Abstract
Background:
Kidney transplantation is the most effective and optimal treatment for end-stage renal disease.
Objective:
To investigate the association between serially measured ultrasound indices during the early post-operative period to determine severe acute tubular necrosis (ATN) in kidney allografts.
Methods:
In a prospective study, we assessed sonographic renal indices including interlobar arteries peak systolic velocity (PSV), end-diastolic velocity (EDV), resistance index (RI), pulsatility index (PI), power doppler grading (PDG), acceleration time (AT), and renal volume on the 3rd and 9th days after kidney transplantation in 46 adult recipients who had no other significant complications except ATN. Biopsies were performed in patients with prolonged delayed graft function (DGF) to exclude other pathologies, especially acute rejection.
Results:
12 (20%) recipients experienced biopsy-proven severe ATN. The differences in the ultrasound indices and their measured discrepancies on the 1st and 2nd examinations between the groups were not statistically significant except for the 1st examined RI (p=0.029) and PI (p=0.04). No patient had PDG of >2. The first RI, with a cut-off value of 0.66, had a sensitivity of 91.7% and a specificity of 50% for predicting severe ATN (area under the ROC curve = 0.71). To compensate for the low specificity of this index, we suggest using the first PDG scale of equal to 2 with a specificity of 85.3%. Overall sensitivity, specificity, and positive and negative predictive values in established severe ATN throughout early post-operative days for a 3rd day RI >0.66 and PDG = 2, were 38%, 92.5%, 64.1%, and 80.9%, respectively.
Conclusions:
The RI and the PDG measured on the 3rd day after renal transplantation are useful indices for the diagnosis of established severe ATN in kidney allografts. Furthermore, donor characteristics, post-harvesting organ preservation status, main renal vascular anastomosis, and early post-operative recipient’s clinical situations may also influence the incidence of severe ATN. Although the 1st ultrasound examination on the 3rd day in early post-transplantation provides important diagnostic and prognostic information, repeated assessment about one week later provides no more valuable information.
Key Words: Renal transplantation, Sonography, Acute tubular necrosis
INTRODUCTION
Increasing number of patients with end-stage renal disease creates a great demand within the health services for the diagnosis and treatment of the disease in and long-term follow-up of an aging population. In an ideal world, transplantation is undoubtedly the most effective and optimal method of treatment for many patients with end-stage renal failure [1-3]. Beyond donor shortage, a significant decrease in morbidity, mortality and also improvement in quality of life of recipients in conjunction with its being an extremely cost-effective procedure, necessitates the development of tools capable of objectively assessing kidney graft in an intense monitoring program to help ensure a successful outcome [1, 3, 4]. These patients are susceptible to complications that may ensure in impaired renal function or graft loss, especially in the early days after transplantation. The main allograft parenchymal complications are rejection, acute tubular necrosis, cyclosporine toxicity, and infection [1, 5].
The importance of delayed graft function in the short- and long-term results of kidney transplantation has been underscored in the transplant literature for at least 30 years [7-9]. Its effects on subsequent rejection, increased morbidity and mortality, and increase in late graft loss have all been reported [7, 10-12].
The early prediction of acute kidney injury by current clinical and laboratory methods remains inadequate [7]. However, it is necessary to follow and screen these recipients properly in the early post-operative period for early onset complications and dysfunctions and implement adequate therapeutic intervention as soon as possible [5, 6]. Ultrasound and radionuclide scan are the primary and major imaging modalities used to screen early graft complications that endanger graft viability. They allow assessment of renal hemodynamics and the modifications induced by several situations that justify initial graft dysfunction [4]. Because of limitation of these techniques in clearly differentiating acute tubular necrosis (ATN) from acute rejection, and because their treatment is different, an early, accurate diagnosis using ultrasound-guided biopsy and histology is usually required [1]. Ultrasound is an inexpensive, noninvasive, non-nephrotoxic, rapid, simple, and readily available imaging method that serves as an excellent tool to assess the baseline anatomic details and vascular status in the immediate post-transplantation period [5, 6, 12, 15]. It has also been proven to be the most valuable aid in further transplant monitoring; the follow-up scans are obtained as necessary based on the renal functional tests and clinical symptoms [1, 5].
ATN is the most common cause of impaired renal function in the early post-transplantation period [5]. It usually occurs right after the transplantation and resolves within two weeks, but can last for up to three months. About 10%–30% of these patients require dialysis in the early stages [1, 5]. The initial cause of ATN in transplant recipients is usually related to the process of transplantation itself that causes ischemia to the kidney. In addition, reperfusion after transplantation may lead to oxygen free radical injury [19].
The current study sought to determine the association between serially measured ultrasound indices measured in renal allografts during the early post-operative period and establishment of severe ATN.
PATIENTS AND METHODS
Study Population
From August 2016 until February 2017, we prospectively studied 61 consecutive unrelated kidney transplantations from living or deceased donors which were performed at Hashemi-Nejad Kidney Disease Center, Iran University of Medical Sciences, Tehran, Iran.
Forty-six (29 male and 17 female) enrolled recipients meeting the inclusion criteria were evaluated using serially ultrasound examination on the third and ninth post-operative days. Clinical and paraclinical evaluations were performed daily; urinary output was measured carefully. Paraclinical evaluations included routine serum biochemical parameters. Twelve (20%) patients encountered prolonged delayed graft function due to severe ATN diagnosed based on their daily biochemical renal function tests, urine volume chart and requirement for dialysis on early post-transplantation phase that were finally confirmed by histology. Patients without any abnormal clinical or paraclinical findings were followed for two weeks.
Patients were then allocated into two groups according to their outcomes: a control group (normal evolution), recipients who did not require dialysis during early post-transplantation days and showed normal graft evolution and those with severe ATN (delayed functional allografts), patients with inadequate diuresis, serum creatinine level more than 2.5 mg/dL on the 4th post-operative day and /or need for dialysis during the first week after transplantation. All these patients underwent renal allograft biopsy. The control group was composed of rapid, normal and slow functional allografts.
Biopsy Indications
Ultrasound-guided biopsy of the transplanted kidney was carried out in patients with clinical suspicion of acute rejection according to following criteria: 1) Sustained increase in creatinine of more than 30% in patients with urine output; and 2) recipients with inadequate diuresis in the context of delayed graft function (serum creatinine level >2.5 mg/dL on the 4th post-operative day and /or need for hemodialysis after transplantation) or who were not typical for established ATN or unresponsive to supportive treatment for ATN till the 7th day after transplantation.
Those with biopsy-proven acute rejection, significant hypotension, post-transplantation infections, cyclosporine toxicity (serum cyclosporine levels >400 ng/mL on the 7th day after transplantation), occurrence of major surgical complications (renal vein or renal artery thrombosis, significant renal artery stenosis or urinary leakage, moderate hydronephrosis or more due to ureteral obstruction, perinephric fluid collection >150 mL or collection with compressive effect on allograft ), recipients aged >65 or <16 years, and any causes that might strongly affect the ultrasound indices (e.g., not being able to hold breath) were excluded from the study.
This study was approved by the local Committee of Research Ethics and all subjects were given informed written consent.
Imaging Protocol
The examinations were obtained using one ultrasound system (PHILIPS affiniti 50) with a 3.5 MHz convex array transducer. Examination was done at least 12 hours after the last dose of calcineurin inhibitors and when patients had a heart rate >50 beats/min. All recipients were examined by one experienced uroradiologist in supine position.
The following ultrasound indices based on B-mode, spectral color and Power Doppler information were measured: End-diastolic velocity (EDV), peak systolic velocity (PSV), resistance index (RI) defined as (PSV – EDV)/PSV, pulsatility index (PI) defined as (PSV – EDV)/mean velocity, acceleration time (ACCT) defined as time to PSV, renal volume, and Power Doppler grading (PDG).
Systolic and diastolic velocities were assessed by pulsed Doppler using an insonation angle of 60° at the renal-iliac artery anastomosis or in any other point of turbulent flow (mosaic) revealed by Color Doppler. Doppler RI and PI were calculated when two or three spectrums at different locations on interlobar arteries were obtained. Then the results of these measurements were averaged as the RI and PI values. Power Doppler scanning was performed using the widest scanning angle including the entire kidney. It measures cortical perfusion and parenchymal vascularity that is quantified on a scale of 1– 4: 1) uniform cortical flow, 2) mild peripheral cortical hypoperfusion, 3) cortex and medulla hypoperfusion, and 4) no visible parenchymal flow, with flow seen only in central vessels. For calculating renal volume, after visualization of maximum length in the longitudinal 2D view, the outer kidney borders were traced on a fixed axis by the same radiologist. The rotation steps were 30° revealing six sequential kidney sections. When all contours had been drawn, the volume of the kidney was calculated automatically.
Statistical Analysis
Considering the study of Mariana Moraes Conti, et al. [20], assuming that the mean RI is around 0.65–0.70 in the group with normal evolution and 0.80 in the DGF group to detect a difference of 0.10 with an SD of 0.10, an acceptable type I error of 0.0 5 and study power of 0.80, and estimated incidence of about 30% [1] for significant ATN, the minimum sample size was estimated at 42 patients using G-POWER 3.1.9.2 statistical software. In order to compensate for possible losses due to surgical complications or refusal to participate in the study, the sample size was increased by 10%, totaling to 46 patients.
Continuous variables are expressed as mean±SD, categorical variables as number and percentage. Mann-Whitney U test was used for data with non-normal distribution. Logistic regression analysis was utilized for multivariable analyses. The correlations between ultrasound parameters and clinical variables were assessed with Spearman’s rank test. Receiver operating characteristic (ROC) curves were built to investigate the performance and predictive accuracy of ultrasound parameters in detecting the chance of severe ATN occurrence and to identify their cut-off for selected indices. To determine the significance of the ROC curves, the area under the curve (AUC) was calculated. Diagnostic validity for selected indices was determined by sensitivity, specificity, and positive and negative predictive values. Statistical significance was set at p<0.05. Statistical analyses and graphics were done with SPSS software (Statistical Package for the Social Sciences, ver 18.0, SPSS Inc, Chicago, Ill, USA).
RESULTS
During the study period, 61 patients underwent renal transplantation. Fifteen patients were excluded from the study according to our exclusion criteria. Of 46 kidney recipients included in this study, 34 had an uneventful convalescence in an equal ratio of rapid, normal and slow graft recovery. The remained 12 (20%) patients experienced histology-proven severe ATN.
There were no significant differences in terms of pretransplantation recipients’ mean age, BMI, urine volume, mean arterial pressure, hemoglobin concentration, and time on dialysis between the studied groups. However, there was a non-significant difference in the male to female ratio of recipients between the severe ATN (75%) and control group (59%) (Table 1). On the other hand, regarding to description of donor characteristics, the differences in terms of mean age and BMI between the two groups were significant. Also, in recipients who received their grafts from female or cadaveric donors, the incidence of severe ATN was significantly more than that in others. However, these differences could not statistically be tested because of insufficient recipients in the severe ATN group. Pre-operative demographic characteristics and other underlying variables in kidney recipients and donors are presented in Table 1.
Table 1.
Pre-operative demographic characteristics and other underlying variables in kidney recipients and donors. Values are either mean±SD or n (%).
| Variables | Severe ATN (n=12) | Others (n=34) | Total (n=46) |
|---|---|---|---|
| Recipients | |||
| Age (yrs) | 43.9±11.9 | 40.6±13.8 | 41.4±13.3 |
| Sex (Female/Total) | 3 (25) | 14 (41) | 17 (37) |
| BMI (kg/m2) | 24.5±3.5 | 24.1±4.3 | 24.2± 4.1 |
| Time on dialysis (months) | 13.8±8.0 | 13.6±11.6 | 13.7±10.7 |
| Urine volume (mL) | 691.7±476.2 | 567.7±471.3 | 600.0±470.5 |
| Mean arterial pressure (mm Hg) | 100.8±13.4 | 97.3±9.4 | 98.2±10.5 |
| Hemoglobin concentration (g/dL) | 12.5±1.2 | 12.4±2.3 | 12.4±2.0 |
| Donors | |||
| Age (yrs) | 33.8±9.8 | 29.7±6.2 | 30.7±7.4 |
| Sex (Female/Total) | 5/12 | 7/34 | 12/46 |
| BMI (kg/m2) | 27.8±6.0 | 24.0±2.7 | 25.0±4.1 |
| Donor type (Cadaveric/Living) | 9/12 | 17/34 | 26/46 |
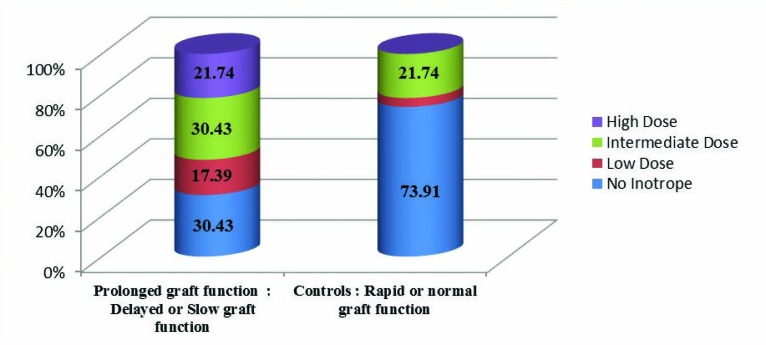
About the cadaveric donors who had received inotropic agents before harvesting their kidneys, especially in a moderate or high dosage, their corresponding recipients experienced slow or delayed graft functional recovery (Fig 1).
Figure 1.
Comparison of donors classified in terms of receiving inotropic agents before the harvest and their recipients with delayed or slow graft function vs controls
Even though the mean number of ATG vials prescribed for recipients, type of the donor (living or cadaveric), the number of cadaveric donors received high or intermediate dose of inotropic agent before harvesting, mean of the first three days post-transplantation recipient’s urine volume, and its difference from mean of the second three days post-transplantation urine volume were significantly different between the severe ATN group and the others, their odds ratios were around 1. Clinical and laboratory data of recipients in early post-operative phase are presented in Table 2.
Table 2.
Clinical and laboratory results of recipients in early phase post-transplantation. Values are either mean±SD or n (%).
| Parameters | Severe ATN (n=12) | Others (n=34) | Total (n=46) |
|---|---|---|---|
| End-to-side arterial anastomosis | 4 (33) | 3 (9) | 7 (15) |
| Hospitalization (day) | 16.7±4.4 | 12.8±4.0 | 13.8±4.4 |
| Number of ATG vials used | 10.0±3.5 | 8.0±5.3 | 8.5±4.9 |
| Mean of first 3 days mean arterial pressure (mm Hg) | 107.7±11.9 | 108.4±11.9 | 108.2±11.8 |
| Hemoglobin concentration drop (g/dL) | 2.2±0.8 | 1.8±0.9 | 1.9±0.9 |
| Variables related to urine volume | |||
| A = Mean of first 3 days urine volume (mL) | 3115± 2836 | 7724±3927 | 6521±4179 |
| B = Mean of second 3 days urine volume (mL) | 3906±2712 | 5783±2852 | 5293±2908 |
| C = Mean of third 3 days urine volume (mL) | 4232±1746 | 4955±2117 | 4766±2033 |
| A – B (mL) | -791±960 | 1941±3084 | 1228±2945 |
| (A – D)/BMI (mL×m2/kg) | 97.6±101.5 | 306.9±182.7 | 252.3±188.8 |
| (B – D)/BMI (mL×m2/kg) | 134.2±104.8 | 222.6±132.6 | 199.6±130.9 |
| Variables related to biochemical graft functional evaluation | |||
| 3rd day eGFR | 21.7±23.5 | 118.8±71.3 | 101.2±75.3 |
| Mean of first 3 days serum creatinine (mg/dL) | 7.0±2.2 | 3.0±1.4 | 4.0±2.4 |
| Mean of second 3 days serum creatinine (mg/dL) | 6.2±2.7 | 1.5±0.5 | 2.7±2.5 |
| Mean of third 3 days serum creatinine (mg/dL) | 4.8±2.3 | 1.3±0.3 | 2.2±1.9 |
ATN: acute tubular necrosis, ATG: anti-thymocyte globulin, D: pretransplant recipients’ urine volume, BMI: body mass index, eGFR: estimated glomerular filtration rate based on Cockraft-Gault formula
The differences in the ultrasound indices and their measured discrepancies on the first and second examination between the groups, using logistic regression analysis, were not statistically significant except for the first examined RI (p=0.029) and PI (p=0.04); it was borderline for the PDG of 2 (p=0.06). No patients had PDG >2. The sonographic B-mode, color and power Doppler indices in each group are presented in Table 3.
Table 3.
Ultrasound allograft indices in the early post-transplantation period. Values are either mean±SD or n (%).
| Parameters | Severe ATN (n=12) | Others (n=34) | p value |
|---|---|---|---|
| End-diastolic velocity 1 (EDV-1) (cm/s) | 9.21±7.31 | 13.16±6.33 | 0.90 |
| EDV – diff (cm/s) | 1.00±7.68 | -0.04±6.16 | 0.63 |
| Peak systolic velocity 1 (PSV-1) (cm/s) | 39.92±20.17 | 43.47±13.06 | 0.48 |
| PSV – diff (cm/s) | -1.08±17.88 | 0.41±16.7 | 0.79 |
| Resistance index 1 (RI-1) | 0.76±0.12 | 0.67±0.10 | 0.03 |
| RI – diff | -0.03±0.12 | 0.01±0.07 | 0.21 |
| Pulsatility index 1 (PI-1) | 1.69±0.6 | 1.34±0.31 | 0.04 |
| PI – diff | -0.14±0.59 | 0.02±0.29 | 0.23 |
| Power doppler grading = 2 (PDG-1) | 5 (42) | 5 (15) | 0.06 |
| Acceleration time 1 (ACCT-1) (m×s) | 30.67±10.85 | 33.06±7.46 | 0.39 |
| ACCT – diff (m×s) | 9.08±19.36 | -0.50±16.55 | 0.14 |
| Transplanted kidney volume 1 (mL) | 181.5±49.0 | 171.4±41.8 | 0.48 |
| Volume – diff (mL) | 22.4±24.6 | 9.6±26.6 | 0.15 |
Index 1 or 2 means the values measured on the first or second examination, respectively.“Index – diff” means the value was calculated as index 1 – index 2.
We found that the first RI with a cut-off value of 0.66 had a sensitivity of 91.7% and a specificity of 50% for predicting severe ATN (area under ROC curve = 0.71). To compensate low specificity of this index we suggest using first power Doppler grading scale of equal to 2 with a specificity of 85.3% (confidence interval = 0.7- 0.93). Overall sensitivity, specificity, and positive and negative predictive values in established severe ATN throughout early post-operative days; for the third day RI > 0.66 and PDG = 2, the test indices were 38%, 92.5%, 64.1%, and 80.9%, respectively.
DISCUSSION
In this study we investigated the role of ultrasound renal allograft indices measured serially on the 3rd and 9th post-operative days to predict and monitor established severe ATN, the most common subgroup of delayed graft function (DGF). In the majority of previous studies the focus was on differentiating the etiology of DGF with non-invasive diagnostic tools to avoid the graft ultrasound-guided biopsy. Considering lack of clinical, biochemical, radioisotope scans and/or imaging evidence to recommend that they can be replaced instead of the histology [1, 29, 30], we preferred to accept the necessity of the biopsy and only evaluate applying serial ultrasound examination in monitoring post-transplantation ATN.
The diagnosis of post-transplantation ATN is based on either inadequate and slow reduction of the serum creatinine level or oliguria through early post-operative phase. However, these two criteria have several limitations and may not be appropriate to predict the severity of ATN [12-17].
Renal hypoperfusion is usually assumed as the main reason of initiating transient acute kidney injury (AKI) may ultimately lead to ATN when it persists. This dichotomy between these two mechanisms has recently been challenged. An early post-mortem series in patients with sepsis with or without AKI revealed that renal tubular injury is common in all patients, that these lesions are focal and that most renal tubular cells appear normal [30]. Therefore, they conclude that AKI seems to be a continuum, and its duration, consequences, and reversibility are more probably related to the severity and duration of the injury and to interaction between different pathophysiological mechanisms including decreased renal perfusion, tubular injury, and interstitial hypertension and edema or renal vasoconstriction resulting from tubuloglomerular feedback [23, 31]. In this context, the development of a marker allowing early detection of AKI may help clinicians prevent or attenuate persistent AKI in patients with transient AKI and is thus considered a research priority [16].
In agreement with previous studies [1], the incidence of severe ATN in our study was about 20%. Gray scale sonogram can successfully detect anatomic changes in the allografts but it is less successful in evaluating functional abnormalities. Renal Doppler is valuable for assessing large arterial or venous abnormalities and has been suggested for evaluating changes in intrarenal perfusion arising from diseases of the renal parenchyma [6, 32]. It is now frequently used as the first-line screening test for early evaluation of the kidney vasculature and function.
Several studies have indicated that some clinical parameters such as mean arterial pressure [12, 29], pulse pressure [24, 25], the degree of atherosclerosis in the donor, and the recipient (their age and vascular compliance) [24, 25], oxygen level [28], intra-abdominal pressure or the existence of collections that compress the graft [27], the use of hypotensive drugs or calcineurin inhibitors influences Doppler-based RI. These findings were not taken into account in most studies. These numerous confounding factors may lead to two opposite views. A pessimistic view may be that RI is unlikely to accurately assess renal perfusion. A more optimistic view may however, arise from the results of clinical and experimental studies suggesting an adequate diagnostic performance of RI as an integrated index in evaluating the risk of renal dysfunction or its reversibility [12].
It seems that RI assesses concomitantly renal preclinical dysfunction, renal vascular damages, and acute changes in renal vascular compliance and resistance. As a consequence, it may help in assessing risk of subsequent renal dysfunction or renal dysfunction reversibility while being a poor candidate in assessing renal perfusion [27].
Choi, et al, reported elevated RI values in abnormal renal transplant recipients. They found a sensitivity of 88% and a specificity of 58%, for a cut-off point of 0.6. They preferred a range of 0.6–0.7 for RI to evaluate graft function, rather than a definitive set cut-off value as a standard [21]. Radmehr, et al, using a cut-off point of 0.63 for RI on the third day, showed a sensitivity of 87% and specificity of 61% [6]. In the current study, considering a cut-off value of 0.66 for the RI on the third day, we obtained a sensitivity of 91.6% and a specificity of 50%, which were relatively similar to the same values reported by Choi and Radmehr and their associates. The differences might because of DGF cases due to etiologies other than ATN included in their (and in almost all other) studies.
Kahraman, et al, also showed a significant decline in allograft function among cases with either RI ≥ 0.7 or PI ≥ 1.1. Patients with impaired allograft function have higher RI and PI values. They concluded that performing Doppler ultrasound examination during the early post-transplantation period with calculation of RI and PI may have a predictive value to predict early and late outcomes of uncomplicated kidney transplants.
Increasing RI in successive studies, associated with function deterioration, is suggestive of an acute rejection episode [4, 33, 34]. On the other hand, high RI in the immediate post-transplantation period associated with delayed creatinine decrease suggests ATN [4]. Thereafter, Doppler ultrasonography allows monitoring of the development of ATN and response to treatment for acute rejection. Gomez, et al, reported that RI, per se, does not have enough predictive value for the diagnosis of kidney transplant dysfunction (cut-off value of 0.66; p = .046). We came to a p value of 0.029 using the same cut-off value. In contrast, we saw the renal indices of repeated ultrasound examination on the 9th post-operative day is completely comparable to that obtained from the last examination; there was no significant difference, especially through recipients with severe ATN, and normal and rapid graft functional recovery, but not in those with slow graft recovery subgroup. Therefore, we concluded that in the absence of acute rejection or anatomic abnormality, one time baseline sonography on the 3rd post-operative day can provide enough data to evaluate the anatomy and predict the function of renal allografts without need for repetition through the first 10 days after transplantation. In these recipients, clinical and biochemical monitoring may be sufficient for clinicians. As previously clarified, appropriate decision timing in terms of the need to perform biopsy, or checking for drug level or infections, are the key in the diagnosis and management of delayed or slow graft function.
As reported in previous studies, we saw a nearly linear correlation between the RIs and corresponding PIs calculated in ultrasound examinations; so we chose RI for further analyses because of its slightly higher prognostic value. On the other hand, in agreement with Conti, et al’s study [20], we saw notable discrepancy of worse PDG between recipients who suffered severe ATN and others.
Additionally, in our study, the hospital stay length and size of the kidney were found not significantly increased in those with severe ATN than others. Female sex, deceased donation, higher BMI and mean age of donors, and higher percentage of end-to-side anastomosis of the main renal artery were more common in those with severe ATN than others.
The present study had a number of limitations. The first was the absence of protocol biopsy to define the histology of slow graft function (SGF) recipients. All SGF cases were probably due to ATN because total renal function recovery occurred during post-transplantation follow-up. Performed protocol biopsy for all included prolonged DGF ruled out rejection.
The second limitation was the known deficiencies of ultrasound, depending on the operator and patient. Bowel gas shadowing and obesity affect the quality of the Doppler signals. Additionally the variability in the operators expertise, the ultrasound systems, and targeted intrarenal vessels; may limit the generalizability of the present findings.
The third limitation was that we were unable to match or omit other medications used by recipients, especially antihypertensive agents and some other confounding factors along with the weak correlation between intrarenal vascular indices and the function of the allograft. To the best of our knowledge, there is no previous report in terms of observed significant correlation between medications, except for calcineurin inhibitors or inotropic agents, and the incidence of ATN or change in the intrarenal vascular indices early after kidney transplantation. Additionally, the difference in the mean age and arterial pressure, before and during the first three days after transplantation, were not significant between the two groups. Therefore, we believe that this difference could not have significantly influenced the results of this study.
The last limitation was the limited number of recipients with severe ATN. It might lead to an overestimation of the effect size. Before implementing this tool to clinical practice, larger multicenter and adequately powered prospective studies should be conducted to clarify its performance and the optimal cut-off value.
On the other hand, the study’s strengths included its prospective design, exclusively focusing on the establishment of post-transplantation ATN, as a most common cause of DGF, and on the finding that considering PDG after calculating an RI > 0.66 as a more specific ultrasound perfusion parameter for the detection of severe ATN, in the absence of acute rejection, anatomic abnormality and drug toxicity, early after kidney transplantation. It is noteworthy that this index is less frequently cited.
The present study suggested that the renal allograft elevated interlobar arteries’ RI (or PI) and also PDG of 2, measured on the third post-operative day, were associated with establishment of severe ATN. These findings confirmed that this easy-to-apply tool would be a promising technique to enhance prediction of renal function and also as a risk stratification approach in assessing chances for renal recovery in the absence of acute rejection and drug toxicity. Additionally, our study revealed that special donor characteristics, organ preservation after harvesting, surgical technique of renal arterial anastomosis, and some of the early post-operative recipient’s clinical data, were important and might have predictive values for the establishment of severe ATN in renal allografts.
CONFLICTS OF INTEREST:
None declared.
FINANCIAL SUPPORT:
This study was financially supported by Iran University of Medical Sciences.
References
- 1.Baxter GM, Rodger RS. Doppler ultrasound in renal transplantation. Nephrol Dial Transplant. 1997;12:2449–51. doi: 10.1093/ndt/12.11.2449. [DOI] [PubMed] [Google Scholar]
- 2.Valderrabano F, Jones EHP, Mallick NP. Report on the management of renal failure in Europe XXIV,1993. Nephrol Dial Transplant. 1995;10(Suppl.51):1–25. doi: 10.1093/ndt/10.supp5.1. [DOI] [PubMed] [Google Scholar]
- 3.Gore SM, Cable DJ, Holland AJ. Organ donation from intensive care units in England and Wales - two year confidential audit of deaths in intensive care. Br Med J. 1992;304:349–55. doi: 10.1136/bmj.304.6823.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez V, Orosa A, Rivera M, et al. Resistance Index Determination in the Pre and Post Kidney Transplantation Time Points in Graft Dysfunction Diagnosis. Transplant Proc. 2015;47:34–7. doi: 10.1016/j.transproceed.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Irshad A, Ackerman S, Sosnouski D, et al. A review of sonographic evaluation of renal transplant complications. Curr Probl Diagn Radiol. 2008;37:67–9. doi: 10.1067/j.cpradiol.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Radmehr A, Babaei Jandaghi A, Hashemi Taheri AP, Shakiba M. Serial Resistive Index and Pulsatility Index for Diagnosing Renal Complications in the Early Posttransplant Phase: Improving Diagnostic Efficacy by considering maximum values. Exp Clin Transplant. 2008;6:161–7. [PubMed] [Google Scholar]
- 7.Cansu A, Kupeli A, Kul S, et al. Evaluation of the relationship between renal function and renal volume-vascular indices using 3D power Doppler ultrasound. Europ J Radiol. 2014:1080–85. doi: 10.1016/j.ejrad.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Prigent A. Monitoring renal function and limitations of renal function tests. Semin Nucl Med. 2008;38:32–46. doi: 10.1053/j.semnuclmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Mileti´c D, Fuckar Z, Susti´c A, et al. Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound. 1998;26:185–89. doi: 10.1002/(sici)1097-0096(199805)26:4<185::aid-jcu1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Muto NS, Kamishima T, Harris AA, et al. Renal cortical volume measured using automatic contouring software for computed tomography and its relationship with BMI, age and renal function. Eur J Radiol. 2011;78:151–6. doi: 10.1016/j.ejrad.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Ninet S, Schnell D, Dewitte A, et al. Doppler-based renal resistive index for prediction of renal dysfunction reversibility: a systematic review and metaanalysis. J Crit Care. 2015;30:629–35. doi: 10.1016/j.jcrc.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Pons B, Lautrette A, Oziel J, et al. Diagnostic accuracy of early urinary index changes in differentiating transient from persistent acute kidney injury in critically ill patients: multicenter cohort study. Crit Care. 2013;17 doi: 10.1186/cc12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darmon M, Vincent F, Dellamonica J, et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: amulticenter cohort study. Crit Care. 2011;15:R178. doi: 10.1186/cc10327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmon M, Schortgen F, Vargas F, et al. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011;37:68–76. doi: 10.1007/s00134-010-2050-y. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Schneider AG, Bellomo R. Urinalysis and pre-renal acute kidney injury: time to move on. Crit Care. 2013;17:141. doi: 10.1186/cc12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.H Cano, DA Castañeda, N Patiño, et al. Resistance Index Measured by Doppler Ultrasound as a Predictor of Graft Function After Kidney Transplantation. Transplant Proc. 2014;46:2972–4. doi: 10.1016/j.transproceed.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Faubel S, Patel NU, Lockhart ME, Cadnapaphornchai MA. Renal Relevant Radiology: Use of Ultrasonography in Patients with AKI. Clin J Am Soc Nephrol. 2014;9:382–94. doi: 10.2215/CJN.04840513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contti MM, Garcia PD, Kojima CA, et al. Quantified power Doppler as a predictor of delayed graft function after renal transplantation. Int Urol Nephrol. 2015;47:405–12. doi: 10.1007/s11255-014-0896-6. [DOI] [PubMed] [Google Scholar]
- 21.Choi CS, Lee S, Kim JS, et al. Usefulness of the resistive index for the evaluation of transplanted kidneys. Transplant Proc. 1998;30:3074–5. doi: 10.1016/s0041-1345(98)00936-1. [DOI] [PubMed] [Google Scholar]
- 22.Nezami N, Tarzamni MK, Argani H, Nourifar M. Doppler Ultrasonographic Indices After Renal Transplantation as Renal Function Predictors. Transplant Proc. 2008;40:94–9. doi: 10.1016/j.transproceed.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Schneider AG, Bellomo R. Urinalysis and pre-renal acute kidney injury: time tomove on. Crit Care. 2013;17 doi: 10.1186/cc12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211:411–7. doi: 10.1148/radiology.211.2.r99ma48411. [DOI] [PubMed] [Google Scholar]
- 25.Murphy ME, Tublin ME. Understanding the Doppler RI: impact of renal arterial distensibility on the RI in a hydronephrotic ex vivo rabbit kidney model. J Ultrasound Med. 2000;19:303–14. [PubMed] [Google Scholar]
- 26.Kirkpatrick AW, Colistro R, Laupland KB, et al. Renal arterial resistive index response to intraabdominal hypertension in a porcine model. Crit Care Med. 2007;35:207–13. doi: 10.1097/01.CCM.0000249824.48222.B7. [DOI] [PubMed] [Google Scholar]
- 27.Naesens M, Heylen L, Lerut E, et al. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369:1797–806. doi: 10.1056/NEJMoa1301064. [DOI] [PubMed] [Google Scholar]
- 28.Darmon M, Schortgen F, Leon R, et al. Impact of mild hypoxemia on renal function and renal resistive index during mechanical ventilation. Intensive Care Med. 2009;35:1031–8. doi: 10.1007/s00134-008-1372-5. [DOI] [PubMed] [Google Scholar]
- 29.Lerolle N, Guérot E, Faisy C, et al. Renal failure in septic shock: predictive value of Doppler-based renal arterial resistive index. Intensive Care Med. 2006;32:1553–9. doi: 10.1007/s00134-006-0360-x. [DOI] [PubMed] [Google Scholar]
- 30.Takasu O, Gaut JP, Watanabe E, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–17. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrler T, Tischer A, Meyer A, et al. The intrinsic renal compartment syndrome: new perspectives in kidney transplantation. Transplantation. 2010;89:40–6. doi: 10.1097/TP.0b013e3181c40aba. [DOI] [PubMed] [Google Scholar]
- 32.Krumme B, Grotz W, Kirste G, et al. Determinants of intrarenal Doppler indices in stable renal allografts. J Am Soc Nephrol. 1997;8:813–6. doi: 10.1681/ASN.V85813. [DOI] [PubMed] [Google Scholar]
- 33.Burgos FJ, Marcen R, Pascual J, et al. Utilidad de la Ecografía y el Eco-doppler en el trasplante renal. Arch Esp Urol. 2006;59:343–52. doi: 10.4321/s0004-06142006000400004. [DOI] [PubMed] [Google Scholar]
- 34.Schwenger V, Hinkel UP, Nahm AM, et al. Color Doppler ultrasonography in the diagnostic evaluation of renal allografts. Nephron Clin Pract. 2006;104:107–12. doi: 10.1159/000094445. [DOI] [PubMed] [Google Scholar]
- 35.Kahraman S, Genctoy G, Cil B, et al. Prediction of renal allograft function with early Doppler ultrasonography. Transplant Proc. 2004;36:1348. doi: 10.1016/j.transproceed.2004.05.030. [DOI] [PubMed] [Google Scholar]