Abstract
Interventional oncology is a subspecialty field of interventional radiology that addresses the diagnosis and treatment of cancer and cancer-related problems by using targeted minimally invasive procedures performed with image guidance. Immuno-oncology is an innovative area of cancer research and practice that seeks to help the patient’s own immune system fight cancer. Both interventional oncology and immuno-oncology can potentially play a pivotal role in cancer management plans when used alongside medical, surgical, and radiation oncology in the care of cancer patients.
© RSNA, 2019
Summary
We provide a concise report on the current state of the synergies that exist between interventional oncology and immuno-oncology, as well as the future directions of these fields.
Introduction
Interventional oncology (IO) is a subspecialty field of interventional radiology that addresses the diagnosis and treatment of cancer and cancer-related problems by using targeted minimally invasive procedures performed with image guidance. With this specialization, interventional oncologists observe patients in the clinic, admit patients to hospitals, serve on tumor review boards and multidisciplinary treatment teams, and have active roles in the diagnosis and management of patients with cancer (1). The interventional oncologist is an essential member of the treatment team for the patient with cancer (2–5). Interventional oncologists can identify safe approaches for performing minimally invasive tumor biopsies to obtain the necessary genetic or proteomic material that is needed to precisely tailor the chemotherapeutic agents expected to elicit the greatest treatment effect (6). Immuno-oncology is an innovative area of cancer research and practice that seeks to help the patient’s own immune system fight cancer. In November 2016, the Society of Interventional Oncology formally commissioned a white paper to explore the synergies between IO and immuno-oncology. A panel of 18 expert interventional oncologists and immuno-oncologists were selected and invited by the Society of Interventional Oncology to identify essential elements of the emerging field of immuno-oncology for interventional oncologists with the goal of issuing this consensus document. This panel met on January 23, 2017, for a full-day meeting in New York at Memorial Sloan-Kettering Cancer Center and had multiple subsequent teleconferences to evaluate key areas in immuno-oncology considered integral to the interventional oncologist’s practice. This paper represents a consensus report by the panel on the current state of the synergies between IO and immuno-oncology as well as the future directions of the fields, which was formally ratified by the Society of Interventional Oncology in September 2018.
Stimulating the Immune System by Using IO Techniques
In IO, a large variety of in situ tumor destruction techniques such as thermal ablation, chemo- or radioembolization, irreversible electroporation, and high-intensity focused ultrasound have been successfully used for the treatment of an array of malignancies. Whereas ablative techniques are diverse in technology and the mechanism of inducing cell death, they share one key feature: creating in situ availability of the ablated tumor material (7). The ablated material can induce immune responses leading to the infrequently observed abscopal effect (8,9). The ability to stimulate the immune system upon scavenging antigens from dead tumor cells has led to the concept that in situ tumor destruction can be used to achieve systemic so-called in vivo vaccination against tumors. Several studies (10–12) have demonstrated that a tumor can ultimately serve as its own antigenic vaccine after ablation, provided that additional contextual signals are provided via immunotherapy.
Immune Response to Ablation
Data are limited regarding the different contextual immune responses between the various ablation methods. To our knowledge, it remains unknown regarding which technique results in the most effective release of tumor antigens, creates the most immunostimulatory environment from a molecular perspective, or combines most effectively with optimally timed immune-stimulating therapies.
Cryoablation has been shown to induce greater increases in the plasma level of some cytokines compared with other heat-based therapies such as radiofrequency ablation (RFA), suggesting that a greater postprocedure immune response with this technique can be achieved compared with other thermal-based techniques (13–17). Mechanical high-intensity focused ultrasound (settings, such as boiling histotripsy, are unique in that they result in emulsified acellular (tumor) debris that can be more effectively removed via drainage or absorbed as part of the physiologic healing response (18,19). Each ablation technique generates a unique antigenic fingerprint. This is illustrated by the variability in desmoplastic response among ablative modalities. This fingerprint interacts with the existing T cell pool preablation to determine the final tumor-directed T cell repertoire. Clonal analysis of the T cell repertoire following cryoablation shows that 18% of T cells undergo clonal expansion, demonstrating diversification and remodeling of the intratumoral T cell reactivity (20). Identification and further understanding of the unique antigenic fingerprint expressed during various ablation techniques will be crucial for optimizing therapies for each tumor type and stage of cancer.
Immune Response to Transarterial Chemoembolization
At present, the largest application of transarterial chemoembolization (TACE) of tumors is for hepatocellular carcinoma (HCC). Several lines of evidence support a role for targeting the immune system in HCC. Patients with HCC exhibit a 66% increase in circulating regulatory T cells (Tregs) compared with healthy control patients, in part through tumor-secreted transforming growth factor (TGF) β mediated expression of forkhead box P3 (known as FOXP3) (21). The percentage of Tregs has been shown to correlate with disease stage, with increasing Tregs observed in more advanced stages of HCC (22–24), suppressing the immune system’s ability to recognize tumor as foreign. Tumor-infiltrating lymphocytes have been shown to represent the largest share of Tregs in patients with HCC (25). A high percentage of Tregs have been associated with a poorer survival prognosis (26). In addition, a high tumor-infiltrating macrophage density predicts poor prognosis in patients with HCC (hazard ratio, 1.6) (27). However, a proinflammatory antitumor microenvironment is associated with superior survival in patients with HCC (28).
Transarterial embolization induces tumor-associated antigen (TAA)–specific responses, with an increase in the diversity and strength of TAA immune responses after embolization (25,29). This so-called neo-antigenic expansion after TACE can be augmented by blocking cytotoxic T-lymphocyte antigen-4 (CTLA-4), resulting in an unmasking of TAA-specific immune responses (29). TAA-specific T cell responses after TACE have been demonstrated to be associated with patient survival (30,31). TACE has also been demonstrated to increase circulating Th17 cells in some patients with HCC, and this increase correlates with overall survival and time to progression (32). Furthermore, changes in T cell populations have been shown after bland hepatic transarterial artery embolization and TACE. After transarterial artery embolization, Treg fractions decrease 32% in the peripheral blood (33). In patients with HCC who were treated with TACE, the ratio of CD4/CD8 T lymphocytes increased, whereas the percentage of CD4+CD25+ Treg cells decreased 1 month after treatment, suggesting that the immune function of patients with HCC was improved after TACE (34). Finally, there are complex changes in inflammatory cytokine profiles after TACE, which are likely to influence the global immune response in the days and weeks after TACE (35).
Immune Response to Yttrium 90 Radioembolization
Yttrium 90 (90Y) transarterial radioembolization (TARE) (also called selective internal radiation therapy) has become a commonly used approach for treating HCC (36–40) and other cancers (41–45). The antitumor efficacy of ionizing radiation has been largely attributed to its capacity to induce direct tumor cell death through DNA damage of cancer cells (46). Besides its local-regional anticancer efficacy, ionizing radiation may also induce immune-mediated antitumor responses distant to the targeted area, known as the abscopal effect (47). Strong evidence supports the ability of ionizing radiation to induce an immune response, providing the rationale to use radiation therapy in combination with immune-based strategies (48,49). Once administered into the hepatic artery, the 90Y microspheres are preferentially trapped into the tumor microvasculature, allowing for selective tumor targeting while relatively sparing normal parenchyma (50). TARE is able to generate an inflammatory reaction, which is particularly evident at cross-sectional imaging performed after the procedure (51). 90Y-microsphere administration is followed by an increase in proinflammatory and oxidative stress biomarkers (52–54).
The core mechanism is that ionizing radiation–induced cell death generates a flood of preserved tumor-associated antigens, creating an effective in situ tumor vaccine (55,56). Among many interactions, radiation improves dendritic cell priming of antitumor T cells and increases major histocompatibility complex class-1 expression, thereby facilitating tumor antigen manifestation to T cells (57–59). High-mobility group box 1 protein (known as HMGB1) released from irradiated dying tumor cells activates toll-like receptor–4 pathways, which increase tumor antigen recognition (60). Local radiation therapy modulates the tumor microenvironment by prompting macrophages to secrete nitric oxide, which promotes vascular normalization, secretion of chemokines, T cell recruitment, higher endothelial expression of vascular adhesion molecule-1, improved tumor recognition, and killing by CD8+ T cells (61). A sustained increase of interleukin (IL)-6 and IL-8 occurring 6–8 weeks after therapy has been reported (52,54). A selective approach permits maximization of the administered dose and decreased toxicity, and also enables repeated TARE (62–64), but is currently applied only to patients with limited disease.
In addition to promotion of immunity, radiation may exhibit certain immuno-inhibitory effects. Radiation upregulates TGF-β1 and galectin-1, which have been shown to indirectly suppress T cell activity (61). Although TARE does not rely on the embolic effect of microspheres, there is a 28% Hounsfield unit reduction at delayed arterial-phase CT after TARE, which suggests a potential decrease in local oxygen supply (65). Transient lymphopenia has been previously observed (66) in patients undergoing TARE, which is usually of no clinical consequence, but this effect may interfere with the immune response. Whereas radiation induces tumor vascular normalization, it can also exacerbate local hypoxia via endothelial damage and veno-occlusive–associated interstitial hypertension, which theoretically abates local immune responses (67,68). Although preclinical models support hypofractionated external beam therapy to maximize the effective antitumor immune response, to our knowledge, the equivalent dosimetry of brachytherapy has not been studied specifically for this purpose (69). Similar studies are necessary for brachytherapies because the clinical expansion of external radiation therapy to treat liver tumors has been limited by the marked radiosensitivity of nontumoral liver parenchyma even with technologic improvements to deliver radiation dose to the target volume (70).
Tumor Stimulatory Effects of IO Therapies
There is increasing clinical and experimental evidence that IO therapies can induce local and systemic secondary biologic reactions that promote aggressive tumor biology and stimulate tumor growth (71–73), which could potentially counteract any of the beneficial immune effects of interventional radiology procedures. These effects can be related to changes in tumor cells and/or normal tissue that are manifest within the treatment zone (71,72). Cell populations and molecular pathways that are responsible for these stimulatory effects may share common links with immunogenic pathways linked to IO therapies in part on the basis of the type of therapy, organ, and primary tumor type (71,73–82). Furthermore, the clinical evidence includes findings regarding the upregulation and expression of key cytokines and pathways that are associated with tumor growth and stimulation. Retrospective studies (75,83) suggest a higher incidence of tumor progression after IO therapy and studies link patient outcomes to key expressions of tumor markers and upregulation of oncogenic pathways after treatment (83).
Many of the commonly used IO therapies, including transarterial embolization, TACE, TARE, and tumor ablation, can incite secondary reactions generating growth factors and cytokines that have roles in both immunogenic and pro-oncogenic pathways (84–87). Increased tumor expression of vascular endothelial growth factor (VEGF), VEGF receptor, hypoxia-inducible factor 1-α (HIF-1-α), matrix metalloproteinases (known as MMPs), cluster of differentiation 147 (CD147), and mammalian target of rapamycin (known as mTOR), and genes for cellular proliferation, tissue remodeling, and inflammation have all been reported after TACE (86–89). Similarly, increased growth factor expression (VEGF and platelet derived growth factor–BB) and increased markers of inflammation (including IL-6 and IL-8), oxidative stress, and endothelial damage have been observed after TARE (84,85). Finally, there are also a number of studies that demonstrate the potential tumorigenic effects of tumor ablation, including ablation-induced local and systemic inflammation (including cytokines such as IL-6 and heat shock proteins), and upregulation of pro-oncogenic growth factors (such as HIF-1-α, VEGF, hepatocyte growth factor, and hepatocyte growth factor receptor [c-Met]) (71,74,80,90). In some cases, local hepatic thermal ablation has been linked to a higher rate of distant intrahepatic tumor development, and increases in inflammation (IL-6) and growth factors (ie, hepatocyte growth factor) have been directly linked to overall worse patient outcomes (72,83,90). Experimental and clinical studies (75,81,82) further suggest that such effects occur for many of the currently used ablative technologies, especially for sublethal or so-called stunned tumors, and that varying ablation parameters for the same technology may incite or reduce so-called off-target effects.
Specific clinical evidence of the potential importance of off-target, tumor stimulatory effects of IO therapies, in particular regarding tumor ablation, also exists. In a study (91) of 580 patients with small (<3 cm) HCC, thermal ablation led to a higher 5-year cumulative distant intrahepatic new tumor rate (62.7% vs 36.6%) and worse disease-free survival rate (31.7% vs 61.1%) compared with surgical resection. Those investigators also detected separate new HCC tumors in the same segment in up to 15% of patients after thermal ablation of the primary HCC (76). In recent randomized controlled trials that compared thermal ablation with surgical resection for colorectal liver metastases, ablation led to a 39% increase in new, separate intrahepatic metastases compared with surgical resection (73,83). Three large studies representing over 550 patients treated with RFA for small (<3 cm) HCC had significantly higher rates (73%–80%) of new distant tumors 5 years after RFA compared with an anticipated cumulative incidence of 26%–55% for a similar population not treated with RFA (92–95). There are also increasing reports of postablation phenomena in clinical ablation scenarios beyond liver cancer. Recent National Institutes of Health data regarding RFA of renal tumors provides the most direct in-patient evidence of tumorigenic effects (96). In their series of 63 patients with multiple hereditary renal cell carcinomas in which one tumor was treated with RFA, untreated tumors manifest at the time of ablation had an accelerated growth rate (fourfold) after RFA in 26 patients. Clearly, studies that explore the immunogenic effect of interventional radiology procedures should also take into consideration any systemic pro-oncogenic effects that may be concomitantly created.
Human Clinical Research on the Immune Effects of IO Therapies
Efforts are currently underway to better understand the effect of embolization and ablation on the immune system through several lines of human clinical research. Most of these studies have assessed changes in peripheral blood lymphocyte populations. After thermal ablation, CD8 T cell fractions increase by 10% and CD8-to-Treg ratios increase by 15% in the peripheral blood (97). These changes are most pronounced for heating-based ablation versus cooling-based ablation. After cryoablation of early stage breast cancer, proliferating (Ki67+) and activated (ICOS+) CD4 and CD8 T cells were identified in the peripheral blood (98). More importantly, in the study’s ablate-and-resect model, increases in CD8-to-Treg ratios were noted in the resected specimen. Deep sequencing of T cell receptors from patients in this cohort showed that whereas high magnitude clonal expansion (>1000 copies) did occur with cryoablation alone, the number of clones that exhibited high magnitude clonal expansion doubled with the addition of ipilimumab (20). RFA of HCC also produces increases in CD4 cells, with a 19% increase in Th1 populations, which aid in CD8 cell costimulation (99). RFA of HCC also leads to a nearly 60% increase in natural killer (NK) cell populations in humans, and the functionality of these NK cells (as indicated by inferon (INF) γ production) strongly predicts survival after RFA (100). Following hepatic artery embolization, both Th1 and Treg populations decrease in the peripheral blood (33).
The immune microenvironment of tumors has a major role in the response to image-guided therapy. Tumor microenvironments can be locally assessed via biopsy or systemically by determining the concentration of peripheral blood mononuclear cells. Both neutrophil-to-lymphocyte ratio and the lymphocyte-to-monocyte ratio are thought to reflect immunogenic (lymphocytic) versus inflammatory (neutrophilic) environments and have been found to be prognostic of outcomes following image-guided intervention. These indicators may reflect the precarious balance between tumorigenic inflammatory influences and helpful immunogenic responses. Patients with small HCCs undergoing RFA with a decreased neutrophil-to-lymphocyte ratio showed better survival than those with increased neutrophil-to-lymphocyte ratio; moreover, postoperative neutrophil-to-lymphocyte ratio change was almost as predictive of survival as tumor size (hazard ratio, 2.39 vs 2.68, respectively) (101). Elevated neutrophil-to-lymphocyte ratio (>5) is an independent predictor of worse survival in patients who undergo 90Y TARE for colorectal metastases in liver (102). High preoperative neutrophil-to-lymphocyte ratio (>2.79) and elevated postoperative neutrophil-to-lymphocyte ratio was associated with a significant increase in risk of local recurrence and distant metastasis after RFA of renal cell cancer (103). However, lymphocyte-to-monocyte ratio is predictive of survival after RFA for colorectal liver metastases (104). Further studies will be critical to better understand the immune microenvironment, determine optimal neutrophil-to-lymphocyte and lymphocyte-to-monocyte ratios related to various interventional techniques, and ultimately aid the timing and selection of future image-guided therapies.
Combining IO Therapies with Immunotherapy
Several groups (105) have investigated combining immunotherapy approaches with transarterial embolization. Ito et al reported safety and limited response combining Freund’s adjuvant with recombinant IL-2 for TACE in HCC. By using a pulsed hepatic arterial infusion approach, Lygidakis et al (106) reported a median survival of 18 months in a group of 20 patients with HCC who underwent direct infusion of IL-2 and IFN-γ into the hepatic and splenic arteries. They went on to report that postresection recurrence was reduced from 55% to 0% in a group of 40 patients with HCC prospectively randomized to receiving the therapy in an adjuvant setting after resection (107). In another prospective randomized study in patients with colorectal cancer with liver metastases, Lygidakis et al (108) demonstrated addition of IL-2 and IFN-γ into the standard hepatic arterial infusion chemotherapy regimen led to a median survival of 20.3 versus 9.9 months in the chemotherapy-only hepatic arterial infusion group. Whether the hepatic arterial infusion results reported will translate into a more conventional TACE regimen is unknown, though some early studies suggested that immunotherapy in combination with TACE may yield improved survival. Kanai et al (109) combined OK-432 with embolization with a prolongation of disease-free survival compared with embolization alone. Yuen et al (110) reported a median survival of 15.9 months by combining IFN-γ with embolization. Valsecchi et al (111) demonstrated a 4.3-month survival benefit in treating liver metastases from uveal melanoma by combining lipiodol and granulocyte-macrophage colony-stimulating factor (known as GM-CSF) with embolization compared with embolization alone.
Other groups have sought to combine arterial infusion of activated immune cells with embolization (adoptive immunotherapy). Nakamoto et al (112) administered autologous dendritic cells during transarterial artery embolization, resulting in the induction of tumor antigen-specific T lymphocyte responses without a statistically significant change in survival rate. However, they did demonstrate a prolonged recurrence-free survival of patients with HCC by combining hepatic artery infused OK-432-stimulated dendritic cells with transarterial artery embolization, and the induction of proinflammatory peripheral cytokines was consistent with an antitumor immune response (113). Hao et al (114) reported a median overall survival of 31 months in patients with unresectable HCC by using TACE combined with cytokine-induced killer cells compared with 10 months by using TACE alone. They used an activation cocktail of CD3, IL-2, and IFN-γ to activate peripheral blood mononuclear cells from the patients during pretreatment. Huang et al (115) reported an overall survival of 56 versus 31 months in a retrospective nonrandomized analysis of patients who underwent either TACE or RFA with cytokine-induced killer compared to TACE or RFA alone.
Finally, the advent of checkpoint inhibitors in HCC management is likely to change the treatment landscape considerably. The programmed cell death protein 1 inhibitor nivolumab (Opdivo; Bristol-Myers Squibb) represents the first checkpoint inhibitor approved for HCC (116). A multicenter phase I pilot study is currently underway to evaluate the use of drug-eluting bead TACE with nivolumab and the study is scheduled to be completed in April 2019 (NCT03143270). Well-designed phase II and III trials that examine a combination approach of embolization and checkpoint inhibition will be necessary.
Some cancers such as HCC are suitable for TARE but not all cancer types are radiosensitive. Ionizing radiation has demonstrated the ability to effectively recruit effector T cells, converting poorly inflamed tumors (so-called immune deserts or nonimmunoresponsive tumors) into immunogenic tumors (117). Administered doses are a major determinant of treatment efficacy and the potential immunomodulatory activities of TARE are likely to be dose-dependent (118). 90Y has a short half-life (2.67 days) and a majority (>95%) of radiation is delivered into the targeted area within 2 weeks after administration (119). Thus, both the dose and timing of evaluation are fundamental to understanding the immunologic events after TARE. Although TARE has immunogenic potential and cases of abscopal effect have been reported (8), a procedure-induced immune response is unlikely to be of sufficient magnitude to cause sustained regression of distant metastases. Future studies are needed to determine the optimal administered radiation dose eliciting an adequate immune response and to define the ideal immunomodulatory agent, schedule, and route of delivery. Dual-checkpoint blockade such as anticytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein ligand 1 antibodies combined with radiation have demonstrated a 60% survival rate versus 25% for immunotherapy alone in a preclinical model (48).
To maximize the potential additive or synergistic benefits of combining local-regional therapies with immunomodulation, optimal treatment sequencing and timing will also be essential (120,121). In addition to exploiting treatment-triggered immunomodulation after local-regional therapies (75,81,82), manipulations such as priming (122) or potentiation (74,123–130) have been attempted. Much of the published data are preclinical (74,81,82,122–125,127,128,130), but human data (75,129) and even pilot trial results (124,126) have begun to emerge. Approximately 50 combination immunotherapy and local-regional therapy trials are listed as active in ClinicalTrials.gov and combine ablation or embolic therapy with cellular or pharmacological immunotherapy, but only a handful have incorporated the timing or sequencing of therapies as a systematic test variable. One report specifically found a clear advantage of priming with immunotherapy before ablation compared with concurrent or delayed immunotherapy in a murine breast cancer model (122). Priming was associated with enhanced suppression of tumor macrophages and expansion of CD8 T cells. It was postulated that thermal ablation exerted such a drastic mechanical destruction of the tumor microenvironment and alteration in immune phenotype due to inflammation that it rendered concurrent or delayed immunotherapy less effective. With further investigation and mechanistic understanding, it is clear that the sequence and timing of combined immunotherapies with interventions will have an important role in optimized treatments.
Development and Optimization of Noninvasive Methods to Monitor Effects of Immunotherapy
In addition to the necessity to determine optimal treatment sequencing in the development of combination therapies, the growth of various immunotherapy approaches and their combinations with local-regional therapy approaches will cause a shift in diagnostic radiologists’ assessment of tumor response and plans for follow-up imaging. Current techniques and imaging response criteria (eg, response evaluation criteria in solid tumors [known as RECIST], modified RECIST [known as mRECIST], and quantitative European Association for the Study of the Liver [known as qEASL] measured at CT, MRI, and conventional fluorodeoxyglucose PET imaging, PET Response Criteria in Solid Tumors [known as PERCIST]) were initially developed to assess the effects of conventional cytotoxic chemotherapies. These criteria are on the basis of evaluation of the changes in tumor size and enhancement after treatment. Because immunotherapy mostly facilitates or modulates the inflammatory response rather than causing tumor cell death through cytotoxic effects, to our knowledge no specific guidelines exist to evaluate changes in tumor imaging appearance after such treatment. Several response criteria have been developed to evaluate patients treated with systemic immunotherapy. Unidimensional immune-related RECIST provides a feasible and reproducible alternative that is highly concordant with immune-related response criteria (131). The bidimensional immune-related response criteria are a modification of the World Health Organization criteria and recommend consecutive follow-up imaging with a prolonged interval of at least 4 weeks to capture delayed therapy response (132). Immune-related adverse events commonly observed after immunotherapy often resemble and must be distinguished from tumor progression at imaging. Indeed, the term pseudoprogression refers to when a tumor in a patient treated with immunotherapy initially increases in size, but then later decreases (131). However, none of the response criteria described above have been validated with intratumoral delivery methods or approaches combining loco-regional therapy (percutaneous and transarterial) with systemically delivered immune-reactive agents, which will be essential before they are widely implemented to successfully evaluate treatment response.
Future Directions and Recommendations
The role of immunotherapy in cancer care is rapidly developing, and immunotherapy will continue to be a key player in improving cancer care. As our role as interventional oncologists in relation to immunomodulation continues to take shape, multiple questions and challenges exist for determining some of the optimal approaches and implementing immunotherapy into practice, and these are outlined in the Table. Some of these challenges include the cost of preclinical basic science studies, correlating serum and tumor markers to outcomes, incorporating more extensive proteomic and genomic sampling and testing, and achieving appropriate patient and tumor selection to account for biologic variability. In experimental studies, there are challenges in the selection of representative animal models in which the underlying organ physiology matches the human condition and the selection of models in which the IO therapy can be scaled down in size but is still representative of the human condition.
Future Directions and Recommendations for Immunotherapy and Interventional Oncology
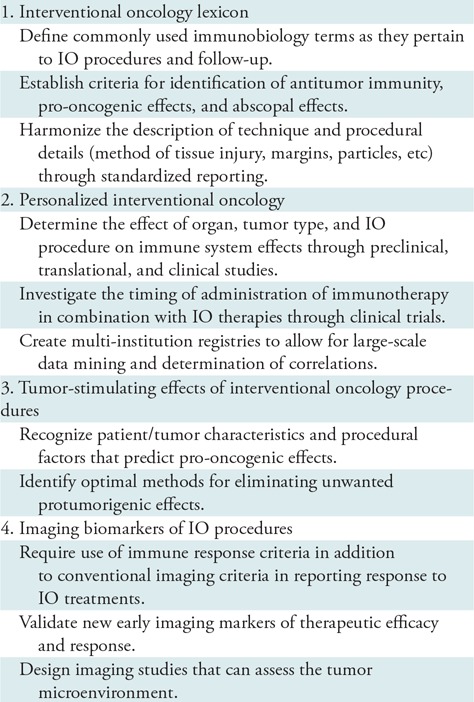
Note.—IO = interventional oncology.
IO Lexicon
As additional research in immuno-oncology is conducted in relation to IO, development of a standard lexicon will become necessary. Various terms have been used to describe the immune response, such as immunogenic, abscopal, and anti-tumor immunity, with multiple descriptors considered to be equivalent. The term off-target effect has also been used and refers to situations in which the end site of action or stimulation is in tumor, tissues, or organs that are physically separate from the treatment zone. In the future, key distinctions will likely be required between off-target effects, which denote effects occurring at the treatment site, effects that influence tumor biology at a separate site, and those that occur within a partially treated tumor (either from partial embolization or partial ablation). Technique and procedure-related differences for ablative therapies have shown variable pro-oncogenic effects on the basis of the method of ablation, method of tissue injury, extent of ablative margin, and differences in thermal heating pattern, which likely also have a role in transarterial embolizations, necessitating specific terminology. Standardized reports will have an important role in this endeavor.
Personalized IO
Future research will need to identify whether observed immune system effects vary on the basis of organ system, tumor type, IO therapy and administration, and timing of secondary effects after treatment. We will need better insights into how best to recognize patients and tumors that may benefit from particular interventions or combination therapies, optimal timing of therapies, and patients who are at risk for tumorigenic effects. The range of immunomodulatory effects (both cellular and humoral) will further need to be identified and characterized for each treatment modality. Further investigations to determine how best to control the immune response as it relates to the individual, combination therapies, and sequencing of therapies will be crucial if IO therapies are to be optimized for strong immune effects. Identification of whether immunotherapies should be sequenced before (ie, priming), concurrently, or after (ie, potentiating) interventions will be essential and the precise sequences may vary according to tumor type, immune status, and treatment modality. Large multi-institution trials and registries will be helpful in elucidating the optimal strategies.
Tumor Stimulating Effects of IO Procedures
Significant issues regarding the tumor stimulating effects of local therapies remain, and they include the following: (a) when these effects occur on the basis of organ, tumor type, specifics of IO therapy and administration, and timing of secondary effects after treatment; (b) how best to recognize patients and tumors that are at risk for such effects; and (c) when, how, and in whom additional measures should be taken to reduce potential unintended tumor stimulation. Understanding these effects can be challenging because many current studies are focused on local treatment efficacy, and therefore patients are not tracked and/or data are not collected that would account for the manifestation of some of these effects. Additionally, in many patients, markers of effects may be biochemical in the local treated tissues and/or systemic circulation, and are therefore never sampled. Prospective large comparative studies, in particular those that assess outcomes, will ultimately be required where variability in patient and tumor biology is accounted for to determine ideal treatment regimens.
Imaging Biomarkers of IO Procedures
Imaging of relevant elements of the immune system will also be essential as the evaluation of tumor response to immunotherapies begins to play a regular role in clinical care for cancer patients. Several imaging methods are currently being developed to capture clinically relevant elements of the tumor microenvironment including the immune system such as biosensor imaging of redundant deviation in shifts and phosphorous 31 MR spectroscopy (133). Techniques that use chemical exchange saturation transfer for imaging various compounds (eg, lactate), that are indirectly viewed through water signal by circumventing labeling or radioactive isotopes (134) are also in development. The application of new and reliable imaging techniques in diagnostic and follow-up imaging will be critical for identifying therapeutic efficacy and nonresponders early in the course of treatment. First, one or a combination of several elements of the immune system must be identified as imageable biomarkers for determining susceptibility to treatment, and for assessing therapy success and response. These might include target molecules (eg, programmed cell death protein 1 receptor status and c-Met status), cell secretions such as granzyme B (13), immune cell populations (ie, dendritic cells, M1 macrophages, and T cell subpopulations), and tumor microenvironment (with elements such as extracellular pH and tissue hypoxia) (132). Second, proper molecular imaging techniques (ie, molecular probes, nanoparticles, specific contrast agents, and tracers) must be developed for use in MRI or PET systems to image relevant factors that measure immune system activity. Third, prospective clinical trials including longitudinal imaging must be conducted to systematically apply and evaluate the clinical value of the developed methods with the ultimate goal of developing novel and objective tumor response criteria.
Conclusion
Regardless of the challenges we face in investigating and incorporating immuno-oncology into an interventional oncology (IO) practice, immunotherapy is destined to become an integral part of IO care and the integration is therefore essential for the future of IO. Logical steps will be necessary to progressively implement and assimilate this evolving field into our practices. Both preclinical and clinical studies are already underway for evaluating the role of IO in the field of immuno-oncology. Further clinical trials involving local-regional therapy in combination with systemic immunotherapies (T cell and checkpoint) will be critical to optimize treatment regimens, beginning with phase I pilot studies that evaluate safety end points and comprehensive immune profiling (both genetic and pathologic). Ultimately, phase II and III studies with efficacy end points and tailored immune profiling will be required. We are at the beginning of an exciting revolution in cancer care with the advent of immunotherapy. The role that IO will play in immunotherapy will depend on our collective efforts to address rational questions regarding the fundamental immune effects of local and regional image-guided interventions.
Acknowledgments
Acknowledgments
The authors acknowledge the contributions of J. Bussink, MD, PhD, Johan J. Futterer, MD, PhD, and J. Frank Nijsen, PhD (Radboud University Medical Center, Nijmegen, the Netherlands), and Lynn J. Savic, MD (Yale University School of Medicine) to the manuscript.
Footnotes
Disclosures of Conflicts of Interest: J.P.E. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for advisory board membership from AstraZeneca and from BTG. Other relationships: disclosed no relevant relationships. G.C.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for service on a prelitigation panel; disclosed money to author for manuscript preparation from EBSCO Health Dynamed Plus; disclosed patent pending for image processing and minimally invasive devices; disclosed travel and accommodations paid for by Terumo Interventional Services for a clinical course. Other relationships: disclosed no relevant relationships. G.J.A. Activities related to the present article: disclosed grant from the Dutch Cancer Society. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. M.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from Agile Devices; disclosed money to author’s institution for grants/grants pending from GE Healthcare; disclosed money paid to author for scientific expert panel from Replimune. Other relationships: disclosed no relevant relationships. J.C. disclosed no relevant relationships. M.d.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed employment by Triskelion BV. Other relationships: disclosed no relevant relationships. R.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from BTG/Biocompatibles; disclosed money paid to author’s institution for grants/grants pending from Society of Interventional Oncology, Guerbet, and BTG/Biocompatibles. Other relationships: disclosed no relevant relationships. S.J.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants and personal fees from BTG, Inc. Other relationships: disclosed no relevant relationships. D.T.J. disclosed no relevant relationships. J.R. disclosed no relevant relationships. D.Y.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for consultancy from AstraZeneca, Boston Scientific, Bristol Myers Squibb, BTG, Eisai, Embolx, W.L. Gore, and Terumo; disclosed stock/stock options in Confluent Medical, Proteus Digital Health, Koli Medical, and RadiAction Medical; disclosed industry-sponsored clinical trial participation sponsored by BTG, Merit Medical, and SIR-Tex. Other relationships: disclosed no relevant relationships. B.B.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for board membership from Vivos; disclosed money paid to author for consultancy from BTG, Boston Scientific, and Ethicon. Other relationships: disclosed no relevant relationships. B.J.W. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author’s institution for grants/grants pending from BTG Biocompatibles; disclosed money to author’s institution for travel/accommodations from NIH. Other relationships: disclosed that NIH has research and development agreements with Philips, BTG Biocompatibles, Celsion, Xact Robotics, and Siemens; disclosed patents in the field of image-guided therapy, and that author receives royalties from Philips/Invivo unrelated to this work. D.W. Activities related to the present article: disclosed money paid to author for consulting fee or honorarium from BTG Medical. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. S.N.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed unrelated consulting on ablation devices for Angiodynamics, Cosman Company. Other relationships: disclosed no relevant relationships.
References
- 1.Adam A, Kenny LM. Interventional oncology in multidisciplinary cancer treatment in the 21(st) century. Nat Rev Clin Oncol 2015;12(2):105–113. [DOI] [PubMed] [Google Scholar]
- 2.Duka E, Ierardi AM, Floridi C, Terrana A, Fontana F, Carrafiello G. The role of interventional oncology in the management of lung cancer. Cardiovasc Intervent Radiol 2017;40(2):153–165. [DOI] [PubMed] [Google Scholar]
- 3.Paul D, Ostwal V, Bose S, Basu S, Gupta S. Personalized treatment approach to gastroenteropancreatic neuroendocrine tumors: a medical oncologist’s perspective. Eur J Gastroenterol Hepatol 2016;28(9):985–990. [DOI] [PubMed] [Google Scholar]
- 4.Yakar D, Debats OA, Bomers JG, et al. Predictive value of MRI in the localization, staging, volume estimation, assessment of aggressiveness, and guidance of radiotherapy and biopsies in prostate cancer. J Magn Reson Imaging 2012;35(1):20–31. [DOI] [PubMed] [Google Scholar]
- 5.Brown DB, Gonsalves CF. Percutaneous biopsy before interventional oncologic therapy: current status. J Vasc Interv Radiol 2008;19(7):973–979. [DOI] [PubMed] [Google Scholar]
- 6.Tam AL, Lim HJ, Wistuba, et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the Society of Interventional Radiology research consensus panel. J Vasc Interv Radiol 2016;27(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 2004;64(11):4024–4029. [DOI] [PubMed] [Google Scholar]
- 8.Ghodadra A, Bhatt S, Camacho JC, Kim HS. Abscopal effects and yttrium-90 radioembolization. Cardiovasc Intervent Radiol 2016;39(7):1076–1080. [DOI] [PubMed] [Google Scholar]
- 9.Rao P, Escudier B, de Baere T. Spontaneous regression of multiple pulmonary metastases after radiofrequency ablation of a single metastasis. Cardiovasc Intervent Radiol 2011;34(2):424–430. [DOI] [PubMed] [Google Scholar]
- 10.den Brok MH, Nierkens S, Wagenaars JA, et al. Saponin-based adjuvants create a highly effective anti-tumor vaccine when combined with in situ tumor destruction. Vaccine 2012;30(4):737–744. [DOI] [PubMed] [Google Scholar]
- 11.den Brok MH, Sutmuller RP, Nierkens S, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res 2006;66(14):7285–7292. [DOI] [PubMed] [Google Scholar]
- 12.Waitz R, Solomon SB, Petre EN, et al. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res 2012;72(2):430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larimer BM, Wehrenberg-Klee E, Dubois F, et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res 2017;77(9):2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad F, Gravante G, Bhardwaj N, et al. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg 2010;200(4):500–506. [DOI] [PubMed] [Google Scholar]
- 15.Jansen MC, van Hillegersberg R, Schoots IG, et al. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 2010;147(5):686–695. [DOI] [PubMed] [Google Scholar]
- 16.Chapman WC, Debelak JP, Wright Pinson C, et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg 2000;231(5):752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.den Brok MH, Sutmuller RP, Nierkens S, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006;95(7):896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall TL, Kieran K, Ives K, Fowlkes JB, Cain CA, Roberts WW. Histotripsy of rabbit renal tissue in vivo: temporal histologic trends. J Endourol 2007;21(10):1159–1166. [DOI] [PubMed] [Google Scholar]
- 19.Hoogenboom M, Eikelenboom DC, van den Bijgaart RJE, et al. Impact of MR-guided boiling histotripsy in distinct murine tumor models. Ultrason Sonochem 2017;38:1–8. [DOI] [PubMed] [Google Scholar]
- 20.Page DB, Yuan J, Redmond D, et al. Deep sequencing of T-cell receptor DNA as a biomarker of clonally expanded TILs in breast cancer after immunotherapy. Cancer Immunol Res 2016;4(10):835–844 [Published correction appears in Cancer Immunol Res 2017;5(3):269.] 10.1158/2326-6066.CIR-16-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Deng B, Tang W, Liu T, Shen X. TGF-β1 secreted by hepatocellular carcinoma induces the expression of the Foxp3 gene and suppresses antitumor immunity in the tumor microenvironment. Dig Dis Sci 2013;58(6):1644–1652. [DOI] [PubMed] [Google Scholar]
- 22.Cao M, Cabrera R, Xu Y, et al. , Hepatocellular carcinoma cell supernatants increase expansion and function of CD4(+)CD25(+) regulatory T cells. Lab Invest 2007;87(6):582–590. [DOI] [PubMed] [Google Scholar]
- 23.Yang ZQ, Yang ZY, Zhang LD, et al. Increased liver-infiltrating CD8+FoxP3+ regulatory T cells are associated with tumor stage in hepatocellular carcinoma patients. Hum Immunol 2010;71(12):1180–1186. [DOI] [PubMed] [Google Scholar]
- 24.Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol 2010;136(11):1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayaru L, Pereira SP, Alisa A, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. J Immunol 2007;178(3):1914–1922. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Guo Z, Lizée G, Yu H, Wang H, Si T. Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clin Chem Lab Med 2014;52(9):1357–1365. [DOI] [PubMed] [Google Scholar]
- 27.Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol 2009;40(3):381–389. [DOI] [PubMed] [Google Scholar]
- 28.Chew V, Tow C, Teo M, et al. Inflammatory tumour microenvironment is associated with superior survival in hepatocellular carcinoma patients. J Hepatol 2010;52(3):370–379. [DOI] [PubMed] [Google Scholar]
- 29.Mizukoshi E, Nakamoto Y, Arai K, et al. Comparative analysis of various tumor-associated antigen-specific T-cell responses in patients with hepatocellular carcinoma. Hepatology 2011;53(4):1206–1216. [DOI] [PubMed] [Google Scholar]
- 30.Hiroishi K, Eguchi J, Baba T, et al. Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol 2010;45(4):451–458. [DOI] [PubMed] [Google Scholar]
- 31.Flecken T, Schmidt N, Hild S, et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014;59(4):1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao Y, Wang B, Huang ZL, et al. Increased circulating Th17 cells after transarterial chemoembolization correlate with improved survival in stage III hepatocellular carcinoma: a prospective study. PLoS One 2013;8(4):e60444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaki H, Imai N, Contessa TT, et al. Peripheral blood regulatory T-Cell and type 1 helper T-cell population decrease after hepatic artery embolization. J Vasc Interv Radiol 2016;27(10):1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep 2015;12(4):6065–6071. [DOI] [PubMed] [Google Scholar]
- 35.Kim MJ, Jang JW, Oh BS, et al. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine 2013;64(2):516–522. [DOI] [PubMed] [Google Scholar]
- 36.Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52(5):1741–1749. [DOI] [PubMed] [Google Scholar]
- 37.Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140(2):497–507.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138(1):52–64. [DOI] [PubMed] [Google Scholar]
- 39.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54(3):868–878. [DOI] [PubMed] [Google Scholar]
- 40.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47(1):71–81. [DOI] [PubMed] [Google Scholar]
- 41.Gordon AC, Gradishar WJ, Kaklamani VG, et al. Yttrium-90 radioembolization stops progression of targeted breast cancer liver metastases after failed chemotherapy. J Vasc Interv Radiol 2014;25(10):1523–1532, 1532.e1–1532.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Memon K, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys 2012;83(3):887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol 2011;196(2):468–473. [DOI] [PubMed] [Google Scholar]
- 44.Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med 2016;57(5):665–671. [DOI] [PubMed] [Google Scholar]
- 45.van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol 2016;34(15):1723–1731. [DOI] [PubMed] [Google Scholar]
- 46.Ross GM. Induction of cell death by radiotherapy. Endocr Relat Cancer 1999;6(1):41–44. [DOI] [PubMed] [Google Scholar]
- 47.Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26(305):234–241. [DOI] [PubMed] [Google Scholar]
- 48.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520(7547):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017;67(1):65–85. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of (90)Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys 2004;60(5):1552–1563. [DOI] [PubMed] [Google Scholar]
- 51.Bester L, Hobbins PG, Wang SC, Salem R. Imaging characteristics following 90yttrium microsphere treatment for unresectable liver cancer. J Med Imaging Radiat Oncol 2011;55(2):111–118. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Ros N, Iñarrairaegui M, Paramo JA, et al. Radioembolization of hepatocellular carcinoma activates liver regeneration, induces inflammation and endothelial stress and activates coagulation. Liver Int 2015;35(5):1590–1596. [DOI] [PubMed] [Google Scholar]
- 53.Wickremesekera JK, Chen W, Cannan RJ, Stubbs RS. Serum proinflammatory cytokine response in patients with advanced liver tumors following selective internal radiation therapy (SIRT) with (90)Yttrium microspheres. Int J Radiat Oncol Biol Phys 2001;49(4):1015–1021. [DOI] [PubMed] [Google Scholar]
- 54.Seidensticker M, Powerski M, Seidensticker R, et al. Cytokines and 90Y-radioembolization: relation to liver function and overall survival. Cardiovasc Intervent Radiol 2017;40(8):1185–1195. [DOI] [PubMed] [Google Scholar]
- 55.Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res 2013;1(1):11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Curr Opin Oncol 2009;21(1):71–76. [DOI] [PubMed] [Google Scholar]
- 57.Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014;41(5):843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009;15(10):1170–1178. [DOI] [PubMed] [Google Scholar]
- 59.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203(5):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007;13(9):1050–1059. [DOI] [PubMed] [Google Scholar]
- 61.Demaria S, Golden EB, Formenti SC. Role of Local radiation therapy in cancer immunotherapy. JAMA Oncol 2015;1(9):1325–1332. [DOI] [PubMed] [Google Scholar]
- 62.Garin E, Rolland Y, Edeline J, et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med 2015;56(3):339–346. [DOI] [PubMed] [Google Scholar]
- 63.Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 2011;79(1):163–171. [DOI] [PubMed] [Google Scholar]
- 64.Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology 2014;60(1):192–201. [DOI] [PubMed] [Google Scholar]
- 65.Pellerin O, Lin M, Bhagat N, Shao W, Geschwind JF. Can C-arm cone-beam CT detect a micro-embolic effect after TheraSphere radioembolization of neuroendocrine and carcinoid liver metastasis? Cancer Biother Radiopharm 2013;28(6):459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol 2005;16(12):1627–1639. [DOI] [PubMed] [Google Scholar]
- 67.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177(3):311–327. [DOI] [PubMed] [Google Scholar]
- 68.Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol 2014;4(4):332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15(17):5379–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dawson LA, Normolle D, Balter JM, McGinn CJ, Lawrence TS, Ten Haken RK. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys 2002;53(4):810–821. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed M, Kumar G, Moussa M, et al. Hepatic radiofrequency ablation-induced stimulation of distant tumor growth is suppressed by c-met inhibition. Radiology 2016;279(1):103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rozenblum N, Zeira E, Scaiewicz V, et al. Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology 2015;276(2):426–432. [DOI] [PubMed] [Google Scholar]
- 73.Tanis E, Nordlinger B, Mauer M, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer 2014;50(5):912–919. [DOI] [PubMed] [Google Scholar]
- 74.Ahmed M, Kumar G, Navarro G, et al. Systemic siRNA nanoparticle-based drugs combined with radiofrequency ablation for cancer therapy. PLoS One 2015;10(7):e0128910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erinjeri JP, Thomas CT, Samoilia A, et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol 2013;24(8):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang TW, Lim HK, Lee MW, et al. Aggressive intrasegmental recurrence of hepatocellular carcinoma after radiofrequency ablation: risk factors and clinical significance. Radiology 2015;276(1):274–285. [DOI] [PubMed] [Google Scholar]
- 77.Kumar G, Goldberg SN, Gourevitch S, et al. Targeting STAT3 to suppress systemic pro-oncogenic effects from hepatic radiofrequency ablation. Radiology 2018;286(2):524–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar G, Goldberg SN, Wang Y, et al. Hepatic radiofrequency ablation: markedly reduced systemic effects by modulating periablational inflammation via cyclooxygenase-2 inhibition. Eur Radiol 2017;27(3):1238–1247. [DOI] [PubMed] [Google Scholar]
- 79.Nijkamp MW, van der Bilt JD, Snoeren N, et al. Prolonged portal triad clamping during liver surgery for colorectal liver metastases is associated with decreased time to hepatic tumour recurrence. Eur J Surg Oncol 2010;36(2):182–188. [DOI] [PubMed] [Google Scholar]
- 80.Nikfarjam M, Muralidharan V, Christophi C. Altered growth patterns of colorectal liver metastases after thermal ablation. Surgery 2006;139(1):73–81. [DOI] [PubMed] [Google Scholar]
- 81.Bulvik BE, Rozenblum N, Gourevich S, et al. Irreversible electroporation versus radiofrequency ablation: a comparison of local and systemic effects in a small-animal model. Radiology 2016;280(2):413–424. [DOI] [PubMed] [Google Scholar]
- 82.Velez E, Goldberg SN, Kumar G, et al. Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology 2016;281(3):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hinz S, Tepel J, Röder C, Kalthoff H, Becker T. Profile of serum factors and disseminated tumor cells before and after radiofrequency ablation compared to resection of colorectal liver metastases - a pilot study. Anticancer Res 2015;35(5):2961–2967. [PubMed] [Google Scholar]
- 84.Carpizo DR, Gensure RH, Yu X, et al. Pilot study of angiogenic response to yttrium-90 radioembolization with resin microspheres. J Vasc Interv Radiol 2014;25(2):297–306.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewandowski RJ, Andreoli JM, Hickey R, et al. Angiogenic response following radioembolization: results from a randomized pilot study of yttrium-90 with or without sorafenib. J Vasc Interv Radiol 2016;27(9):1329–1336. [DOI] [PubMed] [Google Scholar]
- 86.Gou X, Tang X, Kong DK, et al. CD147 is increased in HCC cells under starvation and reduces cell death through upregulating p-mTOR in vitro. Apoptosis 2016;21(1):110–119. [DOI] [PubMed] [Google Scholar]
- 87.Chao Y, Wu CY, Kuo CY, et al. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int 2013;7(3):883–892. [DOI] [PubMed] [Google Scholar]
- 88.He X, Guo X, Zhang H, Kong X, Yang F, Zheng C. Mechanism of action and efficacy of LY2109761, a TGF-β receptor inhibitor, targeting tumor microenvironment in liver cancer after TACE. Oncotarget 2017;9(1):1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao ZW, Fan XX, Song JJ, et al. ShRNA knock-down of CXCR7 inhibits tumour invasion and metastasis in hepatocellular carcinoma after transcatheter arterial chemoembolization. J Cell Mol Med 2017;21(9):1989–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nijkamp MW, van der Bilt JD, de Bruijn MT, et al. Accelerated perinecrotic outgrowth of colorectal liver metastases following radiofrequency ablation is a hypoxia-driven phenomenon. Ann Surg 2009;249(5):814–823. [DOI] [PubMed] [Google Scholar]
- 91.Kang TW, Kim JM, Rhim H, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology 2015;275(3):908–919. [DOI] [PubMed] [Google Scholar]
- 92.Lee DH, Lee JM, Lee JY, Kim SH, Han JK, Choi BI. Radiofrequency ablation for intrahepatic recurrent hepatocellular carcinoma: long-term results and prognostic factors in 168 patients with cirrhosis. Cardiovasc Intervent Radiol 2014;37(3):705–715. [DOI] [PubMed] [Google Scholar]
- 93.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234(3):961–967. [DOI] [PubMed] [Google Scholar]
- 94.N’Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50(5):1475–1483. [DOI] [PubMed] [Google Scholar]
- 95.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47(Suppl):S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor MA, Rothwax J, Linehan WM, Wood BJ, Metwalli AR. Accelerated growth rate of multifocal renal tumors after initial radiofrequency ablation. Society of Urologic Oncology. http://suonet.org/pdf/ProgramBooks/2014-SUO-Program-Book.pdf:101. Published 2014. Accessed DATE.
- 97.Takaki H, Imai N, Thomas CT, et al. Changes in peripheral blood T-cell balance after percutaneous tumor ablation. Minim Invasive Ther Allied Technol 2017;26(6):331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McArthur HL, Diab A, Page DB, et al. A pilot study of preoperative single-dose ipilimumab and/or cryoablation in women with early-stage breast cancer with comprehensive immune profiling. Clin Cancer Res 2016;22(23):5729–5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y, Xu WG, Liu H, Yan K, Ma L, Guo W. Effects of radiofrequency ablation on lymphocyte subsets and type 1/type 2 T cell subpopulations in patients with hepatocellular carcinoma. Chin J Cancer Res 2009;21(4):310–317. [Google Scholar]
- 100.Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 2010;138(5):1931–1942. [DOI] [PubMed] [Google Scholar]
- 101.Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One 2013;8(3):e58184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sukato DC, Tohme S, Chalhoub D, et al. The prognostic role of neutrophil-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol 2015;26(6):816–824.e1. [DOI] [PubMed] [Google Scholar]
- 103.Chang X, Zhang F, Liu T, Wang W, Guo H. Neutrophil-to-lymphocyte ratio as an independent predictor for survival in patients with localized clear cell renal cell carcinoma after radiofrequency ablation: a propensity score matching analysis. Int Urol Nephrol 2017;49(6):967–974. [DOI] [PubMed] [Google Scholar]
- 104.Facciorusso A, Del Prete V, Crucinio N, Serviddio G, Vendemiale G, Muscatiello N. Lymphocyte-to-monocyte ratio predicts survival after radiofrequency ablation for colorectal liver metastases. World J Gastroenterol 2016;22(16):4211–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ito T, Ikeda N, Sue K, et al. Immunotherapy using Freund’s adjuvant and recombinant interleukin-2 combined with transarterial chemoembolization for hepatocellular carcinoma. Gastroenterol Jpn 1989;24(4):386–392. [DOI] [PubMed] [Google Scholar]
- 106.Lygidakis NJ, Kosmidis P, Ziras N, Parissis J, Kyparidou E. Combined transarterial targeting locoregional immunotherapy-chemotherapy for patients with unresectable hepatocellular carcinoma: a new alternative for an old problem. J Interferon Cytokine Res 1995;15(5):467–472. [DOI] [PubMed] [Google Scholar]
- 107.Lygidakis NJ, Pothoulakis J, Konstantinidou AE, Spanos H. Hepatocellular carcinoma: surgical resection versus surgical resection combined with pre- and post-operative locoregional immunotherapy-chemotherapy. A prospective randomized study. Anticancer Res 1995;15(2):543–550. [PubMed] [Google Scholar]
- 108.Lygidakis NJ, Stringaris K, Kokinis K, Lyberopoulos K, Raptis S. Locoregional chemotherapy versus locoregional combined immuno-chemotherapy for patients with advanced metastatic liver disease of colorectal origin: a prospective randomized study. Hepatogastroenterology 1996;43(7):212–220. [PubMed] [Google Scholar]
- 109.Kanai T, Monden M, Sakon M, et al. New development of transarterial immunoembolization (TIE) for therapy of hepatocellular carcinoma with intrahepatic metastases. Cancer Chemother Pharmacol 1994;33(Suppl):S48–S54. [DOI] [PubMed] [Google Scholar]
- 110.Yuen MF, Ooi CG, Hui CK, et al. A pilot study of transcatheter arterial interferon embolization for patients with hepatocellular carcinoma. Cancer 2003;97(11):2776–2782. [DOI] [PubMed] [Google Scholar]
- 111.Valsecchi ME, Terai M, Eschelman DJ, et al. Double-blinded, randomized phase II study using embolization with or without granulocyte-macrophage colony-stimulating factor in uveal melanoma with hepatic metastases. J Vasc Interv Radiol 2015;26(4):523–532.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nakamoto Y, Mizukoshi E, Tsuji H, et al. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin Exp Immunol 2007;147(2):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakamoto Y, Mizukoshi E, Kitahara M, et al. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol 2011;163(2):165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hao MZ, Lin HL, Chen Q, Ye YB, Chen QZ, Chen MS. Efficacy of transcatheter arterial chemoembolization combined with cytokine-induced killer cell therapy on hepatocellular carcinoma: a comparative study. Chin J Cancer 2010;29(2):172–177. [DOI] [PubMed] [Google Scholar]
- 115.Huang ZM, Li W, Li S, et al. Cytokine-induced killer cells in combination with transcatheter arterial chemoembolization and radiofrequency ablation for hepatocellular carcinoma patients. J Immunother 2013;36(5):287–293. [DOI] [PubMed] [Google Scholar]
- 116.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008;181(5):3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cao M, Cabrera R, Xu Y, Liu C, Nelson D. Different radiosensitivity of CD4(+)CD25(+) regulatory T cells and effector T cells to low dose gamma irradiation in vitro. Int J Radiat Biol 2011;87(1):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology 2013;58(6):2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aerts M, Benteyn D, Van Vlierberghe H, Thielemans K, Reynaert H. Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J Gastroenterol 2016;22(1):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mehta A, Oklu R, Sheth RA. Thermal ablative therapies and immune checkpoint modulation: can locoregional approaches effect a systemic response? Gastroenterol Res Pract 2016;2016:9251375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Silvestrini MT, Ingham ES, Mahakian LM, et al. Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2017;2(6):e90521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kudo-Saito C, Fuwa T, Kawakami Y. Targeting ALCAM in the cryo-treated tumour microenvironment successfully induces systemic anti-tumour immunity. Eur J Cancer 2016;62:54–61. [DOI] [PubMed] [Google Scholar]
- 124.Thakur A, Littrup P, Paul EN, Adam B, Heilbrun LK, Lum LG. Induction of specific cellular and humoral responses against renal cell carcinoma after combination therapy with cryoablation and granulocyte-macrophage colony stimulating factor: a pilot study. J Immunother 2011;34(5):457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast Cancer Res Treat 2009;114(3):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Niu L, Chen J, He L, et al. Combination treatment with comprehensive cryoablation and immunotherapy in metastatic pancreatic cancer. Pancreas 2013;42(7):1143–1149. [DOI] [PubMed] [Google Scholar]
- 127.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun 2016;7(1):13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jiang T, Zhang X, Ding J, Duan B, Lu S. Inflammation and cancer: inhibiting the progression of residual hepatic VX2 carcinoma by anti-inflammatory drug after incomplete radiofrequency ablation. Int J Clin Exp Pathol 2015;8(11):13945–13956. [PMC free article] [PubMed] [Google Scholar]
- 129.Fietta AM, Morosini M, Passadore I, et al. Systemic inflammatory response and downmodulation of peripheral CD25+Foxp3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum Immunol 2009;70(7):477–486. [DOI] [PubMed] [Google Scholar]
- 130.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med 2013;210(11):2435–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013;19(14):3936–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 133.Coman D, Huang Y, Rao JU, et al. Imaging the intratumoral-peritumoral extracellular pH gradient of gliomas. NMR Biomed 2016;29(3):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.DeBrosse C, Nanga RP, Bagga P, et al. Lactate chemical exchange saturation transfer (LATEST) imaging in vivo a biomarker for LDH activity. Sci Rep 2016;6(1):19517 [Published correction appears in Sci Rep 2016;6:21813.]. [DOI] [PMC free article] [PubMed] [Google Scholar]


