Abstract
Increased aggression is common after traumatic brain injuries and may persist after cognitive recovery. Maladaptive aggression and violence are associated with dysfunction in the prefrontal and temporal cortex, but such dysfunctional behaviors are typically measured by explicit scales and history. However, it is well known that answers on explicit scales on sensitive topics—such as aggressive thoughts and behaviors—may not reveal true tendencies. Here, we investigated the neural basis of implicit attitudes toward aggression in humans using a modified version of the Implicit Association Task (IAT) with a unique sample of 112 Vietnam War veterans who suffered penetrating brain injury and 33 healthy controls who also served in combat in Vietnam but had no history of brain injury. We hypothesized that dorsolateral prefrontal cortex (dlPFC) lesions, due to the crucial role of the dlPFC in response inhibition, could influence performance on the IAT. In addition, we investigated the causal contribution of specific brain areas to implicit attitudes toward violence. We found a more positive implicit attitude toward aggression among individuals with lesions to the dlPFC and inferior posterior temporal cortex (ipTC). Furthermore, executive functions were critically involved in regulating implicit attitudes toward violence and aggression. Our findings complement existing evidence on the neural basis of explicit aggression centered on the ventromedial prefrontal cortex. These findings highlight that dlPFC and ipTC play a causal role in modulating implicit attitudes about violence and are crucially involved in the pathogenesis of aggressive behavior.
SIGNIFICANCE STATEMENT Maladaptive aggression and violence can lead to interpersonal conflict and criminal behavior. Surprisingly little is known about implicit attitudes toward violence and aggression. Here, we used a range of techniques, including voxel-based lesion–symptom mapping, to examine the causal role of brain structures underpinning implicit attitudes toward aggression in a unique sample of combat veterans with traumatic brain injury. We found that damage to the dorsolateral prefrontal cortex (dlPFC) led to a more positive implicit attitude toward violence that under most normal situations would be considered inappropriate. These results suggest that treatments aimed at increasing cognitive control using cognitive behavioral therapies dependent on the intact dlPFC could treat aggressive and violent behavior.
Keywords: aggression, implicit attitudes, traumatic brain injury
Introduction
Maladaptive aggression and violence can lead to interpersonal conflict and criminal behavior, which endangers societal stability and incurs substantial financial costs (McCollister et al., 2010). In 2013, ∼3 million people in the United States were victims of violent crimes (Truman, 2010). Multiple factors contribute to aggression, including brain function, genetic predisposition, and environmental provocation (Nelson and Trainor, 2007; de Almeida et al., 2015). Studying the neural basis of aggressive behavior can inform targeted interventions to prevent and control aggression and changes in public policy.
The incidence of aggression and violence after traumatic brain injury (TBI) ranges between 35% and 90% (Tateno et al., 2003). Such aggressive behavior is generally reactive without premeditation and nonpurposeful (Silver et al., 2005). The aggression reported in TBI patients tends to be threats, yelling, throwing things, and, less frequently, physical assaults (Grafman et al., 1996). Damage to the limbic system, ventromedial prefrontal cortex (vmPFC), and temporal lobes has been associated with aggressive behavior (Silver and Yudofsky, 1994). Aggressive behaviors are most linked to dysfunction in the frontal lobes, which are responsible for executive function and complex social behavior (Anderson and Bushman, 2002; Forbes and Grafman, 2010). A meta-analysis on neuroimaging studies showed diminished functionality in the dorsolateral prefrontal cortex (dlPFC), anterior cingulate cortex, and orbitofrontal cortex (OFC), in antisocial/violent individuals (Yang and Raine, 2009). Studies in violent individuals and patients with temporal lobe epilepsy suggested that dysfunction in temporal lobe structures can also lead to increased aggression (Seidenwurm et al., 1997). Interestingly, one study reported decreased frontal gray matter in temporal lobe epilepsy patients with affective aggression (Woermann et al., 2000). Therefore, frontotemporal regions appear to coordinate in regulating aggressive impulses. Most research on aggressive behavior has relied upon explicit reports, yet a large proportion of human social cognition occurs outside of consciousness. Implicit measures such as the Implicit Association Test (IAT) have been used to study beliefs that people may wish to disguise, for example, negative views about women or racial groups (Greenwald et al., 1998).
The two central premises of our study are as follows: (1) implicit attitudes toward violence predict dysfunctional violent behavior and (2) “dysfunctional” violence is qualitatively different from adaptive violence. A recent study showed no differences on explicit violence measures between male perpetrators of intimate partner violence and nonviolent individuals (Robertson and Murachver, 2007). However, implicit measures revealed that violent men showed a more rapid association between women and violence than nonviolent individuals (Eckhardt et al., 2012).
To date, only one behavioral study has investigated implicit attitudes toward violence and aggression. This evidence indicated that explicit measures of violence are not as sensitive as the implicit measures in predicting aggression related to criminal activity (Gray et al., 2003; Banse et al., 2014). For example, psychopathic murderers showed a much more positive implicit attitude toward violence than nonpsychopathic murderers (Gray et al., 2003). Nevertheless, the neural basis of implicit, automatically driven attitudes toward aggression and violence has remained unexplored.
Here, we investigated the neural basis of implicit attitudes toward aggression and violence in patients with penetrating TBI (pTBI) using violence and weapons IATs (see Fig. 1a). We used voxel-based lesion-symptom mapping (VLSM) to identify brain regions causally involved in implicit attitudes toward aggression. We examined the association between implicit and explicit aggression in patients with lesions to different brain regions. We hypothesized that lesions in the dlPFC and temporal cortices—regions that may be critical for modulating implicit attitudes toward violence and are distinct from those previously involved in explicit measures of aggressive behavior—would be associated with a more positive attitude toward violence and aggression. Although vmPFC has been associated with aggressive behavior, those studies were based on self-reported or observer-reported questionnaires, not implicit tasks (Grafman et al., 1996), and our hypothesis focused on response inhibition with regard to implicit violence attitude. Finally, using a hypothesis-driven approach, we tested whether executive functions mediate the interaction between dlPFC and implicit attitudes toward aggression.
Figure 1.
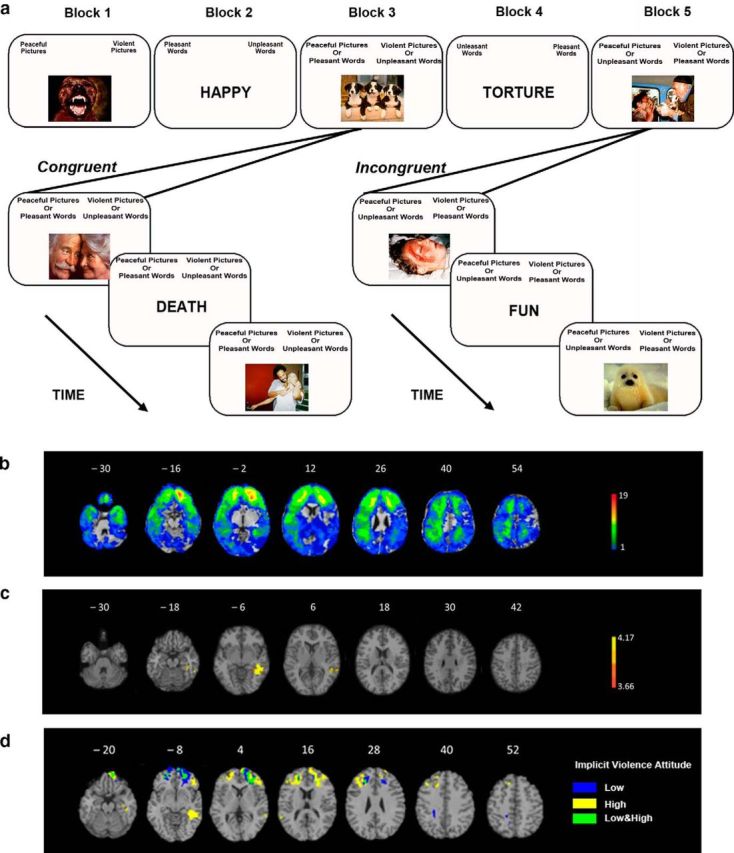
Violence IAT design (a) and lesion analysis results (b–d). a, Violence IAT design. Participants completed five blocks, including one congruent and one incongruent condition. b, Lesion overlay of all 112 patients with focal penetrating traumatic brain injuries. The color indicates the number of patients with damage to a given voxel. The greatest lesion overlap is in red and the least lesion overlap is in blue. c, VLSM of violence IAT performance (D-score). Lesions within the left inferior posterior temporal cortex were associated with lower D-scores (i.e., more positive implicit attitude toward violence) on the violence IAT. The statistical map is corrected at 5% FDR, with minimum cluster size of 10 voxels. The color indicates the z-value: yellow represents the highest z-value and orange represents the lowest z-value. d, Conjunction and disjunction lesion overlay of the Violence IAT low and high score groups. Blue indicates regions associated with high D-score only (i.e., a less positive implicit attitude toward violence), yellow indicates regions associated with low D-score only (i.e., a more positive implicit attitude toward violence), and green indicates regions associated with both high and low D-score (i.e., overlapping regions of more and less positive implicit attitude toward violence). Patients with lower D-score (i.e., a more positive implicit attitude toward violence) sustained lesions that affected the left inferior posterior temporal cortex and bilateral dlPFC, whereas patients with higher D-scores (i.e., a less positive implicit attitude toward violence) had more lesions in the middle and superior orbitofrontal cortex. Only voxels with a minimum of four patients are displayed. All brain figures are in radiologic convention: the right hemisphere is on the reader's left.
Materials and Methods
Participants.
Participants were male combat veterans recruited from the W.F. Caveness Vietnam Head Injury Study Registry (VHIS) during Phase 4, conducted between 2009 and 2012 at the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health in Bethesda. Given its large sample size and wealth of preinjury and postinjury data, the VHIS provides a unique opportunity to investigate brain–behavior relationships using lesion-mapping methods. Our sample consisted of 112 veterans with pTBI and 33 healthy controls (HCs), who also served in combat in Vietnam but had no history of brain injury or other neurological disorders. All pTBIs and HCs were recruited from the VHIS. The two groups were matched on key demographic variables including age, sex, education, and war experiences. Very few participants (pTBIs and HCs) reported a diagnosis of substance abuse of cannabis, anxiolytics, stimulants, opioids, cocaine, PCPs, or other substances. Only four HCs and six pTBIs (distributed in the different groups) reported lifetime prevalence of cannabis abuse. For the other substances, less than three participants (pTBIs and HCs) reported lifetime prevalence. However, there were no differences in prevalence [χ2 (2) = 0.07, p = 0.97] and diagnosis (Fisher's exact test, p = 0.58) of alcohol abuse between pTBIs and HCs. Concerning the legal and criminal history of the participants, a total of 16 pTBIs versus eight HCs reported minor criminal activity. There was no association between participant group (HCs vs pTBIs) and criminal activity [χ2 (1) = 0.01, p = 0.92)]. There were no career military members who retired after a life-long career; all pTBIs were retired after their tour of duty ended or injury mandated it. All HCs spent time in Vietnam but all retired shortly afterward.
Participants were evaluated ∼40 years after injury, so it can be assumed that they were stable because most of the compensatory mechanisms observed after TBI had likely occurred in the 3 years immediately after injury (Han et al., 2007). Table 1 reports pTBI and HC group demographics and results from selected neuropsychological tests that were administered over a 5 d testing period. The Institutional Review Board at the National Institute of Neurological Disorders and Stroke in Bethesda approved all study procedures and participants provided written consent for inclusion in the study.
Table 1.
Demographics, neurobehavioral data (mean ± SD), and Mann-Whitney U test statistics for pTBI patients and HCs
| pTBI (n = 112) | HC (n = 33) | Statistics | |
|---|---|---|---|
| Age (y) | 63.29 ± 2.97 | 63.33 ± 3.79 | U = 1766, Z = −0.38, p = 0.69 |
| Education (y) | 14.6 ± 2.2 | 15.06 ± 2.12 | U = 1646, Z = −0.97, p = 0.33 |
| Preinjury AFQT percentile | 66.05 ± 22.76 | 72.9 ± 17.05 | U = 906, Z = −1.29, p = 0.19 |
| Postinjury AFQT percentile | 57.29 ± 24.99 | 71.39 ± 19.54 | U = 1229, Z = −2.92, p = 0.003 |
| VOSP silhouettes | 20.17 ± 4.84 | 21 ± 3.62 | U = 1741, Z = −0.5, p = 0.61 |
| BNT (BNT) | 53.59 ± 7.09 | 55.75 ± 3.79 | U = 1514, Z = −1.58, p = 0.11 |
| Trail making (D-KEFS) | 9.54 ± 3.67 | 10.84 ± 2.53 | U = 1502, Z = −1.64, p = 0.1 |
| Verbal fluency (D-KEFS) | 9.70 ± 3.11 | 10.58 ± 3.79 | U = 2059, Z = 1.45, p = 0.15 |
| Token test (TT) | 97.64 ± 5.26 | 98.39 ± 1.95 | U = 1793, Z = −0.26, p = 0.78 |
| BIS (BIS) | 61.22 ± 10.03 | 63.45 ± 11.73 | t = 1.08, p = 0.282 |
| M-PTSD | 78.68 ± 21.48 | 83.66 ± 22.88 | U = 1617, Z = −1.08, p = 0.27 |
| Combat exposure | 2.91 ± 1.68 | 2.72 ± 1.23 | U = 1663, Z = −0.91, p = 0.35 |
“Combat exposure” is the frequency of exposure to enemy contact where 0 = support unit (no action), 1 = combat support (occasional mortar attack), 2 = combat support (intermittent enemy contact), 3 = combat unit (intermittent enemy contact), and 4 = combat unit (constant enemy contact).
*For HC, preinjury and postinjury refer to preservice and postservice.
Neuropsychological tests.
An extensive battery of neurobehavioral tests was administered to participants. For the purposes of this study, we report on a subset of those tests including the Armed Forces Qualification Test (AFQT) (United States Department of Defense, 1960), which is highly correlated with existing measures of intelligence and on which we have preinjury data (Grafman et al., 1988). Other tests results that we report on include: the Delis–Kaplan Executive Function System (D-KEFS; Delis et al., 2001), including the trail-making test for cognitive set shifting, and verbal fluency for word fluency; the Visual Object and Space Perception Battery (VOSP; Warrington and James, 1991) for perceptual ability; the Boston Naming Test (BNT; Kaplan et al., 1983) for object naming; the Token Task (TT; McNeil and Prescott, 1994) for verbal comprehension; the Beck Depression Inventory (BDI; Beck et al., 1996) for depression; the Barratt Impulsivity Scale (BIS-11; Patton et al., 1995) for impulsive tendencies; and the Mississippi Scale (MPTSD; Hyer et al., 1991) for posttraumatic stress disorder (PTSD). Participants also reported their degree of combat exposure in terms of the frequency of enemy contact during combat (Koenigs et al., 2008). The measure of combat exposure was based on unit assignment (e.g., support unit, combat service support unit, combat unit; see (Koenigs et al., 2008). In our sample, combat exposure relied only on unit assignment records because it was difficult to quantify and fully document military experience in the Vietnam War.
IAT.
The IAT measures implicit attitudes using the associations of two target categories (e.g., male/female) with two attribute categories (e.g., strong/weak). More strongly associated target–attribute pair elicits a more rapid response revealing implicit biases. We adapted the IAT method (Greenwald et al., 1998; Gozzi et al., 2009) to study implicit attitudes toward violence. To accomplish this aim, we developed two versions of the IAT: the Weapons IAT and the Violence IAT. Both IATs contained five blocks each. In Block 1, target stimuli were presented in the center of the screen, and participants categorized them as a Flower or Weapon (Weapons IAT) or as Peaceful or Violent (Violence IAT) by pressing the corresponding key. In Block 2 and Block 4, attribute stimuli were presented in the center of the screen and participants categorized them as Pleasant or Unpleasant (Weapons IAT and Violence IAT). The categories assigned to the response keys were reversed between Block 2 and Block 4 and the order counterbalanced across participants. In Block 3 and Block 5, all stimuli were presented in random order in the center of the screen and participants performed combined classification into each stimulus's corresponding category. In the congruent block, the Pleasant/Flower (Weapons IAT) or Pleasant/Peaceful (Violence IAT) categories shared one response key, whereas the Unpleasant/Weapon (Weapons IAT) or Unpleasant/Violent (Violence IAT) categories shared the other response key. In the incongruent block, the category combination was reversed, such that the Pleasant/Weapon (Weapons IAT) or Pleasant/Violent (Violence IAT) categories shared one response key, whereas the Unpleasant/Flower (Weapons IAT) or Unpleasant/Peaceful (Violence IAT) categories shared the other response key. The order of congruent and incongruent blocks was counterbalanced across participants. See Figure 1a for a schematic of the experimental procedure for the Violence IAT.
In the Weapons IAT, the target category stimuli included names of flowers (e.g., “rose,” “daffodil”) and names of weapons (e.g., “pistol,” “sword”); in the Violence IAT, the target category stimuli included peaceful images (i.e., women dancing, puppies) and violent images (e.g., bloody hand, robbery). In both the Violence IAT and the Weapons IAT, the attribute categories included pleasant words (e.g., “fun,” “love”), and unpleasant words (e.g., “pain,” “afraid”). Pleasant and unpleasant words were selected from the Affective Norms for English Words (Bradley and Lang, 1999), whereas peaceful and violent images were obtained from the International Affective Picture System (Lang et al., 2008). A total of 40 stimuli (4 categories × 10 stimuli per category) were used in each IAT. In both IAT designs, stimuli were presented in random order within each block. The stimuli were viewed on a PC and administered using E-Prime version 1.2 software (Psychology Software Tools). Participants were instructed to classify each word or image according to the categories given on the left or right sides of their screen by pressing one of two corresponding keys. Participants were asked to respond as quickly and accurately as possible. In each trial, a fixation cross was presented for 1000 ms, followed by a stimulus. The stimulus remained on the screen until the participant selected a response. Immediately after a response, a feedback cross “X” appeared for 1500 ms if the participant pressed the wrong key.
Blocks 1, 2, and 4 contained 20 trials each, whereas Blocks 3 and 5 (congruent and incongruent blocks) contained 40 trials each. Words and/or images within each block were presented in random order. Participants viewed all words and images presented in the IAT before beginning each task. Participants completed 20 practice trials before Block 3 or Block 5.
The IAT has been primarily used to measure association strengths of stereotypical attributes (Rudman et al., 2001; Greenwald et al., 2003; Hofmann et al., 2005; for review, see Amodio, 2014). Decreased response latency represents stronger association between target concept and attribute in that condition. We calculated the D-score to index the IAT effect for each task using the scoring algorithm advocated by Greenwald et al. (2003). According to this algorithm, the IAT effect is calculated by subtracting the reaction time (RT) in the congruent trials (i.e., violent images and unpleasant words) from those in the incongruent trials (i.e., violent images and pleasant words) and dividing the difference by the pooled SDs after eliminating trials with extreme latencies and replacing error latencies. Normally, violence is more strongly associated with Unpleasant rather than with Pleasant attributes; therefore, Violence/Unpleasant is defined as the congruent condition and Violence/Pleasant as the Incongruent condition (Gray et al., 2003). In our study, a decreased IAT effect indicated a faster response to the incongruent Violence/Pleasant condition, reflecting a more positive implicit attitude toward violence (i.e., indicating a stronger association between violence and pleasantness).
The order of congruent and incongruent blocks in both IATs was counterbalanced across participants in the subgroups (Violence: χ2 = 3.97, p = 0.41; Weapons: χ2 = 3.35, p = 0.50).
Explicit measures of violence and aggression.
We administered self-report questionnaires to measure explicit attitudes toward violence and aggression (Table 2) such as the Aggression Questionnaire (AQ; Buss and Warren, 2000), the Attitudes Toward Guns and Violence (AGVQ; Shapiro, 2000), and the State-Trait Anger Expression Inventory (STAXI; Spielberger, 1988; Spielberger and Sydeman, 1994). The AQ (Buss and Warren, 2000) is made up of 34 items assessing five domains of aggression. These domains include physical aggression (e.g., “I may hit someone if he or she provokes me”), verbal aggression (e.g., “My friends say that I argue a lot”), anger (e.g., “I have trouble controlling my temper”), hostility (e.g., “At times I feel I have gotten a raw deal out of life”), and indirect aggression (e.g., “When people are bossy, I take my time doing what they want, just to show them”). AQ total score is a summary measure of the overall level of anger and aggression of the participants. Participants rated these statements using a Likert scale ranging from 1 (not at all like me) to 5 (completely like me). Higher scores reflected increased presence of aggressive behaviors in the five domains of aggression and in the total score.
Table 2.
Implicit and explicit measures of aggression (mean ± SD) and Mann-Whitney U test statistics for pTBIs and HCs
| pTBIs (n = 112) | HCs (n = 33) | Statistics | |
|---|---|---|---|
| Violence IAT D-score | 1.13 ± 0.31 | 1.16 ± 0.26 | U = 1744, Z = −0.1, p = 0.91 |
| Congruent RT (ms) | 964 ± 411 | 805 ± 160 | U = 1370, Z = −2.0, p = 0.045 |
| Incongruent RT (ms) | 1641 ± 710 | 1381 ± 348 | U = 1472, Z = −1.51, p = 0.13 |
| Weapons IAT D-score | 0.95 ± 0.4 | 0.95 ± 0.38 | U = 1776, Z = −0.1, p = 0.91 |
| Congruent RT (ms) | 923 ± 342 | 858 ± 189 | U = 1714, Z = −0.48, p = 0.63 |
| Incongruent RT (ms) | 1322 ± 543 | 1234 ± 309 | U = 1802, Z = −0.062, p = 0.95 |
| AQ (total) | 48.77 ± 10.04 | 53.61 ± 10.03 | U = 2375, Z = 2.58, p = 0.01 |
| Physical | 50.59 ± 8.93 | 52.79 ± 11.2 | U = 2161, Z = 1.57, p = 0.15 |
| Verbal | 48.92 ± 10.78 | 50.94 ± 12.37 | U = 2027, Z = 0.93, p = 0.35 |
| Anger | 48.69 ± 12.06 | 50.67 ± 12.65 | U = 2037, Z = 0.98, p = 0.32 |
| Hostility | 50.33 ± 11.03 | 54.67 ± 11.82 | U = 2343, Z = 2.44, p = 0.01 |
| Indirect | 48.69 ± 11.91 | 51.48 ± 12.11 | U = 2138, Z = 1.46, p = 0.14 |
| AGVQ (total) | 20.74 ± 14.98 | 17.82 ± 9.94 | U = 1837, Z = −0.05, p = 0.95 |
| Shame | 8.67 ± 16.64 | 4.36 ± 10.4 | U = 1848, Z = 0.08, p = 0.93 |
| Comfort | 14.13 ± 18.03 | 8.7 ± 11.43 | U = 1532, Z = −1.42, p = 0.15 |
| Excite | 8.84 ± 19.02 | 3.67 ± 10.53 | U = 1798, Z = −0.18, p = 0.85 |
| Power | 12.74 ± 18.22 | 8.64 ± 12.65 | U = 1802, Z = −0.13, p = 0.89 |
| STAXI | 44.93 ± 9.81 | 48.42 ± 11.34 | t = 1.74, p = 0.085 |
AQ and AGVQ include total scores and subscores.
A second questionnaire, the AGVQ (Shapiro, 2000), was administered to assess beliefs regarding the use of guns in society and personally held views related to violent versus nonviolent conflict resolution. The measure contains 26 statements related to violence, guns, and conflict behavior (e.g., “I would feel awful inside if someone laughed at me and I didn't fight them,” “I'd like to have my own gun,” and “Carrying a gun makes people feel powerful and strong.”). Participants responded using a Likert scale ranging from 1 (Agree), 2 (Not Sure), to 3 (Disagree). Four subscale scores are obtained from the AGVQ: Aggressive Response to Shame, Comfort with Aggression, Excitement, and Power/Safety. Importantly, the scores on this measure reflect attitudes toward guns and violence, not just the frequency of violent behaviors.
The State-Trait Anger Expression Inventory (STAXI; Spielberger, 1988; Spielberger and Sydeman, 1994) was also administered to assess anger. The STAXI offers a self-reported measure of the experience and expression of anger in 44 items (i.e., “When I get mad, I say nasty things”). Participants answered on a 4-point Likert scale to assess either the intensity of their angry feelings or the frequency of experienced, expressed, or controlled angry episodes.
Lesion analysis and mapping.
CT scans were obtained for all patients from a General Electric Medical System Light Speed Plus CT scanner in helical mode due to the nature of patients' penetrating brain injuries and the likelihood of retained intracranial metal fragments. CT scans were acquired during Phase III of the VHIS study (∼6 years before Phase IV). However, a clinical reading of CT scans acquired during Phase IV indicated that the lesions were unchanged from Phase III and no additional aging or pathological effects were observed.
CT images were reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1 mm slice interval. Using the Analysis of Brain Lesion (ABLe) software version 2.8b (Makale et al., 2002; Solomon et al., 2007) implemented in MEDx (Medical Numerics) version 3.44, we calculated lesion location and volume loss. To identify the location of each lesion, a neuropsychiatrist (V.R.) trained in interpreting CT scans manually outlined the lesion area on every slice in native space. Results were reviewed by another investigator (J.G.) who was blind to the clinical evaluation. Volume loss was determined by summing the area of all traced lesions and then multiplying by slice thickness. Each CT scan was then spatially normalized to a CT brain image in Montreal Neurological Institute (MNI) space (Collins et al., 1994). To improve registration accuracy, spatial normalization was performed using the automated image registration algorithm (Woods et al., 1998) with a 12-parameter affine fit. This procedure enabled us to calculate brain volume loss across the entire brain and within ROIs defined using the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) and MNI coordinates, including the vmPFC, vlPFC, dmPFC, and dlPFC, as well as the inferior posterior temporal cortex (ipTC) (see Koenigs et al., 2008 for a similar procedure; Gozzi et al., 2009).
Through the VLSM analysis, we compared for each voxel scores from patients with a lesion in a given voxel against patients without a lesion at that given voxel. We applied a false discovery rate (FDR) correction of 0.05 and a minimum cluster size of 10 voxels with our patients. VLSM analyses were only conducted in voxels with a minimum of four patients with a lesion in a given voxel. The FDR correction of 0.05 was based on previous VLSM studies (Kimberg and Farah, 1993; Gläscher et al., 2009; Tsuchida and Fellows, 2012; Dal Monte et al., 2013). The minimum of four patients with damage to a given voxel is sufficient to detect significant differences (Gläscher et al., 2009). The AAL atlas for gray matter (Tzourio-Mazoyer et al., 2002) and the ICBM-DTI-81 atlas for white matter (Mori et al., 2008) were used to identify the anatomical locations of significant clusters.
We determined whether the weapons and violence IAT D-scores were associated with regional gray and white matter using VLSM (Bates et al., 2003). Through VLSM analysis, we were able to identify brain regions that played a causal role in modulating task performance (Barbey et al., 2014).
Statistical analysis.
We performed all statistical analyses using SPSS 21.0 with the significance level set to p < 0.05, two-tailed. We checked normality of data using the Kolmogorov–Smirnov test and conducted parametric or nonparametric statistical tests as appropriate. Furthermore, we performed a hierarchical multiple regression analysis to examine potential predictors of the violence D-score. We entered lesion size in violence-related cortical areas as regressors, including PFC subregions (vmPFC, vlPFC, dmPFC, and dlPFC) and ipTC (Gozzi et al., 2009) while adjusting for preinjury intelligence, PTSD, impulsivity, visual discrimination, verbal comprehension, and total percentage of brain volume loss. Preinjury intelligence and total volume loss are important factors influencing recovery after TBI (Raymont et al., 2011), whereas PTSD and impulsivity measures were included due to their implication in aggressive behaviors (Hoptman et al., 2002; Koenigs and Grafman, 2009). Finally, we controlled for visual discrimination due to the visual nature of our task. We performed Spearman correlations between implicit and explicit measures of violence and between implicit attitudes about violence and executive functions in the pTBI and HC groups. A Kruskal–Wallis test was used for the confirmatory group analysis and we performed planned follow-up pairwise comparisons.
Group analysis.
Based on our previous hypothesis about the role of vmPFC in modulating aggressive attitudes and on the results predicted by the regression analysis (dlPFC and ipTC), we performed a confirmatory group analysis to assess the contribution of specific brain areas to implicit attitudes about violence and to determine whether lesion groups performed differently from controls in the direction predicted by the regression. Our lesion groups were relatively selective, with damage largely restricted to the specified regions.
Each patient's brain scan was normalized to a CT template brain image MNI space. Subsequently, we calculated percentages of Brodmann areas (BAs) intersected by the lesion by analyzing the overlap of the spatially normalized lesion images with the BA atlas. We defined ROIs based on BAs and divided participants into four lesion groups (dlPFC, vmPFC, ipTC, and other lesions) according to percentage of lesions in the ROIs (see Fig. 2a for a lesion overlay of each group). The dlPFC ROI included BA9 and BA46; 14 patients had lesions mostly confined to the dlPFC. We decided to include only BA9 and BA46 because BA44 and BA45 are also involved in language processing and lesions in those regions could confound the results. It is also more difficult to categorize patients with posterior PFC lesions, such as in BA44 and BA45, into a specific lesion group because they also had more extended lesions to the temporal/parietal cortex. We based our grouping criteria on a previous research study that used a similar IAT task (Forbes et al., 2012b). The vmPFC ROI included BA10, BA11, BA25, and BA32; 19 patients had lesions mostly confined to the vmPFC. The ipTC ROI included BA20 and BA21; 13 patients had lesions mostly confined to the ipTC. Finally, we selected a control patient group consisting of 30 pTBI patients referred to as the “other lesions group”; patients in this group did not have damage to any of the previously selected BAs.
Figure 2.
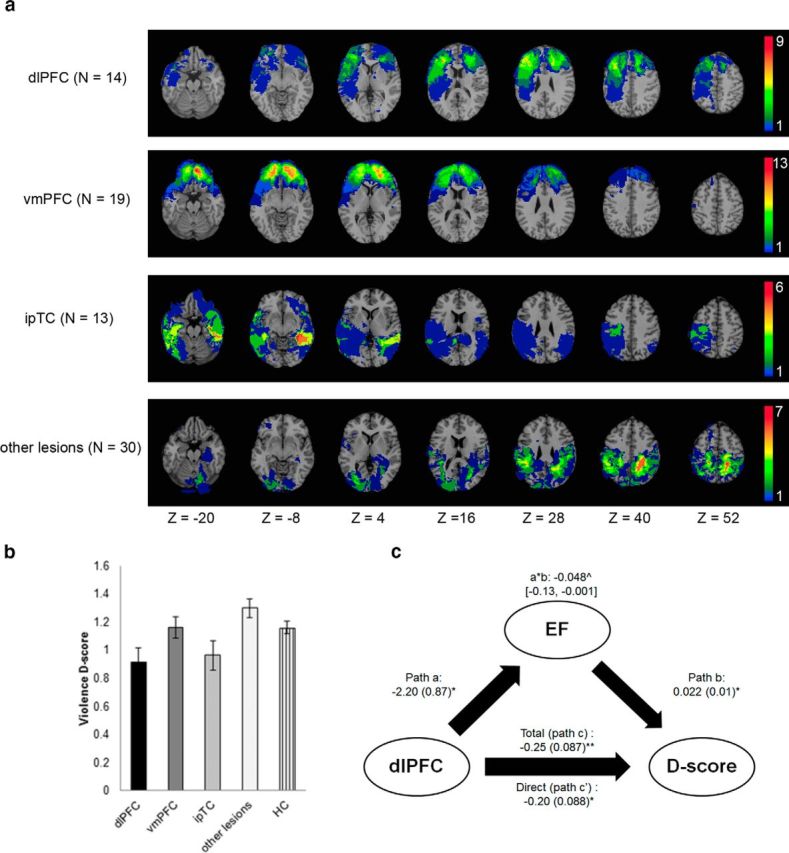
Group lesion overlays (a) and group analysis results (b, c). a, Lesion overlays of the dlPFC (BA46, BA9), vmPFC (BA10, BA11, BA25, and BA32), ipTC (BA20 and BA21), and other lesions group (no dlPFC, no vmPFC, no ipTC). Color bar indicates the number of patients with overlapping lesions in each voxel. The figure is in radiologic convention: the right hemisphere is on the reader's left. b, Average violence IAT D-scores for the five groups: dlPFC (n = 14), vmPFC (n = 19), ipTC (n = 13), and other lesions (n = 30) and HCs (n = 33). The dlPFC group had a lower violence D-score (i.e., a more positive implicit attitude toward violence) compared with the HC (p = 0.03), vmPFC (p = 0.05), and other lesions (p < 0.01) groups. The ipTC group had a lower violence D-score compared with the other lesions group (p < 0.01). Error bars indicate ± SEM. c, Results of the mediation analysis testing the relationship between dlPFC lesion, executive function (EF, verbal fluency), and implicit attitude toward violence (D-score). The diagram shows the coefficients ± SE of the path model significant at **p < 0.01 and *p < 0.05. ∧Indirect path coefficient (lower and upper limit of the confidence interval).
Lesion groups were defined for each region that significantly predicted IAT performance in the regression analysis (e.g., dlPFC and ipTC) or plays an important role in aggression (e.g., vmPFC) based on previous findings. In addition, we selected a control group of patients who had lesions not involving vmPFC, dlPFC, or ipTC. We based our inclusion criteria to a specific lesion group on previous studies (Tranel et al., 2005; Koenigs et al., 2008). A patient was included in a lesion group if his lesion involved at least 15% of that region. Patients with brain damage to more than one region were classified based on the region with the greatest percentage of brain damage (Gozzi et al., 2009). We used this procedure to guarantee that patients in a lesion group not only had brain damage to that region, but also had greater brain damage to that region than to the other regions under investigation (Mah et al., 2004). In addition, patients with brain damage >15% in more than one region or whose damage was <15% were excluded from this confirmatory analysis.
Concerning legal and criminal history of the participants, only one participant (vmPFC) reported major/serious criminal activity. In addition, we performed a group analysis and found that the dlPFC (Fisher's exact test, p = 0.58), vmPFC (Fisher's exact test, p = 0.52), and ipTL (Fisher's exact test, p = 0.71) groups did not differ in criminal activity compared with HCs.
We performed Kruskal–Wallis tests to compare implicit attitudes and explicit measures about violence between the lesion subgroups and HCs. Finally, we analyzed the percentage of white matter tracts damaged in the pTBI subgroups.
Mediation analysis.
We performed a mediation analysis to determine whether the association between violence D-score and dlPFC lesion was mediated by executive function (i.e., verbal fluency). Mediation analyses were performed for all pTBI patients, entering as an independent variable different brain regions. However, we found significant results only with a dlPFC lesion as the independent variable. We used the PROCESS macro (Hayes, 2013) implemented in SPSS 21 to estimate the mediation model. PROCESS uses a boot-strapping approach (1000 iterations) to evaluate the 95% confidence intervals of the size of the model-specified indirect effect. The mediation analysis was based on a three-variable path model (see Figure 2c). Following standard assumptions (Baron and Kenny, 1986), we required that three tests reach significance in the mediation analysis. First, the independent variable (dlPFC lesion) must be related to the mediating variable (EF, verbal fluency) (Figure 2c, path a). Second, the mediating variable must be related to the outcome variable (violence D-score) after controlling for the independent variable (Fig. 2c, path b). Third, the mediation effect defined as product of the indirect paths (a * b) must be statistically significant. We tested for the hypothesis that including the mediating variable in the model significantly reduces the relationship between predictor (dlPFC) and outcome (violence D-score).
Results
Behavioral results
Demographics and neuropsychological testing results of the participants are described in Table 1. As a whole, the pTBI and HC groups did not differ on demographics and their performance was similar on most of the reported neuropsychological measures. The pTBI group did have a lower postinjury AFQT score (AFQT-7A, 1960) than the HC group, but their scores were within the normal range (Grafman et al., 1988).
The Gender IAT was administered in Phase 3, ∼5 years before Phase 4, and the Violence and Weapons IAT were administered in Phase 4. All tests and questionnaires were counterbalanced across participants.
We calculated the D-score to index the IAT effect; that is, the RT differences between incongruent and congruent trials for each task, using the scoring algorithm advocated by Greenwald et al. (2003). In our study, a decreased IAT effect indicated a faster response to the incongruent Violence/Pleasant condition, reflecting a more positive implicit attitude toward violence (i.e., indicating a stronger association between violence and pleasantness). The combined pTBI and HC groups did not differ in their violence and weapons D-scores. pTBI participants had significantly slower RTs than HCs in the congruent block of the Violence IAT (U = 1370, Z = −2.0, p = 0.045). In the pTBI group, there was no correlation between accuracy in either condition and the IAT D-score (incongruent: ρ = 0.001, p = 0.992; congruent: ρ = 0.152, p = 0.116); in the Weapons IAT, there was a modest correlation between D-score and congruent block accuracy (ρ = 0.207, p = 0.03), but not incongruent block accuracy (ρ = −0.036, p = 0.712). In both IATs, the overall accuracy did not correlate with D-score (Violence IAT: ρ = 0.018, p = 0.85; Weapons IAT: ρ = 0.050, p = 0.607).
Concerning the explicit aggression measures, HCs reported higher total AQ total aggression and hostility compared with the pTBI group (Table 2). Nevertheless, both pTBIs and HCs had AQ scores in the normal range (i.e., between 45 and 55; Buss and Warren, 2000).
We next investigated the correlation between implicit and explicit measures and executive functions.
Whereas the pTBI group showed a modest negative correlation between implicit and explicit measures of aggression (ρ = −0.20, p = 0.019, two-tailed), the same effect was not found in the HC group (ρ = −0.11, p = 0.275, two-tailed). To investigate the relationship between executive functions and violence D-score, we calculated correlations between the violence D-score and the executive function subtests of the D-KEFS in the pTBI and HC groups. In the pTBI group, we found modest positive correlations between the violence D-score and trail making (ρ = 0.207, p = 0.016) and verbal fluency (ρ = 0.182, p = 0.030) tests, indicating that a higher D-score (i.e., a less positive implicit attitude toward violence) was associated with better executive functions. No significant correlations were found in the HC group on these same measures. Fisher's z test (z = 0.42, n.s.) showed no difference in implicit–explicit correlations between the two groups. A moderation analysis did not show a significant moderator effect of pTBIs on the association between implicit and explicit measures.
Finally, we performed a supplementary correlation analysis between D-scores and a caregiver-rated neurobehavioral scale. In both pTBIs and HCs, there was no correlation between Violence or Weapon D-scores with the Neurobehavioral Rating Scale (NBRS) (Levin et al., 1987) scores on hostility/uncooperative, agitation, or anxiety.
To determine whether other factors could contribute to implicit violent attitudes, we performed more fine-grained analyses between predisposition factors (such as education, intelligence, depression) and D-scores. The results of these analyses are summarized in Table 3.
Table 3.
Spearman rank correlations between violence D-score and the predisposition factors of education, intelligence (post-AFQT), and depression (BDI-II) for the pTBI patients and HCs
| pTBI (n = 112) |
HCs (n = 33) |
|||||
|---|---|---|---|---|---|---|
| Education | Post-AFQT | BDI-II | Education | Post-AFQT | BDI-II | |
| D-score | rho = −0.143 (p = 0.143) | rho = −0.035 (p = 0.719) | rho = −0.115 (p = 0.238) | rho = −0.093 (p = 0.605) | rho = −0.075 (p = 0.677) | rho = −0.162 (p = 0.369) |
VLSM results
Figure 1b illustrates the overall distribution of lesions in our pTBI sample (n = 112) by overlapping spatially normalized lesion images. Lesions within the left posterior inferior temporal cortex (ipTC) were associated with lower D-scores on the violence IAT (Fig. 1c, Table 4). As a reminder, a lower D-score indicated a faster RT to the incongruent Violence/Pleasant condition and implied a more positive implicit attitude toward violence. Unlike the violence IAT task, we did not find significant clusters associated with the weapons D-score. As a result, subsequent analyses only used the violence D-score.
Table 4.
VLSM results
| Region | MNI |
Cluster size | z-value threshold | z-value | Cohen's d | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Gray matter | −38 | −34 | −12 | 693 | 3.44 | ||
| Left middle temporal gyrus | 4.17 | 2.01 | |||||
| Left inferior temporal gyrus | 4.17 | 2.2 | |||||
| Left parahippocampal gyrus | 3.66 | 1.95 | |||||
| Left fusiform gyrus | 4.17 | 2.2 | |||||
| Left lingual gyrus | 3.8 | 2.03 | |||||
| Left hippocampus | 3.9 | 2.03 | |||||
| White matter | |||||||
| Left sagittal stratum | 3.7 | 1.97 | |||||
| Left posterior thalamic radiation | 4.17 | 2.03 | |||||
To further examine the brain regions associated with the violence D-score, we divided all patients into quartiles based on their D-scores and generated lesion overlay maps of participants in the highest (top 25%, n = 27) and lowest (bottom 25%, n = 27) scoring quartiles (Fig. 1d). Patients with lower D-scores (i.e., a more positive implicit attitude toward violence; Fig. 1d, yellow) sustained lesions that affected the left ipTC, bilateral dlPFC, and bilateral OFC, whereas patients with higher D-scores (i.e., a less positive implicit attitude toward violence; Fig. 1d, blue) sustained lesions that affected the middle and superior OFC. The lesion overlap between participants with higher and lower implicit attitude toward violence is shown in green in Figure 1d (i.e., participants in the highest and lowest scoring quartiles). The lesion overlap in these two groups of patients was primarily found in the OFC. Because this overlay was based on participants' lesion location, the finding of common overlapping regions in the two groups was expected.
Hierarchical multiple regression
Next, we performed hierarchical multiple regression analyses to examine the predictive power of lesion size in different brain regions on violence D-scores while adjusting for damage to other areas and neuropsychological covariates that were correlated with violence D-scores. In the hierarchical multiple regression analysis, the lesion sizes of different regions were entered in one model.
The ROIs used in the multiple regression analysis were anatomically defined based on the AAL atlas (Gozzi et al., 2009). Based on previous knowledge of frontal and temporal lobe involvement in aggression, we entered as regressors prefrontal cortex subregions (which were not identified in the VLSM) and temporal lobe subregions including the superior and inferior anterior temporal lobes, inferior posterior temporal lobes, medial temporal lobes, and amygdala.
After controlling for preinjury AFQT score and total percentage lesion volume loss, we entered lesion percentage in different subregions within the temporal and prefrontal cortex as predictors. Lesions to the dlPFC (β = −0.66, t(83) = −3.48, p = 0.001) and the ipTC (β = −0.41, t(83) = −2.64, p = 0.010) predicted lower violence D-scores (F(11,83) = 2.20, p = 0.022, Adjusted R2 = 12.3%). When we controlled for neuropsychological covariates including a diagnosis of MPTSD (Hyer et al., 1991), TT (McNeil and Prescott, 1994), BIS (Patton et al., 1995), and D-KEFS (Delis et al., 2001) trail-making test scores, the lesion percentage in the dlPFC remained the only significant predictor (β = −0.56, t(79) = −2.95, p = 0.004), with larger dlPFC lesions predicting lower violence D-scores (F(15,79) = 2.29, p = 0.01, adjusted R2 = 17%).
Group analysis
We next performed a confirmatory group analysis by selecting four groups of pTBI patients with lesions to the dlPFC (n = 14), vmPFC (n = 19), ipTC (n = 13), and other lesions (i.e., brain lesion control group, not including previously selected areas, n = 30) and comparing them with the HCs (n = 33) on the violence D-score and explicit measures of aggression and violence (see Fig. 2a for lesion overlays of the groups). The remaining 36 participants were excluded from the group analysis due to their extensive lesions, which made it impossible to include them in a selective lesion group. Table 5 and Table 6 report the scores on the neuropsychological measures and implicit and explicit measures of aggression. To maintain sufficient power, we used a planned-comparisons approach to analyze the data.
Table 5.
Demographics, neurobehavioral data (mean ± SD), and Kruskal–Wallis test statistics for pTBI patients and HCs
| dlPFC (n = 14) | vmPFC (n = 19) | ipTC (n = 13) | Other lesions (n = 30) | HC (n = 33) | Statistics | |
|---|---|---|---|---|---|---|
| Age (y) | 62.29 ± 1.43 | 62.26 ± 2.07 | 64.0 ± 2.53 | 63.24 ± 1.94 | 63.33 ± 3.79 | H = 8.57, p = 0.073 |
| Education (y) | 13.43 ± 1.69 | 13.47 ± 1.98 | 15.46 ± 2.22 | 15.08 ± 2.23 | 15.06 ± 2.12 | H = 13.99, p < 0.01 |
| Preinjury AFQT percentile | 65.14 ± 25.56 | 67.58 ± 19.30 | 68.92 ± 15.60 | 62.24 ± 29.47 | 72.9 ± 17.05 | H = 1.57, p = 0.813 |
| Postinjury AFQT percentile | 56.57 ± 23.50 | 58.47 ± 23.69 | 52.83 ± 24.14 | 55.08 ± 27.78 | 71.39 ± 19.54 | H = 11.12, p = 0.025 |
| VOSP silhouettes | 20.29 ± 3.36 | 21.74 ± 4.54 | 20.08 ± 3.66 | 20.71 ± 4.87 | 21 ± 3.62 | H = 2.60, p = 0.627 |
| BNT | 51.43 ± 4.81 | 54.89 ± 4.34 | 52.67 ± 6.85 | 55.40 ± 4.57 | 55.75 ± 3.79 | H = 12.05, p = 0.017 |
| Trail making (D-KEFS) | 8.93 ± 3.07 | 10.53 ± 2.43 | 8.58 ± 4.88 | 8.72 ± 4.05 | 10.84 ± 2.53 | H = 5.89, p = 0.207 |
| Verbal fluency (D-KEFS) | 7.46 ± 3.45 | 10.58 ± 2.93 | 8.38 ± 2.46 | 9.40 ± 2.60 | 10.58 ± 3.79 | H = 13.16, p = 0.011 |
| TT | 97.5 ± 2.9 | 98.63 ± 1.34 | 96.25 ± 4.26 | 98.56 ± 2.08 | 98.39 ± 1.95 | H = 3.82, p = 0.430 |
| BIS | 65.79 ± 11.3 | 59.29 ± 9.64 | 63.75 ± 13.12 | 58.8 ± 8.8 | 63.45 ± 11.73 | H = 7.97, p = 0.093 |
| M-PTSD | 81.64 ± 22.25 | 70.53 ± 17.75 | 88.75 ± 22.82 | 74.14 ± 21.38 | 83.66 ± 22.88 | H = 11.66, p = 0.020 |
| Combat exposure | 3.14 ± 1.29 | 2.95 ± 1.22 | 3.08 ± 2.5 | 3.0 ± 1.52 | 2.72 ± 1.23 | H = 1.40, p = 0.843 |
“Combat exposure” is the frequency of exposure to enemy contact where 0 = support unit (no action), 1 = combat support (occasional mortar attack), 2 = combat support (intermittent enemy contact), 3 = combat unit (intermittent enemy contact), and 4 = combat unit (constant enemy contact).
Table 6.
Implicit and explicit measures of aggression (mean ± SD) and Kruskal–Wallis test statistics for pTBIs and HCsa
| dlPFC (n = 14) | vmPFC (n = 19) | ipTC (n = 13) | Other lesions (n = 30) | HC (n = 33) | Statistics | |
|---|---|---|---|---|---|---|
| Violence IAT D-score | 0.91 ± 0.36 | 1.17 ± 0.32 | 0.94 ± 0.37 | 1.29 ± 0.18 | 1.16 ± 0.26 | H = 17.61, p < 0.01 |
| Congruent RT (ms) | 1225 ± 463 | 949 ± 300 | 1211 ± 747 | 824 ± 348 | 805 ± 160 | H = 23.35, p < 0.01 |
| Incongruent RT (ms) | 2134 ± 693 | 1461 ± 446 | 1705 ± 899 | 1597 ± 753 | 1381 ± 348 | H = 14.98, p < 0.01 |
| Weapons IAT D-score | 0.94 ± 0.37 | 0.96 ± 0.38 | 0.85 ± 0.43 | 1.02 ± 0.42 | 0.95 ± 0.38 | H = 2.03, p = 0.730 |
| Congruent RT (ms) | 1025 ± 237 | 846 ± 150 | 1160 ± 450 | 867 ± 436 | 858 ± 189 | H = 15.60, p = 0.004 |
| Incongruent RT (ms) | 1664 ± 671 | 1291 ± 412 | 1591 ± 725 | 1210 ± 595 | 1234 ± 309 | F = 13.13, p = 0.011 |
| AQ (Total) | 51.43 ± 13.35 | 46.53 ± 7.63 | 52.77 ± 7.72 | 46.0 ± 11.0 | 53.61 ± 10.03 | H = 13.11, p = 0.011 |
| Physical | 50.64 ± 12.39 | 51.16 ± 6.27 | 51.85 ± 7.61 | 47.32 ± 9.2 | 52.79 ± 11.2 | H = 6.76, p = 0.149 |
| Verbal | 53.50 ± 11.89 | 47.95 ± 9.23 | 51.15 ± 7.93 | 46.52 ± 12.86 | 50.94 ± 12.37 | H = 3.21, p = 0.523 |
| Anger | 50.93 ± 14.21 | 45.21 ± 11.35 | 53.15 ± 9.6 | 44.81 ± 13.39 | 50.67 ± 12.65 | H = 6.59, p = 0.159 |
| Hostility | 53.21 ± 14.13 | 48.58 ± 7.91 | 53.69 ± 8.22 | 45.84 ± 13.8 | 54.67 ± 11.82 | H = 12.67, p = 0.013 |
| Indirect | 51.86 ± 9.64 | 46.95 ± 9.27 | 55.15 ± 8.92 | 44.94 ± 12.53 | 51.48 ± 12.11 | H = 14.19, p < 0.01 |
| AGVQ (total) | 19.93 ± 17.73 | 16.21 ± 10.78 | 19.77 ± 14.93 | 20.97 ± 13.39 | 17.82 ± 9.94 | H = 2.03, p = 0.730 |
| Shame | 6.79 ± 18.47 | 5.74 ± 13.94 | 8.69 ± 16.17 | 9.06 ± 17.4 | 4.36 ± 10.4 | H = 4.66, p = 0.324 |
| Comfort | 1.14 ± 10.66 | 10.26 ± 14.07 | 13.46 ± 19.8 | 14.94 ± 17.07 | 8.7 ± 11.43 | H = 2.91, p = 0.573 |
| Excite | 6.5 ± 22.61 | 5.74 ± 15.76 | 9.08 ± 20.39 | 9.81 ± 18.95 | 3.67 ± 10.53 | H = 1.84, p = 0.765 |
| Power | 9.09 ± 17.79 | 8.74 ± 12.26 | 11.54 ± 17.64 | 14.42 ± 19.38 | 8.64 ± 12.65 | H = 3.02, p = 0.55 |
| STAXI | 45.57 ± 10.26 | 42.42 ± 9.55 | 47.85 ± 7.72 | 42.0 ± 8.94 | 48.42 ± 11.34 | H = 8.29, p = 0.081 |
aFor HC scores, see Table 2.
A Kruskal–Wallis test of the violence D-score between the five groups (dlPFC, vmPFC ipTC, other lesions, and HCs) show a significant main effect (H = 17.61, df = 4, p < 0.001, ηp2 = 0.183). Planned comparisons using Mann–Whitney U tests revealed that patients with lesions to the dlPFC had a significantly lower violence D-score (M = 0.91, SD = 0.36) compared with the HCs (M = 1.16, SD = 0.26, U = 141, Z = −2.09, p = 0.03, d = 0.796), the vmPFC (M = 1.16, SD = 0.32, U = 81, Z = −1.89, p = 0.05, d = 0.734), or the other lesions groups (M = 1.29, SD = 0. 18, U = 73, Z = −3.53, p < 0.01, d = 1.33), but not compared with the ipTC group (M = 0.96, SD = 0.37, U = 84, Z = −0.34 p > 0.250, d = 0.136). Conversely, patients with lesions to the ipTC had significantly lower violence D-score compared with the other lesions group (U = 80, Z = −3.12, p = 0.002, d = 1.031), but not compared with the dlPFC (U = 84, Z = −0.34, p > 0.250, d = 0.136), the vmPFC (U = 91, Z = −1.24, p = 0.22, d = 0.578), or the HC groups (U = 148, Z = −1.62, p = 0.10, d = 0.625; Fig. 2b). Across the groups, there was no correlation between education and Violence IAT D-score (ρ = 0.079, p = 0.41), so the level of education did not affect the difference in D-scores among the groups.
To verify that the observed result was a manifestation of attitudes about violence rather than a more general executive impairment in an RT-based task, we compared group performance in a previous Gender IAT task that was explored in the same population of patients (Gozzi et al., 2009). This analysis showed no difference between groups in terms of D-score performance on the Gender IAT (F = 0.93, df = 4, p = 0.48, ηp2 = 0.043), confirming the specificity of dlPFC and ipTC involvement in the violence IAT.
We next compared explicit aggression measures and executive functions between these subgroups. A Kruskal–Wallis test of the explicit measures of violence and aggression between the five groups (dlPFC, vmPFC, ipTC, other lesions, and HCs) showed a significant main effect in the AQ total score (H = 13.13, df = 4, p = 0.011, ηp2 = 0.019), AQ Hostility subtest (H = 12.67, df = 4, p = 0.013, ηp2 = 0.032) and AQ Indirect Aggression subtest (H = 14.16, df = 4, p < 0.01, ηp2 = 0.096), but planned comparisons revealed no significant results. See Table 5 for average scores on the tests. A Kruskal–Wallis test of the D-KEFS tests between the five groups (dlPFC, vmPFC, ipTC, other lesions, and HCs) showed a significant main effect for the verbal fluency test (H = 13.16, df = 4, p = 0.011, ηp2 = 0.020). Planned comparisons revealed that only patients with lesions to the dlPFC had a significantly lower verbal fluency score (U = 132, Z = −2.31, p = 0.02, d = 0.861) compared with the HCs. See Table 5 for average scores in the tests.
Given that dlPFC lesions were associated with both a reduced violence D-score and executive function (i.e., verbal fluency), we next conducted a mediation analysis to test whether executive function performance might mediate the association between dlPFC lesion and implicit attitude toward violence (D-score). Figure 2c shows the results of the mediation analysis, indicating that verbal fluency is a partial mediator of the relationship between implicit attitude toward violence and dlPFC lesion. To determine whether the mediating role of verbal fluency in the violence D-score was specific to the dlPFC, we repeated the mediation analyses for the vmPFC, ipTC, and other lesions groups, but did not find any significant mediation effect.
In addition, we assessed white matter tract damage in the pTBI subgroups. We found that the superior corona radiata, extreme capsule, cingulum bundle, superior longitudinal fasciculus, and uncinate fasciculus were more damaged in the dlPFC compared with the vmPFC group (see Table 7 for a summary of the results).
Table 7.
Percentage of white matter tract damaged in the dlPFC, vmPFC, ipTC, and other lesions subgroups
| dlPFC | vmPFC | ipTC | Other lesions | |
|---|---|---|---|---|
| ACR | 87.62% | 88.45% | 4.27% | 0.06% |
| SCR | 51.27% | 8.03% | 13.75% | 26.67% |
| EC | 47.74% | 7.34% | 9.33% | 3.53% |
| CGC | 14.18% | 2.67% | 7.13% | 13.80% |
| SLF | 46.31% | 7.35% | 46.98% | 80.60% |
| UNC | 37.23% | 1.06% | 24.75% | 0% |
ACR, Anterior corona radiate; SCR, superior corona radiate; EC, external capsule; CGC, cingulum; SLF, superior longitudinal fasciculus; UNC, uncinate fasciculus.
Finally, to confirm that the IAT effect that we observed was not an artifact of general response compatibility inhibition, we compared group performance in the Multi-Source Inhibition Task (Bush et al., 2003). There was no difference between the lesion groups in RT or accuracy (RT: H = 2.08, df = 4, p = 0.72; accuracy: H = 3.77, df = 4, p = 0.44). In addition, to verify that that the observed result was a manifestation of attitudes about violence rather than a more general executive impairment in an RT-based task, we compared group performance in a previous Gender IAT task that was explored in the same population of patients (Gozzi et al., 2009). This analysis showed no difference between groups in the Gender IAT D-score (F = 0.93, df = 4, p = 0.48, ηp2 = 0.043), confirming the specificity of dlPFC and ipTC involvement in the violence IAT.
Discussion
The goal of this study was to investigate the neural bases of implicit attitudes toward violence and aggression. Our results indicated that the dlPFC and ipTC are required to constrain implicit attitudes toward violence. Lesions involving either area were associated with a more positive implicit attitude toward violence. However, after controlling for PTSD, impulsivity, verbal comprehension, executive function, and total lesion size, the dlPFC was the only significant predictor of a more positive implicit attitude toward violence. This finding suggests that damage to the dlPFC impairs top-down mechanisms regulating attitudes about violence. This result is corroborated by a mediation analysis showing that verbal fluency partially mediated the association between dlPFC and implicit attitudes toward aggression.
In our study, we found that damage to both the dlPFC and ipTC affected implicit attitudes toward violence. The inferior temporal cortex is known to relay visual information in the ventral stream. Lesion and imaging studies have shown left ipTC involvement in generating and retrieving visual representations (Gross, 1992). The involvement of posterior cortices in IAT studies has been found previously (Forbes et al., 2012a). We hypothesize that the ipTC regions identified in our analysis may be involved in the rapid automatic perceptual processing of violent stimuli conveying visual information to cortices concerned with inhibition and decision making. The dlPFC is a region crucial to cognitive control, inhibition, and conflict processing (Koechlin et al., 2003; Forbes and Grafman, 2010). It is involved in top-down modulation of stereotypic social attitudes such as racial bias through its extensive connection to the limbic system and other associative cortices (Knutson et al., 2007; Forbes et al., 2012a). Therefore, we speculate that a lesion to the dlPFC would disrupt the inhibition of a positive implicit attitude toward violence, thereby releasing the expression of such implicit attitudes. In our study, patients with lesions to the dlPFC performed worse on a verbal fluency test that requires cognitive control, suggesting that impairments in verbal fluency may be a functional explanation for the enhancement of a more positive implicit attitude toward violence. Other studies have suggested that the dlPFC modulates dietary control via vmPFC (Hare et al., 2014). Therefore, reciprocal associative relationships between knowledge concerned with explicit behavioral control stored in the dlPFC and implicit reinforced routines stored in the vmPFC may be a general feature of PFC and subject to dissociative impairments after brain damage. This was confirmed by mediation analysis, which demonstrated that verbal fluency partially mediates the interaction between dlPFC lesions and attitudes toward violence.
Consistent with previous studies on implicit and explicit attitudes, we did not find a significant difference on the explicit measure of aggression among the dlPFC group, the ipTC group, and the other lesions groups. This suggests that the self-report questionnaire may not reflect automatic aggression tendencies due to either self-representation concerns or a lack of awareness, which can be a prominent issue with frontal lobe brain damage. In contrast, implicit measures are derived from more automatic processes that are resistant to biases in, or limitations of, explicit deliberation. Therefore, implicit and explicit test scores do not necessarily correlate. As a result, differences that are masked by controlled, explicit measures can potentially be revealed by automatic, implicit tests (Milne and Grafman, 2001; Gozzi et al., 2009). In interpreting IAT results, caution is always necessary because any correlation between implicit and explicit measures is moderated by such factors as experience, agency, number of exposures, familiarity with the stimuli, and other task demands (Nosek, 2005; Quadflieg et al., 2009). As noted above, implicit, automatic attitudes such as the kind measured with the IAT may better inform how accepting certain groups of individuals are toward violence. In our modified version of the IAT, the fact that the implicit measures correlated with the explicit measures of aggression in the pTBI group suggested good external validity of the test. We did not find significant correlations between the D-scores and observed agitation, anxiety, or hostility using the NBRS (Levin et al., 1987). Further research is needed to detail the relationship between implicit and observed explicit violence.
HCs reported higher explicit aggression (e.g., total AQ total aggression and hostility scores) compared with the pTBI group. Nevertheless, both pTBIs and HCs had AQ scores in the normal range (i.e., between 45 and 55; Buss and Warren, 2000). It is possible that HCs were more aware of their aggressive behaviors and able to explicitly report them.
Although we found that self-report questionnaires did not correlate with implicit violent attitudes, we think that they could still provide useful information, especially if used in combination with implicit measures. Individuals are prone to social desirability bias when they have to disclose personal and sensitive information about themselves, such as negative behaviors like aggression. In addition to the implicit measures, observer reports from caregivers could complete the diagnostic clinical picture of a violent/aggressive patient. Therefore, a combination of implicit and explicit measures may provide useful tools to better monitor violent individuals and prevent future violent behaviors.
Our results add to existing theories of aggression in the human brain. The dlPFC, which is responsible for explicit deliberative thinking about violent contexts/scenarios, could downregulate the vmPFC that stores implicit attitude about violence reinforced through learning and repetition (Grafman et al., 1996). The vmPFC interacts with the anterior temporal lobe, which would likely be a storehouse for social semantics about violence (Zahn et al., 2007), and the posterior temporal lobe, which processes visual information pertaining to violence or aggression. Why did we not find any results linked to the vmPFC lesions? One possible explanation for this is that almost all of the prior studies of aggression linking it to the vmPFC is based upon reports about explicit aggressive behavior (Grafman et al., 1996) rather than an implicit tendency toward aggressive behavior, as investigated in the current study. Implicit aggression could be associated with dlPFC lesions because the dlPFC is associated with poor behavioral control over more automatic behaviors. Therefore, a lesion to the dlPFC would be more likely to lessen inhibition over automatic (i.e., implicit) aggressive reactions to perceived provocation. These automatic aggressive behaviors, we suggest, are more likely to be mediated by vmPFC and to be “released” after a dlPFC lesion.
In our study, we found selective white matter tracts were more damaged in the dlPFC group compared with the vmPFC group. These white matter tract lesions in the dlPFC group could further reduce the regulatory effects of spared dlPFC on the vmPFC (Hare et al., 2009) and other structures contained in an aggression network, leading to less inhibition of implicit attitudes about violence.
Remarkably, we did not find any significant behavioral or lesion-mapping effects for the Weapons IAT. It could be argued that finding results only in the Violence IAT could hamper our overall interpretation of the results. However, we stress that our sample was a retired military population and therefore they might have perceived the guns as a tool for protection and defense. In addition, the difference in results between the Weapon and Violence IAT might be due to different cognitive systems involved in processing in the two tasks. The Weapons IAT used only word stimuli in both attribute and target categories, whereas the violence IAT used both word and picture stimuli.
Our study provides novel insight into the understanding and control of aggressive behaviors. Moreover, our findings are applicable to soldiers who have recently returned from the battlefield given their perspectives on aggression and higher incidence of domestic violence (Finley et al., 2010). Implicit measures, such as the violence IAT, could be applied to detect hidden attitudes toward violence and aggression.
Our sample has the unique advantage of uniformity of age, sex, and education, as well as the availability of preinjury intelligence data (Raymont et al., 2011). However, all our participants were males ∼60 years old during the administration of the tasks. To assess the generalizability of our results, examining implicit attitudes toward violence in a younger population of male and female veterans and nonveterans with and without brain damage is necessary. The signal-to-noise ratio of the effects reported in the confirmatory group analysis (Fig. 2b) might be biased because those effects are based on lesion subgroups derived from the whole-brain VLSM analysis (Fig. 1c).
The dlPFC has a pivotal role in downregulating behaviors (Koechlin et al., 2003). Our results are consistent with this idea. We found that damage to the dlPFC leads to a more positive implicit attitude toward violence that, under most situations, would be considered inappropriate. We suspect that this susceptibility toward an aggressive reaction would be the most apparent in novel or ambiguous circumstances that relied upon a rapid response (e.g., if a driver in another car rolled down their window and made an obscene gesture when complaining about the patient's driving). These results suggest that treatments aimed at increasing cognitive control using cognitive behavioral therapies that depend on the intact dlPFC could treat aggressive and violent behavior (Landenberger and Lipsey, 2005).
Footnotes
We thank our dedicated Vietnam veterans for their invaluable participation in the study; J. Solomon for assistance with ABLe; V. Raymont, S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for testing and evaluating participants; and the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke for their support and provision of their facilities. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government.
The authors declare no competing financial interests.
References
- Amodio DM. The neuroscience of prejudice and stereotyping. Nat Rev Neurosci. 2014;15:670–682. doi: 10.1038/nrn3800. [DOI] [PubMed] [Google Scholar]
- Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27–51. doi: 10.1146/annurev.psych.53.100901.135231. [DOI] [PubMed] [Google Scholar]
- Banse R, Messer M, Fischer I. Predicting aggressive behavior with the aggressiveness-IAT. Aggress Behav. 2014 doi: 10.1002/AB.21574. In press. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Paul EJ, Chau A, Solomon J, Grafman JH. Lesion mapping of social problem solving. Brain. 2014;137:2823–2833. doi: 10.1093/brain/awu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- Bradley MM, Lang PJ. Technical Report C-1. Gainesville, FL: Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): instruction manual and affective ratings. [Google Scholar]
- Bush G, Shin LM, Holmes J, Rosen BR, Vogt BA. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Buss AH, Warren WL. Aggression questionnaire: manual. Los Angeles: Western Psychological Services; 2000. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. doi: 10.1097/00004728-199403000-00005. [DOI] [PubMed] [Google Scholar]
- Dal Monte O, Krueger F, Solomon JM, Schintu S, Knutson KM, Strenziok M, Pardini M, Leopold A, Raymont V, Grafman J. A voxel-based lesion study on facial emotion recognition after penetrating brain injury. Soc Cogn Affect Neurosci. 2013;8:632–639. doi: 10.1093/scan/nss041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RM, Cabral JC, Narvaes R. Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiol Behav. 2015;143:121–135. doi: 10.1016/j.physbeh.2015.02.053. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis–Kaplan Executive Function System: examiner's manual. San Antonio, TX: Psychological Corp; 2001. [Google Scholar]
- Eckhardt CI, Samper R, Suhr L, Holtzworth-Munroe A. Implicit attitudes toward violence among male perpetrators of intimate partner violence: a preliminary investigation. J Interpers Violence. 2012;27:471–491. doi: 10.1177/0886260511421677. [DOI] [PubMed] [Google Scholar]
- Finley EP, Baker M, Pugh MJ, Peterson A. Patterns and perceptions of intimate partner violence committed by returning veterans with post-traumatic stress disorder. J Fam Viol. 2010;25:737–743. doi: 10.1007/s10896-010-9331-7. [DOI] [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Cameron KA, Grafman J, Barbey A, Solomon J, Ritter W, Ruchkin DS. Identifying temporal and causal contributions of neural processes underlying the Implicit Association Test (IAT) Front Hum Neurosci. 2012a;6:320. doi: 10.3389/fnhum.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes CE, Poore JC, Barbey AK, Krueger F, Solomon J, Lipsky RH, Hodgkinson CA, Goldman D, Grafman J. BDNF polymorphism-dependent OFC and DLPFC plasticity differentially moderates implicit and explicit bias. Cereb Cortex. 2012b;22:2602–2609. doi: 10.1093/cercor/bhr337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi M, Raymont V, Solomon J, Koenigs M, Grafman J. Dissociable effects of prefrontal and anterior temporal cortical lesions on stereotypical gender attitudes. Neuropsychologia. 2009;47:2125–2132. doi: 10.1016/j.neuropsychologia.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Jonas BS, Martin A, Salazar AM, Weingartner H, Ludlow C, Smutok MA, Vance SC. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111:169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–1238. doi: 10.1212/WNL.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Gray NS, MacCulloch MJ, Smith J, Morris M, Snowden RJ. Forensic psychology: violence viewed by psychopathic murderers. Nature. 2003;423:497–498. doi: 10.1038/423497a. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JL. Measuring individual differences in implicit cognition: the implicit association test. J Pers Soc Psychol. 1998;74:1464–1480. doi: 10.1037/0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Gross CG. Representation of visual stimuli in inferior temporal cortex. Philos Trans R Soc London B Biol Sci. 1992;335:3–10. doi: 10.1098/rstb.1992.0001. [DOI] [PubMed] [Google Scholar]
- Han SD, Drake AI, Cessante LM, Jak AJ, Houston WS, Delis DC, Filoteo JV, Bondi MW. Apolipoprotein E and traumatic brain injury in a military population: evidence of a neuropsychological compensatory mechanism? J Neurol Neurosurg Psychiatry. 2007;78:1103–1108. doi: 10.1136/jnnp.2006.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, Hakimi S, Rangel A. Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Front Neurosci. 2014;8:50. doi: 10.3389/fnins.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford; 2013. [Google Scholar]
- Hofmann W, Gawronski B, Gschwendner T, Le H, Schmitt M. A meta-analysis on the correlation between the Implicit Association Test and explicit self-report measures. Pers Soc Psychol Bull. 2005;31:1369–1385. doi: 10.1177/0146167205275613. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/S0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Hyer L, Davis H, Boudewyns P, Woods MG. A short form of the Mississippi scale for combat-related PTSD. J Clin Psychol. 1991;47:510–518. doi: 10.1002/1097-4679(199107)47:4<510::AID-JCLP2270470407>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lee & Febiger; 1983. [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal lobe damage: the role of working memory in complex, organized behavior. J Exp Psychol Gen. 1993;122:411–428. doi: 10.1037/0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landenberger NA, Lipsey MW. The positive effects of cognitive–behavioral programs for offenders: a meta-analysis of factors associated with effective treatment. J Exp Criminol. 2005;1:451–476. doi: 10.1007/s11292-005-3541-7. [DOI] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical report A-8. Gainesville, FL: University of Florida; 2008. International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Levin HS, High WM, Goethe KE, Sisson RA, Overall JE, Rhoades HM, Eisenberg HM, Kalisky Z, Gary HE. The neurobehavioural rating scale: assessment of the behavioural sequelae of head injury by the clinician. J Neurol Neurosurg Psychiatry. 1987;50:183–193. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. Am J Psychiatry. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput. 2002;34:6–18. doi: 10.3758/BF03195419. [DOI] [PubMed] [Google Scholar]
- McCollister KE, French MT, Fang H. The cost of crime to society: new crime-specific estimates for policy and program evaluation. Drug and alcohol dependence. 2010;108:98–109. doi: 10.1016/j.drugalcdep.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil MM, Prescott TE. Revised Token Test. Los Angeles: Western Psychological Services; 1994. [Google Scholar]
- Milne E, Grafman J. Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. J Neurosci. 2001;21:RC150. doi: 10.1523/JNEUROSCI.21-12-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Nosek BA. Moderators of the relationship between implicit and explicit evaluation. J Exp Psychol Gen. 2005;134:565–584. doi: 10.1037/0096-3445.134.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Quadflieg S, Turk DJ, Waiter GD, Mitchell JP, Jenkins AC, Macrae CN. Exploring the neural correlates of social stereotyping. J Cogn Neurosci. 2009;21:1560–1570. doi: 10.1162/jocn.2009.21091. [DOI] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds”: the Vietnam head injury study and 40 years of brain injury research. Front Neurol. 2011;2:15. doi: 10.3389/fneur.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Murachver T. Correlates of partner violence for incarcerated women and men. J Interpers Violence. 2007;22:639–655. doi: 10.1177/0886260506298835. [DOI] [PubMed] [Google Scholar]
- Rudman LA, Greenwald AG, McGhee DE. Implicit self-concept and evaluative implicit gender stereotypes: self and ingroup share desirable traits. Person Soc Psychol Bull. 2001;27:1164–1178. doi: 10.1177/0146167201279009. [DOI] [Google Scholar]
- Seidenwurm D, Pounds TR, Globus A, Valk PE. Abnormal temporal lobe metabolism in violent subjects: correlation of imaging and neuropsychiatric findings. Am J Neuroradiol. 1997;18:625–631. [PMC free article] [PubMed] [Google Scholar]
- Shapiro JP. Attitudes Towards Guns and Violence Questionnaire: manual. Los Angeles: Western Psychological Services; 2000. [Google Scholar]
- Silver E, Arseneault L, Langley J, Caspi A, Moffitt TE. Mental disorder and violent victimization in a total birth cohort. Am J Public Health. 2005;95:2015–2021. doi: 10.2105/AJPH.2003.021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JM, Yudofsky SC. Aggressive disorders. In: Silver JM, Yudofsky SC, Hales RE, editors. Neuropsychiatry of traumatic brain injury. Washington, DC: American Psychiatric; 1994. pp. 313–353. [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Comput Methods Programs Biomed. 2007;86:245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the state-trait anger expression inventory (STAXI) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Spielberger CD, Sydeman SJ. State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ: Lawrence Erlbaum Associates; 1994. pp. 292–321. [Google Scholar]
- Tateno A, Jorge RE, Robinson RG. Clinical correlates of aggressive behavior after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2003;15:155–160. doi: 10.1176/jnp.15.2.155. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Truman JL. Criminal victimization. Washington, DC: Bureau of Justice Statistics, US Dept of Justice; 2010. [Google Scholar]
- Tsuchida A, Fellows LK. Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex. 2012;22:2904–2912. doi: 10.1093/cercor/bhr370. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- United States Department of Defense. Armed Forces Qualification Test (AFQT-7A). Form 1293. Washington, DC: United States Department of Defense; 1960. [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St. Edmunds, UK: Thames Valley Test Company; 1991. [Google Scholar]
- Woermann FG, van Elst LT, Koepp MJ, Free SL, Thompson PJ, Trimble MR, Duncan JS. Reduction of frontal neocortical grey matter associated with affective aggression in patients with temporal lobe epilepsy: an objective voxel by voxel analysis of automatically segmented MRI. J Neurol Neurosurg Psychiatry. 2000;68:162–169. doi: 10.1136/jnnp.68.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]


