Abstract
Background
Mature myeloid cells play a crucial role in Crohn's disease (CD) but the molecular players that regulate their functions in CD are not fully characterized. We and others have shown that TRIM33 is involved in the innate immune response and in the inflammatory response but TRIM33 role in intestinal inflammation is not known. In this study, we investigated the role of TRIM33 in myeloid cells during dextran sulfate sodium (DSS)-induced colitis.
Methods
We study the role of TRIM33 during DSS-induced colitis which mimics intestinal inflammation using mice deleted for Trim33 only in mature myeloid cells (Trim33−/− mice)
Findings
We first show that Trim33 mRNA level is decreased in CD patient's blood monocytes suggesting a role of TRIM33 in CD. Using Trim33−/− mice, we show that these mice display an impaired resolution of colonic inflammation with an increased number of blood and colon monocytes and a decreased number of colonic macrophages. Trim33−/− monocytes are less competent for recruitment and macrophage differentiation. Finally, during resolution of inflammation, Trim33−/− colonic macrophages display an impaired M1/M2 switch and express a low level of membrane-bound TNF that is associated with an increased number of colonic neutrophils.
Interpretation
Our study shows an important role of TRIM33 in monocytes/macrophages during DSS-induced colitis and suggests that the decreased expression of TRIM33 in CD patient's blood monocytes might not be a consequence but might be involved in CD progression.
Fund
La Ligue contre le Cancer (équipe labelisée), INSERM, CEA, Université Paris-Diderot, Université Paris-Sud.
Keywords: Colonic inflammation, Macrophage, Monocyte, TRIM33, TNF
Research in context.
Evidence before this study
Inflammatory bowel diseases (IBDs) include Crohn's disease (CD) and ulcerative colitis (UC) and are characterized by chronic inflammation of the gastrointestinal tract. It's generally accepted that IBD results from an aberrant immune response to commensal microflora in genetically susceptible individuals. However, only limited data are available on the role of mature myeloid cells and how the functions of these mature myeloid cells are regulated in IBDs. Recent studies demonstrate the involvement of TRIM33 in immune response by regulating the production and the functions of macrophages but any role of TRIM33 in monocytes/macrophages during intestinal inflammation is not known.
Add value of this study
In the present study, we first showed that TRIM33 mRNA level is decreased in blood monocytes of CD patients. Using a mouse model of IBDs, the dextran sulfate sodium (DSS)-induced colitis, we showed that TRIM33 expression in monocytes/macrophages is necessary for the resolution of intestinal inflammation. Indeed, TRIM33 expression in monocytes/macrophages was required for three essential steps of resolution of inflammation: recruitment of blood monocytes to the inflammatory site, differentiation of monocytes into M2 macrophages in the colon and activation of membrane-bound TNF on macrophages associated with apoptosis of colonic neutrophils.
Implications of all the available evidence
These data suggest that TRIM33 expression in monocytes/macrophages regulates the progression of IBDs and may become a novel indicator for disease activity of IBDs.
Alt-text: Unlabelled Box
1. Introduction
Inflammatory bowel disease (IBD) comprises a group of inflammatory conditions of the gastrointestinal tract [1] affecting an increasing number of patients worldwide [2]. The two forms of IBD, Crohn's disease (CD) and ulcerative colitis (UC), result from alterations in the immune homeostasis of the intestinal tissue leading to local uncontrolled inflammation [3,4]. IBDs generally result in epithelial cell death, loss of crypt architecture, submucosal edema and mucosal ulceration [4]. Although the etiology of IBDs remains unclear, it has been shown that abnormal functions of myeloid cells in the intestinal lamina propria contribute to the gut unresolved inflammation [5]. Gut contains a self-maintaining population of macrophages [6] but, during inflammation, these resident gut macrophages are replaced by macrophages produced by incoming blood monocytes that acquire a pro-inflammatory or tissue-protective signature [[7], [8], [9]]. These blood monocytes, also called “inflammatory” monocytes are characterized by a high expression of Ly6C and represent a transient inflammatory stage from blood monocytes to tissue macrophages.
Resident and newly produced macrophages play a crucial role in maintaining gut homeostasis [10] and are thought to be key players in IBD. Although there are several macrophage subsets, they can be divided into two main groups. M1 (or activated macrophages) produce nitric oxide, reactive oxygen species, and pro-inflammatory cytokines such as tumor necrosis factor α (TNF), interleukin 6 (IL-6), interleukin 1β (IL-1β), and interleukin 23 (IL-23), leading to tissue injury. Conversely, M2 (or regulatory macrophages) produce high levels of interleukin 10 (IL-10) and transforming growth factor-β (TGF-β) and low levels of TNF that function in constructive processes such as wound healing and tissue repair [11].
The tripartite motif (TRIM) family of proteins plays important roles in innate immunity and antimicrobial infection. TRIM33 (TIF1γ, Ectodermin) belongs to a sub-family of chromatin binding TRIM proteins that also includes TRIM24 (TIF1α), TRIM28 (TIF1β, KAP1) and TRIM66 (TIF1δ) [12]. Recently, we showed that TRIM33 switches off Ifnβ1 gene transcription during the late phase of macrophage activation [13], regulates the production of macrophages and is involved in the innate immune response [14]. In zebrafish, macrophages of TRIM33-deficient embryos display a lack of a response to inflammatory recruitment signals [15] but the role of TRIM33 in monocytes/macrophages during inflammatory responses in vivo is not known.
In this report, we use a model of human IBD, the dextran sulfate sodium (DSS) induced colitis, in mice deleted for Trim33 only in mature myeloid cells (Trim33−/− mice), to study the role of TRIM33 in mature myeloid cells during this colonic inflammation. TRIM33 deficiency in mature myeloid cells results in a significant accumulation of neutrophils and monocytes in blood and colon but a decrease number of colonic macrophages. Using competitive bone marrow transplantation, adoptive transfer of monocytes and in vitro studies, we show an inefficient recruitment and differentiation of Trim33−/− blood monocytes in the inflammatory colon. In addition, Trim33−/− colonic macrophages display deficiencies that may participate to the unresolved colonic inflammation. Altogether, these results showed a critical role of TRIM33 expression by monocyte/macrophage during colonic inflammation.
2. Materials and methods
2.1. Ethics statments
2.1.1. Mice
Experiments were performed in compliance with European legislation and with the Ethics Committee of the French Ministry of Agriculture (Agreement D9203202; reference 2015012911496673 (APAFIS#190)).
2.1.2. Human
Experiments were conducted on a prospective review of Crohn's disease patients at the Gastroenterology department of Lyon Sud University Hospital (Clinical Trial: NCT03712826).
2.2. Mice and DSS treatment
2.2.1. Mice
Trim33−/− mice were previously described [14]. In all in vivo experiments, 11-to 13 weeks-old female mice were used.
2.2.2. DSS-induced colitis
To induce acute colitis, mice were given drinking water containing 2% Dextran Sulfate Sodium (DSS) (molecular weight: 36,000–50,000; MP Biomedicals) for 6 days with repeat at 3 day [16]. After 6 days of treatment, mice were given normal drinking water.
2.2.3. Inflammation assessment
Inflammation assay daily were measured by weight loss of mice, the variation of colon width and length and analysis of myeloid blood cells. The peak of inflammation and resolution of inflammation in WT mice were determined by the percentage of blood myeloid cells and by width and length colon.
2.3. Histopathological analysis
The colon was perfused with cold PBS and was opened longitudinally. Distal colon was Swiss-rolled and fixed with 4% paraformaldehyde (PFA). The fixed tissue was then embedded in paraffin. Five-micrometer tissue sections were sliced and stained with hematoxylin and eosin (H&E). Cross sections were view with OLYMPUS BX51 microscope and recorded by Archimed software.
2.4. Isolation of murine lamina propria cells from colonic tissues
To isolate colonic cells, the large intestines of mice were excised and soaked in cold PBS. After removing all excess fat and faeces, the colon was opened longitudinally, washed in cold PBS on ice. The mucus was removed by scraped the tissue into petri dishes and cut into 1 cm pieces. Epithelial cells and mucus were removed by two incubations of 15 min with 5 ml of predigestion solution containing HBSS (without Ca2+ and Mg2+), 5% FBS, 2 mM EDTA, and 0.15 mg/ml (1 mM) DTT at 37 °C shaking at 250 rpm. The intestinal tissue was washed by adding cold PBS (with Ca2+ and Mg2+), and cut into 5 mm pieces on ice. Colon pieces were then digested in PBS (with Ca2+ and Mg2+) containing 5% FBS, 1 mg/ml Collagenase VIII (Sigma), and 0.1 mg/ml DNase I (Roche) for 20 min at 37 °C shaking at 250 rpm. The solution was centrifuged, the supernatant was kept in a new falcon with 1 ml of cold FBS, then the colon pieces was incubated with a new digestion solution for 20 min at 37 °C at 250 rpm. The resulting cell suspension and the supernatant were passed through a 70 μm cell strainer washed with PBS and passed through 40 μm cell strainer. Cells were centrifuged, counted and kept on ice until flow cytometry analysis [17].
2.5. Flow cytometry
Blood cells were depleted of red blood cells using an ammonium chloride solution (STEMCELL™) for 15 min and stop with PBS. Blood cells were incubated with anti-CD11b (M1/70) for myeloid cells, anti-CD115 (AFS98), anti-Ly6C (HK1.4) for monocytes and anti-Ly6G (1A8) (Ozyme) for neutrophils and anti-CD3 (17A2) and anti-B220 (RA3-6B2) (Ozyme) for lymphocytes T and B for 20 min at 4 °C. Lamina propria cells were preincubated with a commercial Fc-blocking antibody (anti-CD16/32, ebioscience) for 10 min at 4 °C and then with a cocktail of anti-bodies against various lineage markers, including anti-CD45.2 (104), anti-CD11b (M1/70), anti-IA-IE (CMHII: M5/114.15.2), anti-Ly6C (HK1.4), anti-F480 (BM8), anti-CD64 (X54-5/7.1), anti-Ly6G(1A8) and anti-CD206 (C068C2) (Biologend) for M1/M2 macrophages. Sorting cells were performed on FACS Aria II (BD Biosciences).
For TNF intracellular staining, BD Cytofix/Cytoperm kit (BD Biosciences) was used. Briefly, after membrane staining lamina propria cells were incubated with 500 μl Cytofix/Cytoperm during 20 min at 4 °C and washed with PermWash and stained with antibody specific to TNF (MP6-XT22) (Biolegend) or appropriate Ig control for 1 h on ice.
For membrane-bound TNF staining, lamina propria cells were fixed with 500 μl of 0.5% (PFA) in PBS for 45 min, washed three times with PBS and incubated with anti-bodies against macrophages including anti-TNF (MP6-XT22) or appropriate Ig control for 20 min at 4 °C.
Spleen cells were depleted of red blood cell with ammonium chloride solution (STEMCELL™) for 15 min and stopped with PBS and then were incubated for 20 min at 4 °C with different antibodies described in methods for blood monocytes and neutrophils.
For Ki67 staining, blood cells and lamina propria cells were incubated with antibodies for monocytes staining. BD cytofix/cytoperm kit (BD Bioscience) was used to do the cells fixation and permeabilization and next staining with anti-Ki67 and Hoechst.
For Annexin V staining, cells apoptosis was quantified by the expression of membrane phosphatidylserine (PS) detected by Annexin V binding using Annexin V-APC Apoptosis detection kit as recommended by manufacturer (ebioscience) and analyzed by flow cytometry. Flow cytometry data were collected with the FACS LSR II (BD Biosciences) or FACS Canto II (BD Biosciences) and they were analyzed by Flow Jo software (version 7.6.5).
2.6. Cell culture
2.6.1. Monocytes differentiation
Mouse bone marrow (BM) was flushed out of the tibia, femur and humerus from WT and Trim33−/− mice using a syringe with PBS, filtered through a 70-μm cell strainer to remove debris and pelleted by centrifugation. Monocytes from BM or blood were isolated by negative selection using Mouse Monocyte Isolation Kit (EasySep™; STEMCELLTM) and obtained around 90% of purity. Monocytes differentiation in culture was triggered by association of 50 ng/ml mouse M-CSF (Miltenyi Biotec) and RPMI Medium 1640 (Gibco) supplemented with 10% FBS (Sigma) and 1% Penicillin – Streptomycin (Gibco) during 3 to 4 days. After removing nonadherent cells, cells were detached with non enzymatic solution (Cell dissociation from Sigma) and after with cold PBS. Cells were incubated with anti-CD16/32 and then with a cocktail of antibodies (anti-CD45.2, anti-CD11b, anti-Ly6C and anti F4/80) for detected differentiation of monocytes into macrophages by flow cytometry.
2.6.2. Monocytes co-culture
It was the same protocol that monocytes differentiation. After negative selection of BM monocytes, Trim33−/− monocytes were labeled with CFSE, 70% of Trim33−/− monocytes with 30% of WT monocytes were put in the same well with M-CSF during 3 at 4 days and conversely 70% of WT monocytes with 30% of Trim33−/− monocytes were used.
2.7. Adoptive transfert of monocytes
WT or Trim33−/− monocytes were isolated from BM after DSS treatment (day 16) and marked with CFSE. About 4.106 WT or Trim33−/− monocytes CFSE were injected in intravenous (i.v.) in WT mice treated with DSS (day 16). Percentage of CFSE in blood of WT recipient mice were analysis at different time after adoptive transfer by flow cytometry. 72 h post adoptive transfer, percentage of CFSE in colon cells were analysis by flow cytometry, precisely in monocytes intermediate and macrophages.
2.8. Competitive BM transplantation
WT BM CD45.1 (2.5·106 cells) and Trim33−/− BM CD45.2 (2.5·106 cells) were injected in WT recipient mice CD45.1.2 irradiated at 10 Gy. 3 months after transplantation, WT recipient mice were treated by DSS. At day 16, colon and blood cells were analyzed by flow cytometry.
2.9. Microarray
Ly6Chigh inflammatory blood monocytes RNA was extracted with the RNeasy Micro kit (QIAGEN). Gene expression profiling was performed in biological duplicates on Mouse GE 8x60K Microarrays (Agilent) according to the manufacturer's instructions. The accession number in the Gene Expression Omnibus (GEO) database for the microarrays data is GSE124778.
2.10. Quantitative RT-PCR
Total RNA was extracted with the RNeasy Plus Micro kit (Qiagen) and reverse transcribed with random primers and Superscript III (Life Technologies). Quantitative PCR was performed using the Power SYBR green PCR master mix (Applied Biosystems) in the 7900HT Fast Real-Time or the StepOne PCR Systems (Applied Biosystems). Hprt or β2m mRNA levels were used as an internal reference for mouse and human experiments respectively. Primer sequences are listed below.
2.11. Primer sequences
The following primer sequences were used in quantitative RT-PCR.
2.11.1. Human
β2m F: 5′-CACAGCCCAAGATAGTTAAGT-3′; R: 5′-CCAGCCCTCCTAGAGC-3′; Trim33 F: 5′-AGCAACGGCGACATCCA-3′; R: 5′-CCAGCCCTCCTAGAGC-3′.
2.11.2. Mouse
Hprt F: 5′-GCTGGTGAAAAGGACCTCT-3′; R: 5′-CACAGGACTAGAACACCTGC-3′; Stfa1 F: 5′ AAACTCAAGTCGTCGCTGGAGAA-3′; R: 5′-GTCAGTCTGGTAACCATGAAGTTC-3′; Emp1 F: 5′-ATTGCCACTGCCATTATGCT-3′; R: 5′-TTGCGTAATCTGCAACCATC-3′; Retnla F: 5′-GAACTTCTTGCCAATCCAG-3′; R: 5′-TCCAGTCAACGAGTAAGC-3′; Tnf F: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′; R: 5′-TGGGAGTAGACAAGGTACAACCC-3′; Il6 F: CCGGAGAGGAGACTTCACAG; R: TCCACGATTTCCCAGAGAAC. Il1β F: TCGTGCTGTCGGACCCATAT; R: GTCGTTGCTTGGTTCTCCTTGT.
2.12. Irradiation mice
WT and Trim33−/− head and neck mice were irradiated at 16 Gy and the mice were monitoring during 21 days. Parkin's score was evaluated [18].
2.13. Isolation of human blood monocytes
Peripheral blood was obtained from healthy adults and from patients with Crohn's disease, peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Ficoll (Eurobio). Blood monocytes were isolated from PBMC by MACS sorting with PE anti-CD14 (rmC5-3) (Miltenyi). The purity of enriched monocytes samples were evaluated by flow cytometry.
2.14. Immunofluorescence analysis
The colon was perfused with cold PBS and was opened longitudinally. Distal colon was Swiss-rolled and fixed with 4% PFA. OCT-embedded colon sections (20 μm) were fixated with PFA 1% for 10 min at room temperature (RT), rehydrated in HBSS for 2 min at RT, permeabilized with 0.1% Tween in HBSS for 2 min at RT, blocked with 10% normal donkey serum and 0.01% saponin in HBSS overnight at 4 °C, washed 3 times with HBSS for 5 min and then incubated with A488 anti-F4/80 (1/150, BM8, eBiosciences), PE anti-CD31 (1/200, MEC13.3, OZYME) and Ax700 anti-Ly6C (1/200 HK1.4, Ozyme) 1-1 h30 at RT. The tissue sections were then washed 3 times with HBSS for 5 min, treated with DAPI (/3000) for 15mins at RT, washed 3 times with HBSS for 5 min mounted in ProLong Gold antifade reagent (Molecular Probes) and visualized by Leica SP8 confocal microscope.
The intensities of tissues staining were analyzed and illustrated using ImageJ.
2.15. Histopathological analysis
The spleen was fixed with 4% PFA and then, was embedded in paraffin. Five-micrometer tissue sections were sliced and stained with hematoxylin and eosin. Cross sections were view with OLYMPUS BX51 microscope and recorded thanks to Archimed software.
2.16. Statistics
Statistical comparisons were performed with either a Student's t-test or a Mann-Whitney test, as appropriate (*p < 0.05; **p < 0.008; ***p < 0.0007; ****p < 0.00001).
3. Results
3.1. Trim33 mRNA level is decreased in monocytes of patients suffering from Crohn's disease
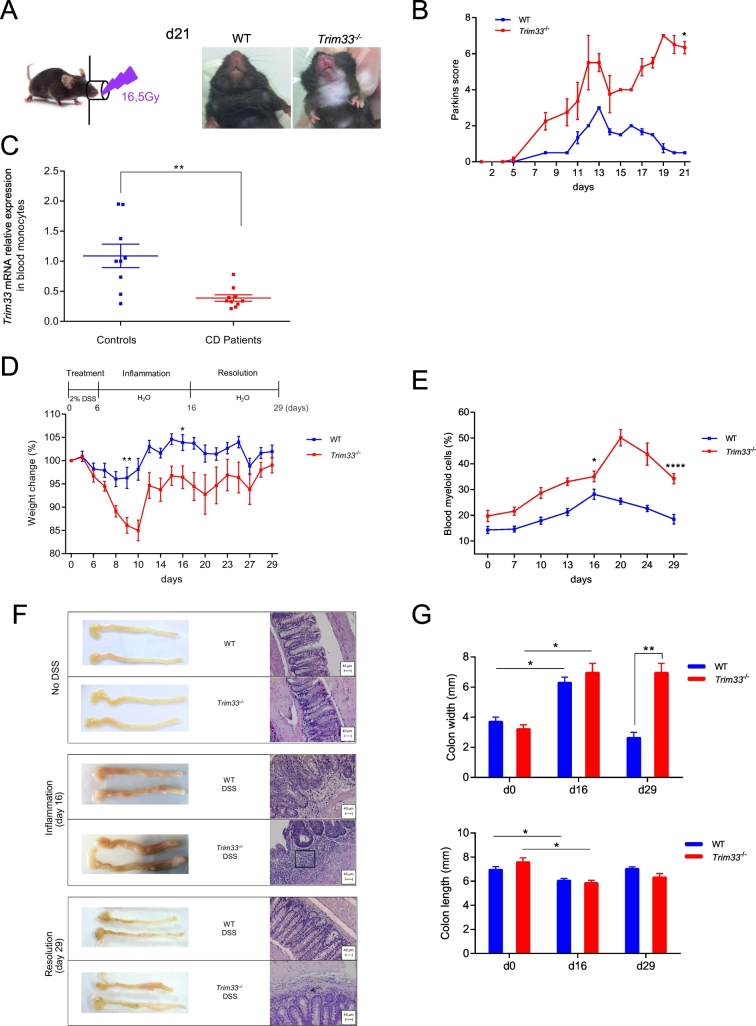
Using LyzCre/Trim33 mice (hereafter referred to as Trim33−/− mice), in which Trim33 is deleted only in mature myeloid cells, we have shown that TRIM33 deficiency in mature myeloid cells decreased these mice survival after lipopolysaccharide (LPS) challenge [14]. The role of TRIM33 in myeloid cells during inflammation was then studied using a local inflammation induced by irradiation of mice in the head-and-neck area. Trim33−/− mice had a continuous inflammation shown by a high Parkin's score until day 21 whereas wild type (WT) mice resolved this radiation-induced inflammation (Fig. 1A and B). Altogether, these results show a role of TRIM33 in mature myeloid cells during inflammation.
Fig. 1.
TRIM33 deficiency in mature myeloid cells worsens the inflammatory response in DSS-induced colitis.
A. 16.5Gy irradiation of the head-and-neck area of WT and Trim33−/− mice (left panel). Persistent inflammation 21 days after the 16.5 Gy local irradiation of Trim33−/− mice (right panels). B. Parkins score monitoring of inflammation after the 16.5 Gy local irradiation of WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 4–5 mice per group. C. Trim33 mRNA levels in purified blood monocytes from healthy (controls) people and Crohn's disease patients using qRT-PCR. Mean ± SEM are performed with n = 9–10 people per group. qRT-PCR data are normalized to b2m. D–G. WT or Trim33−/− mice drank water containing 2% DSS for 6 days (day 6) and normal water for another 23 days (day 29) and the inflammatory response was monitored during this DSS-induced colitis. D. Weight-loss curves of WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 15–22 mice per group. E. Percentage of blood myeloid cells (CD11b+) in WT (blue) and Trim33−/− (red) mice. F. Macroscopic aspect of colon and representative colon sections stained with hematoxylin and eosin (H&E) in WT and Trim33−/− mice. Square and arrow represent cell infiltration. G. Colon width (upper panel) and length (lower panel) in WT (blue) and Trim33−/− (red) mice. Statistical comparisons were performed with Mann-Whitney test. (*p < 0.03; **p < 0.004; ****p < 0.0001).
To get some insights into any role of TRIM33 in inflammatory conditions of the gastrointestinal tract, we studied Trim33 mRNA level in blood monocytes of CD patients as mounting evidence suggests that the pathogenesis of CD involves an impaired acute inflammatory response [19]. We found a decreased mRNA level of TRIM33 (Supplementary Table S1 and Fig. 1C) in CD patients' monocytes suggesting a role of TRIM33 in myeloid cells of CD patients.
3.2. TRIM33 deficiency in mature myeloid cells impairs resolution of DSS-induced colitis
The decreased mRNA level of Trim33 in blood monocytes of CD patients led us to use a mouse model of IBD, the DSS-induced colitis, to determine if this decreased expression was associated with or played a role in CD. At the end of DSS treatment (day 6), WT and Trim33−/− mice begin to lose weight. WT mice regained normal weight 10 days after the end of DSS treatment (hereafter named day 16) but Trim33−/− mice lost more weight and regained normal weight only 23 days after the end of DSS treatment (hereafter named day 29) (Fig. 1D). In WT mice, the highest percentage of circulating myeloid cells was found around day 16, i.e. the peak of inflammation, and this percentage returned to baseline at day 29, i.e. the end of inflammation (Fig. 1E). In Trim33−/− mice, the increase of circulating myeloid cells was always higher and was still high at day 29 suggesting persistent inflammation in these mice (Fig. 1E).
At day 16, DSS treatment resulted in a decreased colon length and an increased colon width in WT and Trim33−/− mice (Fig. 1F and G). In WT and Trim33−/− mice colons showed an inflammatory cell infiltration in lamina propria and this infiltration extensively spread out in Trim33−/− mouse sub-mucosa (Fig. 1F). At day 29, only Trim33−/− mice have an increased colon width (Fig. 1F and G) with an accumulation of inflammatory cells (Fig. 1F) indicating a defect in the resolution of DSS-induced colitis in Trim33−/− mice. This phenotype was associated with a splenomegaly (Supplementary Fig. 1A) and a destruction of splenic follicule structures (Supplementary Fig. 1B) in Trim33−/− mice.
Altogether, these results indicate that TRIM33 deficiency in mature myeloid cells was associated with an abnormal inflammation termination during DSS-induced colitis.
3.3. Impaired recruitment and differentiation of Trim33−/− monocytes during DSS-induced colitis
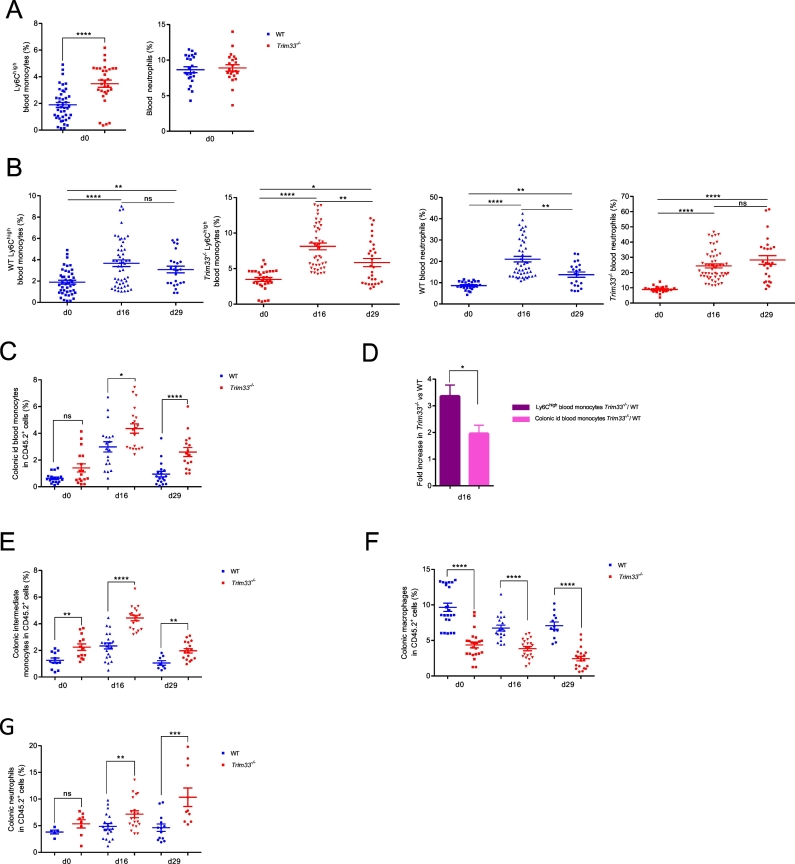
Compared to WT mice, Trim33−/− mice had a higher percentage of circulating Ly6Chigh inflammatory monocytes but a lower percentage of B-lymphocytes and same percentages of Ly6Clow blood monocytes, neutrophils and T-lymphocytes (Fig. 2A and Supplementary Fig. 2A). The higher percentage of blood Ly6Chigh inflammatory monocytes in Trim33−/− mice was associated with decrease apoptosis (Supplementary Fig. 2B). During DSS-induced colitis (day 16), the percentages of circulating Ly6Chigh inflammatory monocytes and neutrophils similarly increased two-fold in Trim33−/− and WT mice (Fig. 2B, day 16 and Supplementary Fig. 2C). We found no difference in the percentages of Ly6Clow monocytes and T-lymphocytes and the same lower percentage of B-lymphocytes in WT and Trim33−/− mice (Supplementary Fig. 2A). At day 29, the percentage of Ly6Chigh inflammatory blood monocytes decreased but remained high in WT and Trim33−/− mice (Fig. 2B, left panels, day 29 and Supplementary Fig. 2C). A high percentage of circulating neutrophils was found only in Trim33−/− mice and this high percentage was not associated with decreased apoptosis of Trim33−/− neutrophils (Fig. 2B, right panels, day 29 and Supplementary Fig. 2D). Furthermore, this increased percentage of blood neutrophils in Trim33−/− at day 29 was not associated with increased percentages of myeloid progenitors/precursors and mature myeloid cells in BM (data not shown) but with an increased percentage of neutrophils in spleen (Supplementary Fig. 2E).
Fig. 2.
TRIM33 deficiency in mature myeloid cells modifies monocytes, macrophages and neutrophils numbers in colon of DSS-treated mice.
A. Percentages of Ly6Chigh inflammatory blood monocytes (Cd11b+ CD115+ Ly6Chigh) (left panel) and blood neutrophils (CD11b+ Ly6Cmed Ly6G+) (right panel) without DSS treatment (d0) in WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 30–40 mice per group. B–F. Myeloid sub-population during DSS-induced colitis. C–F. Of note the number of colonic cells did not change during DSS-induced colitis in WT and Trim33−/− mice (Supplementary Fig. 2G) and thus percentages reflected the number of cells. B. Percentages of Ly6Chigh inflammatory blood monocytes (left panels) and blood neutrophils (right panels) in WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 30–40 mice per group. C. Percentage of id blood monocytes (CD45.2+ CD11b+ CMHII− Ly6Chigh) from colon of WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 10–20 mice per group. D. Fold increase of Trim33−/− over WT inflammatory blood monocytes (purple) and fold increase of Trim33−/− over WT colonic id blood monocytes (pink) at day 16. Mean ± SEM are performed with n = 30–40 mice per group. E. Percentage of intermediate monocytes (CD45.2+ CD11b+ CMHII+ Ly6C+ F480− CD64+) from colon of WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 10–20 mice per group. F. Percentage of macrophages (CD45.2+ CD11b+ CMHII+ Ly6C− F480+ CD64+) from colon of WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 10–20 mice per group. G. Percentage of neutrophils (CD45.2+ CD11bhigh CMHII− Ly6Cmed Ly6G+) from colon of WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 6–20 mice per group. Statistical comparisons were performed with Mann-Whitney test (*p < 0.02; **p < 0.0003; ***p < 0.0006; ****p < 0.0001).
In absence of inflammation, Ly6Chigh inflammatory blood monocytes can enter the intestinal mucosa (and are then named id blood monocytes), mature into intermediate monocytes which can differentiate into F4/80 macrophages (Supplementary Fig. 2F) [20]. Before DSS challenge (day 0), the percentage of id blood monocytes in colon was similar in WT and Trim33−/− mice (Fig. 2C, day 0 and Supplementary Fig. 2H). In contrast, the percentage of intermediate monocytes was higher in Trim33−/− mice compared to WT mice (Fig. 2E, day 0 and Supplementary Fig. 2I) and this difference was associated with a lower percentage of colonic macrophages (Fig. 2F, day 0 and Supplementary Fig. 2 J) suggesting a differentiation defect of Trim33−/− intermediate monocytes into colonic macrophages.
At day 16, an increased percentage of id blood monocytes was found in the colon of WT and Trim33−/− mice as already reported [21] (Fig. 2C, day 0 vs day 16 and Supplementary Fig. 2H). However, the fold increase of Trim33−/− id blood monocytes compared to WT was two-fold lower than the fold increase of Ly6Chigh inflammatory blood monocytes suggesting a decreased capacity of Trim33−/− inflammatory blood monocytes to be recruited to the colon during DSS-induced colitis (Fig. 2D). The increased percentage of WT and Trim33−/−intermediate monocytes compared to day 0 (Fig. 2E, day 0 vs day 16 and Supplementary Fig. 2I) was associated with a decreased or stable percentage of WT and Trim33−/− colonic macrophages respectively, a consequence of the known decreased intermediate monocytes differentiation into macrophages during colonic inflammation [20] (Fig. 2F, day 0 and day 16 and Supplementary Fig. 2J). The increased percentages of id blood monocytes and intermediate monocytes and the diminished percentage of colonic macrophages in Trim33−/− mice at day 16 were neither accounted for by differences in apoptosis or cell cycle of these populations (Supplementary Fig. 2K) nor by a different localization of Ly6C monocytes and F4/80 macrophages in colon tissue from WT or Trim33−/− mice (Supplementary Fig. 2L).
At day 29, the percentage of id blood monocytes returned to their basal level only in the colon of WT mice (Fig. 2C, day 0 vs day 29 and Supplementary Fig. 2H); the percentage of intermediate monocytes returned to their basal level in WT and Trim33−/− mice (Fig. 2E, day 29 and Supplementary Fig. 2I) and the percentage of colonic macrophages decreased only in Trim33−/− mice (Fig. 2F, day 29 and Supplementary Fig. 2J).
Altogether, these results showed a decreased capacity of Trim33−/− blood monocytes to be recruited to the inflammatory colon and a differentiation defect of Trim33−/− intermediate monocytes into colonic macrophages.
Whereas no difference in percentages of colonic neutrophils between WT and Trim33−/− mice was found at day 0 (Fig. 2F, day 0), a continuous increase percentage of infiltrated neutrophils was found in the colon of Trim33−/− mice compared to WT mice during DSS-mediated colitis (Fig. 2F, day 16 and day 29) and might be associated with the impaired resolution of DSS-induced colitis in Trim33−/− mice.
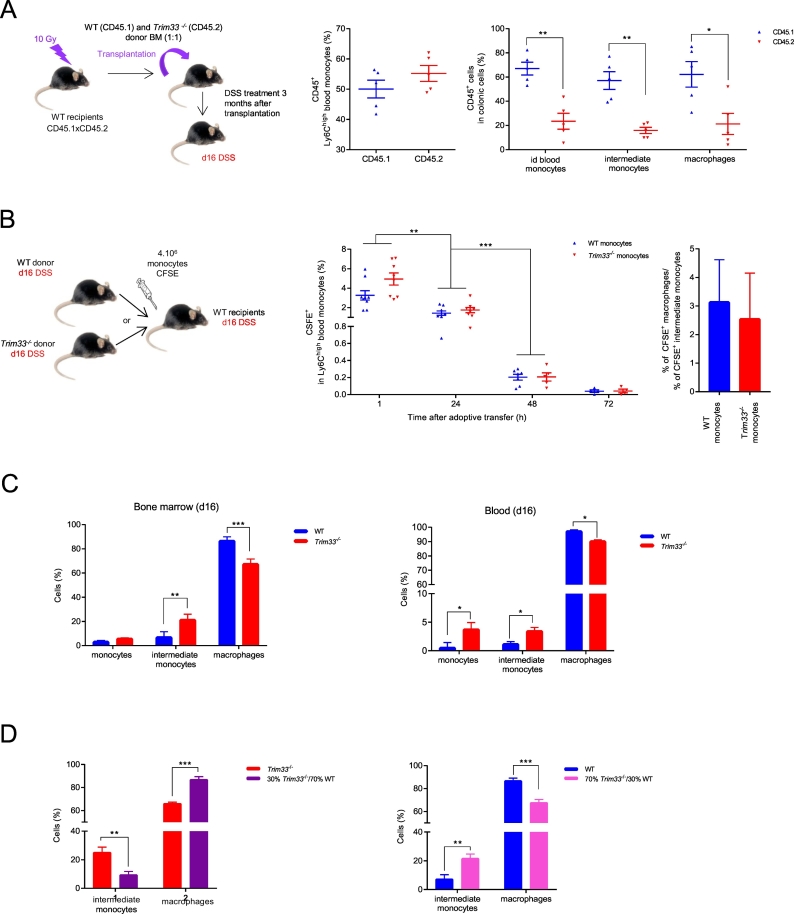
3.4. Cell autonomous deficiency of Trim33−/− monocytes recruitment and differentiation during DSS-induced colitis
In most cases of CD, the macrophages recruitment from blood monocytes is affected [19]. To compare Trim33−/− and WT monocytes recruitment and differentiation during DSS-induced colitis, we performed competitive BM transplantation in WT recipient mice followed by DSS-induced colitis. A 1:1 ratio of WT and Trim33−/− BM was transplanted into lethally irradiated WT recipient mice and 3 months after transplantation, mice were treated by DSS and sacrified at day 16 (Fig. 3A, left scheme). Whereas a 1:1 chimerism was found in Ly6Chigh inflammatory blood monocytes of recipient mice (Fig. 3A, middle panel), three-fold decreased percentages of Trim33−/− id blood monocytes, intermediate monocytes and macrophages were found in the colon (Fig. 3A, right panel) indicating a cell-autonomous deficiency of Trim33−/− monocytes recruitment.
Fig. 3.
Cell-autonomous deficiency of recruitment and differentiation of Trim33−/− monocytes in colon of DSS-treated mice.
A. Scheme of competitive BM transplantation followed by DSS-induced colitis (left scheme). Ly6Chigh blood inflammatory monocytes chimerism of mice reconstituted with CD45.1 WT (blue) and CD45.2 Trim33−/− (red) BM (1:1) and treated with DSS (day16) (middle panel). Percentages of colonic CD45.1+ and CD45.2+ monocytes and macrophages at day 16 (right panel). Mean ± SEM are performed with n = 4–5 mice per group. B. Scheme of adoptive transfer of CFSE-labeled BM monocytes (4 × 106 cells) from WT or Trim33−/− mice treated with DSS (day16) and injected into WT recipient mice treated with DSS (day16) (left scheme). Kinetics of CFSE-monocytes in blood after adoptive transfer of WT (blue) or Trim33−/− (red) BM monocytes (middle panel). Fold increase of CFSE colonic macrophages over CFSE colonic intermediate monocytes in WT mice injected with WT (blue) or Trim33−/− (red) monocytes 72 h post adoptive transfer (right panel). Mean ± SEM are performed with n = 5–9 mice per group. C. Percentages of monocytes and macrophages after M-CSF induced.
differentiation of purified BM (left panel) and blood (right panel) monocytes from WT (blue) or Trim33−/− (red) mice treated by DSS (day 16). Mean ± SEM are performed with n = 15–20 mice per group. D. Percentages of intermediate monocytes and macrophages after M-CSF induced differentiation of CSFE-labeled Trim33−/− monocytes co-cultured with WT monocytes (30/70) (left panel in purple) and of WT monocytes co-cultured with CSFE-labeled Trim33−/− monocytes (30/70) (right panel in pink). Mean ± SEM are performed with n = 6–12 mice per group. Statistical comparisons were performed with either a Student's t-test or a Mann-Whitney test as appropriate (*p < 0.05; **p < 0.008; ***p < 0.0007).
The deficient differentiation of colonic Trim33−/− intermediate monocytes into macrophages can be rescued by a WT environment as same percentages of Trim33−/− intermediate monocytes and macrophages were found after Trim33−/− BM transplantation into WT mice (Fig. 3A, right panel). We then performed adoptive transfer of CFSE labeled inflammatory monocytes from BM of Trim33−/− or WT mice into WT mice at day 16 of DSS treatment (Fig. 3B, left scheme). As previously shown [22], 24 h after adoptive transfer, a significant decrease of injected Ly6Chigh inflammatory blood monocytes was found, followed by a complete disappearance of the injected monocytes 72 h after adoptive transfer (Fig. 3B, middle panel). Of note, 72 h after adoptive transfer, the same percentage of colonic macrophages were observed and a similar fold increase of colonic macrophages over intermediate monocytes was detected in WT recipient mice injected with WT monocytes or Trim33−/− monocytes (Fig. 3B, right panel and Supplementary Fig. 3A) strengthening the positive effect of a WT inflammatory environment on the differentiation of Trim33−/− intermediate monocytes into colonic macrophages during DSS-induced colitis.
Monocytes differentiation into macrophages is dependent on the binding of colony-stimulating factor 1 (CSF1) to its receptor (CSF1-R) [23,24]. Quantification of the CFS1-R (CD115) on the surface of circulating inflammatory monocytes showed no difference between WT and Trim33−/− monocytes (Supplementary Fig. 3B) indicating that impaired differentiation of Trim33−/− monocytes was not accounted for by a decreased expression of the CSF1-R as reported in the control of mature granulomonocytic differentiation [25]. In vitro differentiation of Trim33−/− monocytes from BM or blood mice not treated (data not shown) or treated with DSS (day 16) with CSF1 (M-CSF) resulted in a decreased amount of macrophages and an increased amount of intermediate monocytes when Trim33−/− monocytes were used (Fig. 3C and Supplementary Fig. 3C) strengthening a cell-autonomous defect for the differentiation of Trim33−/− intermediate monocytes into macrophages. However, addition of WT monocytes from mice treated with DSS (day 16) to Trim33−/− monocytes (day 16) increased the differentiation of Trim33−/− monocytes into macrophages (Fig. 3D, left panel and Supplementary Fig. 3D). Conversely, addition of Trim33−/− monocytes (day 16) to WT monocytes (day 16) decreased differentiation of WT monocytes into macrophages (Fig. 3D, right panel and Supplementary Fig. 3D).
Altogether, these results suggest a decreased capacity of Trim33−/− monocytes to be recruited to the inflammatory site and a cell-autonomous differentiation defect of Trim33−/− monocytes into macrophages. Interestingly, this defect can be rescued or mimicked by cellular contact with WT/Trim33−/− monocytes and/or secretion of soluble factors from WT/Trim33−/− monocytes.
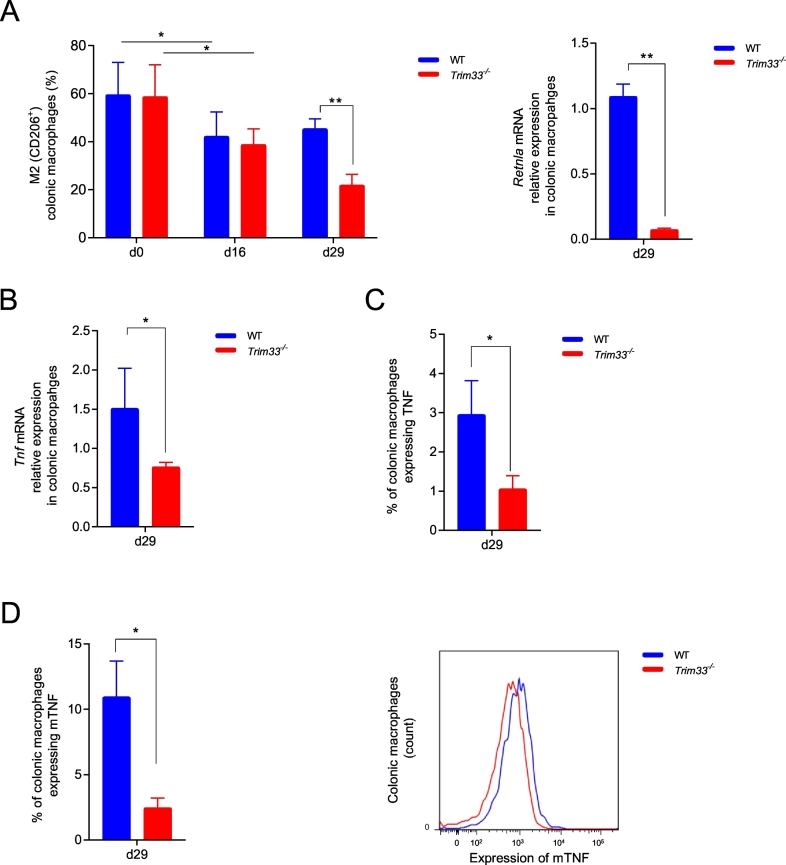
3.5. TRIM33 deficiency in colonic macrophages impaired M2-macrophage polarization and decreased expression of membrane-bound TNF on macrophages during DSS-induced colitis
We finally studied TRIM33 role on colonic macrophage polarization and function during DSS-induced colitis. The percentage of M2 colonic macrophages decreased similarly at day 16 in WT and Trim33−/− mice but, at day 29, the percentage of M2-macrophages decreased only in Trim33−/− mice (Fig. 4A, left panel). Accordingly, in Trim33−/− colonic macrophages at day 29, mRNA level of Retnla (name: resistin like alpha, an M2 gene marker) was decreased (Fig. 4A, right panel). The presence of functional M2-macrophages is important for resolution of inflammation as suggested by the decreased disease in a mouse model of colitis when M2-macrophages but not M1-macrophages were injected [26]. At day 29 after DSS treatment, the decreased percentage of M2-macrophages found in Trim33−/− mice might thus be part of the impaired of resolution of inflammation.
Fig. 4.
Defects of Trim33−/− colonic macrophages during resolution of colitis.
A. Percentages of WT (blue) and Trim33−/− (red) CD206+ M2 colonic macrophages during DSS-induced colitis (left panel). Mean ± SEM are performed with n = 6–9 mice per group. mRNA level of Retnla in WT (blue) and Trim33−/− (red) colonic macrophages at day 29 (right panel). Mean ± SEM are performed with n = 3 mice in each group. qRT-PCR data are normalized to Hprt. B. mRNA level of Tnf in WT (blue) and Trim33−/− (red) colonic macrophages at day 29. Mean ± SEM are performed with n = 3 mice in each group. qRT-PCR data are normalized to Hprt. C. Percentage of colonic macrophages expressing TNF at day 29 in WT (blue) and Trim33−/− (red) mice. Mean ± SEM are performed with n = 6 mice in each group. D. Percentage of colonic macrophages expressing membrane-bound TNF (mTNF) at day 29 in WT (blue) and Trim33−/− (red) mice (left panel). Mean of fluorescence (MFI) of mTNF in WT (blue) and Trim33−/− (red) colonic macrophages at day 29 (right panel). Mean ± SEM are performed with n = 6 mice in each group. Statistical comparisons were performed with either a Student's t-test or a Mann-Whitney test as appropriate (*p < 0.05; **p < 0.003).
The differences in resolution of DSS-induced colitis between WT and Trim33−/− mice might be associated with differences in pro-inflammatory cytokines expression such as IL6 or IL-1β. mRNA levels of these two cytokines at day 29 after DSS treatment were similar in WT and Trim33−/− colonic macrophages (Supplementary Fig. 4A) in contrast with Tnf mRNA level that was decreased in Trim33−/− colonic macrophages (Fig. 4B) indicating an association between Tnf expression and the phenotype observed.
Macrophage membrane-bound TNF (mTNF) induces neutrophils death and its expression is therefore important for the resolution of inflammation [27,28]. As we found an increased percentage of infiltrated neutrophils only in colon of Trim33−/− mice at day 29 after DSS treatment, we studied TNF expression on colonic macrophages. At day 29, less Trim33−/− colonic macrophages expressed TNF and mTNF (Fig. 4C and D, left panel) and Trim33−/− colonic macrophages that expressed mTNF had a lower expression of this protein compared to WT colonic macrophages (Fig. 4D, right panel). In Trim33−/− mice treated by DSS, fewer colonic macrophages produced TNF at day 29 and at a lower level that WT macrophage. This global decreased of mTNF at day 29 by Trim33−/− colonic macrophages was associated with high percentage of infiltrated neutrophils and might be part of the impaired of the resolution of inflammation in Trim33−/− mice.
In conclusion, our results highlight a previously unappreciated function of TRIM33 expression by monocytes/macrophages for the resolution of intestinal inflammation and underscore the importance of TRIM33 expressing myeloid cells in maintaining gut immune homeostasis (Supplementary Fig. 4B).
4. Discussion
Macrophages play a crucial role in the pathogenesis of IBD and may become a meaningful target for the development of drugs that target IBD. Studies using the DSS-induced colitis mouse model that mimics human IBD, have shown the function of myeloid cells in this disease [29] but the macrophage players that are involved in their functions in DSS-induced colitis are not well defined. As TRIM33 regulates the production of bone marrow derived macrophages (BMDM) and peritoneal macrophages (PM), is involved in the innate immune response [14] and regulates Ifnb1 transcription at the late stages of BMDM activation [13], TRIM33 might be an important player of the DSS-induced colitis. In accordance, we show in this study that TRIM33 expression in myeloid cells is required in three essential steps of colitis: recruitment of blood monocytes to the inflammatory site, differentiation of monocytes into macrophages in the tissue and activation of functional macrophages to resolve inflammation.
Monocytes can migrate into tissues and differentiate into heterogeneous cells that include macrophages, dendritic cells, and osteoclasts. To enter the site of inflammation, inflammatory monocytes use adhesion molecules and chemokine receptors such as chemokine (CC motif) receptor 2 (CCR2) that is required for the entry of blood monocytes into the inflamed colonic mucosa [30]. The cell-autonomous impaired capacity of Trim33−/− monocytes to be recruited to the inflammatory site was not associated with deficient CCL2/CCR2 function as Trim33−/− monocytes displayed an unaltered migration of Trim33−/− blood monocytes in the presence of CCL2 (not shown) but might be related to a reduced three-dimensional amoeboid mobility as shown for TRIM33 deficient BMDM [15]. Following extravasion, monocytes can differentiate into inflammatory macrophages. However the molecular players involved in the differentiation of blood monocytes after entering colon is not fully defined [30] and inflammatory monocytes entering the intestine fail to differentiate fully to become mature macrophages in the presence of inflammation that, in turn, results in the accumulation of monocyte-derived cells in the mucosa [20,31]. In the absence of inflammation, a lower percentage of Trim33−/− colonic macrophages were observed and a decreased number of macrophages were also found during resolution of DSS-induced colitis. The impaired differentiation of Trim33−/− blood and intermediate monocytes into tissue macrophages ex vivo is not accounted for by a decreased expression of the CSF1-R as reported in the control of mature granulomonocytic differentiation [25] suggesting a default in the intracellular signaling response. Recently, CSF1–mediated differentiation of monocytes into macrophages requires the activation of caspase-8 in a multimolecular platform [32]. As Trim33−/− inflammatory blood monocytes have an increased expression of both stefin A1 (Stfa1) that regulates caspase activation [33] and epithelial membrane protein 1 (Emp1) that regulates caspase expression [34], the caspase dependent differentiation of monocytes into macrophages might be deficient in Trim33−/− monocytes (Table S2).
Resolution of inflammation is a coordinated and active process that restores tissue integrity and function. An important step of this resolution is the presence of functional M2-macrophages as suggested by the decreased disease associated with decreased colon shortening in a mouse model of colitis when M2-macrophages but not M1-macrophages were injected [26]. Although, in steady state, the percentage of CD206+ M2 colonic macrophages are similar in WT and Trim33−/− mice, a decreased percentage of M2 macrophages was found in Trim33−/− mice during resolution of DSS-induced colitis.
Macrophages are the main source of secreted TNF that displays dose-dependent effects on neutrophil survival but also of mTNF, which can induce neutrophil death [27,28]. The secreted form of TNF is a 17 kD, non-glycosylated protein, which is cleaved from its cell-surface bound precursor (mTNF) by TNF converting enzyme (TACE) [35]. Both the membrane-bound TNF and the soluble TNF have biological activity, with soluble/secreted TNF acting at sites remote from TNF producing cells, whereas the mTNF form acts as a receptor via cell-to-cell contact, transmitting signals to targets when cells are directly in contact with TNF receptor expressing cells [36]. In Trim33−/− mice, fewer colonic macrophages produced TNF at a lower level than WT macrophage during resolution of DSS-induced colitis. The decreased expression of mTNF by Trim33−/− macrophages is associated with high number of infiltrated neutrophils that might be associated with impaired of the resolution of inflammation in Trim33−/− mice. This result is consistent with the therapeutic use of anti-TNF antibodies in CD treatment [37]. Indeed, after binding soluble TNF and membrane-bound TNF, anti-TNF antibodies triggers cytokine suppression and/or apoptosis of monocytes and macrophages [35,38]. Thus, anti-TNF antibodies have a broader action on monocytes and macrophages than just a decreased expression of mTNF on macrophages.
In conclusion, we have shown that TRIM33 deficiency in myeloid cells during colitis is associated with increased production of blood monocytes, a defect in monocytes recruitment and differentiation into functional macrophages leading to failure to resolve intestinal inflammation. These results highlight a previously unappreciated function of TRIM33 expression by monocytes/macrophages in the resolution of intestinal inflammation and underscore the importance of TRIM33 expressing myeloid cells in maintaining gut immune homeostasis.
Acknowledgments
Acknowledgments
We are grateful to the staff of the iRCM animal facility and to N. Dechamps and J. Baijer of the iRCM cytometry platform. The authors thank S.Messiaen for histology analysis, F. Dumont and S. Jacques for microarrays analysis and S. Le Gouvello for critical revision of the manuscript.
Funding sources
This work was supported by La Ligue contre le Cancer (équipe labelisée), INSERM, CEA, University Paris-Diderot, University Paris-Sud.
Declaration of interests
The authors have declared that no conflict of interest exists.
Author contributions
P-H.R. and V·P. designed the project, analyzed data and wrote the manuscript. V.P, A.P, F.F. and C.M organized, performed and analyzed experiments. C.T. and D.L assisted with immunohistochemistry experiments. V.B., A-S.G and M.D. assisted with animal experiments. O.D and G.B performed purification of human blood monocytes. M-C·V performed irradiation experiments. All authors approved the final version of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.05.037.
Contributor Information
Vanessa Petit, Email: vanessa.petit@cea.fr.
Paul-Henri Roméo, Email: paul-henri.romeo@cea.fr.
Appendix A. Supplementary data
Supplementary material
References
- 1.Strober W., Fuss I., Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117(3):514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]; Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117(3):514-21. [DOI] [PMC free article] [PubMed]
- 2.Cosnes J., Gower-Rousseau C., Seksik P., Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]; Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785-94. [DOI] [PubMed]
- 3.Schirbel A., Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. 2010;11(5):266–276. doi: 10.1111/j.1751-2980.2010.00449.x. [DOI] [PubMed] [Google Scholar]; Schirbel A, Fiocchi C. Inflammatory bowel disease: Established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. 2010;11(5):266-76. [DOI] [PubMed]
- 4.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):234–427. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]; Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427-34. [DOI] [PubMed]
- 5.Edelblum K.L., Yan F., Yamaoka T., Polk D.B. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12(5):413–424. doi: 10.1097/01.MIB.0000217334.30689.3e. [DOI] [PubMed] [Google Scholar]; Edelblum KL, Yan F, Yamaoka T, Polk DB. Regulation of apoptosis during homeostasis and disease in the intestinal epithelium. Inflamm Bowel Dis. 2006;12(5):413-24. [DOI] [PubMed]
- 6.De Schepper S., Verheijden S., Aguilera-Lizarraga J., Viola M.F., Boesmans W., Stakenborg N. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175(2):400–415. doi: 10.1016/j.cell.2018.07.048. (e13) [DOI] [PubMed] [Google Scholar]; De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell. 2018. [DOI] [PubMed]
- 7.Hashimoto D., Chow A., Noizat C., Teo P., Beasley M.B., Leboeuf M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792-804. [DOI] [PMC free article] [PubMed]
- 8.Kierdorf K., Prinz M., Geissmann F., Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Semin Immunol. 2015;27(6):369–378. doi: 10.1016/j.smim.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kierdorf K, Prinz M, Geissmann F, Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Semin Immunol. 2015;27(6):369-78. [DOI] [PMC free article] [PubMed]
- 9.Mildner A., Marinkovic G., Jung S. Murine monocytes: origins, subsets, fates, and functions. Microbiol Spectr. 2016;4(5) doi: 10.1128/microbiolspec.MCHD-0033-2016. [DOI] [PubMed] [Google Scholar]; Mildner A, Marinkovic G, Jung S. Murine Monocytes: Origins, Subsets, Fates, and Functions. Microbiol Spectr. 2016;4(5). [DOI] [PubMed]
- 10.Kuhl A.A., Erben U., Kredel L.I., Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. 2015;6:613. doi: 10.3389/fimmu.2015.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kuhl AA, Erben U, Kredel LI, Siegmund B. Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases. Front Immunol. 2015;6:613. [DOI] [PMC free article] [PubMed]
- 11.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]; Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. [DOI] [PMC free article] [PubMed]
- 12.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11(11):792–804. doi: 10.1038/nrc3139. [DOI] [PubMed] [Google Scholar]; Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11(11):792-804. [DOI] [PubMed]
- 13.Ferri F., Parcelier A., Petit V., Gallouet A.S., Lewandowski D., Dalloz M. TRIM33 switches off Ifnb1 gene transcription during the late phase of macrophage activation. Nat Commun. 2015;6:8900. doi: 10.1038/ncomms9900. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ferri F, Parcelier A, Petit V, Gallouet AS, Lewandowski D, Dalloz M, et al. TRIM33 switches off Ifnb1 gene transcription during the late phase of macrophage activation. Nat Commun. 2015;6:8900. [DOI] [PMC free article] [PubMed]
- 14.Gallouet A.S., Ferri F., Petit V., Parcelier A., Lewandowski D., Gault N. Macrophage production and activation are dependent on TRIM33. Oncotarget. 2017;8(3):5111–5122. doi: 10.18632/oncotarget.13872. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gallouet AS, Ferri F, Petit V, Parcelier A, Lewandowski D, Gault N, et al. Macrophage production and activation are dependent on TRIM33. Oncotarget. 2017;8(3):5111-22. [DOI] [PMC free article] [PubMed]
- 15.Demy D.L., Tauzin M., Lancino M., Le Cabec V., Redd M., Murayama E. Trim33 is essential for macrophage and neutrophil mobilization to developmental or inflammatory cues. J Cell Sci. 2017;130(17):2797–2807. doi: 10.1242/jcs.203471. [DOI] [PubMed] [Google Scholar]; Demy DL, Tauzin M, Lancino M, Le Cabec V, Redd M, Murayama E, et al. Trim33 is essential for macrophage and neutrophil mobilization to developmental or inflammatory cues. J Cell Sci. 2017;130(17):2797-807. [DOI] [PubMed]
- 16.Wirtz S., Popp V., Kindermann M., Gerlach K., Weigmann B., Fichtner-Feigl S. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12(7):1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]; Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12(7):1295-309. [DOI] [PubMed]
- 17.Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A.T., Friedlander G. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37(6):1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]; Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, Friedlander G, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37(6):1076-90. [DOI] [PubMed]
- 18.Parkins C.S., Fowler J.F., Yu S. A murine model of lip epidermal/mucosal reactions to X-irradiation. Radiother Oncol. 1983;1(2):159–165. doi: 10.1016/s0167-8140(83)80018-8. [DOI] [PubMed] [Google Scholar]; Parkins CS, Fowler JF, Yu S. A murine model of lip epidermal/mucosal reactions to X-irradiation. Radiother Oncol. 1983;1(2):159-65. [DOI] [PubMed]
- 19.Segal AW. The role of neutrophils in the pathogenesis of Crohn's diseaseEur J Clin Invest. 2018;48(Suppl. 2) doi: 10.1111/eci.12983. [DOI] [PubMed] [Google Scholar]; Segal AW. The role of neutrophils in the pathogenesis of Crohn's disease. Eur J Clin Invest. 2018;48 Suppl 2:e12983. [DOI] [PubMed]
- 20.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102-17. [DOI] [PMC free article] [PubMed]
- 21.Joeris T., Muller-Luda K., Agace W.W., Mowat A.M. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10(4):845–864. doi: 10.1038/mi.2017.22. [DOI] [PubMed] [Google Scholar]; Joeris T, Muller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10(4):845-64. [DOI] [PubMed]
- 22.Yona S., Kim K.W., Wolf Y., Mildner A., Varol D., Breker M. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38(1):79-91. [DOI] [PMC free article] [PubMed]
- 23.Wiktor-Jedrzejczak W., Ratajczak M.Z., Ptasznik A., Sell K.W., Ahmed-Ansari A., Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992;20(8):1004–1010. [PubMed] [Google Scholar]; Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell KW, Ahmed-Ansari A, Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol. 1992;20(8):1004-10. [PubMed]
- 24.Hume D.A., MacDonald K.P. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119(8):1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]; Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119(8):1810-20. [DOI] [PubMed]
- 25.Chretien M.L., Legouge C., Martin R.Z., Hammann A., Trad M., Aucagne R. Trim33/Tif1gamma is involved in late stages of granulomonopoiesis in mice. Exp Hematol. 2016;44(8):727–739 e6. doi: 10.1016/j.exphem.2016.04.009. [DOI] [PubMed] [Google Scholar]; Chretien ML, Legouge C, Martin RZ, Hammann A, Trad M, Aucagne R, et al. Trim33/Tif1gamma is involved in late stages of granulomonopoiesis in mice. Exp Hematol. 2016;44(8):727-39 e6. [DOI] [PubMed]
- 26.Hunter M.M., Wang A., Parhar K.S., Johnston M.J., Van Rooijen N., Beck P.L. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138(4):1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]; Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138(4):1395-405. [DOI] [PubMed]
- 27.Allenbach C., Zufferey C., Perez C., Launois P., Mueller C., Tacchini-Cottier F. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. J Immunol. 2006;176(11):6656–6664. doi: 10.4049/jimmunol.176.11.6656. [DOI] [PubMed] [Google Scholar]; Allenbach C, Zufferey C, Perez C, Launois P, Mueller C, Tacchini-Cottier F. Macrophages induce neutrophil apoptosis through membrane TNF, a process amplified by Leishmania major. J Immunol. 2006;176(11):6656-64. [DOI] [PubMed]
- 28.Meszaros A.J., Reichner J.S., Albina J.E. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165(1):435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]; Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165(1):435-41. [DOI] [PubMed]
- 29.Perse M., Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]; Perse M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [DOI] [PMC free article] [PubMed]
- 30.Bain C.C., Mowat A.M. The monocyte-macrophage axis in the intestine. Cell Immunol. 2014;291(1–2):41–48. doi: 10.1016/j.cellimm.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell Immunol. 2014;291(1-2):41-8. [DOI] [PMC free article] [PubMed]
- 31.Cerovic V., Bain C.C., Mowat A.M., Milling S.W. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 2014;35(6):270–277. doi: 10.1016/j.it.2014.04.003. [DOI] [PubMed] [Google Scholar]; Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 2014;35(6):270-7. [DOI] [PubMed]
- 32.Sordet O., Rebe C., Plenchette S., Zermati Y., Hermine O., Vainchenker W. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100(13):4446–4453. doi: 10.1182/blood-2002-06-1778. [DOI] [PubMed] [Google Scholar]; Sordet O, Rebe C, Plenchette S, Zermati Y, Hermine O, Vainchenker W, et al. Specific involvement of caspases in the differentiation of monocytes into macrophages. Blood. 2002;100(13):4446-53. [DOI] [PubMed]
- 33.Kopitar-Jerala N. Innate immune response in brain, NF-kappa B Signaling and Cystatins. Front Mol Neurosci. 2015;8:73. doi: 10.3389/fnmol.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kopitar-Jerala N. Innate Immune Response in Brain, NF-Kappa B Signaling and Cystatins. Front Mol Neurosci. 2015;8:73. [DOI] [PMC free article] [PubMed]
- 34.Sun G.G., Wang Y.D., Cui D.W., Cheng Y.J., Hu W.N. EMP1 regulates caspase-9 and VEGFC expression and suppresses prostate cancer cell proliferation and invasion. Tumour Biol. 2014;35(4):3455–3462. doi: 10.1007/s13277-013-1456-x. [DOI] [PubMed] [Google Scholar]; Sun GG, Wang YD, Cui DW, Cheng YJ, Hu WN. EMP1 regulates caspase-9 and VEGFC expression and suppresses prostate cancer cell proliferation and invasion. Tumour Biol. 2014;35(4):3455-62. [DOI] [PubMed]
- 35.Horiuchi T., Mitoma H., Harashima S., Tsukamoto H., Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) 2010;49(7):1215–1228. doi: 10.1093/rheumatology/keq031. [DOI] [PMC free article] [PubMed] [Google Scholar]; Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford). 2010;49(7):1215-28. [DOI] [PMC free article] [PubMed]
- 36.Adegbola S.O., Sahnan K., Warusavitarne J., Hart A., Tozer P. Anti-TNF therapy in Crohn's disease. Int J Mol Sci. 2018;19(8) doi: 10.3390/ijms19082244. [DOI] [PMC free article] [PubMed] [Google Scholar]; Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF Therapy in Crohn's Disease. Int J Mol Sci. 2018;19(8). [DOI] [PMC free article] [PubMed]
- 37.Rigby W.F. Drug insight: different mechanisms of action of tumor necrosis factor antagonists-passive-aggressive behavior? Nat Clin Pract Rheumatol. 2007;3(4):227–233. doi: 10.1038/ncprheum0438. [DOI] [PubMed] [Google Scholar]; Rigby WF. Drug insight: different mechanisms of action of tumor necrosis factor antagonists-passive-aggressive behavior? Nat Clin Pract Rheumatol. 2007;3(4):227-33. [DOI] [PubMed]
- 38.Eissner G., Kolch W., Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15(5):353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]; Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15(5):353-66. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material