Abstract
Despite municipal chlorination and secondary disinfection, opportunistic waterborne pathogens (e.g., Legionella spp.) persist in public and private water distribution systems. As a potential source of healthcare-acquired infections, this warrants development of novel pathogen removal and inactivation systems. In this study, electrically heatable carbon nanotube (CNT) point-of-use (POU) filters have been designed to remove and inactivate Legionella pneumophila in water. The CNT/polymer composite membranes effectively removed Legionella (> 99.99%) (i.e., below detection limit) and were able to inactive them on the membrane surface at 100% efficiency within 60 s using ohmic heating at 20 V. The novel POU filters could be used as a final barrier to provide efficient rejection of pathogens and thereby simultaneously eliminate microorganisms in public and private water supplies.
Keywords: Electrically heatable carbon nanotube, Point-of-use filters, Legionella pneumophila, Public and private water supplies
1. Introduction
While Legionella species are ubiquitous in both natural and engineered water systems, most cases of disease are linked back to manmade systems. In particular, cooling towers, potable water systems, evaporative condensers, whirlpool spas, humidifiers, air washers, and decorative fountains, are the most common sources of human infections [1–3]
Legionella infection is caused by the inhalation of water aerosols containing the bacteria. According to data reported to the Centers for Disease Control and Prevention (CDC) through the National Outbreak Reporting System, there have been over 41,000 outbreaks of Legionnaires’ disease in the United States between 1998 and 2014, with the incidence rate thought to be somewhere between 7.0 and 7.9 cases per 100,000 people [4]. Legionella can colonize water distribution lines and building plumbing, contaminating water supplies after it has been centrally treated. Legionella thrive in the temperature range of 25 to 54 °C, a range that includes hot water systems, shower heads and even cold-water systems in warm climates [5–11].
There are several technologies recognized for effective control of Legionella: 1) chlorine [12,13], 2) chlorine dioxide [14,15], 3) monochloramine [16–18], 4) CuO nanoparticles [19] and copper-silver ionization [20–23], 5) ozone [24–28], and 6) ultraviolet (UV)-assisted disinfection [28–31]. The effectiveness of each of these methods can vary. However, some of them have negative effects including formation of disinfection byproducts [32], negative aesthetic effects on water and adverse health effects [33,34], and corrosion of plumbing systems [35].
Legionella pneumophila in water distribution systems poses a potential health risk. An elevated biofilm concentration and growth of L. pneumophila on surfaces were observed with warm tap water (34–39 °C) [36,37]. Therefore, one way could be by combining biofilm removal in premise plumbing and the water systems. However, biofilm removal treatments are not totally efficient and, after a lag period, Legionella species may be able to quickly recolonize in these systems [38–40]. Another approach could be to implement barrier protection at the point-of-use (POU). Numerous studies have investigated the efficacy of POU filters installed in high-risk areas to prevent the transmission of waterborne pathogens to their immunocompromised hosts [41–43]. The POU filtration can limit exposure to pathogens; however, their short lifetime (1 or 2 weeks of continuous use) and membrane clogging (or fouling) have limited their use [44]. Therefore, it is desirable to develop new approaches of cleaning for long-term use of the POU filters.
Recently, carbon nanotube (CNT) composite membranes have been developed for inactivation of Escherichia coli K12 by ohmic heating [45]. However, the roles of surface modification of hydrophobic CNT interfaces and electrical heating conditions such as potential and temperature in separation and inactivation of microorganisms are largely unknown. Therefore, in this study, we engineered geometry and loading of CNT sheet layers on polymeric membranes to study the effects of O2 plasma treatment as a surface modification method of CNTs and ohmic heating conditions on physio-chemical properties of CNT interfaces, resulting in simultaneous separation and inactivation of pathogenic microorganism (i.e. Legionella) from water.
2. Materials and methods
2.1. Fabrication, Surface Modification, and Characterization of Electrically Heatable CNT Composites
To fabricate electrically heatable CNT interfaces on polymeric membranes, spinnable multi-wall CNT arrays that were drawn to form CNT ribbons as described in the previous study [45] directly applied layer-by-layer to the surface of hydrophilic polycarbonate (PC) membrane (nominal pore size = 0.2 μm, diameter = 47 mm, Sterlitech, U.S.A) or hydrophilic polyvinylidene fluoride (PVDF) membrane (nominal pore size = 0.2 μm, diameter = 47 mm, Sterlitech, U.S.A). As shown in Figure S1 (Supporting Information), five layers of CNT ribbon were applied in total and each layer was oriented perpendicular to the layer preceding it. This resulted in a resistive heater with 3 layers connecting the copper electrodes to complete the circuit, and 2 layers aligned parallel to the electrode providing support for the formed CNT sheet, creating a 90° cross-hatch surface pattern. As a final step of the fabrication process, the entire composite was wetted with ethanol in order to increase the adsorption of CNT sheet to the polymeric membranes and improve densification, which occurred after solvent evaporation [45].
Oxygen plasma treatment was successfully applied for functionalization of CNTs [46,47]. In this study, the fabricated CNT composite membranes were hydrophilically treated using atmospheric pressure plasma (Atomflo 400D, Surfx) containing O2 (active gas, 0.25 – 0.75 L/min) and helium (carrier gas, 30 L/min). Finally, two copper tapes (5mm×25 mm) were attached onto the CNT top layer and served as electrodes for ohmic heating (Fig. S1, Supporting Information).
The electrically heatable CNT sheet interfaces were characterized using Raman microscopy (inVia™, Renishaw), X-ray photoelectron spectroscopy (XPS) with Mg K-alpha X-rays at an accelerating voltage of 15.0 kV (PHI 5300, Physical Electronics), Fourier transform infrared spectroscopy (FTIR) (Excalibur FTS 3000, Bio-Rad), and a contact angle analyzer (DSA25E, KRÜSS).
2.2. Preparation of Legionella suspension
L. pneumophila subsp. pneumophila (ATCC 33152) was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Legionella was propagated in Becton Dickinson’s buffered yeast extract (BYE) broth, which is ACES [N-(2-acetamido)-2-aminoethanesulfonic acid (Sigma, St. Louis, Mo.)]-buffered yeast extract broth supplemented with 0.4 g of l-cysteine and 0.135 g of ferric nitrate per Liter (Becton Dickinson and Co.). Cultures were grown at 37°C under shaker conditions (150 rpm). Culture density was measured periodically using Klett Semerson photometer till a Klett Reading of 30 that indicates the log phase of bacterial growth. The cell concentration in the culture was quantitated as colony forming units/ml (CFU/mL) using agar plating procedure based on Buffered charcoal yeast extract (BCYE) agar. To prepare simulated feed water samples containing a well dispersed suspension of Legionella pneumophila, the culture with known CFU/mL was diluted to a desired level. For example, Legionella culture at 30 klett reading was quantitated by 10-fold serial dilutions and plating in triplicate up to the 6th dilution (e.g., undiluted, 10−1 through 10−6); the cell count was determined to be 1.54 × 107 CFU/mL. For obtaining the final Legionella cell suspension with approximately 20,000 CFU/mL, ~65 μL aliquot of the broth culture was diluted to 50 mL and the downstream experiments were performed using the resulting suspension.
2.3. Separation of Legionella using Electrically Heatable Carbon Nanotube Composites
Two mL aliquot of the final Legionella cell suspension (approximately 20,000 CFU/mL) was filtered through a virgin PC membrane, a virgin PVDF membrane, a CNT sheet/PC composite membrane, or a CNT sheet/PVDF composite membrane using a vacuum filtration holder and manifold (Cole-Parmer) at 0.2 bar vacuum pressure. All experiments were performed in triplicate. The student’s t-test was used to assess the significance of results (employing a 95% confidence interval). Water permeability of the CNT composites was calculated based on Darcy’s law as follows [48]:
| (1) |
where Jv is the permeate flux (L/m2/hr), V is the total volume of permeate (L), A is the effective membrane surface area (m2), and t is filtration time (hr).
Removal efficiency of Legionella by polymeric membranes and the CNT composites (i.e., CNT sheet/PC and CNT sheet/PVDF membranes) calculated using the following equation:
| (2) |
where Np is the number of Legionella cells (CFU per mL) in the membrane permeate and Nf is the number of Legionella cells (CFU per mL) in the feed.
2.4. Electrical Heating of Carbon Nanotube Sheet Composites for Inactivation of Legionella
After filtration of Legionella cell suspension through the CNT/PC or the CNT/PVDF composite membrane (active heating area = 25 mm x 20 mm), the CNT sheet was electrically heated using ohmic heating via a bench top direct current (DC) power supply (E3612A, Hewlett-Packard) at various electric potentials (i.e., 10 V, 15 V, or 20 V) for one-minute (Figure S2, Supporting Information). The surface temperatures were monitored with an infrared (IR) camera (T640, FLIR). FLIR Tools+ software was used to record, plot, and prepare images and reports from the resulting thermal data.
2.5. Staining and Counting of Live/Dead Legionella on the CNT POU filters
To determine the effects of ohmic heating on Legionella viability, the CNT/PC or the CNT/PVDF composite were stained using the Live/Dead BacLight Bacterial Viability Kit (ThermoFisher Scientific, U.S.A) as described in the previous study [45]. In Brief, equal aliquots of SYTO 9 and Propidium iodide were applied onto the membrane surface to stain the cells directly. The membranes were incubated in the dark at room temperature for 15 min and then filtered under vacuum to remove the stain solution. The composite membranes were mounted on a pre-cleaned microscope slide (1.0 mm thick, 1” x 3”, FisherScientific, U.S.A) and a coverslip placed over the BacLight mounting oil drop to perform microscopy. The viability of Legionella was examined within one hour using a fluorescence microscope (Olympus IX51 inverted microscopy system) with green and red filters. To count numbers of live (with green filter) or dead (with red filter) Legionella, image analysis of fluorescence microscopy pictures obtained at 10 different places per sample was performed by using an Image-Pro version 4.5 (Media Cybernetics, Inc. Bethesda, MD). Inactivation efficiency of Legionella by ohmic heating at various electric potentials was calculated using the following equation (3):
| (3) |
where NG is the number of separated Legionella under fluorescent light with green filter and NR is the number of separated Legionella under fluorescent light with red filter before and after ohmic heating.
3. Results
3.1. Characteristics of electrically heatable CNT POU filters
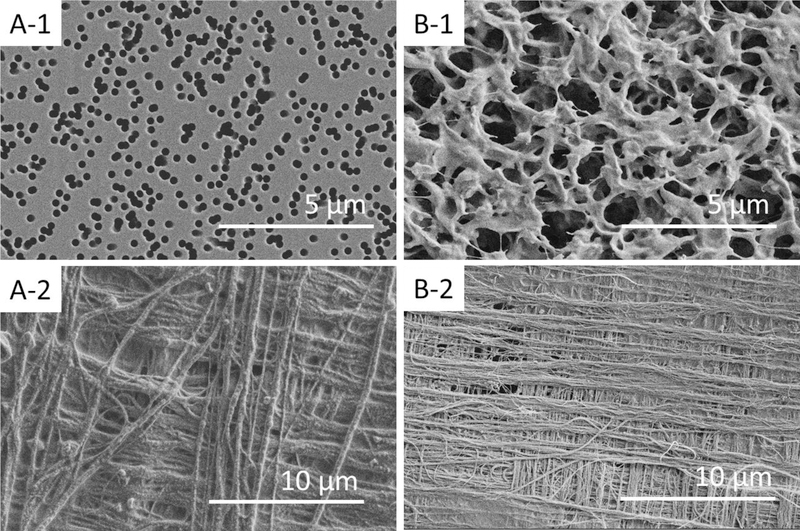
SEM images show that clear pore openings of virgin PC (A-1, Fig. 1) and virgin PVDF (B-1, Fig. 1) membranes, and CNT layers, creating a 90° cross-hatch surface patterns formed on the PC (A-2, Fig. 1) and the PVDF (B-2, Fig. 1) membranes. Cross-sectional images confirmed that thickness of the five-layers CNT on PC or PVDF membranes is approximately 0.5 μm (Fig. S3, Supporting Information).
Fig. 1.
SEM images of a virgin PC membrane (A-1), a CNT/PC composite membrane (A-2), a virgin PVDF membrane (B-1), and a CNT/PVDF composite membrane (B-2).
From the Raman spectra, it was found that intensity of the D-band or the disorder band peak in the spectra relative to the G-band or the graphitic band of the CNTs increased with increasing plasma power (between 100 and 180 W) and exposure time (between 10 and 30 s) reveals that the plasma creates defects in the sidewall of CNTs (Fig. S4, Supporting Information).
XPS analysis of the plasma treated CNT samples shows that plasma treatment has created -C-OH, –C=O, and O=C-OH functionalities on the carbon nanotubes (Fig. S5, Supporting Information). This conclusion is supported by data published by other researchers who have functionalized CNT sheet with plasma [49]. The π-π* shake-up satellite (290.67 eV) is not a functional group, but it is instead evidence of conjugation existing within the pristine CNT sheet.
From the FTIR analysis, it can be seen that there are two distinct features or peaks that are observed for the plasma treated samples in the 1714 cm−1 and 1610–1650 cm−1 regions (Fig. S6, Supporting Information); these peaks can be attributed to the C=O stretching and –OH stretching when it is bonded to a carbonyl group respectively. The stretching around the 1546 cm−1 is common for both functionalized and un-functionalized samples and can be attributed to C=C stretching of the CNT structure. A similar spectrum is also reported by Wang et al. [50] in their study of oxidative treatment of multi-wall CNTs with dielectric barrier discharge plasma. The measured contact angle for the CNT sheet decreased from average 79.6° for the pristine sheet to 14.5° for the plasma treated samples (Fig. S7, Supporting Information), showing the change from hydrophobic to hydrophilic nature of the CNT sheet. This change can be explained on the premise that water droplet is now associated with the CNT sheet through H-bonding with –OH and –COOH groups on the nanotube surface which are generated via the O2 plasma treatment.
3.2. Separation of Legionella using electrically heatable CNT POU filters
Legionella cell suspension (approximately 20,000 CFU/mL) was filtered through four different composite membranes (i.e., virgin PC and PVDF membranes, PC and PVDF membranes with CNT interfaces). Water permeability of virgin PC and CNT sheet/PC membranes was in range of 1502 – 1600 LMH/bar and that of virgin PVDF and CNT sheet/PVDF membranes was in 8166 – 8235 LMH/bar. There was no significant change in water permeability by adding layers of CNT ribbon on polymeric membranes. Regardless of the presence of CNT layers on either PC or PVDF membranes, Legionella was completely removed > 99.99% (i.e., Legionella was not detected in the membrane permeate) (Table S1, Supporting Information).
3.3. Inactivation of Legionella using electrically heatable CNT POU filters
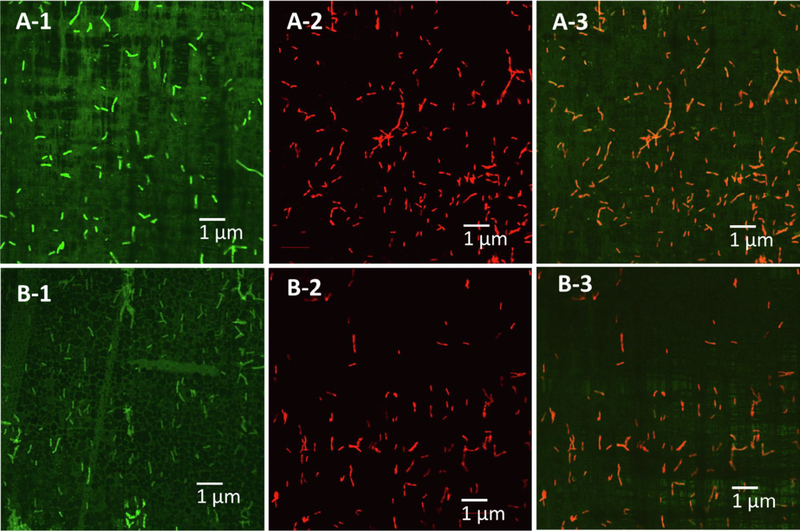
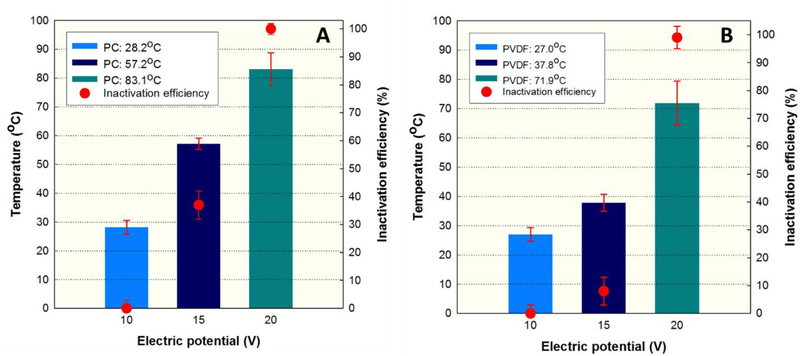
Surface temperature of the CNT/PC and the CNT/PVDF membranes rapidly increased from 28.2 to 83.1 °C and from 27.0 to 71.9 °C as DC electric potential increased from 10 to 20 V within one minute, respectively (Fig. S8, Supporting Information). Consequently, the ohmic heating of the membranes over 70 °C was able to inactive Legionella on the membrane surfaces at 100% inactivation efficiency (Figs. 2 and 3).
Fig. 2.
Live Legionella (green fluorescence filter) on the electrically heatable CNT POU filters before ohmic heating (A-1 and B-1), dead Legionella (red fluorescence filter) on the composite membranes after ohmic heating at 20 V for 60 s (A-2 and B-2), and live/dead Legionella after the ohmic heating (Orange is combination of green and red fluorescence filters, which indicates inactivation of Legionella after the ohmic heating) on the CNT/PC (A-1, 2, and 3) and the CNT/PVDF (B-1, 2, and 3) membranes.
Fig. 3.
Changes in surface temperature and Dead/Live ratio of Legionella on the CNT/PC (A) and the CNT/PVDF (B) membranes by ohmic heating at various potentials for one minute (Current=0.025~0.05 A).
4. Discussion
The developed CNT interfaces pose a self-cleaning option at low energy demand (i.e., 20 V×0.05 A=1 W), which may mitigate membrane fouling and prolong the lifetime of polymeric membranes by reducing chemical cleaning frequency. Techno-economic assessment of the CNT/polymer membranes focusing on repeatability of ohmic heating, physical and chemical stability of the composite membranes, and recovery from membrane biofouling during long-term operation might be the most important step toward scale-up and further applications.
Waterborne pathogens belonging to bacteria, viruses, fungi, and parasites encountered in different water sources including contaminated drinking water have been implicated in several water borne infections [51]. Among the bacterial pathogens, Salmonella typhi, Vibrio cholerae, Legionella spp., Escherichia coli O157:H7, and Campylobacter jejuni have been widely reported in waterborne outbreaks despite improved sanitation techniques. This calls for more advanced and minimally invasive removal and inactivation techniques for these and other waterborne agents of public health significance. In this direction, our electrically heatable CNT POU filter device offers a potential technique to completely separate and annihilate the waterborne pathogens. Though the current study is proof of concept to demonstrate usability based on the waterborne bacterial pathogen Legionella, this technique can be extended to other pathogens of>0.2-μm cell size (which includes almost all bacterial pathogens as well as yeast/fungal and parasitic pathogens).
5. Conclusions
In this study, novel electrically heatable CNT POU filters were developed on commercial PC and PVDF membranes. As a result, it was found that the CNT/polymeric composite membranes were able to effectively remove L. pneumophila from water, and consequently inactivate them at low energy demand. Most cases of legionellosis are the result of exposure to Legionella associated with building water systems (in particular, cooling tower and hospital water systems). The composite membranes have a great potential as a final barrier of Legionella in public and private water systems, reducing environmental and public health concerns.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Interdisciplinary Research Program funding (University Research Council, University of Cincinnati) and the Ohio Water Resources Center funding (Project number: 2017OH518B). This work has been subjected to the U.S. Environmental Protection Agency’s administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the agency; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI:
REFERENCES
- [1].van Heijnsbergen E, Schalk JAC, Euser SM, Brandsema PS, den Boer JW, Husman AMD, Confirmed and Potential Sources of Legionella Reviewed, Environ. Sci. Technol 49 (2015) 4797–4815. [DOI] [PubMed] [Google Scholar]
- [2].Buse HY, Schoen ME, Ashbolt NJ, Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure, Water Res. 46 (2012) 921–933. [DOI] [PubMed] [Google Scholar]
- [3].Sikora A, Wojtowicz-Bobin M, Koziol-Montewka M, Magrys A, Gladysz I, Prevalence of Legionella pneumophila in water distribution systems in hospitals and public buildings of the Lublin region of eastern Poland, Ann. Agr. Env. Med 22 (2015) 195–201. [DOI] [PubMed] [Google Scholar]
- [4].CDC, Legionella (Legionnaires’ Disease and Pontiac Fever), Centers for Disease Control and Prevention, https://www.cdc.gov/legionella/index.html (2017).
- [5].Dilger T, Melzl H, Gessner A, Legionella contamination in warm water systems: A species-level survey, Int. J. Hyg. Environ. Health 221 (2018) 199–210. [DOI] [PubMed] [Google Scholar]
- [6].Caicedo C, Rosenwinkel KH, Nogueira R, Temperature-driven growth of Legionella in lab-scale activated sludge systems and interaction with protozoa, Int. J. Hyg. Environ. Health 221 (2018) 315–322. [DOI] [PubMed] [Google Scholar]
- [7].Rogers J, Dowsett AB, Dennis PJ, Lee JV, Keevil CW, Influence of Temperature and Plumbing Material Selection on Biofilm Formation and Growth of Legionella-Pneumophila in a Model Potable Water-System Containing Complex Microbial-Flora, Appl. Environ. Microbiol 60 (1994) 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mathys W, Stanke J, Harmuth M, Junge-Mathys E, Occurrence of Legionella in hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating, Int. J. Hyg. Environ. Health 211 (2008) 179–185. [DOI] [PubMed] [Google Scholar]
- [9].Proctor CR, Dai D, Edwards MA, Pruden A, Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems, Microbiome 5 (2017) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].van der Kooij D, Brouwer-Hanzens AJ, Veenendaal HR, Wullings BA, Multiplication of Legionella pneumophila Sequence Types 1, 47, and 62 in Buffered Yeast Extract Broth and Biofilms Exposed to Flowing Tap Water at Temperatures of 38 degrees C to 42 degrees C, Appl. Environ. Microbiol 82 (2016) 6691–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Buse HY, Ashbolt NJ, Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing, Lett. Appl. Microbiol 53 (2011) 217–224. [DOI] [PubMed] [Google Scholar]
- [12].Cervero-Arago S, Rodriguez-Martinez S, Puertas-Bennasar A, Araujo RM, Effect of Common Drinking Water Disinfectants, Chlorine and Heat, on Free Legionella and Amoebae-Associated Legionella, PLoS ONE 10 (2015) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sidari FP, Stout JE, Duda S, Grubb D, Neuner A, Maintaining Legionella control in building water systems, J. Am. Water Work Assoc 106 (2014) 24–32. [Google Scholar]
- [14].Al-Bloushi M, Saththasivam J, Al-Sayeghc S, Jeong S, Ng KC, Amy GL, Leiknes T, Performance assessment of oxidants as a biocide for biofouling control in industrial seawater cooling towers, J. Ind. Eng. Chem 59 (2018) 127–133. [Google Scholar]
- [15].Mustapha P, Epalle T, Allegra S, Girardot F, Garraud O, Riffard S, Monitoring of Legionella pneumophila viability after chlorine dioxide treatment using flow cytometry, Res. Microbiol 166 (2015) 215–219. [DOI] [PubMed] [Google Scholar]
- [16].Marchesi I, Ferranti G, Mansi A, Marcelloni AM, Proietto AR, Saini N, Borella P, Bargellini A, Control of Legionella Contamination and Risk of Corrosion in Hospital Water Networks following Various Disinfection Procedures, Appl. Environ. Microbiol 82 (2016) 2959–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Baron JL, Harris JK, Holinger EP, Duda S, Stevens MJ, Robertson CE, Ross KA, Pace NR, Stout JE, Effect of monochloramine treatment on the microbial ecology of Legionella and associated bacterial populations in a hospital hot water system, Syst. Appl. Microbiol 38 (2015) 198–205. [DOI] [PubMed] [Google Scholar]
- [18].Vatansever C, Turetgen I, Survival of Biofilm-Associated Legionella pneumophila Exposed to Various Stressors, Water Environ. Res 87 (2015) 227–232. [DOI] [PubMed] [Google Scholar]
- [19].Lu J, Struewing I, Buse HY, Kou J, Shuman HA, Faucher SP, Ashbolt NJ, Legionella Pneumophila Transcriptional Response following Exposure to CuO Nanoparticles, Appl. Environ. Microbiol 79 (2013) 2713–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barbosa VL, Thompson KC, Controlling Legionella in a UK hospital using copper and silver ionisation-A case study, J. Environ. Chem. Eng 4 (2016) 3330–3337. [Google Scholar]
- [21].Springston JP, Yocavitch L, Existence and control of Legionella bacteria in building water systems: a review, J. Occup. Environ. Hyg 14 (2017) 124–134. [DOI] [PubMed] [Google Scholar]
- [22].Lytle DA, Wahman DG, Schock MR, Nadagouda MN, Harmon S, Webster K, Botkins J, Georgeite: a rare copper mineral with important drinking water implications, Chem. Eng. J 355 (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hwang MG, Katayama H, Ohgaki S, Inactivation of Legionella pneumophila and Pseudomonas aeruginosa: evaluation of the bactericidal ability of silver cations, Water Res. 41 (2007) 4097–4104. [DOI] [PubMed] [Google Scholar]
- [24].Dong SK, Li J, Kim MH, Cho J, Park SJ, Nguyen TH, Eden JG, Deactivation of Legionella Pneumophila in municipal wastewater by ozone generated in arrays of microchannel plasmas, J. Phys. D-Appl. Phys 51 (2018) 9. [Google Scholar]
- [25].Li J, Li XB, Li KQ, Tao T, Plasmas ozone inactivation of Legionella in deionized water and wastewater, Environ. Sci. Pollut. Res 25 (2018) 9697–9707. [DOI] [PubMed] [Google Scholar]
- [26].Li J, Li KQ, Zhou Y, Li XB, Tao T, Kinetic analysis of Legionella inactivation using ozone in wastewater, Chemosphere 168 (2017) 630–637. [DOI] [PubMed] [Google Scholar]
- [27].Edelstein PH, Whittaker RE, Kreiling RL, Howell CL, Efficacy of ozone in eradication of Legionella pneumophila from hospital plumbing fixtures, Appl. Environ. Microbiol 44 (1982) 1330–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ochiai T, Masuko K, Tago S, Nakano R, Niitsu Y, Kobayashi G, Horio K, Nakata K, Murakami T, Hara M, Nojima Y, Kurano M, Serizawa I, Suzuki T, Ikekita M, Morito Y, Fujishima A, Development of a hybrid environmental purification unit by using of excimer VUV lamps with TiO2 coated titanium mesh filter, Chem. Eng. J 218 (2013) 327–332. [Google Scholar]
- [29].Rattanakul S, Oguma K, Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms, Water Res. 130 (2018) 31–37. [DOI] [PubMed] [Google Scholar]
- [30].Ceruero-Arago S, Sommer R, Araujo RM, Effect of UV irradiation (253.7 nm) on free Legionella and Legionella associated with its amoebae hosts, Water Res. 67 (2014) 299–309. [DOI] [PubMed] [Google Scholar]
- [31].Lin YE, Stout JE, Yu VL, Controlling Legionella in Hospital Drinking Water: An Evidence-Based Review of Disinfection Methods, Infect. Control Hosp. Epidemiol 32 (2011) 166–173. [DOI] [PubMed] [Google Scholar]
- [32].USEPA, National, Primary Drinking Water Regulations: Stage 2 Disinfectants and Disinfection Byproducts Rule, Final Rule (2006). [Google Scholar]
- [33].Hong JH, Duncan SE, Dietrich AM, Effect of copper speciation at different pH on temporal sensory attributes of copper, Food Qual. Prefer 21 (2010) 132–139. [Google Scholar]
- [34].Rohr U, Senger M, Selenka F, Turley R, Wilhelm M, Four Years of Experience with Silver-Copper Ionization for Control of Legionella in a German University Hospital Hot Water Plumbing System, Clin. Infect. Dis 29 (1999) 1507–1511. [DOI] [PubMed] [Google Scholar]
- [35].Sarver E, Dodson K, Scardina RP, Lattyak-Slabaugh R, Edwards M, Nguyen C, Copper pitting in chlorinated, high-pH potable water, J. Am. Water Works Assoc 103 (2011) 86–98. [Google Scholar]
- [36].van der Kooij D, Bakker GL, Italiaander R, Veenendaal HR, Wullings BA, Biofilm Composition and Threshold Concentration for Growth of Legionella pneumophila on Surfaces Exposed to Flowing Warm Tap Water without Disinfectant, Appl. Environ. Microbiol 83 (2017) e02737–02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van der Kooij D, Brouwer-Hanzens AJ, Veenendaal HR, Wullings BA, Multiplication of Legionella pneumophila Sequence Types 1, 47, and 62 in Buffered Yeast Extract Broth and Biofilms Exposed to Flowing Tap Water at Temperatures of 38°C to 42°C, Appl. Environ. Microbiol 82 (2016) 6691–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Berjeaud JM, Chevalier S, Schlusselhuber M, Portier E, Loiseau C, Aucher W, Lesouhaitier O, Verdon J, Legionella pneumophila: The Paradox of a Highly Sensitive Opportunistic Waterborne Pathogen Able to Persist in the Environment, Front. Microbiol 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hamilton KA, Haas CN, Critical review of mathematical approaches for quantitative microbial risk assessment (QMRA) of Legionella in engineered water systems: research gaps and a new framework, Environ. Sci.-Wat. Res. Technol 2 (2016) 599–613. [Google Scholar]
- [40].Rose LJ, Rice EW , Inactivation of bacterial biothreat agents in water, a review, J. Water Health 12 (2014) 618–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sheffer PJ, Stout JE, Wagener MM, Muder RR, Efficacy of new point-of-use water filter for preventing exposure to Legionella and waterborne bacteria, Am. J. Infect. Control 33 (2005) S20–S25. [DOI] [PubMed] [Google Scholar]
- [42].Marchesi I, Marchegiano P, Bargellini A, Cencetti S, Frezza G, Miselli M, Borella P, Effectiveness of different methods to control legionella in the water supply: ten-year experience in an Italian university hospital, J. Hosp. Infect 77 (2011) 47–51. [DOI] [PubMed] [Google Scholar]
- [43].Zhou ZY, Hu BJ, Qin L, Lin YE, Watanabe H, Zhou Q, Gao XD, Removal of waterborne pathogens from liver transplant unit water taps in prevention of healthcare-associated infections: a proposal for a cost-effective, proactive infection control strategy, Clin. Microbiol. Infect 20 (2014) 310–314. [DOI] [PubMed] [Google Scholar]
- [44].Baron JL, Peters T, Shafer R, MacMurray B, Stout JE, Field evaluation of a new point-of-use faucet filter for preventing exposure to Legionella and other waterborne pathogens in health care facilities, Am. J. Infect. Control 42 (2014) 1193–1196. [DOI] [PubMed] [Google Scholar]
- [45].Alvarez NT, Noga R, Chae S-R, Sorial GA, Ryu H, Shanov V, Heatable carbon nanotube composite membranes for sustainable recovery from biofouling, Biofouling 33 (2017) 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee J-Y, Jin J-H, Kim JH, Kim MJ, Lee CJ, Min NK, Plasma-activated carbon nanotube-based high sensitivity immunosensors for monitoring Legionella pneumophila by direct detection of maltose binding protein peptidoglycan-associated lipoprotein (MBP-PAL), Biotechnol. Bioeng 109 (2012) 1471–1478. [DOI] [PubMed] [Google Scholar]
- [47].Malik R, McConnell C, Alvarez NT, Haase M, Gbordzoe S, Shanov V, Rapid, in situ plasma functionalization of carbon nanotubes for improved CNT/epoxy composites, RSC Adv. 6 (2016) 108840–108850. [Google Scholar]
- [48].Chae SR, Ahn YT, Kang ST, Shin HS, Mitigated membrane fouling in a vertical submerged membrane bioreactor (VSMBR), J. Membr. Sci 280 (2006) 572–581. [Google Scholar]
- [49].Kolacyak D, Ihde J, Merten C, Hartwig A, Lommatzsch U, Fast functionalization of multi-walled carbon nanotubes by an atmospheric pressure plasma jet, J. Colloid Interface Sci 359 (2011). [DOI] [PubMed] [Google Scholar]
- [50].Wang W-H, Huang B-C, Wang L-S, Ye D-Q, Oxidative treatment of multi-wall carbon nanotubes with oxygen dielectric barrier discharge plasma, Surf. Coat. Technol 205 (2011). [Google Scholar]
- [51].Ramírez-Castillo FY, Loera-Muro A, Jacques M, Garneau P, Avelar-González FJ, Harel J, Guerrero-Barrera AL, Waterborne Pathogens: Detection Methods and Challenges, Pathogens 4 (2015) 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.