Abstract
Background:
Dysregulated expression of co-stimulatory molecules is one of the immune escape mechanisms employed in hematologic malignancies like acute myeloid leukemia (AML).
Objectives:
To evaluate the expression of the CD28 and CTLA-4 molecules in 62 adults with de novo AML and its correlation with the development of acute graft vs host disease (GVHD) after hematopoietic stem-cell transplantation.
Methods:
The relative expression of CD28 and CTLA-4 was measured by quantitative SYBR Green real-time PCR method in a group of patients and controls as well as different risk groups (high, intermediate and favorite risk), M3 vs non-M3 and GVHD vs non-GVHD patients.
Results:
The mRNA expression of CD28 (7.9-fold) and CTLA-4 (5.7-fold) was significantly increased in AML patients compared with healthy controls (p=0.006 and 0.02, respectively). Although the mean expression of both CD28 and CTLA-4 was increased in high-risk group compared with low-risk and intermediate-risk groups, the difference was not statistically significant. Also, the mean expression of the CTLA-4, but not CD28, was significantly higher in M3 patients compared with non-M3 ones (p<0.001). The expression of CD28 was upregulated in GVHD patients, while the expression of CTLA-4 was slightly lower in GVHD patients compared with non-GVHD patients, though the difference was not statistically significant. There was no significant correlation between the expression of CD28 and CTLA-4 and laboratory parameters like white blood cells and platelets counts, and hemoglobin and lactate dehydrogenase level in AML patients.
Conclusions:
CD28 and CTLA-4 molecules are aberrantly expressed in peripheral blood leukocytes of AML patients and might contribute to the development of aGVHD after hematopoietic stem cell transplantation.
Key Words: Acute graft versus host disease (aGVHD), AML, Co-stimulatory molecules, Hematopoietic stem cell transplantation (HSCT)
INTRODUCTION
Acute myeloid leukemia (AML) is the most common acute leukemia in adults that is usually treated with standard chemotherapy regimens and in some cases with allogeneic stem-cell transplantation [1]. Although AML cells express antigens that can be recognized by the host T cells, the host immune system is mostly unable to eradicate the blast cells.
Graft-versus-host disease (GVHD) is a critical complication occurred mostly after allogeneic hematopoietic stem-cell transplantation (allo-HSCT) [2]. Acute GVHD (aGVHD) occurs at the first three months after HSCT and is still the main cause of morbidity and mortality after allo-HSCT; Activation of T lymphocytes has been shown to have a major role in the pathogenesis and severity of this disease [3].
It is well-known that full activation of T cells requires two signals: TCR stimulation (signal 1) and co-stimulatory signals provided by engagement of co-stimulatory molecules and their ligands on the surface of the immune cells (signal 2) [4]. CD28 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are among the most studied co-stimulatory molecules that regulate T cell activation [5]. The role of CD28 and CTLA-4 in the development of aGVHD after HSCT has been studied in animal models [6, 7].
It has been shown that AML blasts constitutively express CTLA-4 and its ligands CD80 and CD86 at the diagnosis, which may favor their escape from T cell activation and effector function [8-11]. However, there is not enough report about the association between the expression of CD28 and CTLA-4 in peripheral blood leukocytes of AML patients and development of aGVHD. We therefore evaluated the expression of the CD28 and CTLA-4 molecules in peripheral blood mononuclear cells (PBMCs) of adult patients with de novo AML at the time of diagnosis prior to chemotherapy, and assessed its correlation with laboratory and clinical parameters. We also investigated whether change in the CD28 and CTLA-4 expression was associated with the development of aGVHD post-HSCT.
PATIENTS AND METHODS
In this cross-sectional study, 62 adults with de novo AML who referred to our central hospital between years 2015 and 2016 were investigated. Fifty healthy age- and sex-matched individuals were also evaluated as normal controls. The AML was diagnosed based on the morphology, cytochemistry and immunophenotyping. Clinical and laboratory data including French-American-British (FAB) subclass, complete blood count, and hemoglobin (Hb) and lactate dehydrogenase (LDH) levels, were also collected. All patients received the standard induction chemotherapy, which consisted of daunorubicin 45 mg/m2 on days 1 to 3 and cytarabine 100–200 mg/m2 on days 1 to 7, followed by high doses of a cytarabine-based consolidation phase (cytarabine 3 g/m2 q12h for 3 days, repeated for 2–3 cycles). For M3 patients, arsenic trioxide (0.15 mg/kg/day iv) was used until bone marrow remission occurs plus ATRA (45 mg/m2/day) in two divided doses in addition to the standard induction chemotherapy regimen.
From all patients, 18 undergone HSCT from related HLA-matched donors; 8 of these 18 patients developed aGvHD; 10 did not. aGvHD was graded according to the classic Glucksberg-Seattle criteria and the International Bone Marrow Transplant Registry [12].
Sample Collection and Ribonucleic Acid Purification
Five mL peripheral blood was collected in ethylene-diamine-tetra-acetic acid (EDTA)-containing tubes from all patients at the time of diagnosis prior to chemotherapy and also from healthy controls. The peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-hypaque (Inno-train, Germany) density gradient centrifugation. Total RNA was extracted by Trizol reagent (Invitrogen, USA). The quantity of the extracted RNA was evaluated by Nanodrop (Thermo Fisher Scientific, USA). Then, total RNA was converted into cDNA using Prime Script RT Reagent kit (Takara, Japan) according to the manufacturer’s instruction in the T100 thermocycler (Bio-Rad Laboratories, USA).
SYBR Green Real-Time PCR
For the quantitative analysis of CD28 and CTLA-4 mRNAs expression, the SYBR Green Real-Time PCR was performed using SYBR® Premix Ex Taq™ II (Tli RNase H Plus) (Takara, Japan) in an iQ5 thermocycler (BioRad Laboratories, USA). The specific primers were designed by Allele ID program. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as the internal control and the expression of CD28 and CTLA-4 mRNAs was normalized to that. Real-time PCR reaction program and primer sequences are summarized in Table 1. The specificity of the reaction was confirmed by melt curve analysis for all genes. The changes in the relative expression levels of CD28 and CTLA-4 mRNAs were calculated by [2∆∆Ct] method, where ∆∆Ct = [∆Ct (patients) – ∆Ct (controls)] and ∆Ct = [Ct (sample) – Ct (housekeeping gene)]. All reactions were performed at least in duplicate wells.
Table 1.
The primers (5’ → 3’) and PCR condition for the CD28 and CTLA-4 and GAPDH transcripts
| Gene | PCR Product length | Program |
|---|---|---|
| CD28 Forward primer: GCGTCTTTCAGTTCCCCTCA Reverse primer: GCTTCACCAAAATCTTGTTTCCTGT |
150-bp | 95°C/2 min, 40 cycles of 95 °C/30 sec, 60 °C/20 sec, and 70 °C/30 sec |
| CTLA-4 Forward primer: TACCCACCGCCATACTACCT Reverse primer: GGCACGGTTCTGGATCAATTA |
70-bp | 95 °C/2 min, 40 cycles of 95 °C/30 sec, 60 °C/20 sec, and 70°C/30 sec |
| GAPDH Forward primer: GGACTCATGACCACAGTCCA Reverse primer: CCAGTAGAGGCAGGGATGAT |
119-bp | 95 °C/2 min, 40 cycles of 95 °C/30 sec, 57.5 °C/20 sec, and 70 °C/30 sec |
Ethics
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed written consent was obtained from all participants.
Statistical Analysis
Data were analyzed by SPSS® for Windows® ver 18. Independent sample Student’s t test and Mann-Whitney U test were used for comparing mean expression of CD28 and CTLA-4 between AML patients and controls, patients with different FAB groups as well as patients with and without aGVHD. Correlation between CD28 and CTLA-4 gene expression and laboratory parameters was assessed by Pearson correlation coefficient. A p value <0.05 was considered statistically significant.
Results
From 62 studied AML patients, 35 (56%) were male and 27 (44%) were female. The mean±SD age of patients was 50.0±2.4 (range 20–81) years. The laboratory data of AML patients are presented in Table 2.
Table 2.
Laboratory data of AML patients
| Variable | Mean±SD |
|---|---|
| WBC count | 50,046±72,511 |
| PLT count | 54,137±52,824 |
| Hb (g/dL) | 8.1±2.3 |
| LDH (U/L) | 1434±1065 |
CD28 and CTLA-4 Gene Expression in AML Patients and Development of aGVHD
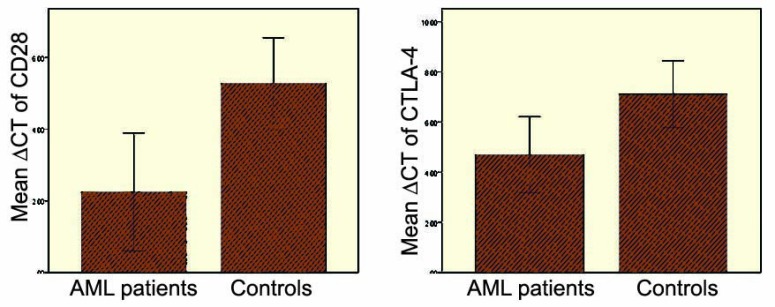
The relative mRNA expression of CD28 and CTLA-4 genes was measured in AML patients and compared with that in healthy controls as ∆Ct values (Fig 1). The expression of CD28 was significantly increased by 7.9-fold in the PBMCs of AML patients compared with healthy controls (p=0.006). CTLA-4 had a significantly higher expression (5.7-fold) in the PBMCs of AML patients than healthy controls (p=0.02). The mean expression of CD28 and CTLA-4 was compared in HSCT patients with and without aGVHD. The mean ΔCt of the CD28 was lower in aGVHD patients compared with those without aGVHD (1.86±2.05 vs 2.24±2.33, respectively), indicating higher expression of CD28 in aGVHD patients compared to non-aGVHD patients. However, the difference was not statistically significant (p=0.9). The mean ΔCt of the CTLA-4 was slightly higher in aGVHD patients compared with non-aGVHD patients (3.58±1.32 vs 3.43±1.4, respectively), showing slightly lower expression of the CTLA-4 in GVHD patients (p=0.94).
Figure 1.
CD28 and CTLA-4 mean ΔCT in AML patients and controls
Association of the CD28 and CTLA-4 Gene Expression and Laboratory Findings
The association between mean ∆Ct of CD28 and CTLA-4 mRNA with laboratory parameters such as white blood cells (WBC) and platelets count, and Hb and LDH levels were evaluated in AML patients. There was no significant association between the mean level of CD28 and CTLA-4 expression and laboratory parameters in AML patients.
The CD28 and CTLA-4 Expression according to AML Risk Stratification and FAB Groups
Based on the 2017 European Leukemia Net (ELN) genetic risk stratification, AML patients were divided into three groups: “favorable,” “intermediate,” and “high-risk” groups [13]. Accordingly, from 62 AML patients studied, 22 were high risk; 26, intermediate; and the remaining 14 had favorable risk. The mean expression of both CD28 and CTLA-4 was increased in high-risk group compared with low-risk and intermediate-risk groups, although the difference was not statistically significant.
The mean CD28 and CTLA-4 expression level was evaluated according to FAB subtype of AML patients. Based on the cytogenetic aberration, AML patients were categorized into M3 and non-M3 groups. Although the mean mRNA level of CD28 expression was increased in M3 patients compared with non-M3 ones, the difference was not statistically significant (p=0.36). The mean expression of CTLA-4 was significantly (p<0.001) increased in M3 patients compared with non-M3 ones (0.12±1.24 vs 5.07±6.06).
DISCUSSION
It is obvious that the co-stimulatory molecules have an indispensable role in providing the second signal needed for full activation of the T cells [4]. Among them, the CD28 and CTLA-4 molecules have been largely implicated in the T cell activation and inhibition, respectively [4].
In this study, we evaluated the mean mRNA expression of CD28 and CTLA-4 molecules in peripheral blood leukocytes of the newly diagnosed adults with AML. We also studied whether a change in the CD28 and CTLA-4 expression was associated with disease outcome like the development of aGVHD in patients who had HSCT. Our results showed that the mean mRNA expression of CD28 (7.9-fold) and CTLA-4 (5.7-fold) was significantly increased in peripheral blood leukocytes of the AML patients compared with healthy controls.
The defective anti-leukemic immune response has been shown in AML patients [14]. Le Dieu, et al, demonstrated that peripheral blood T cells taken from AML patients are dysfunctional and form defective immunological synapses with AML blasts that contribute to the failure of a host anti-leukemic immune response [15]. On the other hand, it has been shown that malignancies like AML exploit different immune evasion strategy to inhibit the generation of the functional anti-tumor immune responses that promotes AML blast survival and prevents their efficient recognition and destruction by the host immune system [14, 16]. Recent studies describe that in addition to cells of the immune system, myeloid leukemic cells can also express co-stimulatory molecules like CTLA-4 and PD-1 to hamper efficient anti-leukemic T cell response [16]. In this regard, expression of the co-stimulatory molecules of B7 superfamily B7-2 (CD86), B7-H2 (inducible co-stimulator ligand, ICOSL) and CTLA-4, have been detected on the surface of AML cell subpopulations [10, 17-20]. Moreover, the presence of B7-2+ and/or B7-H2+ AML cell subpopulations has been shown to have strong negative prognostic value, which has been associated with poor clinical outcomes such as hyperleukocytosis and limited disease-free or relapse-free survival [17-19]. Dolen, et al, revealed increased expression of B7 family molecules on AML cells like B7-H1 (PD-L1) and B7-DC (PD-L2) along with downregulation of B7-H2 (ICOSL) on AML cells, which is associated with AML cells immunosuppressive phenotype, attenuation of helper T-cell responses and promote the differentiation of regulatory T cells, especially through the PD1 pathway [21].
Based on our results, it is proposed that the increased expression of CTLA-4 in peripheral blood leukocytes of AML patients could be associated with defective T cell anti-tumor response and support the immunosuppressive environment observed in AML patients that favor disease progression. Moreover, in addition to conventional T cells, CTLA-4 is also expressed on the surface of regulatory T cells (Tregs) and contributes to the suppressive function of Tregs [22]. Therefore, it can be presumed that Tregs with increased expression of CTLA-4 molecule might have more immunosuppressive activities in AML patients. The reason for increased CD28 expression in our AML patients is unknown. However, it can be hypothesized that higher expression of CD28 in peripheral blood leukocytes of our AML patients might be a natural protective strategy of the immune system to improve recognition of tumor cells by cells of the immune system and fight against tumor cell progression but overexpression of CTLA-4 molecule (which binds to the same ligands as CD28 albeit with higher affinity) might overcome this CD28 positive effect and render immune system to the immunosuppressive condition. This hypothesis needs to be clarified in more complete in vitro and in vivo studies in a larger population of AML patients.
As expected, we found that the expression of the CD28 was higher in patients with GVHD than those without GVHD, albeit the difference was not statistically significant, while the expression of CTLA-4 was slightly lower in GVHD patients compared with non-GVHD ones.
aGVHD is a major deleterious complication after HSCT. Activation of T lymphocytes has been shown to be associated with the pathogenesis and severity of this disease [2]. Using the experimental model of aGVHD, it has been shown that co-stimulatory molecules have a critical role in the development of aGVHD [3, 23]. CD28-deficient mice exhibited reduced severe GVHD, providing that GVHD is at least partially dependent on signaling through CD28 receptor since selective targeting of CD28 with an anti-CD28 blocking antibody prevents donor T cells proliferation and significantly inhibits GVHD [6]. On the other hands, studies in animal models have shown that infusion of CTLA4-Ig (a soluble fusion protein containing the extracellular domain of CTLA-4 linked to an IgG Fc region) diminished GVHD in recipients of mismatched grafts, albeit with a lesser extent than anti-CD28 antibodies [7, 24]. Accordingly, lack of association between CD28 and CTLA-4 expression and development of aGVHD in our AML patients might be attributed to the limited number of HSCT patients who developed aGVHD.
According to the risk stratification of AML patients, the expression of both CD28 and CTLA-4 was augmented in high-risk group compared to the low- and intermediate-risk groups, although the difference was not statistically significant. Another finding of our study was increased expression of CTLA-4 in AML patients with M3 FAB subtypes. Therefore, according to these results, it can be proposed that the dysfunctional immune system may be more pronounced in M3 AML patients compared to other FAB groups as well as in patients included in high-risk group compared to low- and intermediate-risk groups, which need to be confirmed by larger studies. There was no significant association between the mean level of CD28 and CTLA-4 expression and laboratory parameters (WBC and platelets count, and Hb and LDH levels) in AML patients.
It should be noted that in addition to the cells of the immune system, peripheral blood mononuclear cells also constitute a fraction of leukemic blasts, and such changes in CD28 and CTLA-4 gene expression may also be attributed to AML blasts. Therefore, it is better to evaluate the change in these co-stimulatory molecules and also other molecules such as ICOS and PD-1 in an isolated population of T cells as well as blasts (both peripheral blood and bone marrow-derived) from AML patients. To clarify the critical role of CD28 and CTLA-4 gene in the clinical outcome of AML patients (e.g., occurrence of aGVHD), study of a larger population of AML patients, particularly those who developed aGVHD after HSCT, is thus recommended. If so, modulation of these co-stimulatory molecules by administration of drugs targeting these molecules might be beneficial for the management of aGVHD post-HSCT in AML patients.
In conclusion, we found that CD28 and CTLA-4 molecules were aberrantly expressed in peripheral blood leukocytes of AML patients, especially in those with M3 FAB subtypes. Studying other co-stimulatory as well as co-inhibitory molecules in line with increasing the number of AML patients, particularly those who underwent HSCT and developed aGVHD, may provide additional information for using these molecules as a targeted therapy in line with the standard conventional chemotherapy for reducing the risk of aGVHD after HSCT.
CONFLICTS OF INTEREST:
None declared.
FINANCIAL SUPPORT:
This study was financially supported by Shiraz University of Medical Sciences.
References
- 1.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. 2011;117:2307–18. doi: 10.1182/blood-2010-10-265603. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. The Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briones J, Novelli S, Sierra J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone marrow research. 2011;2011:976793. doi: 10.1155/2011/976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002;2:439–46. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 5.Capece D, Verzella D, Fischietti M, et al. Targeting costimulatory molecules to improve antitumor immunity. Biomed Res Int. 2012;2012:926321. doi: 10.1155/2012/926321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X-Z, Martin PJ, Anasetti C. Role of CD28 in acute graft-versus-host disease. Blood. 1998;92:2963–70. [PubMed] [Google Scholar]
- 7.Wallace PM, Johnson JS, MacMaster JF, et al. CTLA4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–10. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Barrett A, Le Blanc K. Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol. 2010;161:223–32. doi: 10.1111/j.1365-2249.2010.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaBelle JL, Hanke CA, Blazar BR, Truitt RL. Negative effect of CTLA-4 on induction of T-cell immunity in vivo to B7-1+, but not B7-2+, murine myelogenous leukemia. Blood. 2002;99:2146–53. doi: 10.1182/blood.v99.6.2146. [DOI] [PubMed] [Google Scholar]
- 10.Pistillo MP, Tazzari PL, Palmisano GL, et al. CTLA-4 is not restricted to the lymphoid cell lineage and can function as a target molecule for apoptosis induction of leukemic cells. Blood. 2003;101:202–9. doi: 10.1182/blood-2002-06-1668. [DOI] [PubMed] [Google Scholar]
- 11.Re F, Arpinati M, Testoni N, et al. Expression of CD86 in acute myelogenous leukemia is a marker of dendritic/monocytic lineage. Exp Hematol. 2002;30:126–34. doi: 10.1016/s0301-472x(01)00768-8. [DOI] [PubMed] [Google Scholar]
- 12.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2016;129:424–47. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin R, Smyth MJ, Lane SW. Harnessing the immune system in acute myeloid leukaemia. Crit Rev Oncol Hematol. 2016;103:62–77. doi: 10.1016/j.critrevonc.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 15.Le Dieu R, Taussig DC, Ramsay AG, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114:3909–16. doi: 10.1182/blood-2009-02-206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1:13. doi: 10.1186/2051-1426-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda A, Yamamoto K, Yamashita K, et al. The expression of co-stimulatory molecules and their relation-ship to the prognosis of human acute myeloid leukaemia: poor prognosis of B7-2-positive leukaemia. Br J Haematol. 1998;102:1257–62. doi: 10.1046/j.1365-2141.1998.00901.x. [DOI] [PubMed] [Google Scholar]
- 18.Tamura H, Dan K, Tamada K, et al. Expression of functional B7-H2 and B7 2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005;11:5708–17. doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- 19.Whiteway A, Corbett T, Anderson R, et al. Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br J Haematol. 2003;120:442–51. doi: 10.1046/j.1365-2141.2003.04085.x. [DOI] [PubMed] [Google Scholar]
- 20.Laurent S, Palmisano GL, Martelli AM, et al. CTLA-4 expressed by chemoresistant, as well as untreated, myeloid leukaemia cells can be targeted with ligands to induce apoptosis. Br J Haematol. 2007;136:597–608. doi: 10.1111/j.1365-2141.2006.06472.x. [DOI] [PubMed] [Google Scholar]
- 21.Dolen Y, Esendagli G. Myeloid leukemia cells with a B7-2+ subpopulation provoke Th-cell responses and become immuno-suppressive through the modulation of B7 ligands. Eur J Immunol. 2013;43:747–57. doi: 10.1002/eji.201242814. [DOI] [PubMed] [Google Scholar]
- 22.Walker LS. Treg and CTLA-4: two intertwining pathways to immune tolerance. J Autoimmun. 2013;45:49–57. doi: 10.1016/j.jaut.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XC, Rothstein DM, Sayegh MH. Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev. 2009;229:271–93. doi: 10.1111/j.1600-065X.2009.00781.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu X-Z, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol. 2000;164:4564–8. doi: 10.4049/jimmunol.164.9.4564. [DOI] [PubMed] [Google Scholar]