There is much evidence to suggest that control of gene expression and protein translation contribute to memory formation and consolidation in the hippocampus (Costa-Mattioli et al., 2007; Cho et al., 2015). Therefore, the intricate coordination of mechanisms that couple extracellular signals to translational regulation is essential for memory stability. Disruption of such pathways might contribute to memory decline in cognitive disorders such as Alzheimer's disease (AD).
Most cases of AD are sporadic, meaning they are not tightly linked to gene mutations. Nonetheless, several genetic risk factors for late-onset, sporadic AD have been reported. The strongest genetic risk factor report is possession of the apolipoprotein E ε4 allele (ApoE4). Compared to the ApoE2 or ApoE3 alleles, ApoE4 appears to promote metabolic impairment, amyloid-β (Aβ) production, and tau aggregation, which are hallmarks of AD (Liu et al., 2013). The debate about the culprits of AD pathology is still open, but mounting evidence indicates that neuronal stress-related mechanisms (initiated by either Aβ, tau, or ApoE4) take place in AD brains to induce synapse dysfunction, memory loss, and neurodegeneration (Lourenco et al., 2015).
Cellular stress signals converge to increase phosphorylation of eukaryotic translation initiation factor 2α (eIF2α-P), resulting in transcriptional changes and protein synthesis attenuation, in a set of mechanisms collectively known as the integrated stress response (ISR). eIF2α can be phosphorylated by four different kinases, namely double-stranded RNA-dependent protein kinase (PKR), PKR-like endoplasmic reticulum kinase (PERK), general control nonderepressible 2 (GCN2), and heme-regulated kinase (HRI). eIF2α-P then acts by blocking its partner, eIF2B, in the translation initiation complex (Buffington et al., 2014). Recent evidence has demonstrated significant roles for ISR elements, including PKR and eIF2α-P, in suppressing memory, in either physiological or AD contexts (for review, see Buffington et al., 2014; Lourenco et al., 2015).
In a recent paper in The Journal of Neuroscience, Segev, Barrera et al. (2015) addressed whether PKR could mediate the deleterious impact of ApoE4 on memory. They used a mouse model harboring two genomic copies of either human ApoE3 or ApoE4 alleles in place of the murine homologs. They initially found that ApoE4 mice exhibited impaired contextual memory in the fear conditioning paradigm, in agreement with their previous observations (Segev et al., 2013). They further demonstrated that young (∼3-month-old) ApoE4 mice had higher hippocampal levels of mRNA encoding activating transcription factor 4 (ATF4) than age-matched ApoE3 or wild-type mice (Segev, Barrera et al., 2015).
This increase in ATF4 levels is interesting for several reasons. ATF4 mRNA translation is selectively induced when eIF2α-P increases, despite general translational attenuation, and this drives ISR-related transcriptional reprogramming (Buffington et al., 2014). Recently, ATF4 was described as essential for synapse development and morphogenesis (Liu et al., 2014), and hippocampal silencing of ATF4 impaired synapse plasticity and spatial memory formation in mice (Pasini et al., 2015). However, ATF4 overexpression has been shown to mediate oxidative stress-induced cell death (Lange et al., 2008) and alter expression of several ISR factors, including chaperones and the pro-apoptotic transcription factor C/EBP-homologous protein (CHOP) in neurons (Galehdar et al., 2010). Earlier reports have also indicated that ATF4 could negatively impact memory, as it was shown to oppose the actions of the cAMP response element binding (CREB) transcription factor in the hippocampus (Chen et al., 2003). Thus, fine tuning of ATF4 signaling appears essential for proper synapse and cognitive function. Furthermore, increased ATF4 levels have been observed in the brains of both AD patients (Baleriola et al., 2014) and mouse models (Ma et al., 2013). A recent study by Baleriola and colleagues (2014) identified ATF4 as a retrograde neurodegenerative signal that propagates from neurons exposed to toxic Aβ oligomers (AβOs), known for their central role in AD. Hence, ATF4 has the potential to explain, at least in part, how AD pathology spreads throughout brain regions.
Segev, Barrera et al. (2015) next demonstrated that PKR inhibition alleviates memory impairment and hippocampal ATF4 upregulation in ApoE4 mice. This is consistent with previous reports that blocking eIF2α kinases is effective in impeding AD-related memory impairment in different animal models (Lourenco et al., 2013; Ma et al., 2013) and further extends the notion that metabolic stress and ISR are integral components of AD pathogenesis.
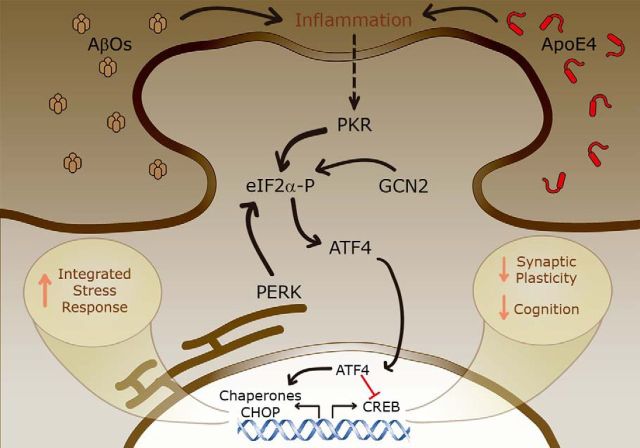
Neuroinflammation has been linked to AD pathogenesis, and some evidence suggests that inflammatory mechanisms drive synapse and cognitive impairment through ISR and metabolic stress (Lourenco et al., 2015). Human ApoE4 carriers have elevated plasma levels of inflammatory markers (Ringman et al., 2012), and ApoE4 has been shown to exacerbate central inflammatory responses that coincide with decreased levels of synaptic markers in mice (Maezawa et al., 2006; Tai et al., 2015). A similar phenomenon is thought to underlie AβO-induced cognitive dysfunction in AD models (Lourenco et al., 2013). Therefore, current evidence suggests that ApoE4 and AβOs may contribute to a toxic process that includes brain inflammation and ISR and impairs synapse and memory (Fig. 1).
Figure 1.
ISR mediates synaptic and memory impairment triggered by ApoE4 and AβOs. ISR comprises the activation of PKR, GCN2, or PERK, resulting in abnormal eIF2α-P. ApoE4 and AβOs promote an inflammatory process, which likely enhances ISR through PKR-dependent eIF2α-P. PERK and GCN2 may further contribute to the increase eIF2α-P levels. This process favors upregulation of ATF4, whose nuclear actions antagonize CREB activity and facilitate stress-related gene transcription, including CHOP and molecular chaperones. Such transcriptional changes result in defective synaptic plasticity and cognition, and may further stimulate ISR. Aberrant ISR may thus comprise a common ground to explain memory loss in AD, possibly offering novel targets for therapeutic intervention.
The precise mechanisms by which ApoE4 acts to increase AD risk remain to be determined, and inflammation-dependent cellular stress pathways might offer a consistent explanation for ApoE4-linked AD cases. The description of higher ATF4 levels in brains of ApoE4 carriers than in noncarriers, and in AD subjects compared to cognitively healthy controls, offers additional support for this possibility (Baleriola et al., 2014; Segev, Barrera et al., 2015). Still, further investigation is required to establish a causal role for ATF4 in ApoE4-induced memory defects.
In addition to upregulation of ATF4, eIF2α-P-dependent disruption of translation appears to mediate memory impairment in AD (Ma et al., 2013), which is consistent with the notion that protein synthesis is essential for synaptic plasticity and memory consolidation (Buffington et al., 2014). One might thus envision that targeting aberrant translational regulation could offer therapeutic benefit for cognitive decline. In fact, ISRIB, a pharmacological agent that counteracts eIF2α-P signaling through eIF2B binding (Sekine et al., 2015), reduces ATF4-dependent gene expression while sustaining protein synthesis and enhancing memory in mice (Sidrauski et al., 2013). Nonetheless, further efforts in preclinical research and drug development are needed to support the promising possibility that ISR could become an effective target in neurodegenerative disorders.
In conclusion, Segev, Barrera et al. (2015) provide evidence that ISR mediates memory impairment caused by the ApoE4 allele, whose carriers are at higher risk of developing AD. This opens the possibility that modulating ISR could represent a potential preventive strategy against ApoE4-related cognitive impairment. Furthermore, such findings offer a novel perspective in which similar mechanisms may drive memory loss induced by different AD-associated agents.
Footnotes
Editor's Note: These short, critical reviews of recent papers in the Journal, written exclusively by graduate students or postdoctoral fellows, are intended to summarize the important findings of the paper and provide additional insight and commentary. For more information on the format and purpose of the Journal Club, please see http://www.jneurosci.org/misc/ifa_features.shtml.
M.M.O. was supported by the Brazilian research funding agencies Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). M.V.L. was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa no Estado do Rio de Janeiro (FAPERJ), respectively.
The authors declare no competing financial interests.
References
- Baleriola J, Walker CA, Jean YY, Crary JF, Troy CM, Nagy PL, Hengst U. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell. 2014;158:1159–1172. doi: 10.1016/j.cell.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/S0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Cho J, Yu NK, Choi JH, Sim SE, Kang SJ, Kwak C, Lee SW, Kim JI, Choi DI, Kim VN, Kaang BK. Multiple repressive mechanisms in the hippocampus during memory formation. Science. 2015;350:82–87. doi: 10.1126/science.aac7368. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjević K, Lacaille JC, Nader K, Sonenberg N. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30:16938–16948. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pasini S, Shelanski ML, Greene LA. Activating transcription factor 4 (ATF4) modulates post-synaptic development and dendritic spine morphology. Front Cell Neurosci. 2014;8:177. doi: 10.3389/fncel.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, Sathler LB, Brito-Moreira J, Amaral OB, Silva CA, Freitas-Correa L, Espírito-Santo S, Campello-Costa P, Houzel JC, Klein WL, Holscher C, Carvalheira JB, Silva AM, Velloso LA, Munoz DP, et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's β-amyloid oligomers in mice and monkeys. Cell Metab. 2013;18:831–843. doi: 10.1016/j.cmet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Lourenco MV, Ferreira ST, De Felice FG. Neuronal stress signaling and eIF2alpha phosphorylation as molecular links between Alzheimer's disease and diabetes. Prog Neurobiol. 2015;129:37–57. doi: 10.1016/j.pneurobio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat Neurosci. 2013;16:1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I, Nivison M, Montine KS, Maeda N, Montine TJ. Neurotoxicity from innate immune response is greatest with targeted replacement of ε4 allele of apolipoprotein E gene and is mediated by microglial p38MAPK. FASEB J. 2006;20:797–799. doi: 10.1096/fj.05-5423fje. [DOI] [PubMed] [Google Scholar]
- Pasini S, Corona C, Liu J, Greene LA, Shelanski ML. Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Rep. 2015;11:183–191. doi: 10.1016/j.celrep.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman JM, Elashoff D, Geschwind DH, Welsh BT, Gylys KH, Lee C, Cummings JL, Cole GM. Plasma signaling proteins in persons at genetic risk for Alzheimer disease: influence of APOE genotype. Arch Neurol. 2012;69:757–764. doi: 10.1001/archneurol.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev Y, Michaelson DM, Rosenblum K. ApoE ε4 is associated with eIF2α phosphorylation and impaired learning in young mice. Neurobiol Aging. 2013;34:863–872. doi: 10.1016/j.neurobiolaging.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Segev Y, Barrera I, Ounallah-Saad H, Wibrand K, Sporild I, Livne A, Rosenberg T, David O, Mints M, Bramham CR, Rosenblum K. PKR inhibition rescues memory deficit and ATF4 overexpression in ApoE epsilon4 human replacement mice. J Neurosci. 2015;35:12986–12993. doi: 10.1523/JNEUROSCI.5241-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Zyryanova A, Crespillo-Casado A, Fischer PM, Harding HP, Ron D. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science. 2015;348:1027–1030. doi: 10.1126/science.aaa6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonenberg N, Walter P. Pharmacological brake-release of mRNA translation enhances cognitive memory. eLife. 2013;2:e00498. doi: 10.7554/eLife.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M, Lei AZ, Bahroos N, Green SJ, Hendrickson B, Van Eldik LJ, LaDu MJ. APOE-modulated Abeta-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective. J Neurochem. 2015;133:465–488. doi: 10.1111/jnc.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]