with the acylium ion and the intermediate cation, the raise of intensities of these signals over the time is quite unlikely to come from reactive species (time needed to transfer sample from ball mill to IR spectrophotometer and acquire spectra are within several minutes, which could be detrimental to reactive species to survive in the open air). These signals are not visible after the standard acidic work-up and further study would require the use of in situ IR spectroscopy in solution [63].
Conclusion
In conclusion, the experimental results demonstrate that Friedel–Crafts acylations could be effectively carried out under solid state ball-milling conditions. The reaction takes place by the initial complexation of the carbonyl group of the acylation reagent with aluminium trichloride.
Supporting Information
Details of experimental procedures, spectroscopic characterization data of compounds and computational procedures.
Acknowledgments
We acknowledge the financial support of the Ministry of Science, Education and Sport of Croatia (Project No. 098-0982933-2920).
This article is part of the thematic issue "Mechanochemistry II" and is dedicated to Professor Koichi Komatsu on the occasion of his 77th birthday.
References
- 1.Olah G A, Reddy V P, Prakash G K S. Kirk-Othmer Encyclopedia of Chemical Technology. New York: Wiley; 2000. Friedel-Crafts Reactions. [DOI] [Google Scholar]
- 2.Sartori G, Maggi R. Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes. Boca Raton, FL, U.S.A.: CRC Press; 2009. [DOI] [Google Scholar]
- 3.Toda F, editor. Organic Solid-State Reactions. Vol. 254. Heidelberg, Germany: Springer; 2005. ((Topics in Current Chemistry)). [DOI] [Google Scholar]
- 4.Tanaka K, Toda F. Chem Rev. 2000;100:1025–1074. doi: 10.1021/cr940089p. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K. Solvent-free Organic Synthesis. Weinheim, Germany: Wiley-VCH; 2003. [DOI] [Google Scholar]
- 6.Begum S, Zehra S Q, Siddiqui B S. Synth Commun. 2006;36:3203–3224. doi: 10.1080/00397910600908900. [DOI] [Google Scholar]
- 7.Matlack A. Introduction to Green Chemistry. Boca Raton: CRC Press; 2010. p. 219. [Google Scholar]
- 8.Pivsa-Art S, Okuro K, Miura M, Murata S, Nomura M. J Chem Soc, Perkin Trans 1. 1994:1703–1707. doi: 10.1039/p19940001703. AlCl3 is the most common Lewis acid employed in FCR. Other LA catalysts were applied in solution reactions. See this reference for: InCl3, SbCl5, TiCl4, FeCl3, SnCl4, ZnCl2. [DOI] [Google Scholar]
- 9.Garkhedkar A M, Senadi G C, Wang J-J. Org Lett. 2017;19:488–491. doi: 10.1021/acs.orglett.6b03642. See for ZnBr2. [DOI] [PubMed] [Google Scholar]
- 10.Makarov A S, Kekhvaeva A E, Hall C J J, Price D R, Trushkov I V, Uchuskin M G. Tetrahedron. 2017;73:7042–7053. doi: 10.1016/j.tet.2017.10.054. See for CuBr2. [DOI] [Google Scholar]
- 11.Ichikawa K, Chano K, Inoue M, Sugita T. Bull Chem Soc Jpn. 1982;55:3039–3040. doi: 10.1246/bcsj.55.3039. See for CuCl2. [DOI] [Google Scholar]
- 12.Peng C, Zhang J, Xue J, Li S, Wang X-N, Chang J. J Org Chem. 2018;83:9256–9266. doi: 10.1021/acs.joc.8b01255. See for ZnI2. [DOI] [PubMed] [Google Scholar]
- 13.Stolle A, Ranu B, editors. Ball Milling Towards Green Synthesis: Applications, Projects, Challenges. Vol. 31. Cambridge, UK: Royal Society of Chemistry; 2015. ((RSC Green Chemistry)). [DOI] [Google Scholar]
- 14.Wang G-W. Chem Soc Rev. 2013;42:7668–7700. doi: 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- 15.Stolle A, Szuppa T, Leonhardt S E S, Ondruschka B. Chem Soc Rev. 2011;40:2317–2329. doi: 10.1039/c0cs00195c. [DOI] [PubMed] [Google Scholar]
- 16.James S L, Adams C J, Bolm C, Braga D, Collier P, Friščić T, Grepioni F, Harris K D M, Hyett G, Jones W, et al. Chem Soc Rev. 2012;41:413–447. doi: 10.1039/c1cs15171a. [DOI] [PubMed] [Google Scholar]
- 17.Kaupp G. CrystEngComm. 2009;11:388–403. doi: 10.1039/b810822f. [DOI] [Google Scholar]
- 18.Margetić D, Štrukil V. Practical Considerations in Mechanochemical Organic Synthesis. Amsterdam, Netherlands: Elsevier; 2016. pp. 1–54. ((Mechanochemical Organic Synthesis)). [DOI] [Google Scholar]
- 19.Troschke E, Grätz S, Lübken T, Borchardt L. Angew Chem, Int Ed. 2017;56:6859–6863. doi: 10.1002/anie.201702303. [DOI] [PubMed] [Google Scholar]
- 20.Briš A, Đud M, Margetić D. Beilstein J Org Chem. 2017;13:1745–1752. doi: 10.3762/bjoc.13.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasovac Z, Trošelj P, Jušinski I, Margetić D, Eckert-Maksić M. Synlett. 2013;24:2540–2544. doi: 10.1055/s-0033-1339876. [DOI] [Google Scholar]
- 22.Štrukil V, Sajko I. Chem Commun. 2017;53:9101–9104. doi: 10.1039/c7cc03510a. [DOI] [PubMed] [Google Scholar]
- 23.Portada T, Margetić D, Štrukil V. Molecules. 2018;23:No. 3163. doi: 10.3390/molecules23123163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Đud M, Margetić D. Int J Org Chem. 2017;7:140–144. doi: 10.4236/ijoc.2017.72011. [DOI] [Google Scholar]
- 25.Caution: Aluminium trichloride dust is very irritant and corrosive and reacts violently with water. For its handling appropriate protection measures should be implemented (Supporting Information File 1).
- 26.Howard J L, Sagatov Y, Browne D L. Tetrahedron. 2018;74:3118–3123. doi: 10.1016/j.tet.2017.11.066. [DOI] [Google Scholar]
- 27.Yu J, Hong Z, Yang X, Jiang Y, Jiang Z, Su W. Beilstein J Org Chem. 2018;14:786–795. doi: 10.3762/bjoc.14.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su W, Yu J, Li Z, Jiang Z. J Org Chem. 2011;76:9144–9150. doi: 10.1021/jo2015533. [DOI] [PubMed] [Google Scholar]
- 29.Friščić T, Trask A V, Jones W, Motherwell W D S. Angew Chem, Int Ed. 2006;45:7546–7550. doi: 10.1002/anie.200603235. [DOI] [PubMed] [Google Scholar]
- 30.Denlinger K L, Ortiz-Trankina L, Carr P, Benson K, Waddell D C, Mack J. Beilstein J Org Chem. 2018;14:688–696. doi: 10.3762/bjoc.14.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonnet L, Tintillier T, Venturini N, Konnert L, Hernandez J-F, Lamaty F, Laconde G, Martinez J, Colacino E. ACS Sustainable Chem Eng. 2017;5:2936–2941. doi: 10.1021/acssuschemeng.6b02439. [DOI] [Google Scholar]
- 32.Howard J L, Brand M C, Browne D L. Angew Chem, Int Ed. 2018;57:16104–16108. doi: 10.1002/anie.201810141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, M.; Wu, L. Faming Zhuanli Shenqing 106905136, Jun 30, 2017. See for FeCl3 employed in FCR acylations with phthalic anhydride in solution.
- 34.Clar E. Chem Ber. 1948;81:169–175. doi: 10.1002/cber.19480810215. See for ZnCl2 employed in FCR acylations with phthalic anhydride in solution. [DOI] [Google Scholar]
- 35.Kehoe T D, Sabnis R W, Balchunis R J, inventors. Oral care compositions with color changing indicator. WO2006105260. PCT Int. Appl. 2006 Oct 5; Chem. Abstr.2006,145, 397365. See for ZnCl2 employed in FCR acylations with phthalic anhydride in solution.
- 36.Nakamura H, Tanaka N, Matsuhashi H. J Jpn Pet Inst. 2010;53:276–282. doi: 10.1627/jpi.53.276. See for sulfated ZrO2 employed in FC acylations with phthalic anhydride in solution. [DOI] [Google Scholar]
- 37.Madje B R, Shelke K F, Sapkal S B, Kakade G K, Shingare M S. Green Chem Lett Rev. 2010;3(4):269–273. doi: 10.1080/17518251003776877. See for sulfated ZrO2 employed in FCR acylations with phthalic anhydride in solution. [DOI] [Google Scholar]
- 38.Maeimi H, Brojerdi S S. Polycyclic Aromat Compd. 2014;34:504–517. doi: 10.1080/10406638.2014.910238. See for SiO2/sulfuric acid employed in FCR acylations with phthalic anhydride in solution. [DOI] [Google Scholar]
- 39.Casas-Solvas J M, Mooibroek T J, Sandramurthy S, Howgego J D, Davis A P. Synlett. 2014;25:2591–2594. doi: 10.1055/s-0034-1379026. [DOI] [Google Scholar]
- 40.Arcamone F, Bernardi L, Patelli B, Giardino P, Di Marco A, Casazza A M, Soranzo C, Pratesi G. Experientia. 1978;34:1255–1257. doi: 10.1007/bf01981401. [DOI] [PubMed] [Google Scholar]
- 41.Andersen J, Mack J. Green Chem. 2018;20:1435–1443. doi: 10.1039/c7gc03797j. [DOI] [Google Scholar]
- 42.Kaupp, G.; Funk, B.; Benz, H. U.; Heupel, A.; Zoz, H. Conference paper APMA-2017. The 4th International Conference on Powder Metallurgy in Asia, Hsinchu, Taiwan, April 9–11, 2017.
- 43.Sato H, Dan T, Onuma E, Tanaka H, Aoki B, Koga H. Chem Pharm Bull. 1991;39:1760–1772. doi: 10.1248/cpb.39.1760. Methyl ether cleavage is a common process in Friedel–Crafts reactions with AlCl3, when acylation occurs at the ortho-position. See also references [40,44–45]. [DOI] [PubMed] [Google Scholar]
- 44.Sato H, Kuromaru K, Ishizawa T, Aoki B, Koga H. Chem Pharm Bull. 1992;40:2597–2601. doi: 10.1248/cpb.40.2597. [DOI] [Google Scholar]
- 45.Saha K, Lajis N H, Abas F, Naji N A, Hamzah A S, Shaari K. Aust J Chem. 2008;61:821–825. doi: 10.1071/ch08084. [DOI] [Google Scholar]
- 46.Ghiaci M, Asghari J. Synth Commun. 1998;28:2213–2220. doi: 10.1080/00397919808007036. [DOI] [Google Scholar]
- 47.Wiznycia A V, Desper J, Levy C J. Dalton Trans. 2007:1520–1527. doi: 10.1039/b700001d. [DOI] [PubMed] [Google Scholar]
- 48.Schoental R. J Chem Soc. 1952:4403–4406. doi: 10.1039/jr9520004403. [DOI] [Google Scholar]
- 49.Breton G W, Vang X. J Chem Educ. 1998;75:81–82. doi: 10.1021/ed075p81. [DOI] [Google Scholar]
- 50.Margetić D, Butler D N, Warrener R N, Murata Y. Tetrahedron. 2011;67:1580–1588. doi: 10.1016/j.tet.2010.12.032. [DOI] [Google Scholar]
- 51.Margetić D, Butler D N, Warrener R N. Synlett. 2013;24:2609–2613. doi: 10.1055/s-0033-1339879. [DOI] [Google Scholar]
- 52.Murata Y, Kato N, Fujiwara K, Komatsu K. J Org Chem. 1999;64:3483–3488. doi: 10.1021/jo990013z. [DOI] [PubMed] [Google Scholar]
- 53.Waddell D C, Mack J. Green Chem. 2009;11:79–82. doi: 10.1039/b810714a. [DOI] [Google Scholar]
- 54.Alibert S, Santelli-Rouvier C, Castaing M, Berthelot M, Spengler G, Molnar J, Barbe J. Eur J Med Chem. 2003;38:253–263. doi: 10.1016/s0223-5234(03)00018-7. [DOI] [PubMed] [Google Scholar]
- 55.Reifenrath W G, Bertelli D J, Micklus M J, Fries D S. Tetrahedron Lett. 1976;17:1959–1962. doi: 10.1016/s0040-4039(00)78089-0. [DOI] [Google Scholar]
- 56.Xu Q, Wang G, Wang X, Wu T, Pan X, Chan A S C, Yang T-K. Tetrahedron: Asymmetry. 2000;11(11):2309–2314. doi: 10.1016/s0957-4166(00)00193-2. [DOI] [Google Scholar]
- 57.Reference [2], p. 43: Product 29 was also prepared by double acylation reaction of p-xylene with phthalic anhydride or with phthaloyl chloride using TfOH.
- 58.Sartori G, Casnati G, Bigi F, Foglio F. Gazz Chim Ital. 1990;120:13–19. [Google Scholar]
- 59.Rosenfeld S, VanDyke S. J Chem Educ. 1991;68:691–692. doi: 10.1021/ed068p691. [DOI] [Google Scholar]
- 60.Gracin D, Štrukil V, Friščić T, Halasz I, Užarević K. Angew Chem, Int Ed. 2014;53(24):6193–6197. doi: 10.1002/anie.201402334. [DOI] [PubMed] [Google Scholar]
- 61.Comparison of signals obtained experimentally was performed with Raman spectra calculated at the B3LYP/6-31G* level and corrected by scaling factor of 0.9614.
- 62.Đud M, Glasovac Z, Margetić D. Tetrahedron. 2019;75:109–115. doi: 10.1016/j.tet.2018.11.038. [DOI] [Google Scholar]
- 63.Huang Z, Jin L, Han H, Lei A. Org Biomol Chem. 2013;11:1810–1814. doi: 10.1039/c3ob27094g. Further study would require in situ IR spectroscopy in solution. See for details. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of experimental procedures, spectroscopic characterization data of compounds and computational procedures.