Abstract
Background and Objectives
To assess whether medically supportive care partners modify the associations of symptomatic chronic conditions with the number of functional disabilities in a cohort of multimorbid older adults with cognitive impairment.
Research Design and Methods
The research design is a prospective study of a nationally representative cohort of Medicare beneficiaries. National Health and Aging Trends Study (NHATS) data were linked with Medicare claims for years 2011–2015. Participants were aged 65 or older and had cognitive impairment with at least 2 chronic conditions (N = 1,003). Annual in-person interviews obtained sociodemographic information at baseline and time-varying variables for caregiving, hospitalization, and 6 activities of daily living (ADL); these variables were merged with Center for Medicare and Medicaid Services data to ascertain 16 time-varying chronic conditions. A care partner was defined as a person who sat with their care recipient during doctor visits in the past year and/or who helped them with prescribed medications in the last month. Chronic condition associations and their potential effect modifications by care partner status were assessed using weighted generalized estimating equations accounting for the complex survey design of the longitudinal analytical sample.
Results
Chronic kidney disease, depression, and heart failure were associated with an increased number of functional disabilities. Among these, only the association of chronic kidney disease with the number of functional disabilities (interaction p value = .001) was weakened by the presence of a care partner.
Discussion and Implications
The presence of care partners showed limited modification of the associations of symptomatic chronic conditions with functional disability.
Keywords: activities of daily living, care partner, chronic conditions, cognitive impairment, effect modification, multimorbidity
Translational Significance.
Among cognitively impaired older adults with kidney disease, presence of a care partner that helps with medical visits or medication regimens is associated with less functional decline.
Persons with cognitive impairment have high rates of functional disability (Aguero-Torres et al., 1998; Sauvaget, Yamada, Fujiwara, Sasaki, & Mimori, 2002). Having cognitive impairment and functional disabilities requires care, much of which is provided by caregivers who are unpaid (Wolff & Spillman, 2014) or paid (National Academies of Science, 2016). Providing “substantial help” for the health care needs of older adults with conditions like these has been shown to be associated with caregiver emotional, physical, and financial difficulties (Wolff, Spillman, Freedman, & Kasper, 2016). Research has also focused more broadly on the functional and behavioral care of persons with cognitive impairment; however, a recent systematic review concluded that there are gaps in the evidence on chronic condition management and service utilization for multimorbid persons with cognitive impairment (Snowden et al., 2017). Many common chronic conditions have been shown to be associated with functional disability, and several are interrelated, such as atrial fibrillation leading to heart failure which has been shown to be associated with both cognitive and functional decline (Deschênes, Burns, & Schmitz, 2015; Fumagalli et al., 2018; Griffith et al., 2017). Diabetes, hypertension, and chronic kidney disease are also interrelated with diabetes and hypertension being risk factors for chronic kidney disease; chronic kidney disease, in turn, is associated with declines in physical function and quality of life for which palliative care is often recommended (Viscogliosi et al., 2019; Wasylynuk & Davison, 2015). However, some studies have only used cross-sectional data; thus, could not estimate the temporal associations between chronic kidney disease and physical function (Roderick et al., 2008; Smyth et al., 2013). It is known that caregiver accompaniment to medical visits is associated with positive patient satisfaction and management outcomes (Cene et al., 2015; Rosland, Piette, Choi, & Heisler, 2011; Wolff & Roter, 2008; Wolff & Spillman, 2014). It is unknown, however, whether caregivers modify the associations of symptomatic chronic conditions with functional disability. Our interest is in a specific type of caregiver, a care partner, that is, a person who sat with their care recipient during doctor visits in the last year or who helped their care recipient with prescribed medications in the last month.
The aim of this article is to use longitudinal data to test whether among multimorbid persons with cognitive impairment having a care partner modifies the impact of any of 13 symptomatic chronic conditions on functional disability (as measured by the number of activities of daily living disabled [ADL]), such that the associations are weakened in those older adults who have caregivers. Only multimorbid older adults with cognitive impairment were included in this study because they may need assistance during doctor visits and taking medications, as multimorbidity increases their medical complexity. Thus, the cohort was selected to address the evidence gaps noted by Snowden and colleagues (2017) by assessing whether having a medically-supportive care partner reduces the strength of associations between symptomatic chronic conditions and ADLs. The occurrence of such hypothesized temporally ordered effect modifications will provide support for interventions aimed at enhancing the role of medically supportive caregiving.
Research Design and Methods
Study Sample
The National Health and Aging Trends Study (NHATS) is a nationally representative sample of Medicare beneficiaries (65 years of age or older) “designed to enhance understanding of trends and trajectories of late-life disability” (Kasper & Freedman, 2014). Study details were published elsewhere (Montaquila, Freedman, Spillman, & Kasper, 2012). All study participants, or their proxies, provided written, informed consent; the Johns Hopkins University Institutional Review Board (IRB) approved the protocol and the Yale IRB approved the use of de-identified data (HIC#:1510016585).
From an original cohort of 7,609 NHATS participants, 3,556 were excluded for having <2 of 16 chronic conditions, 495 were excluded for having insufficient fee-for-service Medicare coverage, 2,530 were excluded for having unimpaired cognition at baseline, and 25 were excluded for failing to have ADL measures through Round 2 of follow-up, yielding an analytical data set of 1,003. Participant-level NHATS data were merged through linkages with Medicare claims (Wolinsky et al., 2007). Claims are divided into files based on billing form and location of care and were updated annually.
Data Collection
Cognitive Assessment
At each annual interview (2011–2015) cognitive impairment was assessed in the NHATS study in three ways: (a) self-reported physician diagnosis of dementia, (b) probable dementia classification scores (score ≤ 2 out of 8 items) from the validated proxy-report, that is, the Alzheimer’s Disease-8 Screening interview, and (c) an impairment classification score from a cognitive test battery (Galvin et al., 2005; Galvin, Roe, Xiong, & Morris, 2006; Kasper, Freedman, & Spillman, 2013). Cognitive tests included domains for orientation (scale: 0–8, cutoff ≤ 3), memory (scale: 0–20, cutoff ≤ 3), and executive function (scale: 0–5, cutoff ≤ 1). Cutoff scores indicating cognitive impairment were defined as 1.5 standard deviations below the mean. Probable dementia was defined by scores below the cutoff in at least two of three domains and possible dementia was defined by scores below the cutoff in one domain. These definitions of probable and possible dementia have been validated against dementia cases in a prior study (sensitivity: 85.7%, 95% CI: 69.7; 95.2; Kasper et al., 2013).
Baseline Variables
Baseline demographic and health status characteristics were collected via interview, with 25.4% having a proxy respondent at baseline. Demographics variables included age (65–74, 75–84, and ≥85), sex, education (≥high school vs. below high school), and race (non-Hispanic white vs. other). Baseline health status was assessed by whether participants were hospitalized in the last 12 months and their higher functioning was assessed by their number of instrumental activities of daily living (IADL; i.e., laundry, shopping for groceries or personal items, making hot meals, handling bills and banking, driving) either done by someone else, or not done in the past month.
Time-Varying Variables
Having any hospitalization during the past year of follow-up based on NHATS interview date was examined as a risk factor of functional disability at the subsequent follow-up. The validated algorithm from the Medicare Chronic Data Condition Warehouse was used to ascertain over time whether participants had the following symptomatic conditions: asthma, atrial fibrillation, benign prostatic hyperplasia, chronic kidney disease, chronic obstructive pulmonary disease, depression, diabetes, heart failure, ischemic heart disease, a myocardial infarction, osteoporosis, rheumatoid arthritis or osteoarthritis, stroke or a transient ischemic attack; or the following nonsymptomatic conditions: hyperlipidemia, hypertension, and hypothyroidism (Chronic Conditions Data Warehouse, 2017; Pope, Ellis, & Ash, 2000).
Assessment of Care Partners and Paid Caregivers
Care partners are defined as people who “sat with their care recipient during doctor visits in the last year” or who “helped their care recipient with prescribed medications in the last month” (National Health & Aging Trends Study, 2019). Participants, who had not visited their doctor in the past year or had not taken prescription medication in the last month, were classified as not having a care partner along with participants who said that no one attended doctor visits with them or assisted them with medications (Wolff & Spillman, 2014). Having a care partner was a time-varying factor.
Outcome Assessment
A time-varying functional disability variable was created using a validated protocol summing six ADLs (bathing, dressing, eating, toileting, transferring from bed, and getting around inside one’s home) that participants were not able to do in the prior month (Freedman, Agree, Cornman, Spillman, & Kasper, 2014; Freedman et al., 2011).
Statistical Analysis
Baseline characteristics of participants were compared according to the presence or absence of having a care partner. Survey weighted adjusted chi-square tests compared characteristics among these groups. The subpopulation command and NHATS weights were used in all analyses (Montaquila, Freedman, Spillman, et al., 2012).
Because the number of functional disabilities has a positively skewed distribution, a negative binomial weighted generalized estimating equation model evaluated associations of the number of functional disabilities with having a care partner and with each symptomatic chronic condition. (Since the negative binomial model uses a natural logarithm link, the outcome of these regression models is the natural logarithm of the number of functional disabilities.) The interactions of the care partner and each symptomatic chronic condition were of special interest. After fitting a model with no interactions, a second multivariable regression model was fit with interaction terms crossing the statistically significant symptomatic chronic conditions identified by the first regression model with the care partner variable as a potential effect modifier. If any of these interaction terms were statistically significant, it was concluded that some degree of effect (measure) modification by care partner status was present. As there were four annual observations, within-person correlation was modeled using a compound symmetry covariance structure. Potential confounding was addressed by including covariates age, sex, race, education, baseline number of disabled ADLs, and having any hospitalization in the year before baseline and time-varying covariates for wave of follow-up, probable (vs. possible) dementia, the number of disabled IADLs and 16 chronic conditions listed above. To obtain national estimates, wave 1 analytic weights along with appropriate strata variables were applied to all regression analyses (Heeringa, West, & Berglund, 2017). These weights account for differential probabilities of selection. Furthermore, we adjusted for potential bias related to unit non-response using inverse probability weighing techniques (Montaquila, Freedman, Edwards, & Kasper, 2012; Montaquila, Freedman, Spillman, et al., 2012). All analyses were performed using Stata, version 15, and p < .05 (two-tailed) was used to indicate statistical significance (StataCorp, 2015).
Results
Baseline Characteristics
Baseline characteristics and national estimates are presented in Table 1 as categorized by the presence or absence of a care partner. Most participants were white, female, and had at least a high school education. Most had probable dementia.
Table 1.
Baseline Characteristics of an Estimated National Health and Aging Trends Study Population With Two or More Chronic Conditions and With Cognitive Impairment (Study Sample Sizea: 1,003)
| Characteristics | Overall sample N = 3,600,000b |
Care partnerc absent (28.9%) N = 1,100,000b |
Care partnerc present (71.1%) N = 2,500,000b |
p Valued |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age (%) | <.0001 | |||
| 65–74 | 20.6 | 35.4 | 14.6 | |
| 75–84 | 40.0 | 36.7 | 41.4 | |
| 85+ | 39.3 | 27.9 | 44.0 | |
| Male (%) | 45.2 | 57.7 | 40.1 | <.001 |
| White, non-Hispanic (%) | 65.9 | 60.0 | 68.3 | .166 |
| ≥ High school (%)e | 54.3 | 59.3 | 52.3 | .192 |
| IADL (mean, (SE))f | 2.59 (0.11) | 0.88 (0.07) | 3.29 (0.12) | <.0001 |
| Utilization (%) | ||||
| Any hospitalization (%) (with 12 months before baseline)e | 42.0 | 25.0 | 48.9 | <.001 |
| Any hospitalization (%) (during past year of follow-up) | 27.0 | 19.6 | 30.0 | .035 |
| Cognitive status (%) | <.0001 | |||
| Possible dementia | 43.2 | 71.0 | 31.8 | |
| Probable dementia | 56.8 | 29.0 | 68.2 | |
| Symptomatic chronic conditions (%) | ||||
| Asthma | 10.2 | 9.8 | 10.4 | .863 |
| Atrial fibrillation | 15.8 | 8.5 | 18.8 | <.001 |
| Benign prostatic hyperplasia | 19.2 | 24.9 | 16.9 | .032 |
| Chronic kidney disease | 32.8 | 20.1 | 37.9 | .002 |
| COPD | 27.5 | 18.0 | 31.3 | .007 |
| Depression | 27.2 | 13.7 | 32.7 | <.001 |
| Diabetes mellitus | 42.3 | 39.5 | 43.5 | .417 |
| Heart failure | 40.6 | 25.7 | 46.7 | <.001 |
| Ischemic heart disease | 54.1 | 46.1 | 57.4 | .033 |
| Myocardial infarction | 7.4 | 6.7 | 7.7 | .750 |
| Osteoporosis | 15.5 | 12.2 | 16.8 | .188 |
| Rheumatoid arthritis or osteoarthritis | 41.9 | 41.0 | 42.2 | .787 |
| Stroke | 13.7 | 6.2 | 16.7 | .005 |
| Nonsymptomatic chronic conditions | ||||
| Hyperlipidemia | 54.7 | 57.6 | 53.5 | .439 |
| Hypertension | 88.8 | 89.0 | 88.8 | .967 |
| Hypothyroidism | 13.3 | 7.8 | 15.6 | .001 |
| No. of chronic conditions | <.001 | |||
| 2–3 | 29.8 | 43.4 | 24.2 | |
| 4–5 | 32.8 | 34.9 | 32.0 | |
| 6+ | 37.4 | 21.7 | 43.8 |
IADL, instrumental activities of daily living; COPD, chronic obstructive pulmonary disease; ADL, activities of daily living.
aThe study sample of 1,003 participants was obtained from the 7,609 persons interviewed in the NHATS study during 2011–2014 and included only persons who have possible or probable dementia and who have at least two of 16 chronic conditions as identified by the Chronic Condition Data Warehouse.
bAnalyses were weighted to produce nationally representative estimates. Taylor series linearized standard errors were obtained using 2011 analytic weights.
cCare partners were defined as people who sat with their care recipient during doctor visits in the last year or who helped their care recipient with prescribed medications in the last month. Study participants who specified they had not visited their doctor in the past year or that they had not taken prescription medication in the last month were classified as not having a care partner along with participants who said that no one attended doctor visits with them or assisted them with medications.
dThe p values for percentages are from Pearson chi-square statistics and for means they are from F tests for negative binomial models without covariates; both are adjusted for the complex survey design.
eThere were 20 missing values on this education variable and four missing values on the baseline any hospitalization variable.
fMeans and standard errors were obtained from an unadjusted negative binominal model accounting for the complex survey design; p values are from an overall model F test with 3 degrees of freedom.
At baseline most of the study participants (37.4%) had six or more chronic conditions, 29.8% had two or three and 32.8% had four or five chronic conditions; the distribution of the number of chronic conditions differed by care partner status (p < 0.001; Table 1). Among the symptomatic chronic conditions, atrial fibrillation, depression, and heart failure differed most by care partner status (p < .001 for all). Other symptomatic conditions also showed significant associations with care partner status, including benign prostatic hyperplasia (p = .032), chronic kidney disease (p = .002), COPD (p = .007), ischemic heart disease (p = .033) and stroke (p = .005). There was great heterogeneity among 600 unique combination of chronic conditions at baseline, with no single unique combination accounting for more than 2.8% of the study sample.
Longitudinal Associations
The median follow-up time in months was 28.3 with an interquartile range of 15.3 to 38.6. Loss to follow-up was common with 289 study participants lost by Round 2, of which 147 were due to death; an additional 228 study participants were lost to follow-up by Round 3, of which 136 were due to death. Hospitalization was common: 182 study participants were hospitalized by Round 2, 82.4% of which had care partners, and an additional 95 were hospitalized by the third round, 76.8% of which had care partners.
Having a care partners was strongly associated with the number of functional disabilities, yielding a regression parameter estimate of 0.54 (p < .001; Table 2, left columns) and an exponentiated parameter estimate or risk ratio (RR) of 1.72 (95% confidence interval (CI): 1.31, 2.27). Chronic kidney disease was somewhat less strongly associated with the number of functional disabilities (parameter estimate = 0.41, p < .001) with an exponentiated parameter estimate or RR of 1.51 (95% CI: 1.28, 1.77), followed by heart failure (parameter estimate = 0.33, p < .001), then depression (parameter estimate = 0.23, p = .005). In a model in which these statistically significant symptomatic chronic conditions (i.e., chronic kidney disease, depression, and heart failure) were crossed with care partner, only chronic kidney disease yielded a statistically significant interaction term (p = .001; Table 2, right columns) indicating effect modification by care partner of the association of chronic kidney disease with the log number of functional disabilities. The main effect indicates that an increase in the log number of functional disabilities for study participants without a care partner (parameter estimate = 1.16) The negative parameter estimate of −0.89 for the interaction term crossing care partner with chronic kidney disease indicates that this increase is substantively negated by the presence of a care partner.
Table 2.
Associations of Care Partner and Symptomatic Chronic Conditions With the Number of Disabled Activities of Daily Living From 2011–2015 Using the National Health and Aging Trends Studya
| Factors | Model without interaction terms | Model with interaction terms | ||||
|---|---|---|---|---|---|---|
| Parameter estimateb |
Parameter p value |
Parameter 95% CI |
Parameter estimateb |
Parameter p value |
Parameter 95% CI |
|
| Main predictors | ||||||
| Probable dementia (vs. possible) | 0.35 | .001 | 0.15, 0.56 | 0.34 | .001 | 0.14, 0.54 |
| Care partnerc (vs. absent) | 0.54 | <.001 | 0.27, 0.82 | 1.16 | <.001 | 0.71, 1.61 |
| Symptomatic chronic conditions | ||||||
| Asthma | 0.04 | .764 | −0.21, 0.29 | 0.07 | .591 | −0.18, 0.32 |
| Atrial fibrillation | 0.11 | .243 | −0.07, 0.29 | 0.09 | .261 | −0.07, 0.26 |
| Benign prostatic hyperplasia | −0.07 | .524 | −0.30, 0.15 | −0.07 | .506 | −0.30, 0.15 |
| Chronic kidney disease | 0.41 | <.001 | 0.25, 0.57 | 1.17 | <.001 | 0.68, 1.65 |
| COPD | 0.05 | .580 | −0.12, 0.22 | 0.02 | .849 | −0.15, 0.18 |
| Depression | 0.23 | .005 | 0.07, 0.39 | 0.61 | .026 | 0.07, 1.15 |
| Diabetes | −0.01 | .926 | −0.16, 0.14 | −0.01 | .938 | −0.15, 0.14 |
| Heart failure | 0.33 | <.001 | 0.17, 0.49 | 0.62 | .012 | 0.14, 1.11 |
| Ischemic heart disease | −0.003 | .970 | −0.15, 0.14 | −0.01 | .890 | −0.16, 0.14 |
| Myocardial infarction | −0.01 | .946 | −0.35, 0.33 | −0.05 | .725 | −0.32, 0.23 |
| Osteoporosis | −0.004 | .962 | −0.17, 0.16 | −0.02 | .749 | −0.17, 0.12 |
| Rheumatoid arthritis or osteoarthritis | −0.02 | .805 | −0.17, 0.13 | −0.02 | .779 | −0.16, 0.12 |
| Stroke | 0.09 | .296 | −0.08, 0.25 | 0.07 | .424 | −0.09, 0.23 |
| Interaction terms | Not applicable | |||||
| Chronic kidney disease × Care partner | −0.89 | .001 | −1.40, −0.38 | |||
| Depression × Care partner | −0.44 | .114 | −0.98, 0.10 | |||
| Heart failure × Care partner | −0.32 | .201 | −0.82, 0.17 |
ADL, activity of daily living; CI, confidence interval; COPD, chronic obstructive pulmonary disease; GEE, generalized estimating equations; IADL, instrumental activity of daily living.
aThe study sample of 1,003 participants was obtained from the 7,609 persons interviewed in the NHATS study during 2011–2015 and included only persons who had cognitive impairment and who had at least 2 of 16 chronic conditions as identified by the Chronic Condition Data Warehouse.
bResults are from a multivariable weighted generalized estimating equations negative binomial regression model with a compound symmetry covariance matrix adjusted for age, sex, race, education, number of baseline ADLs, number of disabled IADLs, any hospitalization (with 12 months prior to baseline), any hospitalization during the past year of follow-up, round of data, sampling stratum, all 13 symptomatic chronic conditions and hyperlipidemia, hypertension, and hypothyroidism.
cCare partners were defined as people who sat with their care recipient during doctor visits in the last year or who helped their care recipient with prescribed medications in the last month. Study participants who specified they had not visited their doctor in the past year or that they had not taken prescription medication in the last month were classified as not having a care partner along with participants who said that they said that no one attended doctor visits with them or assisted them with medications.
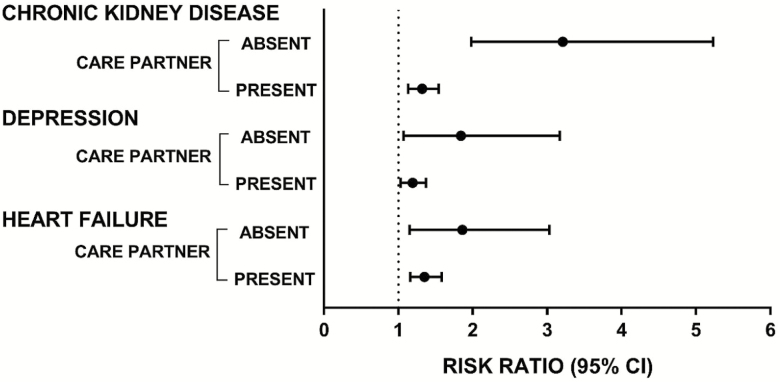
Table 3 displays adjusted mean number of disabled ADLs showing the care partner modification of chronic kidney disease, depression, and heart failure. The difference in mean number of disabled ADLs for those with chronic kidney disease differs only modestly from those without (3.15; 95% CI: 1.97, 4.33) and with (2.76; 95% CI: 2.48, 3.05) a care partner. However, the differences between participants with and without chronic kidney disease are much more pronounced when compared with those with a care partner absent and present. For those without a care partner the difference is 2.17 (=3.15 − 0.98), representing a 221.4% (=2.17 / 0.98 × 100) greater odds. For those with a care partner the difference is 0.67 (=2.76 − 2.09), representing only a 24.3% (=0.67 / 2.76 × 100) difference in odds. Comparable patterns of mean number of disabled ADLs results are shown for depression and heart failure when they are moderated by care partner status, although for these symptomatic chronic conditions the relevant interaction terms are not statistically significant (Table 3). Figure 1 provides a graphical representation of these moderation effects using adjusted risk ratios.
Table 3.
Adjusted Mean Number of Disabled Activities of Daily Living (ADLs)a
| Chronic condition | Care partnerb | Mean number of ADLs disabledc | 95% CI |
|---|---|---|---|
| Chronic kidney disease = yes | Absent | 3.15 | 1.97, 4.33 |
| Chronic kidney disease = no | 0.98 | 0.63, 1.33 | |
| Chronic kidney disease = yes | Present | 2.76 | 2.48, 3.05 |
| Chronic kidney disease = no | 2.09 | 1.88, 2.29 | |
| Depression = yes | Absent | 2.67 | 1.42, 3.92 |
| Depression = no | 1.45 | 0.99, 1.91 | |
| Depression = yes | Present | 2.62 | 2.35, 2.90 |
| Depression = no | 2.20 | 2.01, 2.40 | |
| Heart failure = yes | Absent | 2.45 | 1.62, 3.28 |
| Heart failure = no | 1.31 | 0.71, 1.92 | |
| Heart failure = yes | Present | 2.70 | 2.44, 2.96 |
| Heart failure = no | 2.00 | 1.79, 2.21 |
aThe study sample of 1,003 participants was obtained from the 7,609 persons interviewed in the NHATS study during 2011–2015 and included only persons who had cognitive impairment and who had at least two of 16 chronic conditions as identified by the Chronic Condition Data Warehouse.
bCare partners were defined as people who sat with their care recipient during doctor visits in the last year or who helped their care recipient with prescribed medications in the last month. Study participants who specified they had not visited their doctor in the past year or that they had not taken prescription medication in the last month were classified as not having a care partner along with participants who said that they said that no one attended doctor visits with them or assisted them with medications.
cResults are from a multivariable weighted generalized estimating equations negative binomial regression model with a compound symmetry covariance matrix adjusted for age, sex, race, education, number of baseline ADLs, number of disabled IADLs, any hospitalization (with 12 months before baseline), any hospitalization during the past year of follow-up, round of data, sampling stratum, all 13 symptomatic chronic conditions and hyperlipidemia, hypertension, and hypothyroidism.
Figure 1.
Adjusted risk ratios (i.e., exponentiated parameter estimates from a multivariable regression model) for the number of disabled activities of daily living for symptomatic chronic conditions with statistically significant outcome associations categorized according to the levels of a potential care partner effect modifier.
Discussion and Implications
Key Findings
To our knowledge, previous studies have not evaluated whether care partners modify the associations of symptomatic chronic conditions with functional disability. Evidence of such effect modifications was found in which having a care partner weakened the positive association of chronic kidney disease with functional disability.
Comparison With the Literature
Persons with cognitive impairment have high rates of functional disability (Aguero-Torres et al., 1998; Sauvaget et al., 2002), and cognitive impairment complicates management of chronic conditions, possibly destabilizing one or more conditions leading to decreased function (AARP Public Policy Institute & National Alliance for Caregiving, 2015; Fumagalli et al., 2018; Hayden et al., 2017; Viscogliosi et al., 2019). Roderick and colleagues (2008) studied over 13,000 community-dwelling adults aged 75 and older and found that chronic kidney disease of Stage 3 or worse, was significantly associated with dependence in ≥2 ADLs, cognitive impairment and several chronic conditions. However, their study was cross-sectional and did not consider caregiving. Another cross-sectional study of 3,499 community-dwelling Irish adults aged 50 and older found that mild or worse chronic kidney disease, was significantly associated with increased odds of impairment in ADLs (Smyth et al., 2013). However, their study excluded persons with cognitive impairment and lacked data on caregiving. Our research extends this literature by reporting longitudinal evidence that having a care partner reduces the association between chronic kidney disease and functional disability. Our study differs from many which primarily consider caregivers assisting with ADLs. In this study, at baseline 71.1% had someone who performed one or both medically supportive tasks. However, many care partners may be taking on medical care tasks without medical training (AARP Public Policy Institute & National Alliance for Caregiving, 2015). A recent report found that caregiving tasks have expanded to providing self-care tasks, being a surrogate medical decision maker, and providing specialized medical care. Moreover, 65% of high-need older adults required help with medication management, 20% needed help with medical tasks, and 35% needed help with wound care, yet few were prepared (National Academies of Science, 2016). Training care partners in daily care management strategies for depression, heart failure and chronic kidney disease may lead to longer preservation of physical function.
Limitations and Strengths
Our research article has some limitations. The Center for Medicare and Medicaid Services data linked to NHATS were specific to Medicare Fee-For-Service beneficiaries, which may differ from those choosing managed care. On the other hand, by using the sampling weights, these results reflect older American Medicare Fee-For-Service beneficiaries. Second, it is limited to 4 years of outcome data; however, given the burden of multimorbidity in the setting of cognitive impairment these finding contribute to the knowledge gap (Snowden et al., 2017) highlighted. Third, it is unknown whether the person acting as a care partners changed over time, which could be explored in future studies. Fourth, it is unknown whether care partners had any nursing training. Lastly, these associations with functional disability do not imply causation.
This study has numerous strengths. It followed older, vulnerable adults from a nationally representative cohort using validated algorithms that define cognitive impairment and 16 chronic conditions. This is among the first to investigate whether medically supportive care partners may modify the associations of symptomatic chronic conditions on functional disability. We used sophisticated and rigorous statistical methods that adjusted for losses to follow-up.
Implications for Research and Practice
Although we found a beneficial effect modification of having a care partner on the deleterious association of chronic kidney disease with functional disability, those with a care partner had more disabilities in function than those without (2.72, 95% CI: 2.44, 2.99 vs. 0.40, 95% CI: 0.0.24, 0.56). Gayomali, Sutherland, and Finkelstein (2008) discussed caregiver burden and noted that chronic kidney disease presents with a variety of comorbid conditions that compromise the person’s functional and cognitive capacity. They stated that approximately 40% of patients had ≥5 comorbidities including visual impairment and function impairments such as being unable to drive, and difficulty with ambulation. A recent qualitative study of needs for persons with chronic kidney disease and their caregivers found that understanding the disease, diet, medication and treatments options, mental and physical health consequences, and transportation restrictions are priority areas for future interventions (Donald et al., 2019). Commentaries about caregivers and cognitive impairment infrequently mention how they provide care for care recipients, being primarily focused on caregiver’s burden (Egan et al., 2018). A cross-sectional study of baseline NHATS participants with caregivers, found that the caregivers expressed significantly greater emotional burden as the number of medical care activities increased, as the number of chronic conditions of their care recipient increased, and when their care recipient had dementia (Polenick et al., 2017). Moreover, the caregiver expressed both significantly greater physical burden and caregiver benefits as the number of medical care activities increased (Polenick et al., 2017). Interventions for caregivers may increase feelings of efficacy and decrease feelings of helplessness (aspects of caregiver benefits) associated with caring for multimorbid persons with cognitive impairment.
Future work should examine barriers that care partners face in managing care of multimorbid persons with cognitive impairment in anticipation of creating interventions to overcome these barriers. Interventions that provide care partners with specific skills could potentially stabilize the care of persons with cognitive impairment, as well as improve the well-being of care partners (AARP Public Policy Institute & National Alliance for Caregiving, 2015).
Conclusion
Care partners weakened the positive association of chronic kidney disease with functional disability. Future work developing or applying interventions to care partners may diminish the negative impact of chronic kidney disease, heart failure, and depression on functional disability.
Funding
This work was supported by the National Institute on Aging [R01 AG047891-01A1 to H. G. Allore, P50AG047270 and P30AG021342-16S1 to H. G. Allore, P. H. Van Ness, and J. M. Vrooman], as well as a James Hudson Brown-Alexander Brown Coxe fellowship to J. M. Vrooman. P. H. Van Ness, J. M. Vrooman, B. Vander Wyk, and H. G. Allore receive support from the National Institute on Aging Yale Claude D. Pepper Older Americans Independence Center [P30AG021342]. The National Health and Aging Trends Study (NHATS) is sponsored by the National Institute on Aging [U01AG032947] through a cooperative agreement with the Johns Hopkins Bloomberg School of Public Health.
Conflict of Interest
None reported.
Acknowledgments
Authors’ Contributions: Dr Allore contributed to the concept, design, and analytical plan of the study, the interpretation of results, the preparation of the manuscript, and obtaining funding; Dr Van Ness and MacNeil Vroomen contributed the analysis of data, the interpretation of results, and the preparation of the manuscript; Dr Vander Wyk and Ms Leo-Summers contributed to the management of data and the preparation of the article.
References
- AARP Public Policy Institute, & National Alliance for Caregiving (2015). 2015 Research report: Caregiving in the U.S. Retrieved from http://www.caregiving.org/wp-content/uploads/2015/05/2015_CaregivingintheUS_Final-Report-June-4_WEB.pdf [Google Scholar]
- Aguero-Torres H., Fratiglioni L., Guo Z., Viitanen M., von Strauss E., & Winblad B (1998). Dementia is the major cause of functional dependence in the elderly: 3-Year follow-up data from a population-based study. American Journal of Public Health, 88(10), 1452–1456. doi: 10.2105/AJPH.88.10.1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cené C. W., Haymore L. B., Lin F. C., Laux J., Jones C. D., Wu J. R.,… Corbie-Smith G (2015). Family member accompaniment to routine medical visits is associated with better self-care in heart failure patients. Chronic Illness, 11, 21–32. doi: 10.1177/1742395314532142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronic Conditions Data Warehouse (2017). Chronic Conditions Data Warehouse. Retrieved from http://www.ccwdata.org/web/guest/condition-categories
- Deschênes S. S., Burns R. J., & Schmitz N (2015). Associations between depression, chronic physical health conditions, and disability in a community sample: A focus on the persistence of depression. Journal of Affective Disorders, 179, 6–13. doi: 10.1016/j.jad.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Donald M., Beanlands H., Straus S., Ronksley P., Tam-Tham H., Finlay J., . . . Harwood L (2019). Identifying needs for self-management interventions for adults with CKD and their caregivers: A qualitative study. American Journal of Kidney Diseases. doi: 10.1053/j.ajkd.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Egan K. J., Pinto-Bruno A. C., Bighelli I., Berg-Weger M., van Straten A., Albanese E., & Pot A. M (2018). Online training and support programs designed to improve mental health and reduce burden among caregivers of people with dementia: A systematic review. Journal of the Amercan Medical Directors Association, 19(3), 200.e201–206.e201. doi: 10.1016/j.jamda.2017.10.023 [DOI] [PubMed] [Google Scholar]
- Freedman V. A., Agree E. M., Cornman J. C., Spillman B. C., & Kasper J. D (2014). Reliability and validity of self-care and mobility accommodations measures in the National Health and Aging Trends Study. The Gerontologist, 54, 944–951. doi: 10.1093/geront/gnt104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman V. A., Kasper J. D., Cornman J. C., Agree E. M., Bandeen-Roche K., Mor V.,… Wolf D. A (2011). Validation of new measures of disability and functioning in the National Health and Aging Trends Study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 66, 1013–1021. doi: 10.1093/gerona/glr087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S., Boni S., Pupo S., Migliorini M., Marozzi I., Barghini E.,… Marchionni N (2018). Rate-control vs rhythm-control of atrial fibrillation in elderly patients. From new, age-oriented outcomes to a more complex management strategy. Monaldi Archives for Chest Disease = Archivio Monaldi Per Le Malattie Del Torace, 88, 955. doi: 10.4081/monaldi.2018.955 [DOI] [PubMed] [Google Scholar]
- Galvin J. E., Roe C. M., Powlishta K. K., Coats M. A., Muich S. J., Grant E.,… Morris J. C (2005). The AD8: A brief informant interview to detect dementia. Neurology, 65, 559–564. doi: 10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- Galvin J. E., Roe C. M., Xiong C., & Morris J. C (2006). Validity and reliability of the AD8 informant interview in dementia. Neurology, 67, 1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb [DOI] [PubMed] [Google Scholar]
- Gayomali C., Sutherland S., & Finkelstein F. O (2008). The challenge for the caregiver of the patient with chronic kidney disease. Nephrology, Dialysis, Transplantation, 23, 3749–3751. doi: 10.1093/ndt/gfn577 [DOI] [PubMed] [Google Scholar]
- Griffith L. E., Raina P., Levasseur M., Sohel N., Payette H., Tuokko H.,… Patterson C (2017). Functional disability and social participation restriction associated with chronic conditions in middle-aged and older adults. Journal of Epidemiology and Community Health, 71, 381–389. doi: 10.1136/jech-2016-207982 [DOI] [PubMed] [Google Scholar]
- Hayden D., McCarthy C., Akijian L., Callaly E., Ní Chróinín D., Horgan G.,… Kelly P. J (2017). Renal dysfunction and chronic kidney disease in ischemic stroke and transient ischemic attack: A population-based study. International Journal of Stroke: Official Journal of the International Stroke Society, 12, 761–769. doi: 10.1177/1747493017701148 [DOI] [PubMed] [Google Scholar]
- Heeringa S. G., West B. T., & Berglund P. A (2017). Applied Survey Data Analysis (2nd ed.). Boca Raton, FL: CRC Press. [Google Scholar]
- Kasper J. D., & Freedman V. A (2014). Findings from the 1st round of the National Health and Aging Trends Study (NHATS): Introduction to a special issue. The Journals of Gerontology, Series B: Psychological Science and Social Sciences, 69(Suppl. 1), S1–S7. doi: 10.1093/geronb/gbu125 [DOI] [PubMed] [Google Scholar]
- Kasper J. D., Freedman V. A., & Spillman B. C (2013). Classification of persons by dementia status in the National Health and Aging Trends Study (Technical Paper #5). Retrieved from www.NHATS.org [Google Scholar]
- Montaquila J., Freedman V. A., Edwards B., & Kasper J. D (2012). National Health and Aging Trends Study Round 1 sample design and selection (NHATS Technical Paper #1). Retrieved from www.NHATS.org [Google Scholar]
- Montaquila J., Freedman V. A., Spillman B., & Kasper J. D (2012). National Health and Aging Trends Study development of Round 1 survey weights (NHATS Technical Paper #2). Retrieved from www.NHATS.org [Google Scholar]
- National Academies of Science (2016). Families caring for an aging America. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Health & Aging Trends Study (2019). Data collection instruments. Retrieved from https://www.nhats.org/scripts/DataCollInstrPage.htm [Google Scholar]
- Polenick C. A., Leggett A. N., Webster N. J., Han B. H., Zarit S. H., & Piette J. D (2017). Multiple chronic conditions in spousal caregivers of older adults with functional disability: Associations with caregiving difficulties and gains. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. doi: 10.1093/geronb/gbx118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, G. C., Ellis, R. P., Ash, A. S., Liu, C. F., Ayanian, J. Z., Bates, D. W.,…Ingber, M. J. (2000). Principal inpatient diagnostic cost group model for Medicare risk adjustment. Health care financing review, 21, 93–118. [PMC free article] [PubMed] [Google Scholar]
- Roderick P. J., Atkins R. J., Smeeth L., Nitsch D. M., Hubbard R. B., Fletcher A. E.,… Bulpitt C. J (2008). Detecting chronic kidney disease in older people; what are the implications? Age and Ageing, 37, 179–186. doi: 10.1093/ageing/afm180 [DOI] [PubMed] [Google Scholar]
- Rosland A. M., Piette J. D., Choi H., & Heisler M (2011). Family and friend participation in primary care visits of patients with diabetes or heart failure: Patient and physician determinants and experiences. Medical Care, 49, 37–45. doi: 10.1097/MLR.0b013e3181f37d28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvaget C., Yamada M., Fujiwara S., Sasaki H., & Mimori Y (2002). Dementia as a predictor of functional disability: A four-year follow-up study. Gerontology, 48, 226–233. doi: 10.1159/000058355 [DOI] [PubMed] [Google Scholar]
- Smyth A., Glynn L. G., Murphy A. W., Mulqueen J., Canavan M., Reddan D. N., & O’Donnell M (2013). Mild chronic kidney disease and functional impairment in community-dwelling older adults. Age and Ageing, 42, 488–494. doi: 10.1093/ageing/aft007 [DOI] [PubMed] [Google Scholar]
- Snowden M. B., Steinman L. E., Bryant L. L., Cherrier M. M., Greenlund K. J., Leith K. H.,… Fitzpatrick A. L (2017). Dementia and co-occurring chronic conditions: A systematic literature review to identify what is known and where are the gaps in the evidence? International Journal of Geriatric Psychiatry, 32, 357–371. doi: 10.1002/gps.4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp (2015). Stata Statistical Software: Release 15. College Station, TX: StataCorp LP. [Google Scholar]
- Viscogliosi G., De Nicola L., Vanuzzo D., Giampaoli S., Palmieri L., & Donfrancesco C.; HES Research Group (2019). Mild to moderate chronic kidney disease and functional disability in community-dwelling older adults. The Cardiovascular risk profile in Renal patients of the Italian Health Examination Survey (CARHES) Study. Archives of Gerontology and Geriatrics, 80, 46–52. doi: 10.1016/j.archger.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Wasylynuk B. A., & Davison S. N (2015). Palliative care in patients with advanced chronic kidney disease. CANNT Journal = Journal ACITN, 25, 28–32. [PubMed] [Google Scholar]
- Wolff J. L., & Roter D. L (2008). Hidden in plain sight: Medical visit companions as a resource for vulnerable older adults. Archives of Internal Medicine, 168, 1409–1415. doi: 10.1001/archinte.168.13.1409 [DOI] [PubMed] [Google Scholar]
- Wolff J. L., & Spillman B.(2014). Older adults receiving assistance with physician visits and prescribed medications and their family caregivers: Prevalence, characteristics, and hours of care. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 69(Suppl. 1), S65–S72. doi: 10.1093/geronb/gbu119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. L., Spillman B. C., Freedman V. A., & Kasper J. D (2016). A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Internal Medicine, 176, 372–379. doi: 10.1001/jamainternmed.2015.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky F. D., Miller T. R., An H., Geweke J. F., Wallace R. B., Wright K. B.,… Rosenthal G. E (2007). Hospital episodes and physician visits: The concordance between self-reports and Medicare claims. Medical Care, 45, 300–307. doi: 10.1097/01.mlr.0000254576.26353.09 [DOI] [PMC free article] [PubMed] [Google Scholar]