Abstract
Contrast-enhanced ultrasound is a valuable bedside imaging technique which enables both qualitative and quantitative assessment of cerebral perfusion. In neonates and infants whose fontanelles remain open, the technique is particularly useful as it delineates cerebral pathology with high soft tissue contrast. The technique has the potential to be a valuable alternative to computed tomography (CT) or magnetic resonance imaging (MRI) in critically ill neonates and infants in need of bedside imaging. While further studies are needed to validate the technique, preliminary data in this regard appear promising. The review introduces the current understanding and future potential of brain contrast-enhanced ultrasound.
Keywords: Contrast-enhanced ultrasound, neonate, brain, clinical protocol, application
Introduction
In comparison to conventional brain ultrasound, brain contrast-enhanced ultrasound (CEUS) offers additional information on cerebral perfusion [1–3]. Because perfusion changes accompany brain injury, CEUS can also detect areas of brain injury. Ideally, brain CEUS should follow the standard of care ultrasound to evaluate anatomic detail prior to contrast administration. Microbubbles remain intravascular, in the absence of compromised blood-brain barrier, thereby creating high signal in areas containing large and small intracranial vessels [1, 2]. This permits detection of perfusion abnormalities associated with ischemia, hemorrhage, focal lesions, infection, and intracranial shunts. Its ability to assess cerebral perfusion serially at bedside serves as a major advantage in neonates and infants, whose transport to the magnetic resonance imaging (MRI) suite is inconvenient due to support devices, critical nature of their illness, availability of support staff, and high cost.
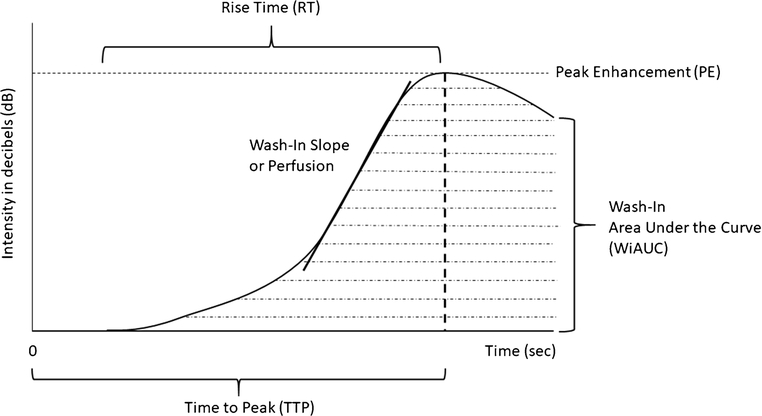
A major advantage of brain CEUS is the ability to quantify cerebral perfusion based on the microbubble flow dynamics [1, 2]. As the microbubbles flow in into a region of interest in the brain, the intensity (dB) of the region of interest changes during the wash-in and wash-out phases from which cerebral perfusion can be quantified. It is based on this intensity change that a time-intensity curve can be generated and various quantification parameters derived. Figure 1 depicts a representative microbubble wash-in graph with a curve fitted to the graph and derivation of the associated quantification parameters. From the curve, standardized CEUS perfusion metrics including peak enhancement (PE), time-to-peak (TTP), rise time (RT), wash-in slope or perfusion (PER), wash-in area under the curve (WiAUC) and perfusion index (PERi; i.e., WiAUC/RT) can be evaluated. PE refers to intensity (dB) at peak enhancement, TTP time from start of cine recording to peak enhancement, RT time from the start of rise in wash-in curve to peak enhancement, WiAUC the total area under the curve during RT, and finally PERi referring to WiAUC over RT.
Figure 1. Microbubble Wash-In Time Intensity Curve (TIC).
The graph represents a time intensity curve generated based on the dynamic microbubble wash-in data. Time 0 represents the time of microbubble injection. Peak enhancement (PE) refers to maximum microbubble intensity (in decibels or dB) during the wash-in phase. The time (sec) it takes from injection to reach PE is called time to peak (TTP). The time from the start of curve rise to peak enhancement is called rise time (RT). The area under the TIC is called wash-in area under the curve (WiAUC) and represents microbubble volume. The slope of the wash-in curve is called wash-in slope or perfusion.
Evaluation of the evolution of cerebral perfusion in the setting of injury is valuable for prognostication and guidance of therapeutic intervention. A study on infants with hypoxic ischemic injury applied Doppler US to perform serial cerebral blood flow measurements and showed that in severe hypoxic ischemic injury as compared to mild or moderate hypoxic ischemic injury, higher reperfusion was seen at immediate post injury period as well as 2 weeks [4]. Post-ischemic hyperperfusion to the basal ganglia and thalamus has been suggested to increase the vulnerability of metabolically active regions.[5, 6]. Previous study with positron emission tomography (PET) performed in infants with hypoxic ischemic injury showed that decreased cerebral uptake at 2 weeks correlated with poor outcome at 2 years [7]. The studies overall suggest that not only hypoperfusion, but also continued or exaggerated post-ischemic hyperperfusion may lead to irreversible brain damage. A better understanding of the relation between the evolution of cerebral perfusion and clinical outcome will therefore be important in developing therapies targeted to minimizing reperfusion injury, promoting vascular remodeling and/or maintaining critical perfusion pressure.
I. Practice Guidelines
Institutional/Food and Drug Administration Approval
Application of contrast-enhanced ultrasound for evaluation of intracranial pathology remains an off-label indication in the United States. This is mainly due to the paucity of published clinical reports and safety data. After Kastler et al [8] from Europe reported a small cohort of neonates and infants evaluated for intracranial pathology with CEUS, Hwang et al introduced feasibility brain CEUS studies on neonates and infants in the United States [1–3]. Given the promise of brain CEUS in the diagnosis of various intracranial pathology not limited to ischemia and absence of documented adverse events, brain CEUS is gaining more interest. With clinical studies, off-label consent form may be used to inform the parent of a child of the technique and safety concerns. With research studies, Institutional Review Board and/or FDA approval for New Investigational Drug (IND) study will be needed to ensure the optimal documentation of the procedures and results for future benefit.
Acoustic Power (Mechanical Index)
There are important safety measures to be aware of in conducting clinical brain CEUS. The minimum safety requirement is to abide by the acoustic power or mechanical index (MI) of <1.9 which is established by the FDA to be the maximum threshold for diagnostic imaging. Typical MI used for CEUS imaging is much lower at 0.05–0.4, and the commercially available scanners usually have MI set at less than 0.1 for the CEUS setting. The absence of bubble growth below the MI value of 0.5 has been reported previously [9]. Moreover, frequent usage of a destruction pulse (MI of 1.0 or higher) which is introduced either by an option on the scanner or switching to a grayscale mode will need to be avoided until safety data is available in regard to the effects of high MI on the integrity of blood-brain barrier. Destruction-replenishment method utilizes induced burst of microbubbles in a region of interest for assessment of reperfusion dynamics without the need for re-injection and can be a useful tool, but its application in the brain of neonates and infants should not be used until there is reasonable confidence that bubble destruction does not result in injury to the blood brain barrier.
Injection Dosage
While the contrast dosage for application in neonatal and infant brains is not established through a carefully designed study, Hwang et al have used the FDA recommended Lumason (Bracco, Inc) dosage of 0.03 cc/kg for pediatric population with optimal image quality [1, 3]. In order to keep the dosage constant across scanners, subjects, and institutions, an adequate understanding of microbubble resonant frequency and ultrasound settings will be needed. The ultrasound parameters that can be adjusted to optimize microbubble signal include MI, gain, frequency, and depth. Depending on the scanner type and probe selection, these settings will need to be adjusted to accommodate for the same injection dosage and image quality. For instance, one scanner type may have varying contrast settings with different transmit frequencies; in such case, the ultrasound settings will have to be adjusted to account for microbubble resonant frequency of 2–3 MHz. If the transmit frequency or probe frequency is much higher at 6–7 MHz, there will be increased attenuation and decreased microbubble oscillation as a result. To account for the loss of microbubble signal, acoustic power may be increased within the limits of safety standards, for instance.
Number of Injections
As with other CEUS exams in pediatric and adult population, brain CEUS in neonates and infants can involve up to two injections per scan. While the second injection may not be necessary, it is beneficial in the early stages of clinical application to confirm reproducibility of exam and validate potential abnormality seen during the first injection. Although the rate of wash-out varies depending on the disease state, with normal subjects demonstrating quicker washout than affected, it is advised that the second injection be performed after natural wash-out rather than employing a destruction pulse to expedite the exam. Based on the preliminary experience, microbubbles reside in the intracranial vasculature for 6–8 minutes after injection in normal subjects and can reside for up to 30 minutes in near brain death cases [2]. Note that not only the wash-in phase but also the wash-out phase can likewise provide important clues to the disease state and will need to be studied.
Injection Parameters
Injection of microbubbles requires proper knowledge on the effects of needle gauge, rate of injection, and site of injection on the interpretation of brain CEUS exams. In neonates and infants, the needle gauge is oftentimes smaller in children, with peripheral intravenous (IV) lines in the scalp or the foot, and injection should be performed at a slow rate to minimize microbubble destruction within the needle. If the peripheral IV line is in the foot, the time from injection to perfusion of intracranial vasculature is increased thereby affecting the time to peak parameter. In the presence of a long intravascular line, moreover, an appropriate saline flush to account for the length of the line should be adopted to adequately clear the microbubbles within the line. In the presence of intravenous in-line filters, microbubbles should be injected above the filters to avoid entrapment. Central lines can be used with sterile technique and should not interfere with the exam interpretation.
II. Clinical Protocol
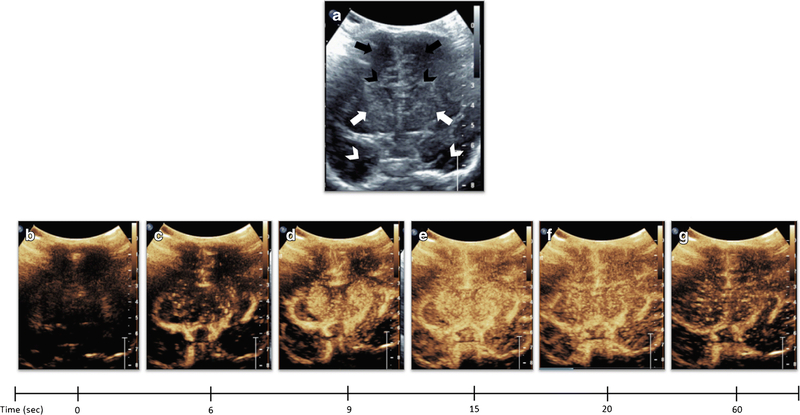
Brain CEUS requires the acquisition of both dynamic and static images of the brain in coronal and sagittal planes during the microbubble wash-in and wash-out phases. The anterior fontanelle is initially used for acoustic window, as this allows delineation of the near entirety of the brain during coronal and sagittal scans. Depending on the operator experience, posterior fontanelle and transmastoid views may be further obtained. Prior to contrast injection, grayscale images of the brain should be obtained as per the clinical protocol. The mid coronal scan plane including the bilateral basal ganglia and frontal horns is then selected for the first contrast injection (Fig. 2). The reason for this slice selection is both for standardization of the initial scan plane for data analysis purposes and to screen for potential injury to both deep and superficial gray white matter. The contrast timer should begin at the time of contrast injection. The ultrasound probe is held over the anterior fontanelle in stable position during a 1-minute cine clip of microbubble wash-in into the mid coronal scan plane. In normal subjects, wash-out may begin toward the end of the 1-minute cine clip. Afterwards, anterior to posterior then posterior to anterior sweeps through the brain in coronal plane is performed to screen for potential perfusion abnormality in the remainder of the brain. Immediately following the coronal sweep, sagittal right to left then left to right sweeps of the brain are performed to assess for perfusion abnormality potentially missed or less evident during the coronal sweep. Delayed wash-out phase is then recorded with intermittent 30-second cine clips, and this recording may occur for longer period in affected cases. Static images can be retrospectively extracted from the cine clips to save time.
Figure 2. Dynamic Microbubble Wash-In on Mid-Coronal Brain Scan in a Normal Subject.
Figure 2A denotes a mid-coronal grayscale ultrasound scan through the brain of a normal subject showing bilateral frontal lobes (orange arrows), frontal horns (yellow arrowheads), basal ganglia (red arrows), and temporal lobes (white arrows). Figure 2B-G demonstrates dynamic microbubble wash-in through the mid-coronal slice through the brain on a contrast specific mode from the time of injection (time 0) to 1 min. Note that the microbubbles flow into the partially visualized Circle of Willis by 13 seconds. By 15 seconds, relatively more avid enhancement to the basal ganglia with respect to the remainder of the brain is seen. Further enhancement of the cortex but with relative hyperenhancement of the basal ganglia is noted at 20 seconds. Wash-out of contrast from both the basal ganglia and cortex begins at 25 seconds and further wash-out noted at 60 seconds. Note that Figure 2 images were obtained with EPIQ scanner (Phillips Healthcare, Bothell, WA) and C5–1 transducer with settings of 12 Hz and MI of 0.06.
III. Clinical Applications
Hypoxic Ischemic Injury (HII)
Brain CEUS enables qualitative and quantitative detection and monitoring of HII. There is paucity of studies in this regard, although emerging evidences demonstrate promise [1–3]. Distinct patterns of perfusion abnormalities associated with HII include 1) focal 2) multifocal 3) diffuse and/or symmetric (Fig. 3). Qualitatively, detection of perfusion abnormality types 1) and 2) is easier than 3) [1]. For accurate and reliable detection of diffuse and/or symmetric perfusion abnormalities accounting for technical differences, standardized quantitative method will be needed. The main challenge behind establishing a quantitative method lies on the difficulty of acquiring the age dependent normative data. While brain CEUS is a relatively safe technique, the contrast administration itself is associated with minimal risk which negates the possibility of recruiting normal subjects for the sole purpose of amassing the normative data. The reasonable approach would be to build a database of normal subjects based on those screened but turned out to have normal clinical and imaging findings, with the latter confirmed with gold-standard methods such as computed tomography (CT) or magnetic resonance imaging (MRI).
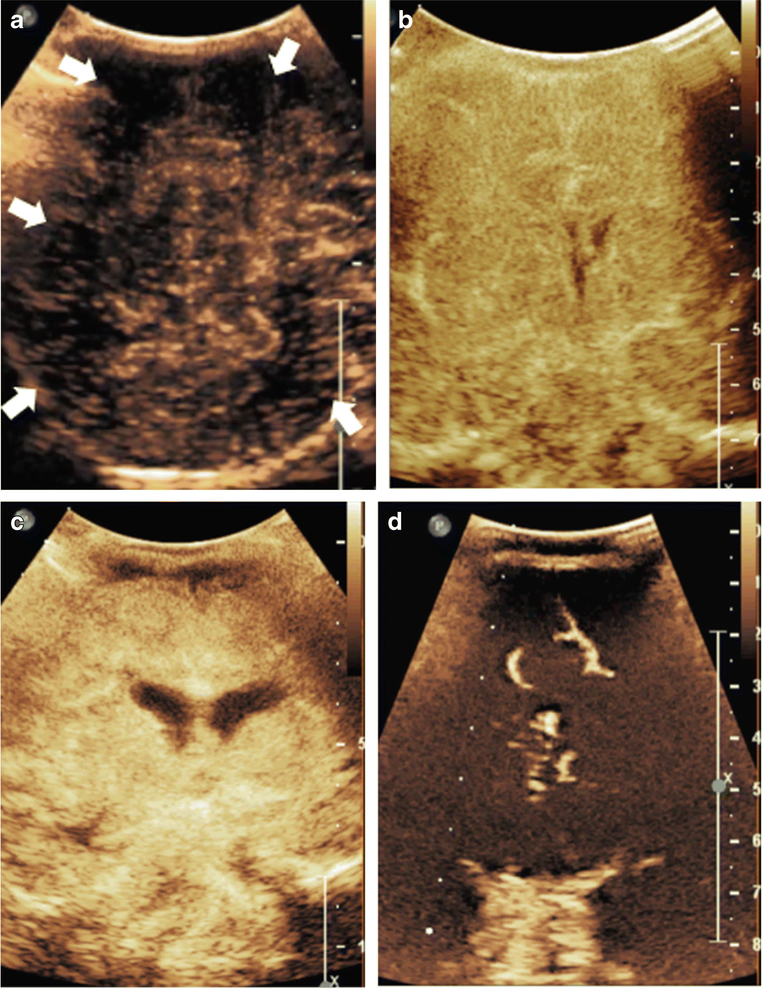
Figure 3. Multifocal and Symmetric, Diffuse Perfusion Abnormalities on CEUS-B.
Figure 3A depicts a coronal scan through the posterior parietooccipital lobes in a neonate with hypoxic ischemic injury. The image was obtained at 27 seconds after microbubble injection at peak intensity. Generalized hypoperfusion with multifocal perfusion abnormalities noted as evidenced by paucity of microbubbles in scattered areas (white arrows). Figure 3B and 3C, both obtained at 18 seconds after microbubble injection, are neonatal cases of symmetric, diffuse hypoxic ischemic injury resulting in generalized hyperperfusion to the brain in the immediate post-injury period. Figure 3D is an infant post prolonged cardiac arrest demonstrating diffuse hypoperfusion to the brain. Note that Figure 3 images were obtained with EPIQ scanner (Phillips Healthcare, Bothell, WA). Figure 3A was obtained with C5–1 transducer and settings of 12 Hz, MI 0.06, Figure 3B with C9–2 transducer and settings of 13 Hz, MI 0.06, Figure 3C with C9–2 transducer and settings of 7 Hz, MI 0.06, and Figure 3D with C9–2 transducer and settings of 12 Hz, MI 0.06. This figure is adapted from Hwang et al.[2] and Shin et al.[27] with permission.
While the quantitative method needs further validation, Hwang et al. suggested several potential approaches for future studies [1, 3]. For focal and multifocal perfusion abnormalities, quantitative parameters of the affected region(s) can be compared to that of the contralateral unaffected region(s) as internal control or to the normative data if available. For diffuse and/or symmetric perfusion abnormalities, establishment of either the normal gray-white matter ratio (GWR) or gray nuclei to cortex (GNC) ratio based on the normative data is necessary. Not only is the perfusion differentially affected in the gray versus white matter, injury can also be isolated to the gray or white matter [12–14]. GWR is obtained by placing the region of interest in the gray and white matter, respectively. GNC is the perfusion ratio obtained of the deep gray nuclei (basal ganglia and thalami) to the remainder of the cortex (Fig. 4). Note that in normal neonates and infants, there is relative hyperperfusion to the basal ganglia and the thalamus as compared to the cerebral cortex [15]. This ratio is especially valuable in detecting either the central or peripheral pattern of HII. Normal neonates and infants would demonstrate GNC greater than 1, but significantly greater as this would be seen in central pattern of hypoxic ischemic injury. In the presence of focal or symmetric peripheral pattern of injury, GNC ratio would be less than 1 and indicative of brain injury [3]. Depending on the size and types of brain injury, the GWR and/or GNC would be altered and quantitatively detected.
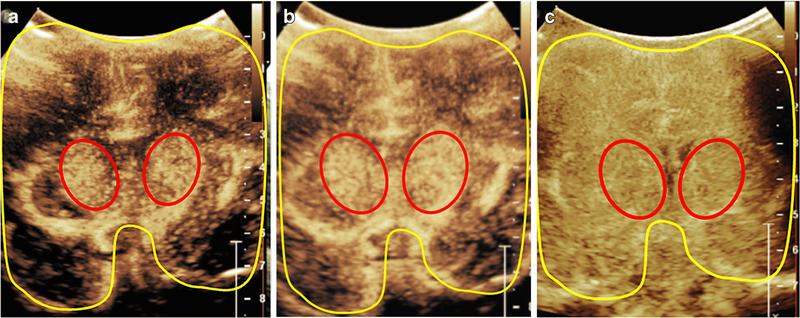
Figure 4. Central Gray Nuclei to Cortex Ratio (GNC).
Central gray nuclei to cortex ratio in a 1-month-old male (a, b) and a 14-day-old male (c). The central gray nuclei (basal ganglia and thalami) (red) and cortex (areas excluding central gray nuclei) (yellow) are outlined on a mid-coronal CEUS. The central gray nuclei to cortex (GNC) ratio equals perfusion in central gray nuclei (red) over perfusion in cortex (yellow). Figure (a) and(b) demonstrates a representative case with the normally seen hyperperfusion to the central gray nuclei, with (a) obtained at 10 seconds after microbubble administration and (b) obtained at 15 seconds after administration at peak enhancement. (c) demonstrates a hypoxic ischemic injury case in which the normally seen GNC ratio greater than 1 is absent due to diffuse hyperperfusion to the brain in the immediate post injury setting. In this case, the GNC ratio is noted to be approximately equal to 1. The image was obtained at 18 seconds after microbubble administration at peak enhancement. Note that all the images were obtained with EPIQ scanner (Philips Healthcare, Bothell, WA) and C5–1 transducer with settings of 12 Hz and mechanical index of 0.06.
Intracranial Hemorrhage
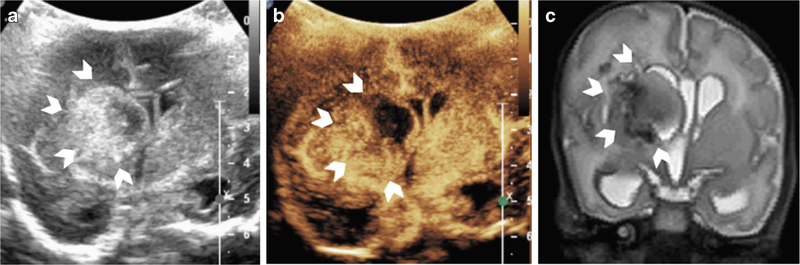
While in the beginning stages of hemorrhage cerebral perfusion is protected by reflex mechanisms, further blood loss can lead to metabolic stress, global hypoperfusion, and neuronal death. It is difficult to discern on grayscale ultrasound whether the cerebral perfusion around the hemorrhage or remainder of the brain is also at risk. In this regard, brain CEUS helps delineate the extent of cerebral perfusion compromise in the setting of intracranial hemorrhage and accurately delineates the hemorrhagic core itself. Figure 5 demonstrates a case in which cerebral perfusion is preserved around the hemorrhage, and the region of heterogeneous hypoperfusion on brain CEUS corresponds to the hyperechogenic hemorrhagic core as seen on the grayscale ultrasound.
Figure 5. Intracranial Hemorrhage on CEUS-B.
Figure 5 is a CEUS-B of a neonate with heterogeneous right germinal matrix hemorrhage with associated periventricular edema/infarct (arrowheads) on grayscale ultrasound (A), CEUS-B (B), T2-weighted MR imaging (C). Heterogeneous hypoperfusion is noted in the right periventricular region in the area of known hemorrhage (B). There is mild leftward midline shift and ventricular dilatation with asymmetric effacement of the right frontal horn due to the hemorrhage. Note that the cerebral perfusion in the remainder of the right hemisphere as well as the left hemisphere is preserved (arrows). Note that Figure 5(B) was obtained with EPIQ scanner (Phillips Healthcare, Bothell, WA) and C5–1 transducer with settings of 12 Hz and MI of 0.06.
Focal Lesions
Various intracranial lesions including brain tumors can be evaluated with brain CEUS for assessment of dynamic perfusion parameters that are of both diagnostic and prognostic value [16, 17]. Vascular malformations including arteriovenous malformation can be assessed for its degree of shunting as well as blood flow and volume with brain CEUS, pre and post endovascular coiling if applicable to assess for treatment response and residual flow. Note that the performance of brain CEUS is most optimized in the setting of open cranial window, such as with burr hole or craniotomy, although thin temporal window permits limited visualization and quantification of microbubbles.
Hydrocephalus
Post-hemorrhagic hydrocephalus (PHH) is a major complication of intraventricular hemorrhage, and risk of intraventricular hemorrhage is very high in premature babies [18]. In order to reduce long-term cognitive and motor disability of premature infants with PHH [19–22], timely decompression of hydrocephalus is needed. Exactly when to place a ventricular shunt for decompression of increased intracranial pressure (ICP) remains a clinical dilemma for neurosurgeons, however. Increase in ventricular size as detected by bedside grayscale ultrasound or ultrafast magnetic resonance imaging (MRI) is an important decision-making factor in shunt placement, despite the lack of reliable association between ventricular size and abnormal ICP [23–26]. Application of brain CEUS may permit improved understanding of perfusion changes that occur with the evolution of PHH and optimal timing of shunting in consideration of long-term cognition and motor function.
IV. Current Limitations and Future Directions
One of the major limitations in advancing brain CEUS to the clinical setting at present is the paucity of data and off-label usage. With further experience and validation of quantitative techniques, brain CEUS will be of significant value in the clinical setting by serving as a safe alternative to CT or MRI in neonates and infants. Another limitation is due to the 2-dimentional (2-D) nature of the exam. The entirety of wash-in and wash-out parameters cannot be obtained of the 3-dimentional (3-D) brain; rather, sweeps through the brain are acquired for visual screening of perfusion abnormality. The mid-coronal section through the brain chosen for cine clip can only be quantitatively evaluated for various wash-in parameters. It is expected, however, that in the next few years there will be increased interest in developing brain CEUS compatible 3-D ultrasound probes that can help assess whole brain perfusion for quantification similar to CT or MRI perfusion techniques. Note also that the artifacts in the superior near field adjacent to the cranium in the provided figures is largely due to the absence of pediatric specific brain contrast ultrasound probe at present. The ultrasound probe used to generate the figures on this paper is designed for imaging adult abdomen and much larger in size than the neonatal anterior fontanelle used as the acoustic window. The presence of such artifacts can interfere with accurate quantification of brain perfusion. The introduction of pediatric specific brain contrast ultrasound probe is expected to occur in the near future, however, which will minimize this concern.
Moreover, the performance and interpretation of brain CEUS exams would require proper training for technologists and radiologists not familiar with the technique. The quantification method can also be daunting at first, as radiologists are used to qualitative rather than quantitative interpretation of brain ultrasound. The region of interest based quantification can be done conveniently on the scanner, although generation of ultrasound perfusion maps with minimal artifactual contribution requires advanced post-processing softwares such as Matlab. In the future, it may be possible that quantification methods such as the central gray matter to cortex ratio can be built into a software that can then instantaneously detect brain injury. Since the quantitative method needs further research and validation, radiologists in the meantime would need to first learn to subjectively interpret brain CEUS exams and be able to detect areas of perfusion abnormality.
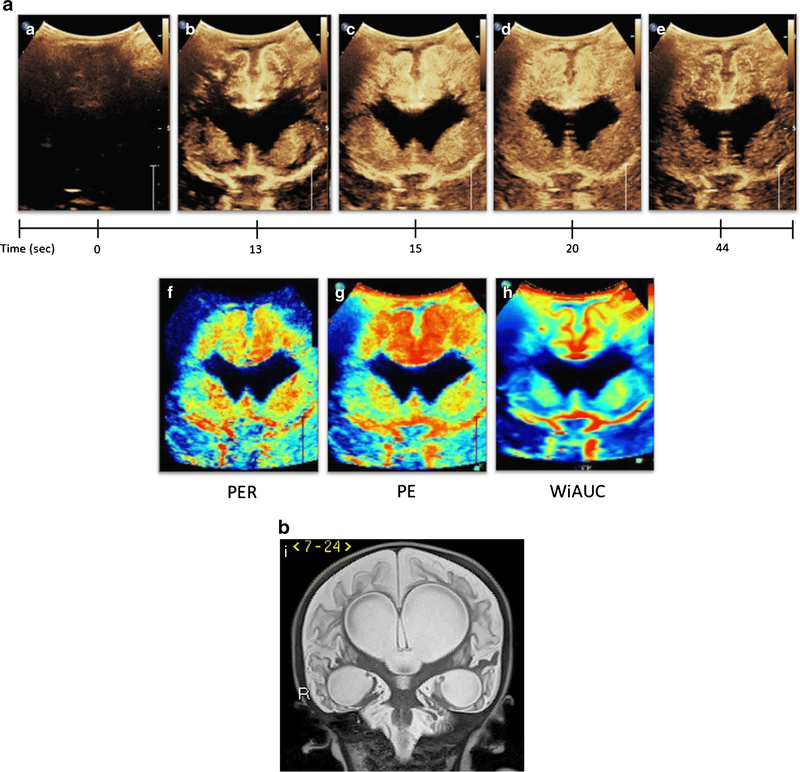
In order to establish brain CEUS as a clinically valuable technique, it will be necessary for future exams to compare brain CEUS parameters with that of gold standard exams such as CT or MRI. Hwang et al have generated ultrasound perfusion maps and demonstrated its comparability to MRI perfusion maps upon qualitative assessment [3]. Ultrasound perfusion maps can be generated of the wash-in slope or perfusion, PE, and WiAUC, representing the rate of wash-in, peak intensity, and blood volume, respectively (Fig. 6). As in CT or MRI, the quantifiable perfusion maps will permit better understanding of the extent of ischemia and tissue at risk in the setting of brain injury. Instantaneous generation of these maps in the presence of normative data will be tremendously helpful in promoting bedside diagnosis and intervention.
Figure 6. Generation of Ultrasound Perfusion Maps from CEUS-B.
Figure 6a (A-E) depicts CEUS-B of an infant status post multiple cardiac arrests and extracorporeal membrane oxygenation. Dynamic CEUS-B images from time 0 to 44 seconds demonstrate abnormally avid perfusion to the cortical and subcortical regions of the cerebral cortex as well as the central gray nuclei, resulting in abnormal GNC ratio Higher peak intensity and area under the curve, as well as delayed washout was seen in both regions as compared to normal subjects. Figure 6a (F-H) represents ultrasound perfusion maps reconstructed from the original CEUS-B. Black denotes no perfusion, blue low perfusion, yellow/green moderate perfusion, and red high perfusion relative to the remainder of the brain. Figure 6a-F is a map of wash-in or perfusion and shows fast rate of wash-in (red) in the cortical/subcortical regions as well as the central gray nuclei. Figure 6a-G is a map of peak enhancement and shows accentuation of increased flow in the cortical/subcortical regions greater than the central gray nuclei. Figure 6a-H is a map of wash-in area under the curve or WiAUC which similarly demonstrates increased flow in the cortical/subcortical regions and central gray nuclei. Figure 6I is a coronal T2-weighted MR sequence of the same patient 4 months following the shown CEUS-B scan and demonstrates interval development of marked encephalomalacia and ventriculomegaly due to the severe hypoxic ischemic injury. This figure is adapted from Shin et al.[27] with permission. Note that Figure 6(A-E) was obtained with EPIQ scanner (Phillips Healthcare, Bothell, WA) and C9–2 transducer with settings of 7 Hz and MI of 0.06.
V. Conclusion
In conclusion, brain CEUS is a potentially valuable technique which enables bedside detection of cerebral pathology. Given the quantifiable nature of the technique, both accurate diagnosis and serial monitoring of perfusion abnormalities is feasible. Given the novelty of its application in the neonate and infant population, more updates are expected in the next few years. Advancements in this regard will likely improve the current clinical algorithm and improve its diagnostic utility in this patient population.
References
- [1].Hwang M, de Jong RM, Herman S et al. (2016) Novel Contrast Ultrasound Evaluation in Neonatal Hypoxic Ischemic Injury: Case Series and Future Directions. J Ultrasound Med 36:2379–2386 [DOI] [PubMed] [Google Scholar]
- [2].Hwang M, Riggs BJ, Katz J et al. (2018) Advanced Pediatric Neurosonography Techniques: Contrast-Enhanced Ultrasonography, Elastography, and Beyond. J Neuroimaging 28:150–157. [DOI] [PubMed] [Google Scholar]
- [3].Hwang M, Sridharan A, Darge K et al. (2018) Novel Quantitative Contrast-Enhanced Ultrasound Detection of Hypoxic Ischemic Injury in Neonates and Infants: Pilot Study I. [DOI] [PubMed]
- [4].Ilves P, Lintrop M, Talvik I et al. (2009) Low cerebral blood flow velocity and head circumference in infants with severe hypoxic ischemic encephalopathy and poor outcome. Acta Paediatr 98:459–465 [DOI] [PubMed] [Google Scholar]
- [5].Okereafor A, Allsop J, Counsell SJ et al. (2008) Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121:906–914 [DOI] [PubMed] [Google Scholar]
- [6].Chugani HT, Phelps ME (1986) Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 231:840–843 [DOI] [PubMed] [Google Scholar]
- [7].Thorngren-Jerneck K, Ohlsson T, Sandell A et al. (2001) Cerebral glucose metabolism measured by positron emission tomography in term newborn infants with hypoxic ischemic encephalopathy. Pediatr Res 49:495–501 [DOI] [PubMed] [Google Scholar]
- [8].Kastler A, Manzoni P, Chapuy S et al. (2014) Transfontanellar contrast enhanced ultrasound in infants: initial experience. J Neuroradiol 41:251–258 [DOI] [PubMed] [Google Scholar]
- [9].Apfel RE, Holland CK (1991) Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound. Ultrasound Med Biol 17:179–185 [DOI] [PubMed] [Google Scholar]
- [10].Talu E, Powell RL, Longo ML et al. (2008) Needle size and injection rate impact microbubble contrast agent population. Ultrasound Med Biol 34:1182–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Eisenbrey JR, Daecher A, Kramer MR, et al. (2015) Effects of needle and catheter size on commercially available ultrasound contrast agents. J Ultrasound Med 34:1961–1968 [DOI] [PubMed] [Google Scholar]
- [12].Koga M, Reutens DC, Wright P et al. (2005) The existence and evolution of diffusion-perfusion mismatched tissue in white and gray matter after acute stroke. Stroke 36:2132–2137 [DOI] [PubMed] [Google Scholar]
- [13].Berner LP, Cho TH, Haesebaert J et al. (2016) MRI Assessment of ischemic lesion evolution within white and gray matter. Cerebrovasc Dis 41:291–297 [DOI] [PubMed] [Google Scholar]
- [14].de Vries LS, Groenendaal F (2010) Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology 52:555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Miranda MJ, Olofsson K, Sidaros K (2006) Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res 60:359–363 [DOI] [PubMed] [Google Scholar]
- [16].Prada F, Perin A, Martegani A, et al. (2014) Intraoperative contrast-enhanced ultrasound for brain tumor surgery. Neurosurgery 74:542–552 [DOI] [PubMed] [Google Scholar]
- [17].Prada F, Mattei L, Del Bene M et al. (2014) Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. Biomed Res Int [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Volpe JJ (2001) Neurology of the Newborn, Philadelphia, [Google Scholar]
- [19].Dykes FD, Dunbar B, Lazarra A et al. (1989) Posthemorrhagic hydrocephalus in high-risk preterm infants: natural history, management, and long-term outcome. J Pediatr 114:611–618 [DOI] [PubMed] [Google Scholar]
- [20].Msall ME, Buck GM, Rogers BT et al. (1991) Risk factors for major neurodevelopmental impairments and need for special education resources in extremely premature infants. J Pediatr 119:606–614 [DOI] [PubMed] [Google Scholar]
- [21].Resch B, Gedermann A, Maurer U, et al. (1996) Neurodevelopmental outcome of hydrocephalus following intra-/periventricular hemorrhage in preterm infants: short- and long-term results. Childs Nerv Syst 12:27–33 [DOI] [PubMed] [Google Scholar]
- [22].International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group (1998) Lancet 352:433–440 [PubMed] [Google Scholar]
- [23].Borgesen SE, Gjerris F (1987) Relationships between intracranial pressure, ventricular size, and resistance to CSF outflow. J Neurosurg 67:535–539 [DOI] [PubMed] [Google Scholar]
- [24].Dahlerup B, Gjerris F, Harmsen A et al. (1985) Severe headache as the only symptom of long-standing shunt dysfunction in hydrocephalic children with normal or slit ventricles revealed by computed tomography. Childs Nerv Syst 1:49–52 [DOI] [PubMed] [Google Scholar]
- [25].Ashley WW, McKinstry RC Jr, Leonard JR et al. (2005) Use of rapid-sequence magnetic resonance imaging for evaluation of hydrocephalus in children. J Neurosurg 103:124–130 [DOI] [PubMed] [Google Scholar]
- [26].Brawanski A, Soerensen N (1985) Increased ICP without ventriculomegaly. Diagnostic and therapeutic problems in a 1-year-old boy. Childs Nerv Syst 1:66–68 [DOI] [PubMed] [Google Scholar]
- [27].Shin SS, Bales JW, Edward Dixon C et al. (2017) Structural imaging of mild traumatic brain injury may not be enough: overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behav 11:591–610 [DOI] [PubMed] [Google Scholar]