Abstract
Lopinavir/ritonavir (LPV/r) is recommended by the World Health Organization (WHO) as first-line treatment for HIV-infected infants and young children. We performed a composite population pharmacokinetic (PK) analysis on LPV plasma concentration data from six pediatric and adult studies to determine maturation and formulation effects from infancy to adulthood. Intensive PK data were available for infants, children, adolescents, and adults (297 intensive profiles/1662 LPV concentrations). LPV PK data included 1 adult, 1 combined pediatric-adult, and 4 pediatric studies (age 6 weeks to 63 years) with 3 formulations (gel-capsule, liquid, melt-extrusion tablets). LPV concentrations were modeled using nonlinear mixed effects modeling (NONMEM v. 7.3) with a one compartment semi-physiologic model. Lopinavir clearance was described by hepatic plasma flow (QHP) times hepatic extraction (EH), with EH estimated from the PK data. Volume was scaled by linear weight (WT/70)1.0. Bioavailability was assessed separately as a function of hepatic extraction (FH) and the fraction absorbed from the GI tract (FABS). The absorption component of bioavailability increased with age and tablet formulation. Monte Carlo simulations of the final model using current WHO weight band dosing recommendations demonstrated that participants younger than 6 months of age had lower AUC (94.8 vs >107.4 mcg•hr/mL) and Cmin (5.0 vs > 7.1 mcg/mL) values compared to older children and adults. Although WHO dosing recommendations include a larger dosage (mg/m2) in infants to account for higher apparent clearance (CL/F), they still result in low LPV concentrations in many infants younger than 6 months of age receiving the liquid formulation.
Keywords: pediatrics, HIV, Lopinavir, Ritonavir, semi-physiologic modeling, population pharmacokinetics
INTRODUCTION
Lopinavir (LPV) is an established antiretroviral (ARV) agent commonly used as part of a first line antiretroviral therapy (ART) in pediatric populations with a liquid formulation suitable for use in term infants as young as 2 weeks of age1. It is the preferred protease inhibitor (PI) for children in the first three years of life. Ritonavir (RTV), also a PI and potent CYP3A4 inhibitor, is used as a component of ART as a pharmacologic enhancer of many PIs. Since LPV is a CYP3A substrate and has a short half-life, it is co-formulated with RTV, as Kaletra® (LPV/r). Several studies described LPV/r pharmacokinetics (PK) in adults and children2–9. However, no prior comprehensive model has described the dynamic changes in LPV/r PK from infancy into adulthood.
Three distinct formulations available for LPV/r include liquid, soft gel capsule, and melt-extrusion tablet. During the Food and Drug Administration’s (FDA) initial approval, LPV/r was formulated as soft gel capsule and liquid. In later years, the capsule formulation was withdrawn from the market and replaced with a heat stable and more bioavailable melt-extrusion tablet formulation10. Due to difficulties swallowing solid medications, infants and younger children receive liquid formulation and the majority of the patients over six years of age along with adults receive the tablet formulation11. In addition, prior studies demonstrated slower rates of viral suppression in younger infants on LPV/r as compared to older infants and children12, 13, thus understanding pharmacokinetic differences across the ages may provide insight into the mechanism.
The current study aimed to characterize PK differences in LPV disposition from birth through childhood, adolescence and into adulthood. In doing so, this analysis will also evaluate the effect of LPV formulation on bioavailability of the drug in children and adults.
METHODS
Participants
Intensive LPV/r PK data were pooled from six completed studies (four pediatric, one adult, and one combined pediatric-adult). Institutional review board (IRB) approval was obtained at each participating site and written informed consent was completed either by the patient (adult subjects) or legal guardian (pediatric subjects) prior to any study specific procedures were initiated.
The pediatric studies were: pediatric AIDS Clinical Trials Group P1030, P1038, P1080, P1083, and a pediatric pharmacology research unit (PPRU) study14–18. The adult data sets were derived from P1080 and a protocol of a national study from California Collaborative Treatment Group (CCTG) study 58518, 19. Individual study characteristics are described below and presented in Table 1.
Table 1.
Summary patient and sampling in the studies
| Study | Location | Study Design | No. of LVP/r subjects | LVP/r maintenance dosage |
Median no. of samples per subject (range) | Median age (yr) at PK visits (range) | Formulation Used |
Median Weight (kg) at PK visits (range) | Total no. of Samples |
|---|---|---|---|---|---|---|---|---|---|
| IMPAACT P103028,b,d | Global (US, Brazil, Puerto Pico) | Pediatric Phase I/II | 30 | 64/16 −192/48 mg BID | 5 (2–5) | 0.49 (0.115–1.289) | Liquid | 6.8 (3.6–12) | 293 |
| IMPAACT P103829,b | Global (US, Puerto Pico) | Pediatric Phase I/II | 21 | 336/84-800/200 mg BID | 6 (5–6) | 15 (7–17) | Gel Capsule Liquid | 45.5 (22.7–71.2) | 264 |
| IMPAACT P108330,c,e | Global (US, Brazil, South Africa, Thailand) | Pediatric Phase II/III | 91 | 120/30–300/75 mg BID | 6(5–6) | 2.19 (0.15–12.90) | Liquid Tablet |
12.2 (4.48–25.3) | 516 |
| PPRU31,a | US | Pediatric Phase IV | 10 | 400/100 mg BID | 7(7–7) | 12.78 (9.99–16.40) | Tablet | 41.95 (30.6–93.8) | 65 |
| CCTG58519,c | US | Adult Phase IV | 17 | 800/200 mg QD | 7(6–7) | 47 (23–63) | Gel Capsule Liquid Tablet |
74.84 (52.6–97.5) | 351 |
| IMPAACT P108018,a,d | Global (US, Puerto Pico) | Pediatric-adult Phase I | 27 | 100/25–400/100 mg QD/BID | 5(4–6) | 12.37(6.29–23.80) | Liquid Tablet |
42.65 (18.6–88.9) | 173 |
| Overall | Global (US, Brazil, Puerto Pico, South Africa, Thailand) | Pediatric-adult Phase I/II/II/IV | 196 | 64/16–400/100 mg QD/BID | 6(2–7) | 7.405(0.115–63) | Gel Capsule Liquid Tablet |
20.5 (3.6 – 97.5) |
1662 |
Majority of subjects had one PK visit
Majority of subjects had two PK visits
Majority of subjects had three PK visits
Based on FDA approved dosing
Based on weight-band dosing
P1030 was a multi-center, phase I/II longitudinal pediatric study of LPV/r in HIV-1-positive children initiating ART between 2 weeks and 6 months of age. Participants were dosed 300/75 mg/m2 in combination with two nucleoside reverse transcriptase inhibitors (NRTIs). Intensive LPV/r PK profiles were assessed at 2 weeks after study enrollment and at one year of age with up to five plasma samples collected throughout the 12-hour dosing interval of an observed dose6, 15.
P1038 was a multi-center, phase I/II longitudinal pediatric study of LPV/r in HIV-1-positive children with viral LPV/r resistance ranging from 2 to 18 years of age failing current ART. Participants received increased doses of LPV/r in combination with two NRTIs. Intensive LPV/r PK profiles were obtained on 2–3 occasions with up to 6 plasma samples collected throughout the 12-hour dosing interval which included a baseline and trough after observed dose16.
P1080 was a multi-center, phase I pediatric and adolescent study which included participants taking psychiatric medications in addition to therapy for HIV-1 (ages ranging 6 to 25 years). LPV/r was dosed in combination with two NRTIs. Intensive LPV/r PK profiles were assessed up to eight weeks after enrollment with up to six plasma samples collected after the dose which included a baseline and trough after observed dose18.
P1083 was a multi-center, phase II/III pediatric longitudinal study which assessed World Health Organization (WHO) LPV/r pediatric weight band dosing guidelines for HIV-1-positive infants and children 3 to 25 kg between 0.1 and 12.8 years of age. Participants received two NRTIs in addition to LPV/r. Intensive LPV/r PK profiles were assessed four weeks after the initial treatment with up to six plasma samples collected during the 12-hour dosing interval which included a baseline and trough after observed dose17.
CCTG585 was a multi-center, phase IV longitudinal study of safety and tolerability of once daily LPV/r dosing (800/200 mg) in HIV-1-positive adults of age 23–63 years sequentially given liquid, capsule and tablet formulations. Participants received two NRTIs in addition to LPV/r. Intensive LPV/r PK profiles were collected after roughly 4 weeks of initial treatment, with up to seven plasma samples collected through the 24-hour dosing interval which included a baseline and trough after observed dose19, 20.
The PPRU study was a randomized, open-label, crossover phase IV study which studied LPV/r bioavailability of crushed vs whole tablets in pediatric participants receiving LPV/r in combination with 2 NRTIs. The intensive LPV/r PK profiles of participants taking intact oral melt-extrusion tablets were used for the analysis, with up to seven plasma samples collected through the 12-hours dosing interval which included a baseline and trough after observed dose14.
LPV concentrations were determined by validated high-performance liquid chromatography (HPLC) methods in the following laboratories: IMPAACT 1030 (University of California, San Diego (UCSD) Pediatric Clinical Pharmacology Laboratory); IMPAACT 1038 (St Jude’s pharmacology laboratory, UCSD Pediatric Clinical Pharmacology Laboratory); IMPAACT 1080 (UCSD Pediatric Clinical Pharmacology Laboratory); IMPAACT 1083 (Chiang Mai University HIV Treatment CRS, UCSD Pediatric Clinical Pharmacology Laboratory); PPRU (UCSD Pediatric Clinical Pharmacology Laboratory). All of the aforementioned laboratories participated in the Division of AIDS (DAIDS) Clinical Pharmacology Quality Assurance (CPQA) program and have their LPV assay method reviewed by CPQA and approved prior to analysis of samples. While the limit of quantification for drug levels varied slightly amongst the labs, all had lower limits of quantification ≤ 0.10 μg/mL for both LPV and RTV. The studies had an assay coefficient of variation of < 15% for the pooled studies.
Pharmacokinetic Analysis
A one-compartment semi-physiologic model using nonlinear mixed-effect modeling was performed using NONMEM® version 7.3 with a GNU Fortan G77 compiler (Gaithersburg LLC, Ellicott City, MD, USA). An open one-compartment model with first order absorption (ADVAN 2, TRANS2 subroutine) and first-order conditional estimation method (FOCE) with interaction of clearance (CL) and volume of distribution (VD) were used to analyze the data. An exponential-normal distribution error model was used to describe between subject variability (BSV), and a combination residual error model was used to describe the residual error that could not be explained by the model and could not be attributed to BSV. Participants with multiple intensive PK visits were categorized as separate for the analysis. All drug concentrations were collected after a minimal duration of at least 2 weeks of therapy and were assumed to be at steady state. Participants who were suspected to be non-adherent (pre-dose levels four-magnitude lower than Cmin) were considered a non-steady state single dose PK for purposes of the analysis.
This semi-physiologic model included hepatic intrinsic clearance which was described by the estimated LVP/r ratio extracted by the hepatocytes (EH) and hepatic plasma flow (QHP) (Figure 1). Hepatic plasma flow was estimated as percentage of plasma (55%) multiplied by typical hepatic blood flow (1500 mL/min or 90 L/hr) or 50 L/hr, and allometrically scaled; QHP = 50 L/hr ⋅ (WT/70)0.75 21. VD was scaled by weight (WT)1.0. Bioavailability (F) is described as the amount absorbed (FABS) and by the ratio escaping hepatic extraction. The model was described by the following equations:
Figure 1.
Hepatic extraction model. LPV/r is absorbed in the gut (FABS), then transferred from the apical to basolateral side of enterocytes and into the blood stream. The amount of drug metabolized (clearance (CL)) is defined by the plasma blood flow (QHP) and the fraction of blood flow extracted (EH) by the liver for metabolism (QHP • EH) as it cycles through the systemic circulation. The overall bioavailability is fraction absorption from the GI tract, FABS, times the fraction that escapes first-pass metabolism ((1- EH) • FABS).
LPV Model with RTV inhibition
The semi-physiological hepatic extraction model was expanded to describe RTV inhibition of LPV CL using observed RTV concentrations. In the model, RTV concentration was assigned an inhibition equation with the concentration of RTV inhibiting LPV metabolism by 50% (IC50) estimated on the EH impacting both CL and bioavailability (FH).
Covariate Screening
Potential covariates (age, sex, and formulation) were tested in the model: age and sex on FABS, EH and VD; formulation was assessed on F. Three age functions were tested in the model (linear, logarithmic, plateau maturation effect model). Potential covariates were added one at a time as either a linear or non-linear function, with covariates that improved the model fitting by change in the objective function value (OFV) of at least 3.84 (p<0.05) being retained in the initial covariate screening. All covariates identified as significant in this phase were then evaluated using a forward selection approach. Covariates found to improve the OFV of at least 8 (p<0.005) were retained in the final model.
Three age functions were tested in the model:
Between Subject Variability (BSV) was assessed for EH and VD. It was not assessed on KA due to limited early sampling nor on FABS due to difficulty separating the BSV from separating the BSV from components of bioavailability (EH and FABS. Empiric Bayesian estimates of individual subject’s PK parameters were generated from the final model using the POSTHOC subroutine. For internal model evaluation, a 1000 sample bootstrap assessment of the final model was performed using Wings 7.3 for NONMEM. A visual predictive check (VPC) was performed using a simulated population of 1000 patients via NONMEM NSUB routine.
Monte Carlo Simulation
Monte Carlo simulations were performed using the final model to generate PK profiles for 5,000 virtual subjects (4,000 pediatric and 1,000 adults (70kg and 40 years of age)). Pediatric subjects were assigned a randomly generated weight based on a uniform distribution with median weights from the CDC-NHAINES age weight distribution22. Subjects with weight less than 35 kg were simulated using WHO weight band dosing and those with weight above 35 kg were assigned adult dosage of 400/100 mg LPV/r tablets23. Virtual subjects aged younger than 6 years were assigned to liquid LPV/r formulation and those aged 6 years of age or older were assigned to the tablet formulation.
RESULTS
Pharmacokinetic Sampling
Intensive PK data were available for 297 infants, children, adolescents, and adults (1662 LPV concentrations) (Figure 2). Table 1 summarizes participant and sampling from intensive PK visits. Participants greater than 10 years of age were assigned solid formulation where formulation information was missing. Specifically, in four participants without formulation data, the participants were assigned gel-capsule formulation as they were all 16 years of age and gel-capsule was the only available solid formulation used in the study. A total of four participants with high pre-dose levels were excluded from the analysis as their self-reported dosing history did not match their PK profile. In addition, 34 subjects (11%) with low pre-dose levels were assumed non-adherent (Cmin was greater than four-fold lower than pre-dose) and were treated as single dose for the analysis.
Figure 2.
Measured non-dose normalized LPV concentrations for the six studies (CCTG 585, IMPAACT P1030, P1038, P1080, P1083, and PPRU). Lines represent local polynomial regression fit for each study. LPV concentrations below 0.1 μg/mL are represented to 0.1 μg/mL. Younger patients from (P1030 participants) had the lowest observed concentrations.
Compartmental Modeling
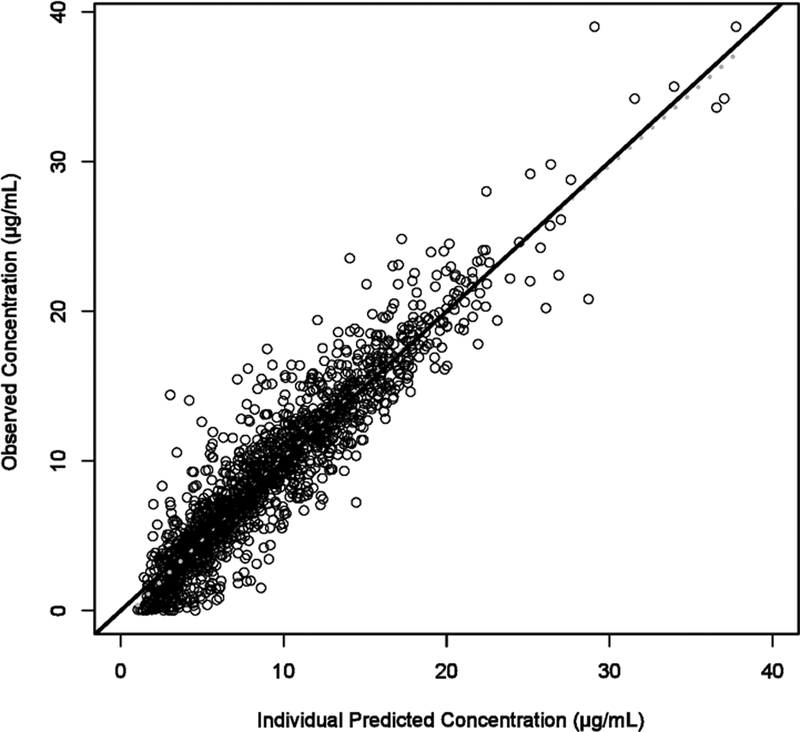
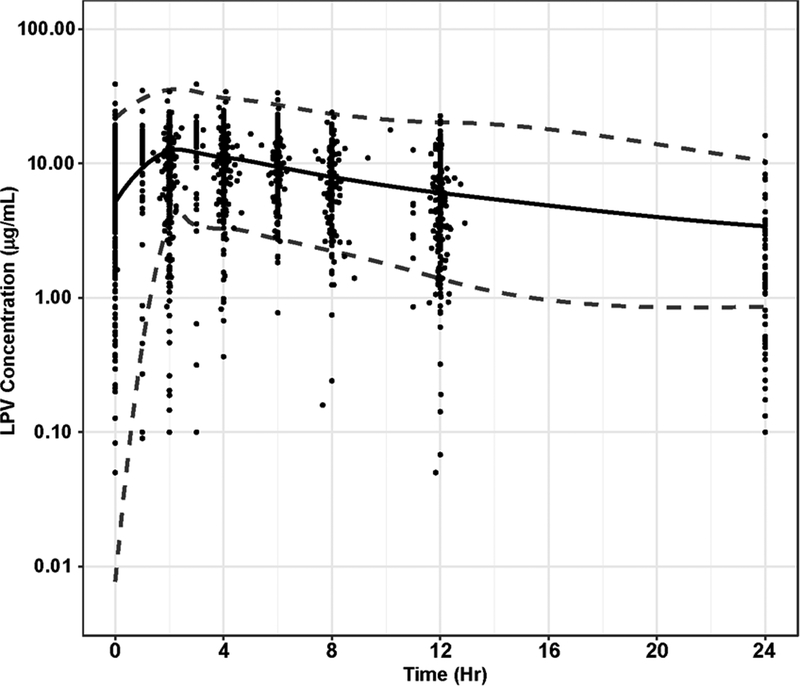
The semi-physiological hepatic extraction model described the data without significant bias (Figure 3A–E). Final PK model parameters and standard error estimates are shown in Table 2. All parameter estimates were within the 95% confidence interval (CI) of the bootstrap data set.
Figure 3A-E.
Goodness of fit plots for hepatic extraction model. A). Intensive LPV concentrations from the six studies were compared with individual predictions from the population PK model. B). Intensive LPV concentrations were compared with population predictions from the population PK model. C). Conditional weighted residual for each sample compared to time after last dose (TALD). D). Conditional weighted residuals compared with population predictions from the population PK model. E). A visual predictive check (VPC) using simulated population of 1000 patients. The dotted lines represent the 95% interval of the simulation. Observed concentrations were graphed onto the simulation for validation. The majority of the observe data falls within the 95% confidence interval of the simulation.
Table 2.
Parameter estimates for final population PK model
| Parameter | Final Value | SE | Median Bootstrap Estimate* (95% Confidence Interval) |
|---|---|---|---|
| Θ1 (EH) | 0.14 | 0.03 | 0.15 (0.10 – 0.22) |
| Θ2 (VD) | 1.61 | 0.34 | 1.63 (1.04 – 2.5) |
| Θ3 (KA) | 0.31 | 0.06 | 0.31 (0.15 – 0.40) |
| Θ4 (Tablet) | 1.23 | 0.11 | 1.23 (1.03 – 1.47) |
| Θ5** (Maturation Effect on F) | 0.97 | 0.46 | 1.09 (0.57 – 2.28) |
| Θ6** (Half-life of Maturation) | 0.98 | 0.63 | 0.97 (0.02 – 6.90) |
| Between Subject Variability (BSV)** | |||
| BSV, EH | 42% | 2% | 41% (35% - 47%) |
| BSV, VD | 48% | 3% | 71% (57% −82%) |
| BSV interaction, (EH-VD) | 38% | 2% | 47% (41% - 53%) |
| Error | |||
| Proportional | 9.4% | 0.03 | 9.6% (0.4% - 15.2%) |
| Additive (μg/mL) | 2.13 | 0.16 | 2.11 (1.71–2.40) |
| EH =Θ1 CL (L/hr) = EH • QHP VD (L) = Θ2 • WT KA (1/hr) = Θ3 | |||
| QHP (L/hr) = | |||
Bootstrap had a convergence rate of 85.1%.
Plateau Maturation Effect is represented by both Maturation Effect on FABS (Θ5) and Half-life of Maturation (Θ6)
BSV was not assessed on FABS or KA.
F represents relative bioavailability with a reference value F=1 for an infant 0.115 years of age receiving the liquid formulation
After allometrically scaling for size, the univariate screen identified age and formulation as a significant covariates on the absorption component on bioavailability (FABS). Age modeled as a rate constant (half-life) best describe maturation changes in FABS (Table 2). These covariates remained significant in the multivariate screen. The final model includes age as a rate constant effect on FABS and formulation as a categorical on F (Figure 4A–B). The final model demonstrates apparent clearance to decrease to half the maximal value at 0.55 years and approaches the adult value at two years of age. Liquid formulation resulted in a 19% reduction in bioavailability compared to other formulations.
Figure 4A-B.
Apparent clearance and volume of distribution vs. age. Individual (A) clearance and (B) volume of distribution predictions from final model were compared to the population estimated clearance. Virtual subjects under 6 years of age were simulated with a lower bioavailable liquid formulation, and subjects over 6 of age were simulated with tablet formulation. All patients greater than 25 years of age were represented as 25 years for the graph. Inset in the upper right are the CL/F or VD/F vs. age for 0.115 to 1 years and represent changes during the first year of life. For CL/F, solid line represents liquid formation while dashed lines represent tablet formulation. The most dramatic changes in CL/F occurs over the first year and plateaus at 0.17 L/hr/kg0.75; the most dramatic changes in VD/F occurs over the first year and diminishes to roughly 0.82 L/kg at age 5 years.
A separate model using individual subjects’ RTV concentrations was also built. RTV inhibition was incorporated in the model as an inhibitory Emax function on EH affecting both CL and F. Using the same inhibitory function for CL and F provided a good fit to the data, thus we did not attempt more complicated models with separate inhibitory functions. The in vivo RTV inhibitory concentration (IC50) on EH was estimated to be 1.62 μg/mL. This resulted in an improved OFV of 61.4, but only marginally reduced BSV from 15.5% to 13.0%. After covariate screening, age and formulation were significant covariates for FABS similar to the model without individual subjects’ RTV concentration. Posthoc analysis of CL/F between the RTV individual observed and RTV individual “ignorant” model yielded a coefficient of determination (R2) value of 0.97 (Supplemental Figure 1). Although the approach utilizing subjects’ RTV concentrations reduced the objective function (Supplemental Table 1, Supplemental Figure 2), it has limited predictive clinical utility as individual subjects’ RTV concentrations are not known a priori. Therefore, the RTV “ignorant” model which incorporated the general RTV “boosting” effects on LPV, but did not require knowledge of the individual RTV concentrations, was used to simulate LPV exposure with WHO dosing.
Monte Carlo Simulation
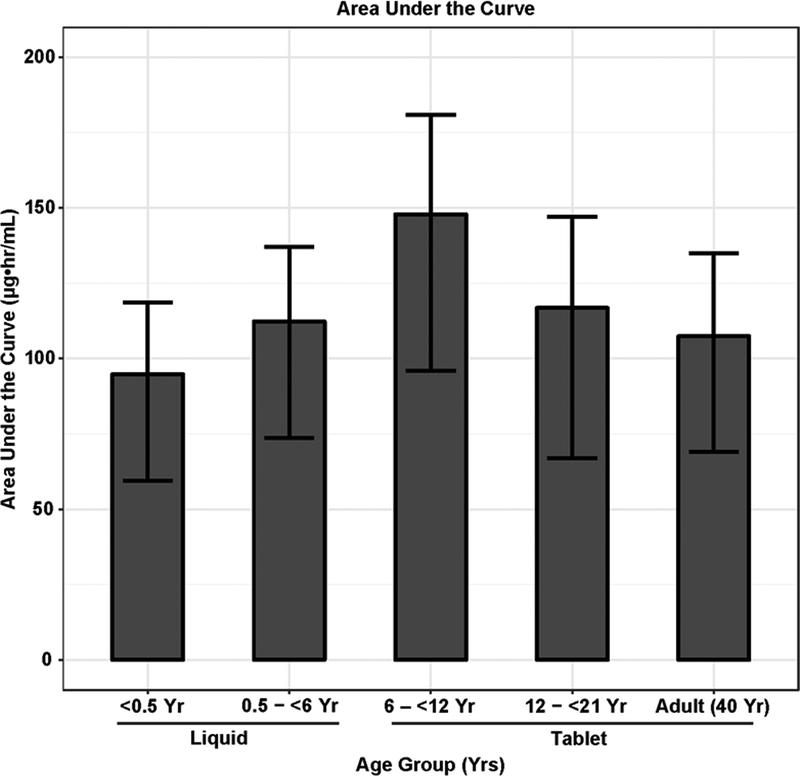
The Monte Carlo simulation comparison of area under the plasma drug concentration-time curve (AUC) and 12-h plasma trough concentrations (Cmin) obtained with the WHO weight band are shown in Figure 5A–B. Subjects older than 6 years of age were transitioned from liquid to tablet dosing. The median AUC values were 94.8, 112.3, 147.9, 116.8 and 107.4 mcg•hr/mL and Cmin were 5.0, 6.5, 9.1, 7.7, and 7.1 mcg/mL for age groups <6 months yr, 6 months - <6 years, 6-;<12 years, 12 - <21 years, and 21–40 years, respectively.
Figure 5.
Monte Carlo simulations based on WHO weight band dosing across different years of age groups: A. Area under the curve (AUC), B. Cmin (trough). Subjects <6 years received liquid and those ≥ 6 years received tablet formulation. AUC and Cmin values in subjects less than <0.5 years were lower than those of other age groups.
DISCUSSION
Multiple studies have evaluated the PK of LPV/r in pediatric and adult patients including the three distinct formulations (liquid, gel capsule, and tablet)1. LPV is well-characterized as a CYP3A substrate and is rapidly metabolized if not administered under cytochrome P450 inhibitory conditions with bio-inactivation via CYP3A(4) and 3A(5)24. LPV PK has a high degree of inter-subject clearance and bioavailability variability especially with the developmental factors seen in childhood. Thus, LPV is co-formulated with low-dose RTV, a PK enhancing agent to decrease the apparent clearance of LPV, prolong LPV exposure, and maintain a longer duration of effect24. Because of its metabolic pathway and co-formulation with RTV, LPV/r has multiple drug-drug interactions with medications commonly used in HIV-infected patients.21
Our study, which pooled data from six prior studies of infants, children and adults, represents the largest population PK analysis of LPV to date. We utilized a semi-physiologic hepatic extraction model rather than the standard PK model with the aim of better characterizing the inhibition of CYP3A(4) enzymes through the administration of this RTV-boosted ARV drug formulation which impacts both bioavailability and clearance. The semi-physiologic hepatic extraction model has the benefit of linking both bioavailability and clearance to hepatic enzyme activity. Our model showed that both age and formulation were independent predictors of bioavailability. The model predicted a decrease in apparent clearance (CL/F) from 0.31 L/hr/kg in the youngest subject receiving liquid formulation to 0.080 L/hr/kg for adults receiving the tablet formulation. Using the median weight (20.5 kg) and age (7.4 years) from the pooled studies, median apparent CL was 0.082 L/hr/kg for a 20.5 kg and 7.4 year average subject. The age effect accounted for a 49% change in CL/F over the range of ages seen in the study and occurred predominantly in the first two years.
The results from our study are reflective of other prior studies (Table 3) of pediatric and adult populations. Using a typical 6-year-old child (weight=20 kg) as a comparator, the hepatic extraction model predicted a weight-adjusted apparent clearance of 0.102 L/hr/kg and 0.082 L/hr/kg for those receiving liquid formulation and tablet formulation, respectively. These results are comparable to those found in other LPV/r pediatric studies. Similar results were seen in adult data studies using a 40-year-old (weight=70 kg) patient as the comparator.
Table 3.
CL/F Comparison with Prior PK Model
| Pediatrics | Study | Total Subjects (Age Range) | Covariates | Final Model | Final Parameterized CL/Fa | CL/F for a typical 6 year old (20 kg) (L/hr/kg) |
| Current Model |
Total Subject
196 Age (range) 7.4 yrs (0.115–63) |
Age Sex Formulation |
Age on VD Age on EH Formulation on F |
CL/F (see
Table 2
for equation)
0.17 L/hr/kg0.75 a CL/F for a typical 20 kg 6 yrs old on liquid formulation: 0.21 L/hr/kg0.75 CL/F for a typical 20 kg 6 yrs old on tablet: 0.17 L/hr/kg0.75 |
Liquid:
0.102 Tablet: 0.082 |
|
| Rakhmanina et al.9 |
Total Subject
50 Median Age (range) 11 yrs (5.3 – 17.5) Weight No provided Formulation capsule, liquid Data Intensive |
Age Sex |
Age on VD |
Model: Not Provided
(Dose/AUC): 0.11 L/hr/kg0.75 |
0.052 | |
| Jullien et al.5 |
Total Subject 157 Median Age (range) 10.2 yrs (0.01–18) Median Weight (range) 27.6 kg (2–73) Formulation capsule, liquid Data Sparse |
Age Sex Body weight (BW) |
BW on CL Sex on CL (age > 12 yr) Nevirapine on CL BW on VD |
CL/F 2.61 L/hra Equation: ≤ 12 yrs CL/F (L/hr) = 2.58 · · 1.34N* ≥12 yrs CL/F (L/hr) = 2.58 · · 1.34N*· 1.39S** *N = 1 if nevirapine was combined with LPV **S = 1 if boy; 0 if girl |
0.112 | |
| Nikanjam et al.6 (subset) |
Total Subject 30 Median Age (range) 0.48 yr (0.117–1.289) Median Weight (range) 4.8 kg (2.9–9.9) Formulation Liquid Data Intensive and Sparse |
Age | Age on F |
CL/F/Kg 0.69 L/hr/kg0.75 a Equation: CL/F/kg0.75 = |
0.122 | |
| Bastiaans et al.7 |
Total Subject 53 Median Age (range; IQR) 11 yrs (4.4–17.7; 8.8–14.7) Median Weight (IQR) 31 kg (23.6–40.0) Formulation Tablet Data Intensive |
None Evaluated | Non-compartmental Analysis | CL/F (L/hr/kg) 0.092 (≥15 to ≤25 kg) 0.085 (>25 to ≤35 kg) 0.089 (>35 kg) |
0.092 | |
| Adult | Study | Total Subjects (Age Range) | Covariates | Final Model | Final Parameterized CL/Fb | Calculation for a 70 kg 40 year old(L/hr/kg)b |
| Current Model | (see above) |
CL/F for a typical 70 kg 40 year old:
0.17 L/hr/kg0.75 |
0.069b | |||
| Dickinson et al.2 |
Total Subject
16 Median Age (range) 42 yr (25 – 55) Median Weight (range) 85 kg (53–115) Formulation Tablet Data Intensive |
Age Sex Eethnicity Bbody weight (BW) Body mass index (BMI) RTV AUC |
Ritonavir AUC on CL |
4.50 L/hr | 0.056b | |
| Molto et al.3 |
Total Subject
78 Median Age (range) 43 ± 8.6 yr (Index set) Median Weight (range) 67.7± 11.5 kg (Index set) Formulation Capsule Data Intensive |
Age Sex Body weight (BW)HCV co-infection, Plasma albumin, α1-acid glycoprotein (AAG) Liver enzymes aspartate aminotransferase and alanine aminotransferase |
Ritonavir AUC on CL AAG on CL AAG on VD |
4.31 L/hr | 0.063b | |
| Crommentyn et al.4 |
Total Subject
122 Median Age (range) 42 yr (IQR: 36–46) Median Weight (range) 72 kg (IQR: 63–80) Formulation Capsule Data Intensive and Sparse |
Age Sex Body weight (BW) Chronic hepatitis C Chronic hepatitis B Liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphate Total bilirubin RTV AUC, Efavirenz, nevirapine, and tenofovir |
RTVAUC on CL efavirenz/ nevirapine |
5.73 L/hr Final Model CL/F = 14.8 ·(1-(AUCRTV/(AUC50 + AUCRTV))) · IND IND = 1.39 when treatment with efavirenz or nevirapine |
0.080b | |
| Aspiroz et al.8 |
Total Subject
263 Median Age (range) 40.3 ± 8.2 yr (Index set) Median Weight (range) 68.12 ± 14.62 kg (Index set) BMI (range) 23.49 ± 4.2 kg/m2 (Index set) Formulation Tablet Data Intensive |
Age Sex Height Body weight (BW) BMI Formulation, Hepatitis C infection Total bilirubin RTV plasma Cmin Hospital of origin, Efavirenz, saquinavir, atazanavir, and tenofovir |
BMI, RTV Cmin efavirenz and atazanavir | 4.11 L/hr Final Model CL/FLPV (L/hr) = 0.216 · BMI · 0.81RTC · 1.25EFV· 0.84ATV |
0.054b Final Model: -BMI of 22 -RTC of 1 mg/L -No EFV nor ATV CL/FLPV = 3.85 L/hr or 0.055 L/hr/kgb |
|
CL/F was calculated using median age and weight
The Monte Carlo simulation of the final model using WHO weight band dosing, demonstrated that both AUC and Cmin increase with age throughout childhood due to increased bioavailability with age and the transition to tablet formation. In this weight band dosing simulation, there was a decreased exposure of LPV in the youngest age group and a significantly lower exposure with the liquid LPV formulation. In addition, a significant number of patients in early infancy (less than 6 months of age and <10 kg) had low LPV trough concentrations (Cmin ≤ 1 μg/mL at 12 hours) compared to older children, adolescents and adults. This could be due to the decreased RTV exposure in those patients which increases LPV clearance. Thus, higher LPV/r doses may be required in infants younger than 6 months of age. This is consistent with the observation that younger infants have slower viral decline following ART therapy12, 13, thus maturational differences in PK likely contribute to this observed clinical effect.
The semi-physiological hepatic extraction model allows the calculation of a RTV in vivo IC50 to determine the degree of CYP450 inhibition. While the final hepatic extraction model used for the simulation did not include individual RTV concentrations, we also developed a model that integrated RTV inhibition based on individual subjects’ RTV concentrations. While the incorporation of RTV inhibition significantly reduced the objective function of the model, it did not lead to the discovery of additional covariates nor significantly reduced BSV. RTV concentrations are not known a priori and thus cannot be used as a predictive factor to optimize LPV/r dosing for individual patients nor can it be used for general dosing recommendations. As such, for clinical interpretation, the final model is RTV concentration “ignorant” and does not require knowledge of the actual ritonavir concentrations for simulations of LPV, but still incorporates the general effect of RTV in inhibiting LPV metabolism.
The extent of the oral bioavailability of LPV/r is dependent on many physiological factors including stability of the formulations, food-drug interactions, transit-time through the gastrointestinal tract, and first-pass metabolism, which are difficult to characterize with the semi-physiologic model. LPV/r can have increased F when taken with a meal, especially with fatty food and the transition from a liquid to solid diet in older infant may account for the majority of the age related changes in FABS and thus CL/F seen in the current study25. Given the variable and unpredictable absorption characteristics, especially in the pediatric population, alternative formulations are currently being explored to replace the less stable liquid LPV/r formulation. A new formulation of LPV/r sprinkles was evaluated in the CHAPAS-2 trial for children aged 3 months to 13 years26. The generic formulation was not bioequivalent to the FDA approved liquid formulation having increased LPV/r levels compared to the liquid formulations, but decreased exposure compared to the melt-extrusion tablet formulation26. The WHO approved this formulation, but it is not currently FDA approved27. The sprinkle formulation was not assessed in the current study.
Prior population PK LPV/r models in adults have evaluated other factors such as albumin, α1-acid glycoprotein levels (AAG), liver function, and potential drug-drug interactions as potential covariates3, 4, 8. Many of these covariates could have a significant effect on LPV PK due to developmental changes from infancy to adolescence. For example, the increased production of albumin and α1-acid glycoprotein during early life could affect LPV/r binding and drug-drug interactions. Since protein binding and concurrent drug were not universally available in the current data sets, these potential effects were not explored. The model was also limited in that it was difficult to separate age from formulation effects as most patients transitioned to tablet formulation around the age of six. In addition, the one study with once daily administration, the LPV concentrations collected 24 hour post dose were over-predicted by the model likely due to waning RTV inhibition. Thus, the current model is representative of LPV/r pharmacokinetics following standard every 12 hour administration. Between-subject variability was not included on KA as there was limited early PK sample collection during the absorption phase. Between subject variability was also not assessed on FABS due to difficulty in parsing to from FH which incorporates BSV from EH. Finally, the typical population blood flow and hematocrit values used in this analysis may vary from those in patients with HIV-infection and which may have biased our estimated of EH21.
CONCLUSIONS
In conclusion, the current population PK analysis represents the most comprehensive analysis of the role of age and formulation on LPV disposition to date, characterizing clearance changes from birth to middle-aged adulthood. In our study, we found a dramatic decrease in CL/F over the first two years of life and an increase in bioavailability with the transition from liquid to tablet formulation. The WHO weight band dosing simulation shows potential for low LPV concentration in early infancy with currently recommended dosing and may require further evaluation for improved dosing recommendations adjusted for newly available LPV/r pediatric formulations such as sprinkles. The current composite pharmacologic model can be used for simulations to better characterize and assess LPV/r dosing regimens across all age groups.
Supplementary Material
ACKNOWLEDGEMENTS
Jincheng Yang received stipend support from Janssen Pharmaceuticals (Pharmaceutical Company of Johnson & Johnson). Dr. Mina Nikanjam received salary support from a National Institutes of Health grant (4T32HL066992 - Academic Training in Hematology) and a Tower Cancer Research Foundation Career Development Award. Additional funding support was provided by a Research in Pediatric and Developmental Pharmacology NIH grant (1U54HD090259-01, Dr. Edmund Capparelli) and the IMPAACT Network grant (5UM1 AI068632–11, Dr. Edmund Capparelli). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
DISCLOSURES: Dr. Edmund Capparelli serves on the data safety and monitoring board for Cempra Pharmaceuticals and The Medicines Company and has received consulting fees from Aptose, Plexxikon, Atox Bio Ltd, Celltrion, and Patara. Dr. Brookie M. Best serves on data safety and monitoring boards for PPD and Celltrion. Dr. Eric Daar receives research support from Gilead, Merck and ViiV as well as is a consultant for Bristol Myers Squibb, Gilead, Janssen, Merck, Theratechnologies, and ViiV.
Data Sharing
Requests for data should be directed to the corresponding author Edmund Capparelli (ecapparelli@ucsd.edu).
REFERENCES
- 1.Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. https://aidsinfo.nih.gov/contentfiles/lvguidelines/PedARV_TablesOnly.pdf. Accessed January 2, 2018. [DOI] [PubMed]
- 2.Dickinson L, Boffito M, Back D, et al. Sequential population pharmacokinetic modeling of lopinavir and ritonavir in healthy volunteers and assessment of different dosing strategies. Antimicrobial agents and chemotherapy. 2011;55(6): 2775–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molto J, Barbanoj MJ, Miranda C, et al. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clinical pharmacokinetics. 2008;47(10): 681–692. [DOI] [PubMed] [Google Scholar]
- 4.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. British journal of clinical pharmacology. 2005;60(4): 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jullien V, Urien S, Hirt D, et al. Population analysis of weight-, age-, and sex-related differences in the pharmacokinetics of lopinavir in children from birth to 18 years. Antimicrobial agents and chemotherapy. 2006;50(11): 3548–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikanjam M, Chadwick EG, Robbins B, et al. Assessment of lopinavir pharmacokinetics with respect to developmental changes in infants and the impact on weight band-based dosing. Clinical pharmacology and therapeutics. 2012;91(2): 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastiaans DE, Forcat S, Lyall H, et al. Pharmacokinetics of pediatric lopinavir/ritonavir tablets in children when administered twice daily according to FDA weight bands. The Pediatric infectious disease journal. 2014;33(3): 301–305. [DOI] [PubMed] [Google Scholar]
- 8.Lopez Aspiroz E, Santos Buelga D, Cabrera Figueroa S, et al. Population pharmacokinetics of lopinavir/ritonavir (Kaletra) in HIV-infected patients. Therapeutic drug monitoring. 2011;33(5): 573–582. [DOI] [PubMed] [Google Scholar]
- 9.Rakhmanina N, van den Anker J, Baghdassarian A, Soldin S, Williams K, Neely MN. Population pharmacokinetics of lopinavir predict suboptimal therapeutic concentrations in treatment-experienced human immunodeficiency virus-infected children. Antimicrobial agents and chemotherapy. 2009;53(6): 2532–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maniruzzaman M, Boateng JS, Snowden MJ, Douroumis D. A review of hot-melt extrusion: process technology to pharmaceutical products. ISRN pharmaceutics. 2012;2012: 436763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Ranmal S, Batchelor HK, et al. Patient-centred pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs. 2014;74(16): 1871–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadwick EG, Yogev R, Alvero CG, et al. Long-term outcomes for HIV-infected infants less than 6 months of age at initiation of lopinavir/ritonavir combination antiretroviral therapy. Aids. 2011;25(5): 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrand RA, Briggs D, Ferguson J, et al. Viral suppression in adolescents on antiretroviral treatment: review of the literature and critical appraisal of methodological challenges. Tropical medicine & international health : TM & IH. 2016;21(3): 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Best BM, Capparelli EV, Diep H, et al. Pharmacokinetics of lopinavir/ritonavir crushed versus whole tablets in children. Journal of acquired immune deficiency syndromes. 2011;58(4): 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadwick EG, Pinto J, Yogev R, et al. Early initiation of lopinavir/ritonavir in infants less than 6 weeks of age: pharmacokinetics and 24-week safety and efficacy. The Pediatric infectious disease journal. 2009;28(3): 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins BL, Capparelli EV, Chadwick EG, et al. Pharmacokinetics of high-dose lopinavir-ritonavir with and without saquinavir or nonnucleoside reverse transcriptase inhibitors in human immunodeficiency virus-infected pediatric and adolescent patients previously treated with protease inhibitors. Antimicrobial agents and chemotherapy. 2008;52(9): 3276–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto JA, Capparelli EV, Warshaw M, et al. A Phase II/III Trial of Lopinavir/Ritonavir Dosed According to the WHO Pediatric Weight Band Dosing Guidelines. The Pediatric infectious disease journal. 2018;37(2): e29–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.IMPAACT P1080: Psychiatric and Antiretroviral Medication Concentrations in HIV-infected and Uninfected Children and Adolescents (IMPAACT P1080) https://clinicaltrials.gov/ct2/show/NCT01232361 Accessed January 2, 2018.
- 19.A Multicenter Study to Assess the Tolerability of Once Daily Lopinavir/Ritonavir (LPV/r) Liquid Versus Capsules https://clinicaltrials.gov/ct2/show/NCT00281606 Accessed January 2, 2018.
- 20.Rieg G, Jain S, Sun S, et al. Switching to once-daily lopinavir/ritonavir liquid, capsules and tablets: A randomized, open-label, cross-over study (CCTG 585). Presented at: 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2009; Cape Town, South Africa. [Google Scholar]
- 21.Pless G, Sauer IM. Bioartificial liver: current status. Transplantation proceedings. 2005;37(9): 3893–3895. [DOI] [PubMed] [Google Scholar]
- 22.Data Table of Infant Weight-for-age Charts https://www.cdc.gov/growthcharts/html_charts/wtageinf.htm Accessed January 2, 2018.
- 23.PRESCRIBING INFORMATION AND WEIGHT-BASED DOSING OF AVAILABLE ARV FORMULATIONS FOR INFANTS AND CHILDREN http://www.who.int/hiv/pub/paediatric/paediatric_arv_dosing.pdf?ua=1 Accessed January 2, 2018.
- 24.Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Therapeutics and clinical risk management. 2008;4(5): 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2003;63(8): 769–802. [DOI] [PubMed] [Google Scholar]
- 26.Kekitiinwa A, Musiime V, Thomason MJ, et al. Acceptability of lopinavir/r pellets (minitabs), tablets and syrups in HIV-infected children. Antiviral therapy. 2016;21(7): 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fact Sheet on Lopinavir and Ritonavir (LPV/r) Oral Pellets http://apps.who.int/iris/bitstream/10665/193543/1/FactsheetIATT_WHO_UNICEF_lopinavir_eng.pdf Accessed January 2, 2018.
- 28.Safety and Effectiveness of Lopinavir/Ritonavir in HIV Infected Infants https://clinicaltrials.gov/ct2/show/NCT00038480 Accessed January 2, 2018.
- 29.Safety of Saquinavir and High Doses of Lopinavir/Ritonavir in Children With HIV https://clinicaltrials.gov/ct2/show/NCT00084058 Accessed January 2, 2018.
- 30.A Phase II/III Trial of Lopinavir/Ritonavir Dosed According to the WHO Pediatric Weight Band Dosing Guidelines https://clinicaltrials.gov/ct2/show/NCT01172535 Accessed January 2, 2018. [DOI] [PMC free article] [PubMed]
- 31.Lopinavir/Ritonavir (Kaletra) PK in Children https://clinicaltrials.gov/ct2/show/NCT00810108 Accessed January 2, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.