Abstract
Background
The association between nonalcoholic fatty liver disease (NAFLD) and sarcopenia has been suggested. We investigated the association between sarcopenia and NAFLD independent of visceral adiposity and searched for the clinical characteristics that affect this association.
Methods
We performed a retrospective study including of 5,989 subjects (mean age, 53.2 years; men, 57.3%) who underwent bioelectrical impedance analysis (BIA) and abdominal ultrasonography in 2012. The appendicular skeletal muscle mass (ASM) was assessed by BIA method. Sarcopenia was defined as ASM/weight (ASM%) <2 standard deviation of the mean for healthy young reference population. NAFLD was diagnosed by ultrasonography.
Results
The prevalence of sarcopenia was 5.3%. The prevalence of NAFLD was significantly higher in subjects with sarcopenia than in those without (69.5% vs. 36.5%, P<0.001). After adjusting with age, sex, visceral fat area, hypertension, diabetes, total and low-density lipoprotein cholesterol, subjects with sarcopenia showed significantly high odds of NAFLD (odds ratio [OR], 1.37; 95% confidence interval [CI], 1.02–1.84; P=0.036). Subjects with sarcopenia have more likely severe grade of NAFLD compared to non-sarcopenic group (OR, 1.58; 95% CI, 1.25–2.00; P<0.001). There was significant interaction for effect modification in the association between sarcopenia and NAFLD by age (P of interaction for effect modification, 0.007).
Conclusion
Sarcopenia was significantly associated with the presence and the severity of ultrasonography-graded NAFLD in our study population independent of visceral fatness and other metabolic confounder. Younger age showed greater magnitude of association between sarcopenia and NAFLD.
Keywords: Sarcopenia, Non-alcoholic fatty liver disease, Skeletal muscle, Intra-abdominal fat
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has becoming the most leading cause of chronic liver disease, with prevalence up to 20%–30% of general population.1 Even though most of NAFLD follows a benign clinical course, simple steatosis can progresses to nonalcoholic steatohepatitis (NASH), 25% of whom may experience progression of liver fibrosis and cirrhosis.2,3 In addition, patients with NASH have been reported to have higher liver-related mortality compared to the general population.4 However, the clinical significance of NAFLD is not limited to liver. NAFLD is also associated with various metabolic conditions such as obesity, dyslipidemia, type 2 diabetes mellitus and cardiovascular diseases.5–7 Insulin resistance is considered to be a shared pathophysiologic mechanism between NAFLD and the other metabolic diseases.8 Alterations in adipokines, adipose tissue inflammation, and increased serum free fatty acids, which are accompanied with the development of visceral obesity, are proposed mechanisms linking insulin resistance and NAFLD and could explain the association between visceral fat and NAFLD.9
Skeletal muscle is one of the important target organs of insulin and previous studies have accumulated evidences for a link between sarcopenia and insulin resistance.10,11 Based on the shared common pathophysiology of insulin resistance, the association of sarcopenia with NAFLD has been studied and the relevance has been reported in several studies.12–16 Recently, not only the presence of NAFLD but also the severity of NAFLD is reported to be associated with sarcopenia.12,15 Also, the existence of effect modification by age, sex, and obesity status, etc. in the association between sarcopenia and NALFD has been suggested in previous studies.16,17 However, the presence or the characteristics of these effect modifiers in the relationship between sarcopenia and NAFLD have not yet been fully elucidated.
In this study, we aimed to investigate the relationship between sarcopenia and NAFLD and tried to find out whether this relationship differs according to subgroups with different clinical characteristics in health checkup examinees.
METHODS
Study population
In this retrospective study, subjects who underwent bioelectrical analysis between January 2012 and December 2012 during health checkup at Seoul National University Hospital Healthcare System Gangnam Center were consecutively enrolled for this study. Initially, a total of 7,239 subjects were enrolled. Among them, 578 subjects with no abdominal sonography data and four subjects without anthropometric measurement were excluded. We also excluded subjects who had potential cause of chronic liver disease; 268 were positive for the hepatitis B virus, 57 were positive for the hepatitis C virus and 343 with significant alcohol intake (>20 g/day for males and >10 g/day for females).18 There was no subjects with pregnancy, cirrhosis or end stage renal disease in this study population. Finally, 5,989 subjects were analyzed.
This study was approved by the Institutional Review Board of the Seoul National University Hospital (IRB No. 1803-009-924). Informed consent was waived because researchers only accessed and analyzed de-identified data.
Clinical and laboratory assessments
Each subject completed a past medical history questionnaire and received an anthropometric assessment and laboratory and radiologic tests on the same day. A current smoker was defined as a person who smoked at least one cigarette per day for the previous 12 months. Systolic and diastolic blood pressure was measured twice, measured twice, and the mean values were reported. The presence of hypertension was defined as a systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg more than twice or taking anti-hypertensive medication. The presence of diabetes was defined as either taking anti-diabetic medications or a fasting serum glucose and glycosylated hemoglobin (HbA1c) level ≥126 mg/dL and ≥6.5%, respectively.
Blood samples were collected before 10:00 AM after a 12-hour overnight fast. The laboratory tests included serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, fasting glucose, HbA1c, uric acid, hepatitis B surface antigen and antibody to hepatitis C virus. All laboratory tests were carried out using standard laboratory methods.
Anthropometric measurements
Body weight and height were measured using a digital scale, and body mass index (BMI) was calculated as weight (kg)/height square (m2). Waist circumference (WC) was measured at the midpoint between the lower costal margin and the anterior superior iliac crest by a well-trained person using a tape. For assessing body composition, bioelectrical impedance analysis (BIA) was performed using the InBody 720 body composition analyzer (InBody, Seoul, Korea). The subjects were in standing position in 5–10 minutes with their legs slightly separated and the arms slightly abducted from the trunk. The subjects were instructed to hold the handles of the analyzer, for contacting with electrodes in each limb. When the measurements stabilized, it provides impedance for each segment including the trunk and the four limbs by performing multi-frequency measurements to estimate the appendicular skeletal muscle mass (ASM).
We used a previously described method to assess the visceral fat area (VFA) on abdominal fat computed tomography (CT) images.19 Briefly, the individuals were examined in the supine position with a 16-detecter CT scanner (Somatom Sensation 16; Siemens Medical Solutions, Forchheim, Germany). The VFA was measured using commercially available CT software (Rapidia 2.8; INFINITT, Seoul, Korea) by setting the attenuation values for adipose tissue area with a range of −250 to −50 Hounsfield units.
Definitions of sarcopenia, obesity and abdominal obesity
ASM (kg) was calculated as the sum of the lean muscle mass in the bilateral upper and lower limbs. ASM% was calculated as ASM/ weight (kg)×100, modified from the Janssen et al.’s study.20 Sarcopenia was defined as ASM% beyond two standard deviation (SD) below the sex-specific mean for young reference population obtained from nationwide health examination in Korean (ASM% <29.0 in men or <22.9 in women).21 Obesity was defined as BMI value ≥25 kg/m2 according to the WHO recommendation for Asian-Pacific region. Abdominal obesity is defined as a WC ≥90 cm for men and ≥85 cm for women according to the guideline by Korean Society for the Study of Obesity.22
Assessment of NAFLD
Hepatic ultrasonography (US; Acuson Sequoia 512, Siemens, Mountain View, CA, USA) was performed by experienced radiologists to diagnose fatty liver. They were blinded to the clinical and laboratory details of the subjects at the time of evaluation. The diagnosis was made with characteristic ultrasonographic findings such as “bright liver” and evident contrast between hepatic and renal parenchyma, vessel blurring, focal sparing, and narrowing of the lumen of the hepatic veins.23,24 The degree of fatty change by US was graded semi-quantitatively as grade 0–3 according to the criteria by Saadeh et al.25 In this study, we referred to grade 0 as normal, grade 1, 2, and 3 as mild, moderate and severe NAFLD, respectively.
Statistical analysis
Continuous variables were expressed as mean±SD, and categorical variables were expressed as number and percent. Comparisons of continuous variables between groups were performed using Student t-test or analysis of variance, and categorical variables were compared using chi-square test or Fisher’s exact tests. ASM% was divided into quartiles according to sex. The group with the highest ASM% value was defined as 1st quartile (Q1), and the group with lowest ASM% value is defined as 4th quartile (Q4). In male, ASM% ranges were Q1 >33.95, >32.35 Q2 ≤33.95, >30.78 Q3 ≤32.35, and Q4 ≤30.78. In female, ASM% ranges were Q1 >29.61, >27.69 Q2 ≤29.61, >25.97 Q3 ≤27.69, and Q4 ≤25.97. The association between Sarcopenia and the severity of NAFLD evaluated by US was evaluated using a chi-square test. Correlations between ASM% and metabolic variables were analyzed using Pearson correlation coefficient. Univariate analysis was performed to identify risk factors associated with NAFLD. Multivariate logistic regression analysis was performed to determine the independent association between sarcopenia and the presence of NAFLD after adjusting for age and sex in model 1; variables in model 1, VFA, hypertension, and diabetes mellitus in model 2; variables in model 2, total cholesterol, and LDL cholesterol in model 3. In this analysis, we also used ASM% quartile (ASM%_Q) as independent variable to identify the dose-response relationship between the muscle mass and NAFLD. An ordinal logistic regression analysis was used to investigate the association between sarcopenia/ASM%_Q and the severity of NAFLD with same adjustment in model 1, 2, and 3.
Stratified analysis was performed in subgroups according to age, sex, obesity and abdominal obesity status and diabetes mellitus. Age was divided into tertiles as follows: T1, 19–49 years old; T2, 50–57 years old; T3, 58–87 years old. A P-value of interaction for effect modification was obtained by putting interaction term (ASM%_Q× subgroups) in the regression model. All statistical analyses were performed using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and R statistical software version 3.2.2 (R Development Core Team; R Foundation for Statistical Computing, Vienna, Austria). The P-values <0.05 were considered statistically significant.
RESULTS
Clinical characteristics according to the sarcopenia status
Among the total of 5,989 subjects, the prevalence of sarcopenia is 5.3% (315/5,989). Mean age of our study population was 53.2± 9.4 and 57.3% was male participants. The clinical characteristics according to the sarcopenia status are summarized in Table 1. The individuals with sarcopenia are older, had a higher prevalence of male, higher BMI, WC and, VFA than the control group (P<0.001). These differences in obesity status and body composition resulted in significantly different metabolic profiles; the individuals with sarcopenia had higher prevalence of diabetes mellitus and hypertension, higher blood pressure, and serum levels of AST, ALT, GGT, total triglycerides, LDL-cholesterol, fasting glucose and HbA1c. The values of ASM% were significantly lower in the sarcopenia group compared to the control group (P<0.05 for all) (Table 1).
Table 1.
Comparison of baseline characteristics according to the presence of sarcopenia
| Variable | Total (n=5,989) | Control (n=5,674) | Sarcopenia (n=315) | P |
|---|---|---|---|---|
| Age (yr) | 53.2±9.4 | 53.0±9.2 | 57.1±11.4 | <0.001 |
|
| ||||
| Male sex | 3,431 (57.3) | 3,201 (56.4) | 230 (73.0) | <0.001 |
|
| ||||
| Height (cm) | 165.7±8.2 | 165.8±8.2 | 163.7±8.4 | <0.001 |
|
| ||||
| Weight (kg) | 64.7±11.7 | 64.1±11.2 | 75.0±14.5 | <0.001 |
|
| ||||
| BMI (kg/m2) | 23.4±3.0 | 23.2±2.8 | 27.8±3.6 | <0.001 |
|
| ||||
| WC (cm) | ||||
| Male | 88.0±7.3 | 87.4±6.7 | 97.3±8.4 | <0.001 |
| Female | 79.7±7.8 | 79.2±7.3 | 92.8±10.0 | <0.001 |
|
| ||||
| VFA (cm2) | 114.4±55.4 | 110.9±53.0 | 179.3±57.7 | <0.001 |
|
| ||||
| ASM (kg) | 19.8±4.6 | 19.8±4.5 | 19.8±4.9 | 0.993 |
|
| ||||
| ASM% | 30.4±3.5 | 30.7±3.3 | 26.2±3.1 | <0.001 |
|
| ||||
| SBP (mmHg) | 116.4±13.7 | 116.0±13.6 | 124.1±14.2 | <0.001 |
|
| ||||
| DBP (mmHg) | 74.3±10.4 | 74.1±10.3 | 78.2±10.8 | <0.001 |
|
| ||||
| AST (IU/L) | 25.3±29.6 | 25.1±30.3 | 28.8±13.4 | 0.034 |
|
| ||||
| ALT (IU/L) | 26.3±34.8 | 25.8±35.3 | 35.9±23.5 | <0.001 |
|
| ||||
| GGT (IU/L) | 35.2±39.1 | 34.5±38.7 | 48.1±42.7 | <0.001 |
|
| ||||
| Cholesterol (mg/dL) | 194.7±35.1 | 194.6±35.3 | 196.5±37.7 | 0.352 |
|
| ||||
| TG (mg/dL) | 107.6±79.9 | 106.5±80.6 | 128.6±62.2 | <0.001 |
|
| ||||
| HDL cholesterol (mg/dL) | ||||
| Male | 48.0±9.8 | 48.1±9.8 | 46.4±8.9 | 0.010 |
| Female | 56.0±11.4 | 56.0±11.4 | 54.9±9.7 | 0.307 |
|
| ||||
| LDL cholesterol (mg/dL) | 121.9±30.7 | 121.6±30.5 | 127.4±33.6 | 0.004 |
|
| ||||
| Fasting glucose (mg/dL) | 97.6±19.7 | 97.1±19.1 | 106.3±27.4 | <0.001 |
|
| ||||
| HbA1c (%) | 5.7±0.6 | 5.7±0.6 | 6.0±0.8 | <0.001 |
|
| ||||
| Uric acid (mg/dL) | 5.5±1.4 | 5.4±1.4 | 6.2±1.6 | <0.001 |
|
| ||||
| Diabetes mellitus | 299 (5.0) | 272 (4.8) | 27 (8.6) | 0.004 |
|
| ||||
| Hypertension | 892 (14.9) | 804 (14.2) | 88 (27.9) | <0.001 |
|
| ||||
| Smoking | <0.050 | |||
| Never | 1,540 (46.5) | 1,489 (47.1) | 51 (34.0) | |
| Former | 1,137 (34.3) | 1,068 (33.8) | 69 (46.0) | |
| Current | 635 (19.2) | 605 (19.1) | 30 (20.0) | |
|
| ||||
| NAFLD | 2,290 (38.2) | 2,071 (36.5) | 219 (69.5) | <0.001 |
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; WC, waist circumference; VFA, visceral fat area; ASM, appendicular skeletal muscle mass; SBP, systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyltransferase; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HbA1c, glycosylated hemoglobin; NAFLD, nonalcoholic fatty liver disease.
Prevalence and severity of NAFLD in sarcopenia
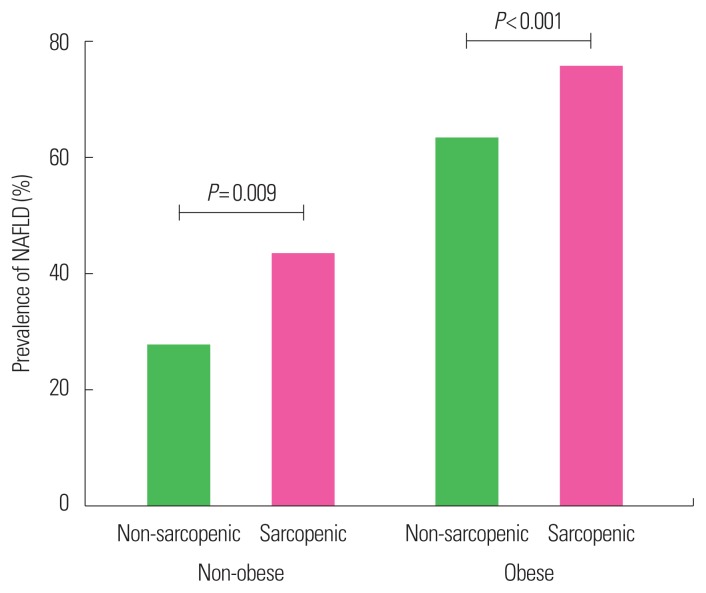
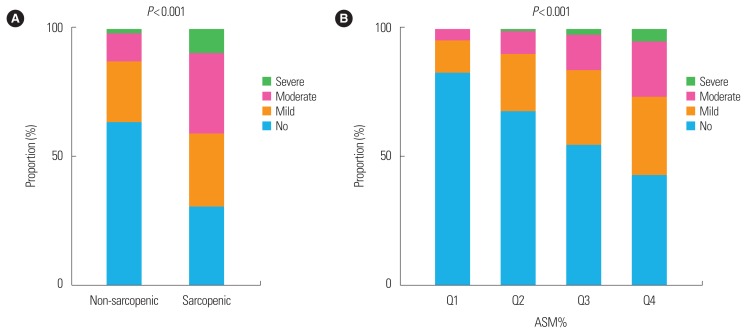
The prevalence of NAFLD was significantly higher in subjects with sarcopenia than in those without (69.5% vs. 36.5%, P<0.001) (Table 1). We stratified subjects into nonobese and obese population based on BMI at baseline. The prevalence of NAFLD in the sarcopenic group was significantly higher than the non-sarcopenic group not only in obese population (sarcopenic vs. non-sarcopenic, 75.3% vs. 62.8%; P<0.001) but also in non-obese population (42.9% vs. 27.1%; P=0.009) (Fig. 1). We evaluated the US-graded severity of NAFLD according to the quartile of ASM% as well as presence of sarcopenia. Subjects with sarcopenia showed significantly greater severity of NAFLD compared to subjects without sarcopenia (P<0.001, by linear association). It was also observed that subjects with the lower the ASM%_Q, the greater the severity of NAFLD (P<0.001, by linear association) (Fig. 2).
Figure 1.
Prevalence of nonalcoholic fatty liver disease (NAFLD) in sarcopenic and non-sarcopenic subjects according to obesity status.
Figure 2.
The severity of nonalcoholic fatty liver disease according to (A) sarcopenia and (B) appendicular skeletal muscle mass (ASM)% quartiles (Qs).
Factors related to ASM% and the presence of NAFLD
A relationship between ASM% and various metabolic parameters were investigated by correlation analysis. ASM% was negatively correlated with age, BMI, WC, VFA, systolic blood pressure, total cholesterol, HDL-cholesterol and LDL-cholesterol. On the other hand, height, weight, diastolic blood pressure and uric acid level showed positive correlation with ASM% (P<0.001 for all) (Supplementary Table 1). Also, we performed univariate analysis to determine risk factors for having NAFLD. For evaluation of the association between sarcopenia and NAFLD, we performed univariate and multivariate analysis of the risk for NAFLD. Age, sex, BMI, WC, VFA, hypertension, diabetes mellitus, triglyceride, HDL-cholesterol, LDL-cholesterol and ALT levels were significantly associated with the risk of NAFLD (P<0.001 for all) (Supplementary Table 2).
Independent impact of sarcopenia on the presence of NAFLD
To verify whether sarcopenia has independent effect on the presence of NAFLD, multiple logistic regression analyses were performed (Table 2). First, the presence of sarcopenia was used as an independent variable. After adjusting with age and sex, subjects with sarcopenia showed significantly high odds of NAFLD (odds ratio [OR], 3.46; 95% confidence interval [CI], 2.68–4.45, P<0.001). With further adjustment with VFA, hypertension and diabetes mellitus, this association attenuated but remained statistically significant (OR, 1.38; 95% CI, 1.03–1.85, P=0.030). Additional adjustment with total cholesterol and LDL cholesterol had little impact on the effect size and the significance of the association (OR, 1.37; 95% CI, 1.02–1.84, P=0.036) (Table 2). Next, ASM%_Q was used as an independent variable to identify the dose response relationship. ASM%_Q was treated as continuous variable, and OR implies a degree of increased risk that NAFLD will accompany each ASM%_Q reduction. Even after adjustment for age, sex, VFA, hypertension, diabetes mellitus, total and LDL-cholesterol, low ASM%_Q is significantly associated with increased with of NAFLD (OR, 1.28; 95% CI, 1.21–1.37; P<0.001) (Table 2). Even after further adjustment with smoking history, triglyceride and HDL cholesterol, which were significantly associated with sarcopenia as well as NAFLD, ORs and statistical significance were maintained for both sarcopenia and ASM%_Q (Supplementary Table 3).
Table 2.
Multivariate analyses of the risk for NAFLD
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Sarcopenia | 3.46 (2.68–4.45) | <0.001 | 1.38 (1.03–1.85) | 0.030 | 1.37 (1.02–1.84) | 0.036 |
|
| ||||||
| ASM%_Q | 1.85 (1.74–1.94) | <0.001 | 1.29 (1.21–1.37) | <0.001 | 1.28 (1.21–1.37) | <0.001 |
Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, VFA, hypertension, and diabetes mellitus; Model 3: adjusted for age, sex, VFA, hypertension and diabetes mellitus, total cholesterol, and low-density lipoprotein cholesterol.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; ASM, appendicular skeletal muscle mass; Q, quartile; VFA, visceral fat area.
Independent impact of sarcopenia on the severity of NAFLD
Next, we evaluated the relationship between sarcopenia and US-graded severity of NAFLD. Subjects with sarcopenia have more likely severe grade of NAFLD with the OR as 1.58 (95% CI, 1.25–2.00) compared to non-sarcopenic group, a statistically significant effect, Wald χ2(1)=16.308, P<0.001 after adjustment with confounding factors. Also, each time the ASM% move from Q1 to Q4, the probability of having a severe NALFD increases by 1.35 times (95% CI, 1.27–1.44) after adjustment with statistical significance (P<0.001) (Table 3). Further adjustment with smoking history, triglyceride and HDL cholesterol showed similar results (Supplementary Table 4).
Table 3.
Ordinal logistic regression analysis for the severity of NAFLD
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Sarcopenia | 3.94 (3.19–4.88) | <0.001 | 1.61 (1.28–2.03) | <0.001 | 1.58 (1.25–2.00) | <0.001 |
|
| ||||||
| ASM%_Q | 1.95 (1.86–2.06) | <0.001 | 1.36 (1.28–1.44) | <0.001 | 1.35 (1.27–1.44) | <0.001 |
Model 1: adjusted for age and sex; Model 2: adjusted for age, sex, VFA, hypertension, and diabetes mellitus; Model 3: adjusted for age, sex, VFA, hypertension and diabetes mellitus, total cholesterol, and low-density lipoprotein cholesterol.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; ASM, appendicular skeletal muscle mass; Q, quartile; VFA, visceral fat area.
Stratified analysis in various subgroups
To investigate the presence of effect modifier, we divided subgroups according to age, sex, obesity and abdominal obesity status, and existence of diabetes mellitus. Age was categorized as tertiles (T1, T2, T3). In the subgroup analysis by age, adjusted OR was higher in lower age groups and there was significant interaction for effect modification (T1 vs. T2 vs. T3: OR, 1.46 vs. 1.31 vs. 1.22, P of interaction for effect modification=0.007). Female showed numerically smaller effect size than male but it was statistically insignificant (female vs. male: OR, 1.17 vs 1.40, P for interaction=0.947). Differences between subgroups according to obesity, abdominal obesity and diabetes mellitus were insignificant (P for interaction >0.05) (Table 4).
Table 4.
Stratified analysis of the risk for NAFLD in various subgroups
| Variable | OR (95% CI) | P for interaction |
|---|---|---|
| Age (yr) | 0.007 | |
| T1 (19–49) | 1.46 (1.30–1.64) | |
| T2 (50–57) | 1.31 (1.18–1.46) | |
| T3 (58–87) | 1.22 (1.09–1.36) | |
|
| ||
| Sex* | 0.947 | |
| Male | 1.40 (1.30–1.52) | |
| Female | 1.17 (1.04–1.31) | |
|
| ||
| Obesity | 0.238 | |
| No | 1.22 (1.12–1.32) | |
| Yes | 1.27 (1.12–1.45) | |
|
| ||
| Abdominal obesity | 0.094 | |
| No | 1.27 (1.12–1.43) | |
| Yes | 1.29 (1.20–1.40) | |
|
| ||
| DM | 0.816 | |
| No | 1.33 (1.25–1.42) | |
| Yes | 1.35 (1.02–1.80) | |
Adjusted for age, sex, and VFA.
Adjusted for age and VFA.
NAFLD, nonalcoholic fatty liver disease; OR, odds ratio; CI, confidence interval; DM, diabetes mellitus; VFA, visceral fat area.
DISCUSSION
We aimed to verify the implication of sarcopenia in the presence and severity of NAFLD evaluated by US. In our study population, sarcopenia is significantly associated with NAFLD even after adjusting possible confounding factors including visceral adiposity. In addition, sarcopenia independently increased severity of NAFLD. In the subgroup analysis, risk of NAFLD according to sarcopenia was significantly higher in younger subjects.
Sarcopenia is characterized as loss of skeletal muscle with low muscle strength and/or physical performance26 and is associated with the increased risk of comorbidity, disability and mortality.27,28 The prevalence of sarcopenia varies significantly ranging 5%–13% in 60- to 70-year-olds, reflecting the differences in study population, the different methods used to define sarcopenia.28,29 In this study, we adopted the definition of (ASM/weight×100), which is modified from the definition by Janssen et al.20 In the previous studies using Janssen’s method performed in Korean elderly subjects, the prevalence of sarcopenia was 5.1% in men and 14.2% in women over 60 years of age10, and 9.7% for men and 11.8% for women in over elderly subjects aged 65 years or older.21 Prevalence of sarcopenia was 5.3% (6.7% in men and 3.3% in women) in our subjects with mean age of 53 years old.
The gold standard for diagnosis of NAFLD requires a liver biopsy for histological analysis. However, it is an invasive method with the risk of rare but sometimes can be fatal complication such as hemorrhage.30 US is an established modality as a screening tool with a sensitivity of 60%–94% and a specificity of 66%–95% in the diagnosis of fatty liver.31 Many studies using US to diagnose NAFLD adopted a three point scoring system for fatty liver (mild, moderate and severe) as in our study, based on the ultrasonographic characteristics.32,33
Several studies have shown that sarcopenia is associated with NAFLD.13,14,17,34 In the studies using predictive index or scoring system to diagnose NAFLD14,17,34, the prevalence of NAFLD was ranged from about 10% to 29%. On the other hand, the prevalence of NAFLD in our study was 38.2% (48.9% in male and 24.0% in female).
The difference in the study population should be considered, but the distinct difference in the prevalence of NAFLD suggests that the sensitivity of noninvasive predictive indices for diagnosing NAFLD might be reduced.
Our findings on the association between sarcopenia and NAFLD are largely consistent with previous studies. Both skeletal muscle index (ASM%) and NAFLD were significantly associated with metabolic parameters such as blood pressure, blood glucose and lipid profile. However, anthropometric parameters such as BMI, WC, and VFA were positively correlated with NAFLD but negatively correlated with muscle index. Therefore, these body fatness indices needed to be adjusted. Considering pathophysiologic relevance with NAFLD, we used VFA as adjustment variable. We also performed sensitivity analysis using BMI instead of VFA, and obtained consistent results (data not shown). Liver enzyme levels were also significantly associated with sarcopenia and NAFLD; however, authors did not consider these as confounders because liver enzymes levels are more likely the outcome than the cause of NAFLD.
The association between sarcopenia and NASH/fibrosis also has been reported.12,15,35 In those studies, advanced liver pathology was estimated by predictive fibrosis indices12 or diagnosed by liver biopsy.15,35 In line with the previous results, sarcopenia independently increased risk of having US-graded severe NAFLD in our study. Even though ultrasonographic diagnosis of NAFLD does not provide reproducible and exact quantitative information, and inaccurate in differentiating fibrosis from steatosis, US-graded severity of NAFLD has been proved to be significantly associated with the risk of metabolic syndrome and subclinical atherosclerosis in previous studies.36,37
The relationship between sarcopenia and NAFLD seems to be relatively consistent throughout the studies regardless of which definition or method they used to diagnose sarcopenia or NAFLD/NASH/fibrosis. However, the question whether there is specific subgroups with stronger association between sarcopenia and NAFLD has been less explored. Kim et al.17 suggested that men ≥45 years of age and premenopausal women has stronger association. However, the 45-year-old cutoff was arbitrary and the rationale for this cutoff has not been stated. We subdivided our study participants into tertiles according to age and obtained the odds of associations in individual groups. As a result, it was observed that the ORs were increased toward the lower tertile, unlike the previous study. Similar trend was observed when the same analysis performed in each sex separately (data not shown). The reason of this observation is unclear so far. If a progressive loss in muscle mass and a gain in fat mass, especially in visceral fat mass, is a part of aging process, we can speculate that young-aged subjects with the same degree of body composition without aging may have genetic or lifestyle contributors, possibly insulin resistance, to cause it.
The mechanism of the close link between sarcopenia and NAFLD is not fully investigated. A previous study that had shown a significant association between sarcopenia and insulin resistance in non-obese population38, suggests the shared mechanism of insulin resistance. The skeletal muscle is considered as the primary tissue responsible for insulin-mediated glucose disposal and recent epidemiological studies have shown that the insulin resistance and mitochondrial dysfunction may have a specific role on skeletal muscle mass.29,39 However, previous studies showed that association between sarcopenia and NAFLD attenuated but persistent even after adjusting insulin resistance implying that there is another mechanism that mediates these two conditions independent of insulin resistance. With many missing variables, we did not adjust insulin resistance in our model and was unable to confirm the significant association between sarcopenia and NAFLD independent of insulin resistance. In addition, the impact of chronic, low-grade inflammation on sarcopenia has been suggested. Inflammatory markers such as tumor necrosis factor-alpha and interleukin-6 are observed quite consistently.40
The advantage of our study is that we performed stratified analysis in subgroups to verify the effect size difference in each subgroup. Also, we used VFA measured by CT scan which is more precise method than the anthropometric measurement or BIA to estimate visceral fatness. This study also has several limitations. First, BIA and US are not gold standard methods to evaluate muscle mass and fatty liver, respectively. Second, we did not evaluate muscle strength or physical performance, which are components for diagnostic criteria of sarcopenia. Third, cross-sectional design of this study limits to verify causality. Fourth, we had not excluded subjects with malignancy. However, the participants in this study included subjects who visited health check-up center, thus the number of subjects with malignancy will be very small. Fifth, some possible confounding factors (e.g., physical activity, nutritional status, and medications, etc.) that could affect the pathophysiology of NAFLD and sarcopenia were not considered in this study. Lastly, study population in our study, who underwent health check-up based on their own initiative, may not represent the general Korean population.
Sarcopenia was significantly associated with not only the presence but also the severity of US-graded NAFLD in our study population independent of visceral fatness and other metabolic confounder. Younger age showed greater magnitude of association between sarcopenia and NAFLD. Further investigation is needed to confirm the differential association according to age and to verify possible mechanisms.
SUPPLEMENTARY MATERIALS
Supplementary Table 1. Correlation between ASM% and metabolic parameters.
Supplementary Table 2. Crude odds ratios for NAFLD.
Supplementary Table 3. Multivariate analyses of the risk for NAFLD with additional covariates.
Supplementary Table 4. Ordinal logistic regression analysis for the severity of NAFLD with additional covariates.
They can be found via https://doi.org/10.7570/jomes.2019.28.2.129.
ACKNOWLEDGMENTS
This research was supported by Research Program 2016 funded by Seoul National University College of Medicine Research Foundation (800-20160446).
The authors are grateful to Boram Park and Yun Hwan Lee for their help with the statistical analysis. We also thank to Dr. Bo Kyung Koo (Seoul National University College of Medicine) for the discussions about the research.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 4.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–8. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 6.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Anstee QM, Tilg H, Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–53. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 8.Khan R, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in NAFLD. Hepatology. 2018 Dec 16; doi: 10.1002/hep.30429. doi: 10.1002/hep.30429. [Epub]. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi Y, Eguchi T, Mizuta T, Ide Y, Yasutake T, Iwakiri R, et al. Visceral fat accumulation and insulin resistance are important factors in nonalcoholic fatty liver disease. J Gastroenterol. 2006;41:462–9. doi: 10.1007/s00535-006-1790-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes (Lond) 2009;33:885–92. doi: 10.1038/ijo.2009.130. [DOI] [PubMed] [Google Scholar]
- 11.Guillet C, Boirie Y. Insulin resistance: a contributing factor to age-related muscle mass loss? Diabetes Metab. 2005;31:5S20–5S26. doi: 10.1016/S1262-3636(05)73648-X. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008–2011) Hepatology. 2016;63:776–86. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 13.Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology. 2014;59:1772–8. doi: 10.1002/hep.26716. [DOI] [PubMed] [Google Scholar]
- 14.Moon JS, Yoon JS, Won KC, Lee HW. The role of skeletal muscle in development of nonalcoholic fatty liver disease. Diabetes Metab J. 2013;37:278–85. doi: 10.4093/dmj.2013.37.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo BK, Kim D, Joo SK, Kim JH, Chang MS, Kim BG, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol. 2017;66:123–31. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Lee MJ, Kim EH, Bae SJ, Kim GA, Park SW, Choe J, et al. Age-related decrease in skeletal muscle mass is an independent risk factor for incident nonalcoholic fatty liver disease: a 10-year retrospective cohort study. Gut Liver. 2019;13:67–76. doi: 10.5009/gnl18070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim HY, Kim CW, Park CH, Choi JY, Han K, Merchant AT, et al. Low skeletal muscle mass is associated with non-alcoholic fatty liver disease in Korean adults: the fifth Korea National Health and Nutrition Examination Survey. Hepatobiliary Pancreat Dis Int. 2016;15:39–47. doi: 10.1016/S1499-3872(15)60030-3. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605–13. doi: 10.1002/hep.25593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, et al. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the fourth Korean National Health and Nutritional Examination Surveys. J Gerontol A Biol Sci Med Sci. 2012;67:1107–13. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- 22.Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. 2014 Clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul) 2014;29:405–9. doi: 10.3803/EnM.2014.29.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 24.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in northern Italy. Ann Intern Med. 2000;132:112–7. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 25.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 26.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Lee JS, Auyeung TW, Kwok T, Lau EM, Leung PC, Woo J. Associated factors and health impact of sarcopenia in older Chinese men and women: a cross-sectional study. Gerontology. 2007;53:404–10. doi: 10.1159/000107355. [DOI] [PubMed] [Google Scholar]
- 28.Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452–6. doi: 10.1007/BF02982705. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int. 2012;12:388–96. doi: 10.1111/j.1447-0594.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 30.Strassburg CP, Manns MP. Approaches to liver biopsy techniques: revisited. Semin Liver Dis. 2006;26:318–27. doi: 10.1055/s-2006-951599. [DOI] [PubMed] [Google Scholar]
- 31.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol. 2009;51:433–45. doi: 10.1016/j.jhep.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011) J Hepatol. 2015;63:486–93. doi: 10.1016/j.jhep.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Petta S, Ciminnisi S, Di Marco V, Cabibi D, Cammà C, Licata A, et al. Sarcopenia is associated with severe liver fibrosis in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2017;45:510–8. doi: 10.1111/apt.13889. [DOI] [PubMed] [Google Scholar]
- 36.Chang Y, Ryu S, Sung KC, Cho YK, Sung E, Kim HN, et al. Alcoholic and non-alcoholic fatty liver disease and associations with coronary artery calcification: evidence from the Kangbuk Samsung Health Study. Gut. 2018 Nov 24; doi: 10.1136/gutjnl-2018-317666. doi: 10.1136/gutjnl-2018-317666. [Epub]. [DOI] [PubMed] [Google Scholar]
- 37.Ryoo JH, Choi JM, Moon SY, Suh YJ, Shin JY, Shin HC, et al. The clinical availability of non alcoholic fatty liver disease as an early predictor of the metabolic syndrome in Korean men: 5-year’s prospective cohort study. Atherosclerosis. 2013;227:398–403. doi: 10.1016/j.atherosclerosis.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Endocr J. 2014;61:61–70. doi: 10.1507/endocrj.EJ13-0244. [DOI] [PubMed] [Google Scholar]
- 39.Abbatecola AM, Paolisso G, Fattoretti P, Evans WJ, Fiore V, Dicioccio L, et al. Discovering pathways of sarcopenia in older adults: a role for insulin resistance on mitochondria dysfunction. J Nutr Health Aging. 2011;15:890–5. doi: 10.1007/s12603-011-0366-0. [DOI] [PubMed] [Google Scholar]
- 40.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Correlation between ASM% and metabolic parameters.
Supplementary Table 2. Crude odds ratios for NAFLD.
Supplementary Table 3. Multivariate analyses of the risk for NAFLD with additional covariates.
Supplementary Table 4. Ordinal logistic regression analysis for the severity of NAFLD with additional covariates.
They can be found via https://doi.org/10.7570/jomes.2019.28.2.129.