Abstract
Background
An excess storage of body fat causes obesity. Since obesity increases risk of chronic diseases, it is important to inhibit excessive storage of fat. Zaluzanin C is a sesquiterpene lactone isolated from Ainsliaea acerifolia. The aim of this study was to demonstrate the effect of zaluzanin C on differentiation of 3T3-L1 preadipocytes into mature adipocytes.
Methods
The cytotoxicity of zaluzanin C and its effect on cell proliferation was determined. For the induction of adipocyte differentiation, 3T3-L1 preadipocytes were treated with differentiating medium containing 10 μg/mL insulin, 115 μg/mL methylisobutylxanthine, and 1 μM dexamethasone. Differentiated 3T3-L1 cells were subjected to Oil red O solution or used for Western blot analysis. Zaluzanin C was added to the cell culture medium at concentrations of 0, 1, 2.5, 5, and 10 μM.
Results
Zaluzanin C did not inhibit cell proliferation and showed no cytotoxicity at 10 μM concentration in 3T3-L1 cells. Therefore, concentration range of 0–10 μM zaluzanin C was used for subsequent experiments. Zaluzanin C inhibited accumulation of lipid droplets in 3T3-L1 adipocytes. To understand the underlying mechanism of zaluzanin C, expression of adipogenesis regulators was determined by Western blot analysis. Zaluzanin C suppressed peroxisome proliferator-activated receptor gamma (PPARγ) expression, an adipogenesis related transcription factor, and inhibited aP2/fatty acid-binding protein-4 expression, a target gene of PPARγ. However, it did not affect expression of CCAAT/enhancer-binding protein alpha related with acquisition of insulin sensitivity.
Conclusion
These data suggest that inhibitory effect of zaluzanin C on adipogenesis of 3T3-L1 adipocytes could be partially caused by suppressing PPARγ.
Keywords: Zaluzanin C, Lactone, 3T3-L1 cell, Proliferator-activated receptor gamma
INTRODUCTION
Prevalence rate of obesity has increased since 1988. Particularly, it was 42.3% among males in South Korea in 2016.1 Obesity is a significant risk factor for many serious illnesses such as gastrointestinal cancer, heart disease, stroke and diabetes.2–5 Increased physical activity, diet modification, drugs, and surgery are common strategies to treat obesity.6 Currently available anti-obesity drugs are associated with several adverse effects such as hyperthyroidism, palpitations, anxiety, insomnia, and diarrhea.7 Therefore, there is a growing demand for the development of new and safe anti-obesity agents.
Imbalance between energy intake and its consumption can lead to an excessive accumulation of lipids in the adipose tissue. Adipose tissue is a key regulator of energy balance, playing an active role in lipid storage and buffering, synthesizing, and secreting a wide range of endocrine products into blood circulation that influence systemic metabolism.8 Therefore, inhibition of adipogenesis in adipocytes could be a good strategy to prevent and treat obesity.
Ainsliaea acerifolia is a perennial plant, which belongs to the family Compositae and has been used in folk medicine for the treatment of rheumatoid arthritis and enteritis.9 It has been reported that zaluzanin C, a sesquiterpene lactone mainly isolated from family Compositae, exhibits anti-fungal, anti-inflammatory and anti-cancer activities, and osteoblastic differentiation potential.10–17 However, its effect on differentiation of 3T3-L1 preadipocytes remains uncertain. Therefore, this study aimed to investigate whether zaluzanin C affects the differentiation of 3T3-L1 preadipocytes into mature adipocytes.
METHODS
Reagents
For cell culture, bovine calf serum (BCS), fetal bovine serum (FBS), penicillin, streptomycin, Dulbecco’s modified Eagle’s medium (DMEM) and 0.5% Trypsin-ethylenediaminetetraacetic acid (EDTA) were purchased from Hyclone (Logan, UT, USA). Methylisobutylxanthine (IBMX), dexamethasone, insulin, and Oil red O solution were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium pyruvate was purchased from Gibco Invitrogen (Carlsbad, CA, USA). Formaldehyde solution was obtained from Junsei chemical (Tokyo, Japan). For protein estimation, Bio-Rad Protein Assay kit was purchased from Bio-Rad Laboratories (Hercules, CA, USA). Anti-aP2/fatty acid binding protein 4 (FABP4) rabbit polyclonal (#2120) and anti-CCAAT/enhancer binding protein alpha (C/EBPα; D56F10, #8178) antibodies were procured from Cell Signaling Technology (Danvers, MA, USA), and anti-peroxisome proliferator-activated receptor gamma (PPARγ; E-8, #sc-7273) and anti-β-actin mouse monoclonal antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and Novus Biologicals (Littleton, CO, USA), respectively.
Isolation of zaluzanin C
Zaluzanin C was extracted and isolated from Ainsliaea acerifolia using a method developed by Lee et al.18 A. acerifolia was purchased from Yeongyang-gun, Gyeongsangbuk-do, Korea in May 2013. A. acerifolia (5.0 kg) was extracted with 70% ethanol (EtOH) at room temperature and the solvent was evaporated in vacuo. The crude EtOH extract was suspended in distilled water (DW), and then partitioned in turn with n-hexane, ethyl acetate (EtOAc) and n-butyl alcohol (n-BuOH) to yield after concentration dried n-hexane (73.8 g), EtOAc (56.0 g), n-BuOH (27.9 g) and H2O-soluble (186.0 g) residues. A portion (20.0 g) of the EtOAC-soluble fraction was chromatographed over a Toyopearl HW 40 (coarse grade; 1.5 cm×51 cm) with H2O containing increased amounts of methanol (MeOH) in a stepwise gradient mode. The H2O eluate was subjected to a combination of chromatography over YMC gel ODS AQ 120S column (1.1 cm×40 cm) with aqueous MeOH to yield pure zaluzanin C (188.4 mg).
Cell line and culture
3T3-L1 mouse embryonic fibroblast cell line is derived from 3T3 cells and is widely used in biological research on adipose tissue.19 3T3-L1 cell line was obtained from the Korean Cell Bank (Seoul, Korea), and was grown in DMEM supplemented with 10% BCS, 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were maintained at 37°C in a humidified incubator with 5% CO2.
Cell differentiation
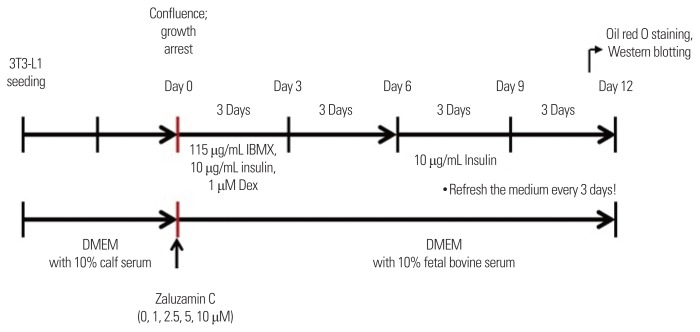
For the induction of adipocyte differentiation, 3T3-L1 preadipocytes were seeded at a density of 5×104 cells/mL/well in a 24-well plate. As shown in Fig. 1, at confluence (day 0), the cultured preadipocytes were induced to differentiate by the addition of differentiating medium (DMEM containing 115 μg/mL IBMX, 10 μg/mL insulin, and 1 μM dexamethasone) from day 0 to day 6. At day 6, medium was changed with medium containing 10 μg/mL insulin for an additional 6 days from day 6 to day 12. The medium was refreshed every 3 days. At day 12, differentiated 3T3-L1 cells were subjected to Oil red O solution or used for Western blot analysis. Zaluzanin C was dissolved in dimethyl sulfoxide (DMSO) and was added to the cell culture medium at concentrations of 0, 1, 2.5, 5, and 10 μM from day 0 to day 12 (Fig. 1). Light microscopy and Oil red O staining were used to monitor the characteristic cell rounding and lipid accumulation during the cell differentiation, respectively.
Figure 1.
Experimental scheme. For the induction of adipocyte differentiation, 3T3-L1 preadipocytes were seeded. At confluence (day 0), the cultured preadipocytes were induced to differentiate by the addition of differentiating medium containing 115 μg/mL methylisobutylxanthine (IBMX), 10 μg/mL insulin, and 1 μM dexamethasone (Dex) from day 0 to day 6. At day 6, medium was changed with medium containing 10 μg/mL insulin for an additional 6 days from day 6 to day 12. The medium was refreshed every 3 days. At day 12, differentiated 3T3-L1 cells were subjected to Oil red O solution or used for Western blot analysis. Zaluzanin C was added to the cell culture medium at concentrations of 0, 1, 2.5, 5, and 10 μM from day 0 to day 12. DMEM, Dulbecco’s modified Eagle’s medium.
Oil red O staining
After cell differentiation, cells were fixed with 1 mL of 10% formaldehyde solution for 1 hour. Fixed cells were washed with 2 mL of 60% isopropyl alcohol and were air dried. One milliliter of Oil red O solution was added to each well and the each well was incubated for 10 minutes. After the removal of Oil red O solution, cells were washed four times with DW and imaged. Accumulated intracellular Oil red O dye was completely extracted by 1 mL of 100% isopropyl alcohol and quantified by measuring its absorbance at 490 nm (Sunrise Tecan, Grödig, Austria).
Cell proliferation and cytotoxicity assay
The cytotoxicity of zaluzanin C and its effect on cell proliferation was determined using CytoTox96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA) and CellTiter96 Aqueous One Solution Assay of cell proliferation (Promega), respectively. 3T3-L1 preadipocytes were seeded at a density of 1×104 cells/200 μL/well in a 96 well plate, and zaluzanin C was added to each well at a concentration range of 0–10 μM in DMEM containing 1% FBS. After 48-hour incubation, cytotoxicity and cell proliferation were measured according to the manufacturer’s instructions. In 0 μM zaluzanin C group, cells were treated with an equal volume of DMSO, maintained at maximum concentration of not more than 0.001% v/v.
Preparation of whole cell lysates
To prepare whole cell lysate, cells were lysed in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 50 mM sodium fluoride (NaF), 30 mM sodium pyrophosphate (Na4P2O7), 1 mM phenylmethanesulfonyl fluoride, 2 mg/mL aprotinin, and 1 mM pervanadate. The lysates were clarified by centrifugation at 12,000×g for 15 minutes at 4°C. The lysates were stored at −80°C until needed.
Western blotting analysis
Proteins in whole cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to Immobilon transfer membranes with pore size of 0.45 μm (Millipore, Billerica, MA, USA). After blocking with 1×blocking buffer (Biofact Biofactory, Daejeon, Korea) for 1 hour, membranes were incubated with the primary antibody at 4°C overnight. Membranes were then washed with Tris buffered saline-Tween 20 solution and were incubated with anti-rabbit or anti-mouse immunoglobulin G conjugated to horseradish peroxidase for 1 hour. After washing, the protein bands were then visualized using enhanced chemiluminescence reagent (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA) and LAS-3000 Lumino Image Analyzer System (Fujifilm, Tokyo, Japan). Densitometric measurements of band intensities were performed using Image J free software (NIH, Bethesda, MA, USA).
Statistical analysis
GraphPad Prism version 5.0 software (GraphPad, San Diego, CA, USA) was used for statistical analysis. The data were expressed as the mean±standard error of the mean of three independent experiments. Differences between the mean values of multiple groups were analyzed using one-way analysis of variance with Dunnett test or Tukey’s test. A P<0.05 was considered as statistically significant.
RESULTS
As part of our ongoing screening program, antiadipogenic potentials of 11 types of bioactive compounds were evaluated.20 According to Kwak’s study20, 10 μM zaluzanin C inhibited lipid accumulation, a characteristic of differentiated adipocytes21,22, in 3T3-L1 adipocytes. Based on this result, antiadipogenic potential of zaluzanin C and its underlying mechanism was evaluated in the subsequent experiments.
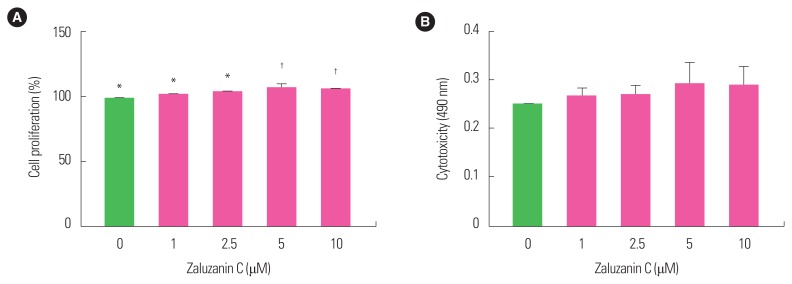
As 10 μM zaluzanin C was shown to inhibit lipid accumulation in 3T3-L1 adipocytes, its effect was examined on cell proliferation and cytotoxicity in 3T3-L1 preadipocytes. Since zaluzanin C did not inhibit cell proliferation and showed no cytotoxicity up to 10 μM concentration (Fig. 2), it was indicated that its inhibitory effect against lipid accumulation was not related to its cytotoxicity or anti-proliferative effect. Therefore, zaluzanin C was used at a concentration range of 0–10 μM for the subsequent experiments.
Figure 2.
Effect of zaluzanin C on cell proliferation and cytotoxicity in 3T3-L1 preadipocyte. 3T3-L1 cells were seeded in a 96 well plate and treated with various concentrations of zaluzanin C (0, 1, 2.5, 5, and 10 μM). After 48 hours, cell proliferation (A) and cytotoxicity (B) were determined using CytoTox 96 non-radioactive cytotoxicity assay and CellTiter 96 Aqueous One Solution assay. The results are presented as mean±standard error of the mean of three independent experiments (n=3). *,†Indicate statistically significant differences between groups.
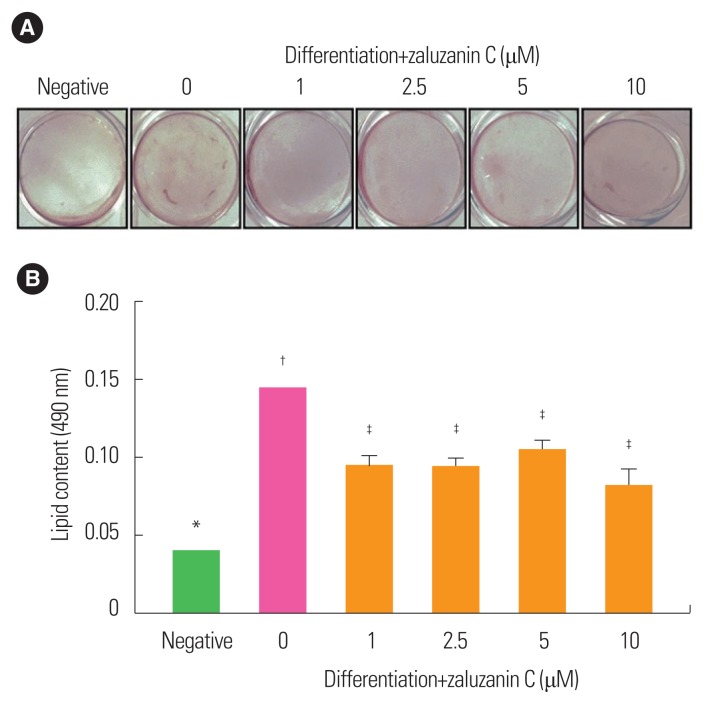
To evaluate the effect of zaluzanin C on lipid accumulation during adipogenesis of 3T3-L1 preadipocytes, intracellular lipid content was measured at day 12 using Oil red O staining. The absorbance value of the negative control was 0.04 and that of 0 μM zaluzanin C was 0.14 (Fig. 3). Differentiation medium induced an increase in absorbance up to 350% in cells treated with 0 μM zaluzanin C compared to the negative control group (Fig. 3).
Figure 3.
Effect of zaluzanin C on lipid accumulation in 3T3-L1 cells. 3T3-L1 cells were treated with differentiation medium in the absence or presence of different concentrations of zaluzanin C (0, 1, 2.5, 5, and 10 μM) for 12 days. (A) On day 12, 3T3-L1 cells were stained with Oil-red O solution and took a photograph. (B) Lipid accumulation was then assessed by extracting Oil-red O from stained 3T3-L1 cells. The results are presented as mean±standard error of the mean of three independent experiments (n=3). *,†,‡Indicate statistically significant differences between groups.
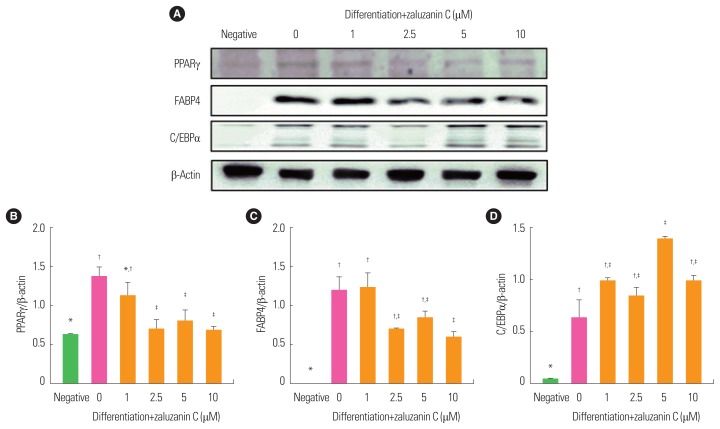
An understanding of the process of adipose formation and the mechanisms that govern adipogenesis is vital in the fight against the growing incidence of obesity.23 Therefore, attenuation of the expression of adipogenic related transcription factors, such as PPARγ and C/EBPα by zaluzanin C was evaluated using Western blot analysis. At day 12, PPARγ expression was significantly increased in 0 μM zaluzanin group compared to the negative control group (Fig. 4). Zaluzanin C effectively inhibited the differentiation medium-induced increase of PPARγ expression in 3T3-L1 adipocytes at concentrations greater than 1 μM. Then, expression of FABP4 protein was determined by Western blot analysis. Similarly, expression of FABP4 was decreased by treatment with zaluzanin C, significantly at 10 μM concentration compared to 0 μM zaluzanin C group. Also C/EBPα protein expression was significantly induced in 0 μM zaluzanin C group compared to negative control cells. However, zaluzanin C did not inhibit expression of C/EBPα.
Figure 4.
Effect of zaluzanin C on the expression of peroxisome proliferator-activated receptor gamma (PPARγ), fatty acid binding protein 4 (FABP4) and CCAAT/enhancer binding protein alpha (C/EBPα) in 3T3-L1 cells. 3T3-L1 cells were treated with differentiation medium in the absence or presence of different concentrations of zaluzanin C (0, 1, 2.5, 5, 10 μM) for 12 days. From each whole cell lysate, 10 μg of proteins was resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis for determination of FABP4, PPARγ and C/EBPα expression. β-actin expression is shown as a loading control. The bands (A) were quantified using image analysis software, and their relative intensities of PPARγ (B), FABP4 (C), or C/EBPα (D) were expressed as target protein/β-actin. The results are presented as mean±standard error of the mean of three independent experiments (n=3). *,†,‡Indicate statistically significant differences between groups.
DISCUSSION
Recently, many studies have been conducted in order to find natural bioactive agents which have antiadipogenic effect in 3T3-L1 preadipocytes.6,7,23,24 Immortalized murine cell lines such as 3T3-L1 preadipocytes are capable of differentiating into mature white adipocytes under appropriate hormonal balance, in presence of FBS, dexamethasone, IBMX, and insulin.22,25 Since 3T3-L1 preadipocyte cell line as an in vitro model of adipogenesis has been proven to be an invaluable resource in elucidating mechanisms of adipocyte differentiation25, these cells were used to investigate the effects of zaluzanin C on adipogenesis. From results of Fig. 3, they indicate that 3T3-L1 preadipocytes differentiate well into mature adipocytes by treatment with differentiation medium. However, treatment with ≥1 μM zaluzanin C inhibited lipid accumulation in 3T3-L1 adipocytes. These results suggest that zaluzanin C has an antiadipogenic effect.
Adipogenesis is the process by which precursor stem cell differentiate into lipid laden adipocytes.21 This process is regulated by transcriptional activators such as PPARγ and C/EBPα.25,26 These transcription factors were known to regulate the middle and late stages of adipocyte differentiation.27 Also, FABP4 is a differentiated adipocyte marker gene that is transcriptionally regulated by PPARγ.28,29 Although C/EBPα is an important factor in terminal differentiation of adipocytes, knockout of C/EBPα in adipocytes did not show insulin sensitivity.30,31 It means that C/EBPα is an essential factor in which adipocyte acquires insulin sensitivity. As obesity and insulin resistance are strongly linked to the accumulation of excessive lipids32, maintenance of C/EBPα by treatment of zaluzanin C has significant meaning in improving insulin resistance that occur by excess lipid accumulation. PPARγ is capable of promoting adipogenesis in C/EBPα-deficient cells, whereas C/EBPα is incapable of promoting adipogenesis in PPARγ-deficient cells. These findings demonstrate that PPARγ is a more important master regulator of adipogenesis than C/EBPα.30
This is the first study to demonstrate that zaluzanin C inhibits differentiation of preadipocytes to mature adipocytes. These data suggest that zaluzanin C particularly affects PPARγ, a key transcription factor for adipogenesis, and does not inhibit the process which acquires insulin sensitivity. Although this study does not reveal how zaluzanin C selectively regulate expression of PPARγ and C/EBPα, previous studies have demonstrated that zaluzanin C exhibited the most potent inhibitory effect in advanced glycation end products, which is valuable therapeutic targets for the regulation of diabetic complications.33 Also, zaluzanin C inhibited α-glucosidase activity which is one of the targets for treatment of diabetes.18 As C/EBPα is an essential factor in which adipocytes acquire insulin sensitivity, maintenance of C/EBPα by treatment of zaluzanin C could positively affect improvement of obesity related diabetes. However, further study is needed to evaluate molecular mechanisms on how zaluzanin C increase expression of C/EBPα. In conclusion, zaluzanin C may be a potential source for the treatment of obesity.
ACKNOWLEDGMENTS
This work was supported and funded by Daegu University (No. 20160438).
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention. Korea Health Statistics 2016: Korea National Health and Nutrition Examination Survey (KNHANES VII-1) Cheongju: Korea Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 2.Murphy N, Jenab M, Gunter MJ. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. 2018;15:659–70. doi: 10.1038/s41575-018-0038-1. [DOI] [PubMed] [Google Scholar]
- 3.Piché ME, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61:103–13. doi: 10.1016/j.pcad.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Reho JJ, Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci (Lond) 2017;131:1689–700. doi: 10.1042/CS20170219. [DOI] [PubMed] [Google Scholar]
- 5.Chukir T, Shukla AP, Saunders KH, Aronne LJ. Pharmacotherapy for obesity in individuals with type 2 diabetes. Expert Opin Pharmacother. 2018;19:223–31. doi: 10.1080/14656566.2018.1428558. [DOI] [PubMed] [Google Scholar]
- 6.Tung YC, Hsieh PH, Pan MH, Ho CT. Cellular models for the evaluation of the antiobesity effect of selected phytochemicals from food and herbs. J Food Drug Anal. 2017;25:100–10. doi: 10.1016/j.jfda.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalilpourfarshbafi M, Gholami K, Murugan DD, Abdul Sattar MZ, Abdullah NA. Differential effects of dietary flavonoids on adipogenesis. Eur J Nutr. 2019;58:5–25. doi: 10.1007/s00394-018-1663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romacho T, Elsen M, Röhrborn D, Eckel J. Adipose tissue and its role in organ crosstalk. Acta Physiol (Oxf) 2014;210:733–53. doi: 10.1111/apha.12246. [DOI] [PubMed] [Google Scholar]
- 9.Jung CM, Kwon HC, Choi SZ, Lee JH, Lee DJ, Ryu SN, et al. Phytochemical constituents of Ainsliaea acerifolia. Korean J Pharmacogn. 2000;31:125–9. [Google Scholar]
- 10.Wedge DE, Galindo JC, Macías FA. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry. 2000;53:747–57. doi: 10.1016/S0031-9422(00)00008-X. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda H, Kagerura T, Toguchida I, Ueda H, Morikawa T, Yoshikawa M. Inhibitory effects of sesquiterpenes from bay leaf on nitric oxide production in lipopolysaccharide-activated macrophages: structure requirement and role of heat shock protein induction. Life Sci. 2000;66:2151–7. doi: 10.1016/S0024-3205(00)00542-7. [DOI] [PubMed] [Google Scholar]
- 12.Shin SG, Kang JK, Lee KR, Lee HW, Han JW, Choi WS. Suppression of inducible nitric oxide synthase and cyclooxygenase-2 expression in RAW 264.7 macrophages by sesquiterpene lactones. J Toxicol Environ Health A. 2005;68:2119–31. doi: 10.1080/15287390591009506. [DOI] [PubMed] [Google Scholar]
- 13.Kang YR, Lee HW, Kim YH. Anti-inflammatory effect of Zaluzanin C on lipopolysaccharide-stimulated murine macrophages. Korean J Food Sci Technol. 2016;48:392–7. doi: 10.9721/KJFST.2016.48.4.392. [DOI] [Google Scholar]
- 14.Lajter I, Pan SP, Nikles S, Ortmann S, Vasas A, Csupor-Löffler B, et al. Inhibition of COX-2 and NF-κB1 gene expression, NO production, 5-LOX, and COX-1 and COX-2 enzymes by extracts and constituents of Onopordum acanthium. Planta Med. 2015;81:1270–6. doi: 10.1055/s-0035-1546242. [DOI] [PubMed] [Google Scholar]
- 15.Choi SZ, Yang MC, Choi SU, Lee KR. Cytotoxic terpenes and lignans from the roots of Ainsliaea acerifolia. Arch Pharm Res. 2006;29:203–8. doi: 10.1007/BF02969394. [DOI] [PubMed] [Google Scholar]
- 16.Csupor-Löffler B, Zupkó I, Molnár J, Forgo P, Hohmann J. Bioactivity-guided isolation of antiproliferative compounds from the roots of Onopordum acanthium. Nat Prod Commun. 2014;9:337–40. [PubMed] [Google Scholar]
- 17.Kim KM, Jang WG. Zaluzanin C (ZC) induces osteoblast differentiation through regulating of osteogenic genes expressions in early stage of differentiation. Bioorg Med Chem Lett. 2017;27:4789–93. doi: 10.1016/j.bmcl.2017.09.061. [DOI] [PubMed] [Google Scholar]
- 18.Lee EW, Kim T, Kim HS, Park YM, Kim SH, Im MH, et al. Antioxidant and α-glucosidase inhibitory effects of ethanolic extract of Ainsliaea acerifolia and organic solvent-soluble fractions. Korean J Food Preserv. 2015;22:275–80. doi: 10.11002/kjfp.2015.22.2.275. [DOI] [Google Scholar]
- 19.Rizzatti V, Boschi F, Pedrotti M, Zoico E, Sbarbati A, Zamboni M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: size and optical density distribution. Eur J Histochem. 2013;57:e24. doi: 10.4081/ejh.2013.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwak SH, Kim TH, Kim YH. Inhibitory effect of natural compounds in differentiation of 3T3-L1 preadipocyte. J Ind Technol. 2018;29:51–5. [Google Scholar]
- 21.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 22.Kuri-Harcuch W, Green H. Increasing activity of enzymes on pathway of triacylglycerol synthesis during adipose conversion of 3T3 cells. J Biol Chem. 1977;252:2158–60. [PubMed] [Google Scholar]
- 23.Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 2016;17:E124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan MH, Tung YC, Yang G, Li S, Ho CT. Molecular mechanisms of the anti-obesity effect of bioactive compounds in tea and coffee. Food Funct. 2016;7:4481–91. doi: 10.1039/C6FO01168C. [DOI] [PubMed] [Google Scholar]
- 25.Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10:3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- 26.MacDougald OA, Lane MD. Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem. 1995;64:345–73. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 27.Johmura Y. Characterization of novel genes regulating adipocyte differentiation. Yakugaku Zasshi. 2007;127:135–42. doi: 10.1248/yakushi.127.135. [DOI] [PubMed] [Google Scholar]
- 28.Thompson GM, Trainor D, Biswas C, LaCerte C, Berger JP, Kelly LJ. A high-capacity assay for PPARgamma ligand regulation of endogenous aP2 expression in 3T3-L1 cells. Anal Biochem. 2004;330:21–8. doi: 10.1016/j.ab.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Kawada T, Goto T, Yamamoto T, Taimatsu A, Matsui N, et al. Dual action of isoprenols from herbal medicines on both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett. 2002;514:315–22. doi: 10.1016/S0014-5793(02)02390-6. [DOI] [PubMed] [Google Scholar]
- 30.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–6. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Jack AK, Hamm JK, Pilch PF, Farmer SR. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARgamma and C/EBPalpha. J Biol Chem. 1999;274:7946–51. doi: 10.1074/jbc.274.12.7946. [DOI] [PubMed] [Google Scholar]
- 32.Finck BN. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes. 2018;67:2485–93. doi: 10.2337/dbi18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong GH, Kim TH. Characterization of anti-advanced glycation end products (AGEs) and radical scavenging constituents from Ainsliaea acerifolia. J Korean Soc Food Sci Nutr. 2017;46:759–64. [Google Scholar]