Abstract
Background
Increased frequency of CCR9+ CD4+ T cells in peripheral blood is linked to several gastrointestinal inflammatory diseases; however, its relationship with necrotizing enterocolitis (NEC) is not understood. We investigated whether the frequencies of CCR9+ CD4+ T cells and related subsets were increased in peripheral blood of both patients and mice with NEC.
Methods
CCR9+ CD4+ T cells and related subsets were evaluated by flow cytometry in peripheral blood collected from both patients and mice with NEC and controls. The suppressive function of CCR9+ regulatory T (Treg) cells in NEC was assessed via in vitro suppression assay. An in vitro T cell polarization assay was performed to investigate the role of proinflammatory cytokines in Treg cell polarization. In vivo Treg cell polarization analysis was performed using NEC mice treated with anti-interleukin-6 (IL-6) receptor antibody.
Findings
A higher proportion of CCR9+ CD4+ T cells occurred in peripheral blood of both patients and mice with NEC than in controls. Elevated CCR9+ CD4+ T cells were primarily CCR9+ IL-17-producing Treg cells, possessing features of conventional Treg cells, but their suppressive activity was seriously impaired and negatively correlated with the severity of intestinal tissue injury. IL-6 promoted polarization of CCR9+ Treg cells to CCR9+ IL-17-producing Treg cells, and blocking IL-6 signalling inhibited this conversion in vitro and ameliorated experimental NEC in vivo.
Interpretation
Collectively, these data suggested that CCR9+ IL-17-producing Treg cells may be a biomarker of severity and highlight the possibility that antibodies targeting IL-6R could ameliorate NEC by modulating lymphocyte balance.
Fund
This work was supported by the Science and Technology Planning Project of Guangdong Province, China (2017A020215100), the Science and Technology Foundation of Guangzhou, China (201704020086 and 201604020154), the Medical Scientific Research Foundation of Guangdong Province, China (A2017304 and A2014704), and the Social Science and Technology Development Foundation of Dongguan, China (2016108101037).
Keywords: CCR9, Interleukin-6, IL-17-producing Treg cell, Necrotizing enterocolitis
Abbreviations: aIL6R, anti-IL6 receptor antibody; ALC, absolute lymphocyte count; FCM, flow cytometry; NEC, necrotising enterocolitis; PBMC, peripheral blood mononuclear cell; STAT, signal transducer and activator of transcription; Th17, interleukin-17 (IL-17)-producing T helper cell; Treg, Foxp3+ regulatory T cell; WBC, white blood cell count
Research in context.
Evidence before this study
The marked elevation of CCR9+ CD4+ T cells in peripheral blood has been linked to several gastrointestinal inflammatory diseases. However, circulating CCR9+ CD4+ T cells have never been investigated in both patients and mice with necrotizing enterocolitis (NEC).
Added value of this study
Findings from this study indicate that peripheral blood CCR9+ IL-17-producing Treg cells were significantly elevated in both patients and mice with NEC and the suppressive activity was seriously impaired. IL-6 could promote the conversion of CCR9+ Treg cells to CCR9+ IL-17+ Treg cells and blocking IL-6 signalling inhibited the above conversion. More importantly, treatment with antibodies targeting IL-6R could ameliorate NEC severity.
Implications of all the available evidence
CCR9+ IL-17-producing Treg cells may be a biomarker of severity, and our research highlights the possibility that antibodies targeting IL-6R could ameliorate NEC by modulating lymphocyte balance.
Alt-text: Unlabelled Box
1. Introduction
Necrotizing enterocolitis (NEC) remains a leading cause of morbidity and mortality in preterm infants [1,2]. The pathophysiology of the disease is characterized by an excessive inflammatory response and necrosis that can affect any part of the gastrointestinal tract, though it often targets the small bowel [1,2]. Moreover, although prematurity, bacterial colonization, formula feeding, and several other factors may predispose the intestinal mucosa to an excessive inflammatory response state in NEC [1,2], the underlying regulatory mechanism of mucosal imbalance remains largely unknown.
An imbalance caused by diminished tolerogenic Foxp3+ regulatory T (Treg) cells and increased proinflammatory interleukin-17 (IL-17)-producing T helper 17 (Th17) cells in lamina propria contributes to the excessive inflammatory response caused by NEC [[3], [4], [5]]. Consistently, a recent study also showed that the infiltration of T cells to intestinal tissue and polarization of these cells towards Th17 cells over Treg cells led to necrosis of intestinal tissue in mice [3]. T cell migration from peripheral blood to small intestine is largely regulated by chemokine (CC motif) receptor 9 (CCR9) [6,7]. Intriguingly, the frequency of circulating CCR9+ CD4+ T cells was found to be increased in patients with gastrointestinal diseases such as small bowel Crohn's disease [8], functional dyspepsia [9], and delayed gastric emptying [10]. These findings reveal the importance of circulating CCR9+ CD4+ T cells in gastrointestinal disease, but whether or not this population has critical role in NEC remains unclear.
Treg and Th17 cells can develop from the same pool of naive T cells under a distinct microenvironment [11,12] and, in some conditions, can also trans-differentiate [[13], [14], [15]]. Further, an intermediate subset of IL-17-producing Treg (IL-17+ Treg) cells can be generated from Treg cells upon polarization by cytokines such as IL-6 [[16], [17], [18]]. Accumulating data demonstrated that circulating IL-17+ Treg cells are increased and play a critical role in inflammatory diseases such as inflammatory bowel disease (IBD) [19], psoriasis [20], and rheumatoid arthritis (RA) [13]. Interestingly, recent studies showed that the levels of IL-6 are significantly elevated in peripheral blood and intestinal tissue of patients with NEC [5,21]. Considering the critical role of circulating CCR9+ CD4+ T cells in gastrointestinal disease [[8], [9], [10]], we hypothesized that increased IL-6 might promote CCR9+ IL-17+ Treg cell polarization and accelerate the development of NEC.
In the present study, we investigated the CCR9+ IL-17+ Treg cells in both patients and mice with NEC and found this population was markedly elevated in peripheral blood. Elevated CCR9+ IL-17+ Treg cells exhibited phenotypic characteristics of Treg and Th17 cells, but the immunosuppressive activity was impaired in vitro. Furthermore, IL-6 promoted the polarization of CCR9+ IL-17+ Treg cells, while blocking IL-6 mediated signalling inhibited this conversion in vitro and ameliorated experimental NEC in vivo. In addition, circulating CCR9+ IL-17+ Treg cells negatively correlated with the the severity of intestinal tissue injury. Thus, we reveal an IL-6- CCR9+ IL-17+ Treg cells regulatory axis in NEC that correlates immune activation and disease progression.
2. Materials and methods
2.1. Study subjects
Peripheral blood samples were collected from 77 preterm neonates diagnosed with NEC (Bell stage I, n = 28; Bell stage II, n = 30; Bell stage III, n = 19) according to the clinical manifestations and radiographic findings using modified Bell's criteria [22], born at the Sixth Affiliated Hospital, Sun Yat-sen University, the Foshan Women and Children's Hospital, the Guangdong Women and Children Hospital, and the Fifth People's Hospital of Dongguan between March 2016 and September 2017. In the 19 patients having Bell stage III NEC, there were 11 that required surgical intervention and one NEC totalis patient who died. Blood specimens from another 80 gestational age (GA)-, birth weight (BW)-, and sex-matched preterm neonates admitted to the neonatal intensive care unit (NICU) without NEC during the same period served as control subjects (Table 1, Table 2). All infants recruited in this study were born at GA < 37 weeks and had no major congenital malformations. Spontaneous intestinal perforation (SIP) was distinguished through clinical and radiological testing and defined as the presence of an isolated intestinal perforation surrounded by apparently normal bowel tissues and the absence of characteristic gross or microscopic features of NEC through a laparotomy [23]. Infants diagnosed with SIP were excluded from our analysis. This study protocol was performed according to the Declaration of Helsinki and was approved by the Ethics Committee of the Sixth Affiliated Hospital, Sun Yat-sen University and the Fifth People's Hospital of Dongguan (2016001). Written informed consent was obtained from the parents of each participant.
Table 1.
Demographic and Clinical Characteristics of NEC and Control Subjects.
| Parameter | Controls | Total NEC | P | NEC Stage |
P | ||
|---|---|---|---|---|---|---|---|
| I | II | III | |||||
| Number | 80 | 77 | – | 28 | 30 | 19 | – |
| Sex (male/female) | 42/38 | 40/37 | 0.945 | 16/12 | 13/17 | 11/8 | 0.481 |
| GA (week)a | 32.14 ± 2.73 | 31.45 ± 2.23 | 0.086 | 31.29 ± 2.17 | 31.82 ± 2.31 | 31.13 ± 2.23 | 0.511 |
| BW (g)a | 1684 ± 431 | 1586 ± 380 | 0.134 | 1616 ± 391 | 1647 ± 396 | 1445 ± 314 | 0.169 |
| Cesarean section, n (%) | 59 (73.75) | 56 (72.73) | 0.885 | 23 (82.14) | 21 (70) | 12 (63.16) | 0.326 |
| 5-min apgar ≤5, n (%) | 7 (8.75) | 6 (7.79) | 0.828 | 2 (7.14) | 2 (6.67) | 2 (10.53) | 0.875 |
| Postnatal age (d)a | 14.80 ± 7.33 | 15.08 ± 7.32 | 0.812 | 15.82 ± 7.90 | 14.00 ± 7.60 | 15.68 ± 6.05 | 0.592 |
| FOBT | 0 | 77 (100) | – | 28 (100) | 30 (100) | 19 (100) | – |
| Blood pathogen culture | 0 | 16 (20.78) | – | 2 (7.14) | 6 (20) | 8 (42.11) | 0.015 |
| E coli | 0 | 8 (50) | – | 2 (12.5) | 3 (18.75) | 3 (18.75) | 0.632 |
| Klebsiella spp | 0 | 4 (25) | – | 0 | 2 (12.5) | 2 (12.5) | 0.251 |
| Enterococcus faecium | 0 | 2 (12.5) | – | 0 | 1 (6.25) | 1 (6.25) | 0.511 |
| Pseudomonas aeruginosa | 0 | 1 (6.26) | – | 0 | 0 | 1 (6.25) | 0.213 |
| Fungi | 0 | 1 (6.25) | – | 0 | 0 | 1 (6.25) | 0.213 |
| Surgical, n (%) | 0 | 11 | – | 0 | 0 | 11 | – |
| NEC totalis, n (%) | 0 | 1 | – | 0 | 0 | 1 | – |
| Died, n (%)b | 0 | 1 | – | 0 | 0 | 1 | – |
NEC, necrotizing enterocolitis; GA, gestational age; BW, Birth weight; FOBT, fecal occult blood testing.
Mean ± SD.
The patient died from NEC totalis.
Table 2.
Demographic and Clinical Characteristics of surgical and nonsurgical patients of Bell stage III.
| Parameter | NEC Stage III |
P | |
|---|---|---|---|
| Surgical | Nonsurgical | ||
| Number | 11 | 8 | |
| Sex (male/female) | 7/4 | 4/4 | 0.658 |
| GA (week)a | 31.10 ± 2.66 | 31.16 ± 1.63 | 0.657 |
| BW (g)a | 1476 ± 347 | 1403 ± 278 | 0.626 |
| Cesarean section, n (%) | 7 (63.63) | 5 (62.50) | 0.663 |
| 5-min apgar ≤ 5, n (%) | 1 (9.09) | 1 (12.50) | 1.0 |
| Postnatal age (d)a | 16.91 ± 7.06 | 14.00 ± 4.41 | 0.314 |
| FOBT | 11 (100) | 8 (100) | – |
| Blood pathogen culture | 5 (45.45) | 3 (37.5) | 0.551 |
| E coli | 2 (25) | 1 (12.5) | 0.624 |
| Klebsiella spp | 1 (12.5) | 1 (12.5) | 0.322 |
| Enterococcus faecium | 1 (12.5) | 0 | 0.579 |
| Pseudomonas aeruginosa | 1 (12.5) | 0 | 0.579 |
| Fungi | 1 (12.5) | 0 | 0.579 |
NEC, necrotizing enterocolitis; GA, gestational age; BW, Birth weight; FOBT, fecal occult blood testing.
Mean ± SD.
2.2. Sample collection
One-millilitre peripheral venous blood samples used for the measurement of cytokines, intestinal barrier integrity biomarkers, and T cell subsets was obtained at the time of diagnosis before the initiation of treatment. For some patients whose peripheral venous blood were also tested for Treg cell polarization, suppressive activity and the genes expression of Foxp3 and RORγt, the lymphocytes were collected from additional 2 ml blood samples which were used for biochemical testing. In the control subjects, blood samples were taken after written informed consent was obtained.
2.3. Isolation of Treg and CCR9+ CD4 T cells
Peripheral blood mononuclear cells (PBMCs) and cord blood mononuclear cells (CBMCs) were isolated by density gradient centrifugation with Ficoll-Hypaque (GE Healthcare, Little Chalfont, UK). Next, Treg cells were isolated from PBMCs and CBMCs using the CD4+ CD25+ CD127dim/− regulatory T cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany), because identifying the expression of CD127 in combination with CD4 and CD25 is a reliable strategy to discriminate Treg cells (CD127dim/−) from conventional T cells [24,25], according to the manufacturer's instructions. CD4+ CD25+ CD127dim/− cells were negatively purified by a cocktail of biotin-conjugated antibodies and anti-biotin monoclonal antibodies conjugated to MicroBeads. Then, CD4+ CD25+ CD127dim/− Treg cells were directly labelled with CD25 MicroBeads and isolated using a selection column (Miltenyi Biotec). Simultaneously, we also separated CD25+ and CD25− T cells from PBMCs according to those described by Yates et al. [26]. For CCR9+ CD4+ T and CCR9+ CD4+ CD25+ CD127dim/− Treg cell isolation, PBMCs, CBMCs or CD4+ CD25+ CD127dim/− Treg cells were stained with fluorochrome-conjugated anti-human antibodies against CD3, CD4, and CCR9 (Table S1) and sorted using the Aria II cell sorter (BD Biosciences, San Jose, CA, USA). The purity of all isolated cell subsets was >90%.
2.4. Treg cell polarization assay
Treg cell polarization assay were cultured according to a previous report [13] and our preliminary experiment (Fig. S1). Briefly, purified CCR9+ CD4+ CD25+ CD127dim/− Treg cells were cultured in TexMACS medium (Miltenyi Biotec) supplemented with 50 μM 2-mercaptoethanol (Thermo Fisher Scientific, Waltham, MA, USA), recombinant human interleukin (IL)-2 (rhIL-2, 100 IU/ml, Peprotech, Rocky Hill, NJ, USA), and penicillin/streptomycin (100 IU/ml, Invitrogen, Carlsbad, CA, USA) and then stimulated with anti-CD3/CD28-coated microbeads (Miltenyi Biotec). For Th17 cell polarization, cells were treated with recombinant human IL-1β (rhIL-1β, 10 ng/ml, Peprotech) recombinant human IL-6 (rhIL-6, 20 ng/ml, Peprotech), or both; when indicated, cells were further treated with neutralizing antibodies against IL-1β (10 μg/ml, R&D Systems, Minneapolis, MN, USA), IL-6 receptor (10 μg/ml, R&D Systems), or both for 4 or 8 days.
2.5. T cell proliferation and suppression assay
Suppression of responder CD4+ T cells proliferation by CD4+ CD25+ Treg cells was assessed using the standard carboxyfluorescein diacetatesuccinimidyl ester (CFSE) dilution method [27]. Briefly, freshly sorted CCR9+ CD4+ CD25+ Treg cells or sorted CCR9+ Treg and CCR9+ IL-17+ Treg cells (5 × 104) from cultured cord blood CCR9+ Treg cells under Th17-polarizing conditions were co-cultured with purified CFSE-labelled CD4+ CD25− responder T cells at a 1:1 ratio in RPMI-1640 medium containing 10% fetal bovine serum (Invitrogen), recombinant human interleukin (IL)-2 (rhIL-2, 100 IU/ml, Peprotech), and penicillin/streptomycin (100 IU/ml, Invitrogen), and then stimulated with anti-CD3/CD28-coated microbeads (Miltenyi Biotec). Activated and non-activated CD4+ CD25− responder T cells without CCR9+ CD4+ CD25+ Treg cells were used as controls. On Day 5, Tresp cell proliferation was determined by flow cytometry.
2.6. Quantitative real-time PCR
Total RNA was extracted from CCR9+ CD4 T cells using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. cDNA was synthesized with M-Mulv Reverse Transcriptase (Promega, Madison, WI, USA) and amplified using the Fast SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in the 7500 real-time PCR system (Applied Biosystems) for each target gene (Table S2). The relative expression of each gene was quantified after normalization against β-actin. All standards and samples were tested in triplicate wells. Data were presented as arbitrary units and calculated as 2(Ct(β-actin – gene of interest)).
2.7. Induction of experimental NEC
All experiments were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University. Ten-day-old C57BL/6 mouse pups were collected from the Experimental Animal Center of Southern Medical University (Guangzhou, China) and NEC-like injury was induced as previously described [28] using formula [Similac Advance infant formula (Abbott Nutrition, Columbus, OH, USA):Esbilac (PetAg) milk replacer for Puppies, 2:1] containing enteric bacteria from a patient with surgical NEC (12.5 μl original stool slurry in 1 ml formula) via gavage five times daily. The mice were simultaneously exposed to hypoxic conditions (5% O2, 95% N2) for 10 min twice daily in a modular chamber (Billups-Rothenberg, San Diego, CA, USA) for 4 days. Pups were fed 50 μl/g body weight gavage over 2–3 min, using a single oral gavage via fine polyethylene tubing. For the inhibition of IL-6 upon the onset of NEC, mice were inoculated with 100 ng anti-IL-6 receptor (NEC + aIL6R group) or control IgG (NEC + cIgG group) antibodies via intraperitoneal injection once daily. According to our preliminary experiment (Fig. S2, a–c), control animals were left with their dams to breastfeed. Animals were euthanized on day 5 after NEC induction, or earlier if they demonstrated moribund signs.
2.8. Tissue collection and injury evaluation
After the animals were sacrificed, the terminal 5 cm of the small intestine (ileum) was removed. The terminal 0.5 cm of each sample was fixed with 10% formalin. Fixed tissues were embedded in paraffin, then sectioned to 5-μm slices, and stained with hematoxylin and eosin (H&E) for histological evaluation. The remaining 4.5 cm of the ileum was used for tissue preparation or isolation of lymphocytes. Two independent pathologists, blinded to the study conditions, determined the severity of mucosal injury. The histological scoring system was graded as follows: grade 0, normal intestine; grade 1, epithelial lifting or separation; grade 2, sloughing of epithelial cells to the midvillus level; and grade 3, necrosis of the entire villus. Tissues with histologic scores ≥ 2 were considered as having NEC [29,30].
2.9. Tissue preparation for immunoblot assay
Total protein from mouse ilea was prepared using a total protein extraction kit (Applygen, Beijing, China) according to the manufacturer's instructions. Protein samples were resolved by SDS-PAGE on pre-cast 4–15% gels (Bio-Rad, Hercules, CA, USA) and transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Burlington, MA, USA) and incubated overnight at 4 °C with rabbit polyclonal antibodies against Foxp3 (ab10901), RORγt (ab207082), STAT3 (ab68153), p-STAT3 (phospho S727, ab30647), STAT5 (ab16276), p-STAT5 (phospho Y694, ab32364), and β-actin (ab179467, Abcam, Cambridge, UK). Horseradish peroxidase-conjugated anti-rabbit polyclonal antibodies (Goat anti-rabbit IgG-HRP, ab6721, Abcam) were used as secondary antibodies and detected using enhanced chemiluminescence (ECL) substrate (Bio-Rad). Band densitometry was performed using Image Lab software (Bio-Rad). The relative index was represented as the ratio of the selected protein/β-actin, and was the average of three biological replicates.
2.10. Preparation of lamina propria mononuclear cells for flow cytometry
To obtain T cell-enriched lamina propria mononuclear cells (LPMCs), the Lamina Propria Dissociation Kit (Miltenyi Biotec) was used according to the manufacturer's instructions. Briefly, mouse ileum specimens were cleaned of mesentery, opened longitudinally, gently fragmented with scissors, and incubated in a pre-digestion solution at 37 °C. Tissues were incubated for 20 min with continuous shaking. Supernatants containing the intraepithelial lymphocytes (IELs) were then discarded. The remaining tissues were incubated in digestion solution at 37 °C for 30 min with continuous shaking. Cells were then washed with PB buffer (PBS with 0.5% BSA) and resuspended in PB buffer for further application.
2.11. ELISA measurement
The concentration of cytokines and intestinal barrier integrity biomarkers in blood plasma was tested using commercial ELISA kits for IL-1β, IL-6, trefoil factor 3 (TFF3), intestinal-fatty acid binding protein (I-FABP), and zonulin (CUSABIO, Wuhan, China) according to the manufacturer's protocols.
2.12. Flow cytometry
For surface staining of immune markers, fresh PBMCs or LPMCs and in vitro cultured Treg cells (1 × 106/ml) were pretreated with Fc-blocking reagent (eBioscience, Waltham, MA, USA) to block nonspecific binding, and then different combinations of fluorochrome-coupled antibodies (Supplementary Table 1) were added and samples were incubated on ice for 20 min. Intracellular detection of mouse Foxp3 and RORγt was performed on fixed and permeabilized cells using Cytofix/Cytoperm (BD Biosciences). For the detection of intracellular cytokine production, PBMCs or in vitro cultured Treg cells were stimulated with the Leukocyte Activation Cocktail (BD Biosciences) in the presence of brefeldin A protein transport inhibitor (BD Biosciences) for 5 h, and then stained with fluorochrome-coupled antibodies against Foxp3, RORγt, IL-10, and IL-17A (Table S3) after fixation and permeabilization. Fluorescence data were acquired using FACS Canto II (BD Biosciences) and analysed with FlowJo software (FlowJo, Ashland, OR, USA).
2.13. Statistical analysis
Unless otherwise specified, data are expressed as the mean ± standard deviation (SD) and were analysed using Prism software version 7.0 (GraphPad, La Jolla, CA, USA) and SPSS software version 21 (IBM, Hampshire, UK). Statistical significance between two groups was analysed using the nonparametric Mann-Whitney U–test or Student's t-test. Differences among three or more groups were evaluated with Kruskal-Wallis with paired comparisons test or one-way ANOVA with Bonferroni multiple comparison test. Potential correlations were examined using Spearman's rank correlation test. A value of P ≤ .05 was considered statistically significant.
3. Results
3.1. CCR9+ IL-17+ Treg cells are elevated in peripheral blood of NEC patients
To determine the change of CCR9+ T cells in the peripheral blood of patients with NEC, we performed quantitative flow cytometric analysis of CCR9 expression in CD3+ T cells and found the frequency of CCR9+ CD3+ T cells was substantially increased in patients with NEC compared to controls (Fig. 1, a and b, top). Furthermore, we found that the increase in CCR9+ CD3+ T cells occurred primarily in CCR9+ CD4+ T cells (Fig. 1, a and b, middle), but not CCR9+ CD8+ T cells (Fig. 1, a and b, bottom). Additionally, we also examined the proportion of CD3, CD4, CD8, and CD19 lymphocyte subsets (Fig. S3a) but found that the absolute numbers and frequencies of the above subsets were not significantly different in NEC patients and controls (Fig. S3b). These data indicate that the frequency of peripheral blood CCR9+ CD4+ T cells is significantly increased in patients with NEC.
Fig. 1.
CCR9+ CD4+ T cells and related subset in peripheral blood from NEC patients and controls. (a and b) CCR9 expression in lymphocytes was assessed by flow cytometry in peripheral blood mononuclear cells from NEC patients (n = 77) and controls (CTRL; n = 80). (a) Representative flow cytometric plots of CCR9 expression in gated CD3+ T cells (top), CD4+ T cells (middle) and CD8+ T cells (bottom). Numbers on the representative flow cytometry graph indicate the percentage of CCR9+ cells in that subset. (b) The absolute numbers (left) and frequencies (right) of CCR9+ CD3+ T cells (top), CCR9+ CD4+ T cells (middle), and CCR9+ CD8+ T cells (bottom). P-values were calculated using Mann-Whitney U–test. (c) The expression of IL-17 and Foxp3 in CCR9+ CD4+ T cells from patients with NEC (n = 6) and controls (CTRL; n = 7) was evaluated by quantitative real-time PCR (qRT-PCR). ns: not significant, *, P < .05. (D - F) The expression of IL-17 and Foxp3 in CCR9+ CD4+ T cells from patients with NEC and controls (CTRL) was evaluated by intracellular cytokine staining and flow cytometry. (d) Representative flow cytometric plots showing IL-17 expression in CCR9+ CD4+ Foxp3+ Treg (CCR9+ Treg) cells in patients with NEC and controls. (e) The frequency of CCR9+ IL-17+ Treg cells in patients with NEC (n = 65) and CTRL (n = 66). ***, P < .001. (f) Spearman correlation (r) between the frequencies of CCR9+ CD4+ T cells and CCR9+ IL-17+ Treg cells in NEC patients (n = 65) and CTRL (n = 66).
Next, we investigated whether CCR9+ IL-17+ Treg cells accounted for the majority increase of CCR9+ CD4+ T cells. Since IL-17+ Treg cells co-express Foxp3 and RORγt [18], we analysed their expression in CCR9+ CD4+ T cells from patients with NEC and controls. Our analysis revealed a slight but not significant decrease in Foxp3, as well as a significant increase in RORγt, in NEC patients compared to controls (Fig. 1c), demonstrating that CCR9+ IL-17+ Treg cells might be elevated in patients with NEC. Flow cytometry confirmed the presence CCR9+ IL-17+ Treg cells within the subset of CCR9+ CD4+ T cells from NEC patients and controls (Fig. 1d), and the frequency and absolute number of CCR9+ IL-17+ Treg cells were significantly higher in patients with NEC than in controls (Fig. 1e and Fig. S4a). More strikingly, in patients with NEC, the frequency and absolute number of CCR9+ IL-17+ Treg cells showed a significant positive correlation with CCR9+ CD4+ T cells (Fig. 1f and Fig. S4b). Indeed, the frequency of CCR9+ RORγt+ Foxp3+ Treg cells, especially CCR9+ IL-17+ RORγt+ Foxp3+ Treg cells, were also significantly elevation in patients with NEC than in controls (Fig. S5, a - c). Collectively, these findings suggest that the primary subset of elevated CCR9+ CD4+ T cells in peripheral blood of NEC patients was CCR9+ IL-17+ Treg cell subset.
3.2. Peripheral CCR9+ IL-17+ Treg cells from NEC patients exhibit Th17- and Treg-like features
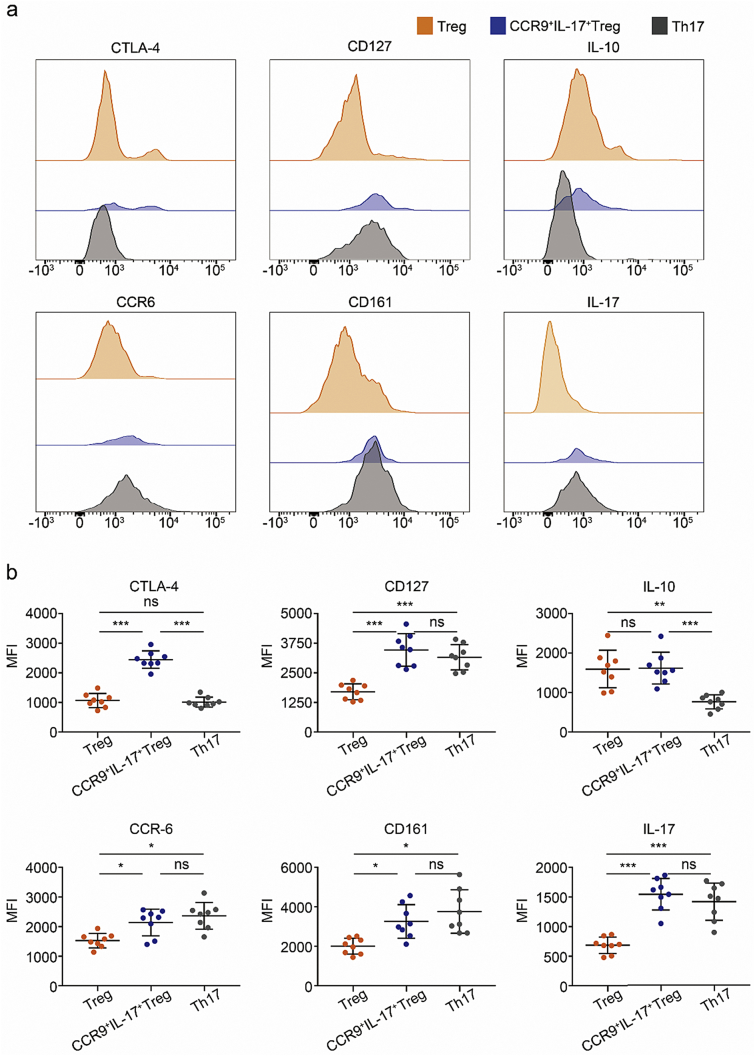
To determine the characteristics of CCR9+ IL-17+ Treg cells in patients with NEC, we first assessed the expression levels of regulatory factors known to be associated with Treg cells, such as CTLA-4 and IL-10 [31]. Notably, CCR9+ IL-17+ Treg cells expressed higher levels of CTLA-4 and similar levels of IL-10 compared to conventional Treg cells (Fig. 2, a and b). Conventional Treg cells are known to express low levels of CD127 [24,25]; furthermore, CCR6, CD161, and IL-17 are human Th17 cell markers [32]. Intriguingly, as in Th17 cells, CCR9+ IL-17+ Treg cells expressed higher levels of CCR6, CD161, IL-17, and CD127 compared to conventional Treg cells (Fig. 2, a and b). These data demonstrate that CCR9+ IL-17+ Treg cells exhibit phenotypes of conventional Th17 and Treg cells.
Fig. 2.
CCR9+ IL-17+ Treg cells are phenotypically similar to Treg and Th17 cells. (a–b) Three subsets of CD4+ T cells defined by the expression of Foxp3, CD127, IL-17, and CCR9: conventional Treg cells (Treg), CCR9− Foxp3+ CD127dim/− cells; conventional Th17 cells (Th17), CCR9− Foxp3− IL-17+ cells; CCR9+ IL-17+ Treg cells (CCR9+ IL-17+ Treg), CCR9+ Foxp3+ CD127dim/− IL-17+ cells. Surface markers or cytokines of CD4+ Foxp3+ Treg cells, CCR9+ IL-17+ Treg cells, and Th17 cells were examined by flow cytometry after stimulation for 5 h with Leukocyte Activation Cocktail in the presence of brefeldin A protein transport inhibitor. Representative histograms (a) and mean fluorescence intensity (b) showing the expression of CTLA-4, CD127, CCR6, and CD161; the expression of the intracellular cytokines IL-10 and IL-17 was measured in the above samples. n = 8; *, P < .05; **, P < .01; ***, P < .001; ns: not significant; MFI, mean fluorescence intensity. P-values were calculated using Kruskal-Wallis with paired comparisons test.
3.3. Peripheral CCR9+ IL-17+ Treg cells from NEC patients exhibit impaired suppressive function
To further investigate whether CCR9+ IL-17+ Treg cells from patients with NEC possessed suppressive function, we sorted CCR9+ CD4+ CD25+ CD127dim/− Treg (CCR9+ Treg) cells from NEC patients and controls. In accordance with our findings (Fig. 1, d and e), the frequency of CCR9+ IL-17+ Treg cells in freshly isolated CCR9+ Treg cells was also significantly increased in patients with NEC compared to controls (Fig. 3, a and b). Considering the limited blood volume in preterm infants and the distinct proportion of CCR9+ IL-17+ Treg cells in CCR9+ Treg cells (Fig. 3, a and b), we analysed the function of CCR9+ Treg cells to reflect CCR9+ IL-17+ Treg cells cells in NEC patients and controls. Interestingly, the CCR9+ Treg cells from NEC patients showed reduced suppression activity on CD4+ CD25− responder T cells compared to controls (Fig. 3, c and d). When we purified CCR9+ Treg and CCR9+ IL-17+ Treg cells from cultured cord blood CCR9+ Treg cells under Th17-polarizing conditions, we also found that the suppression activity of CCR9+ IL-17+ Treg cells was significantly decrease compared to CCR9+ Treg cells (Fig. S6, a and b).
Fig. 3.
The suppression activity of CCR9+ IL-17+ Treg cells is impaired in patients with NEC. (a) Representative intracellular staining for CCR9+ IL-17+ Treg cells in gated freshly isolated CCR9+ CD4+ CD25+ CD127dim/− Treg (CCR9+ Treg) cells in NEC patients (n = 4) and controls (CTRL; n = 4). (b) The frequencies of CCR9+ IL-17+ Treg cells in freshly isolated CCR9+ Treg cells in NEC patients (n = 4) and CTRL (n = 4). *, P < .05. (c) A representative T cell proliferation and suppression assay of freshly sorted CCR9+ Treg cells (5 × 104) isolated from a NEC patient and a CTRL co-cultured with purified CD4+ CD25− responder T cells at a 1:1 ratio. Responder CD4+ T cells were stained with carboxyfluorescein diacetatesuccinimidyl ester (CFSE) and stimulated with anti-CD3/CD28-coated microbeads, and their proliferation in 5 days was determined by flow cytometry. Activated (Teff) and nonactivated (unstimulated) CD4+ T cells without Treg were used as controls. (d) The proliferation of responder CD4+ T cells in different situations. n = 4. P-values were calculated using Student's t-test or one-way ANOVA with Bonferroni multiple comparison test. (e) Distribution of CCR9+ IL-17+ Treg cells in CD127dim/− CCR9+ Treg fractions defined based on their expression of CD45RA and Foxp3. The numbers on the representative flow cytometry graph shows the number of CCR9+ IL-17+ Treg cells in each CCR9+ Treg fraction; percentages (shown in round brackets) indicate the frequencies of CCR9+ IL-17+ Treg cells within each fraction of the total CCR9+ Treg cells from a representative sample. (f) The percentage distribution of CCR9+ IL-17+ Treg cells within each fraction of the total CCR9+ Treg cells. n = 5. Fr: Fraction; Fr I: CD45RA+ Foxp3low resting Treg cells; Fr II: CD45RA− Foxp3hi activating Treg cells; Fr III: CD45RA− Foxp3low non-Treg cells. P-values were calculated using Mann-Whitney U–test or Kruskal-Wallis with paired comparisons test.
A previous study demonstrated that Treg cells could be classified into three functionally distinct subpopulations, CD45RA+ Foxp3low resting Treg cells, CD45RA− Foxp3hi activated Treg cells, both of which have suppressive function in vitro, and cytokine-secreting CD45RA− Foxp3low nonsuppressive T cells [18]. We further investigated the subsets of CCR9+ Treg cells responsible for the secreting of IL-17. We found that over 75% of CCR9+ IL-17+ Treg cells were enriched in the CCR9+ CD45RA− Foxp3low fraction, approximately 20% of cells formed the CCR9+ CD45RA+ Foxp3low fraction, and <5% of cells were in the CCR9+ CD45RA− Foxp3hi fraction (Fig. 3e, f), indicating that most of the CCR9+ IL-17+ Treg cells are nonsuppressive Treg cells. Together, these data indicate that the suppressive activity of CCR9+ IL-17+ Treg cells in NEC patients is markedly impaired.
3.4. IL-6 promotes the polarization of CCR9+ IL-17+ Treg cells from NEC patients
To characterise the inflammatory milieu contributing to the polarization of CCR9+ IL-17+ Treg cells in peripheral blood from patients with NEC, we examined the levels of TGF-β, IL-1β, IL-2, IL-6, IL-21, and IL-23 in plasma samples and found unchanged levels of IL-2, IL-21 and IL-23 but significantly increased levels of IL-1β and IL-6 in NEC patients compared to that of controls (Fig. S7). Since IL-6 and IL-1β together promote the development and expansion of Th17 cells [13,15], increased levels of the two cytokines in patients with NEC may also create the ideal conditions for the expansion of IL-17+ Treg cells [13,33]. To test this hypothesis, we cultured the purified CCR9+ Treg cells from the peripheral blood of patients with NEC and controls for 4 days (Fig. 4a) and identified a relatively low frequency of CCR9+ IL-17+ Treg cells in culture without the addition of IL-6 or IL-1β. In the presence of IL-1β, there was a slight but not marked elevation in the frequency of CCR9+ IL-17+ Treg cells. However, when cells were cultured with IL-6 or IL-6 and IL-1β, the frequency of CCR9+ IL-17+ Treg cells was significantly increased (Fig. 4b). A significant increase in the mean fluorescence intensity (MFI) of RORγt was also seen when cells were cultured with IL-6 or IL-6 with IL-1β (Fig. 4, c and d). Moreover, a neutralizing antibody against IL-6 receptor (anti-IL6R), but not against IL-1β, inhibited the generation of CCR9+ IL-17+ Treg cells when Treg cells were cultured with IL-6 and IL-1β (Fig. 4, e and f). Taken together, these findings indicate that IL-6 plays a crucial role in the polarization of CCR9+ Treg cells to CCR9+ IL-17+ Treg cells.
Fig. 4.
Inducing peripheral CCR9+ Treg cells towards CCR9+ IL-17+ Treg cells polarization under NEC inflammatory conditions. (a–d) CCR9+ CD4+ CD25+ CD127dim/− Treg (CCR9+ Treg) cells were cultured with IL-1β, IL-6, or both in the presence of anti-CD3/CD28 antibody-coated microbeads and IL-2 for 4 days. (a) IL-17 and Foxp3 expression levels in CCR9+ Treg cells from patients with NEC and controls (CTRL). (b) The frequency of CCR9+ IL-17+ Treg cells in patients with NEC and CTRL. Representative histograms (c) and mean fluorescence intensity (d) showing the expression of RORγt in CCR9+ Treg cells in the presence or absence of IL-1β or IL-6. n = 5; MFI, mean fluorescence intensity. (e and f) CCR9+ Treg cells were stimulated with IL-1β, IL-6, IL-2, and anti-CD3/CD28 antibody-coated microbeads in the presence of antibodies targeting IL-6 receptor (aIL-6R), IL-1β, or both for 4 days. (e) Representative flow cytometric plots showing IL-17 and Foxp3 expression levels in CCR9+ Treg cells from patients with NEC. (f) The frequency of CCR9+ IL-17+ Treg cells in the presence or absence of neutralizing antibodies to IL-6R, IL-1β, or both in vitro. n = 5; *, P < .05; **, P < .01; ns: not significant. P-values were calculated using one-way ANOVA with Bonferroni multiple comparison test.
3.5. Treatment with antibodies targeting IL-6 receptor ameliorated NEC in mice
Considering the ability of the anti-IL6R antibody to inhibit IL-17+ Treg cells polarization in vitro (Fig. 4, e and f) and imbalance of Treg/Th17 cells in NEC tissues [3], we assessed whether treatment with this antibody would ameliorate NEC. Notably, in mice with NEC, treatment with the anti-IL6R antibody resulted in a significant reduction in NEC mortality (Fig. 5a), severity (Fig. 5, b and c), and morbidity (Fig. 5d) compared to control IgG antibody treatment. Most significantly, treatment with the anti-IL6R antibody resulted in a significant increase in Treg cells and a reduction in Th17 and IL-17+ Treg cells compared to those with control IgG antibody treatment, as revealed by flow cytometry (Fig. 5, e and f) and immunoblotting (Fig. 5, g and h). Because signal transducer and activator of transcription (STAT) activity plays an important role in the differentiation and balance of Th17 and Treg cells [34], we assessed the phosphorylation of STAT3 and STAT5, which are important for the generation of Th17 and Treg cells, respectively. Immunoblotting indicated that STAT3 phosphorylation was significantly decreased, while STAT5 phosphorylation was markedly increased by anti-IL6R antibody treatment compared to that by control IgG antibody treatment (Fig. 5, g and h). These data suggest the robust therapeutic benefit by anti-IL6R antibody is dependent on the reduced IL-17+ Treg and Th17 cells polarization through IL-6-mediated STAT3 and STAT5 phosphorylation.
Fig. 5.
Inhibiting IL-6R ameliorates NEC by restoring the balance of Treg/Th17 cells in NEC mice. (a) Experimental design (top) and Kaplan-Meier analysis of the survival rate for dam-fed pups (CTRL) and NEC pups treated with control IgG (NEC + cIgG) or anti-IL-6 receptor (NEC + aIL6R) antibodies from the first day of induction of NEC. Data are pooled from three independent experiments; *, P < .05; **, P < .01. (b) Representative intestinal histological changes in CTRL, NEC + cIgG, and NEC + aIL6R pups. Ileal tissues were stained with hematoxylin and eosin. Magnification × 200. (c) NEC severity scores of the histopathological evaluation of mouse ilea (n = 15 for CTRL, 18 for NEC + cIgG, and 22 for NEC + aIL6R groups), graded microscopically by two independent pathologists. *, P < .05; **, P < .01. (d) Incidence of NEC (damage scores >2) in NEC + cIgG and NEC + aIL6R groups. Columns represent the average values of each group (three independent experiments, n = 30); *, P < .05. (e) Representative flow cytometric plots from the analysis of Foxp3 and IL-17 expression in gated CD4+ T cells in CTRL, NEC + cIgG, and NEC + aIL6R groups. (f) The percentages of Treg (left), Th17 (middle) and IL-17 producing CD4+ Foxp3+ Treg cells (IL-17+Treg) (right) in CTRL (n = 6), NEC + cIgG (n = 5), and NEC + aIL6R (n = 6) groups. Data are shown as the mean ± SD; *, P < .05; ***, P < .001. P-values were calculated using one-way ANOVA with Bonferroni multiple comparison test. (g) Representative immunoblot analysis of Foxp3, RORγt, STAT3, p-STAT3, STAT5, and p-STAT5 in ilea of CTRL, NEC + cIgG, and NEC + aIL6R mice. One of three independent experiments is shown. (h) Immunoblot results showing the expression of Foxp3, RORγt, STAT3, p-STAT3, STAT5, and p-STAT5; β-actin was used as an internal control. Data are pooled from three independent experiments (n = 6 per group)**, P < .01; ***, P < .001; ns: not significant. P-values were calculated using one-way ANOVA with Bonferroni multiple comparison test.
3.6. Peripheral CCR9+ IL-17+ Treg cells inversely correlate with histological scoring in NEC mice
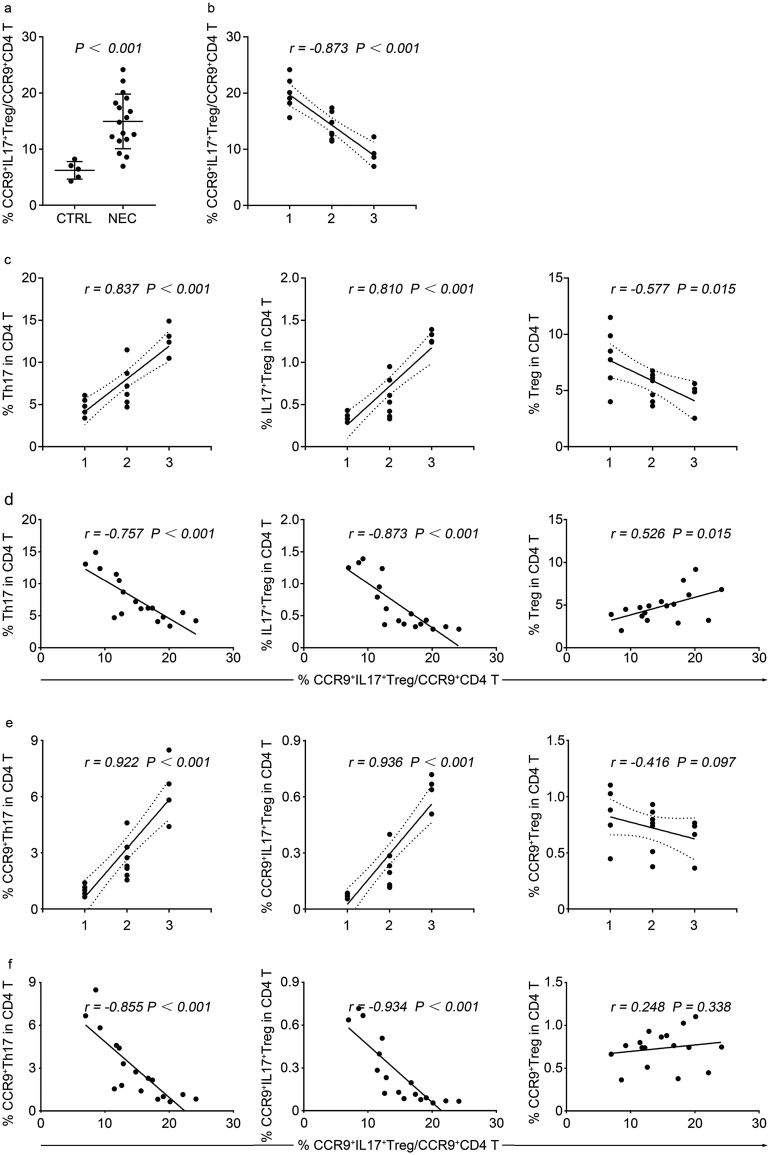
Given the elevated Th17 and IL-17+ Treg cells in experimental NEC tissues (Fig. 5, e and f) and increased CCR9+ IL-17+ Treg cells in peripheral blood of NEC patients (Fig. 1e and Fig. S4a), we sought to determine the frequency of circulating CCR9+ IL-17+ Treg cells in NEC mice. Using flow cytometric analysis of CCR9 expression in IL-17+ Treg cells in peripheral blood, we found that the frequency of circulating CCR9+ IL-17+ Treg cells was substantially increased in NEC mice compared to controls, and was negatively correlated with histological scoring of intestine (Fig. 6, a and b). However, in NEC tissues, we found a significant positive correlation between the histological scoring and the frequencies of Th17 and IL-17+ Treg cells, while the histological scoring showed negative correlation with Treg cells (Fig. 6c).
Fig. 6.
Negative correlation of peripheral CCR9+ IL-17+ Treg cells inversely correlate with histological scoring in NEC mice. (a) The frequencies of circulating CCR9+ IL-17+ Treg cells in normal neonatal mice (CTRL; n = 5) and mice with different grades of intestinal injury (NEC; n = 17). P-values were calculated using Student's t-test. (b) Spearman correlation (r) between the frequencies of circulating CCR9+ IL-17+ Treg cells and histological scoring in NEC mice (n = 17). (c) Spearman correlation (r) between the histological scoring and the frequencies of Th17 cells (left), IL-17+ Treg cells (middle), and Treg cells (right) of intestine in NEC mice (n = 17). (d) Spearman correlation (r) between the frequencies of circulating CCR9+ IL-17+ Treg cells and the frequencies of Th17 cells (left), IL-17+ Treg cells (middle), and Treg cells (right) of intestine in NEC mice (n = 17). (e) Spearman correlation (r) between the histological scoring and the frequencies of CCR9+ Th17 cells (left), CCR9+ IL-17+ Treg cells (middle), and CCR9+ Treg cells (right) of intestine in NEC mice (n = 17). (f) Spearman correlation (r) between the frequencies of circulating CCR9+ IL-17+ Treg cells and the frequencies of CCR9+ Th17 cells (left), CCR9+ IL-17+ Treg cells (middle), and CCR9+ Treg cells (right) of intestine in NEC mice (n = 17).
Next, we considered the possibility that circulating CCR9+ IL-17+ Treg cells could correlate with increased frequencies of intestinal Th17, IL-17+ Treg and Treg cells in NEC mice. Consistent with this, there was a significant negative correlation between circulating CCR9+ IL-17+ Treg cells and the frequencies of intestinal Th17 and IL-17+ Treg cells, and the circulating CCR9+ IL-17+ Treg cells were positive correlated with Treg cells (Fig. 6d). Furthermore, we also analysed intestinal CCR9+ Th17, CCR9+ IL-17+ Treg and CCR9+ Treg cells, and found the same changing trend as Th17, IL-17+ Treg, and Treg cells (Fig. 6e). Indeed, the frequency of circulating CCR9+ IL-17+ Treg cells also inversely correlated with both intestinal CCR9+ Th17 and CCR9+ IL-17+ Treg cells, while no correlation with CCR9+ Treg cells (Fig. 6f). These data demonstrate that the elevated circulating CCR9+ IL-17+ Treg cells negatively correlated with histological scoring could contribute to the elevation of intestinal Th17 and IL-17+ Treg cells.
3.7. Elevated CCR9+ IL-17+ Treg cells inversely correlate with clinical severity in NEC patients
To assess whether the association between the peripheral CCR9+ IL-17+ Treg cells and clinical severity exists in NEC patients, we collected and analysed clinical data from 77 preterm neonates diagnosed with NEC. There were no marked differences among patients with different stages of NEC (Bell stage I, II, and III), or between surgical and nonsurgical patients of Bell stage III in terms of demographic features, delivery method, Apgar score (≤5 at 5 min), and postnatal age of blood collection (Table 1, Table 2). Intriguingly, there was a significant negative correlation between the Bell stage and the frequency of CCR9+ IL-17+ Treg cells (Fig. 7a). Similar to the negative correlation with Bell stage, the frequency of CCR9+ IL-17+ Treg cells were significantly lower in surgical patients than in nonsurgical counterparts of Bell stage III (Fig. 7b). In fact, of the 77 NEC patients, only two NEC IIIA and two NEC IIIB patients experienced moderate feeding intolerance, NEC I, NEC II, NEC IIIA, and NEC IIIB (only for NEC IIIB patients), we also found a significant negative correlation between the Bell stage and the frequency of CCR9+ IL-17+ Treg cells (Fig. 7c). Compared to controls, we also found a significant negative correlation between the expression of intestinal barrier integrity biomarkers (TFF3, IFABP, and zonulin) and the frequency of CCR9+ IL-17+ Treg cells (Fig. 7d).
Fig. 7.
Negative correlation of CCR9+ IL-17+ Treg cells with clinical severity of NEC patients. (a) Spearman correlation (r) between the frequencies of CCR9+ IL-17+ Treg cells and Bell stage in NEC patients (n = 65) and CTRL (n = 66). (b) The frequencies of CCR9+ IL-17+ Treg cells in surgical (n = 11) and nonsurgical (n = 8) patients of Bell stage III. P-values were calculated using Mann-Whitney U–test. (c) The frequencies of CCR9+ IL-17+ Treg cells in two NEC IIIA and two NEC IIIB patients who experienced the course of moderate feeding intolerance (FI), NEC I (I), NEC II (II), NEC IIIA (IIIA), and NEC IIIB (IIIB) (only for NEC IIIB patients). **, P < .01; ***, P < .001. P-values were calculated using one-way ANOVA with Bonferroni multiple comparison test. (d) The frequency of CCR9+ IL-17+ Treg cells were negatively correlated with intestinal barrier integrity biomarkers [TFF3 (left), I-FABP (middle), and zonulin (right)] in NEC patients (n = 65) compared to controls (CTRL; n = 66) analysed by Spearman's rank correlation test. (e–f) Spearman correlation (r) between the frequencies (left) and absolute numbers (right) of CCR9+ IL-17+ Treg cells and gestational age, age and postmenstrual age in NEC patients (n = 65) and CTRL (n = 66).
Finally, we assessed the distribution of peripheral blood CCR9+ IL-17+ Treg cells in NEC patients at different gestational ages. We investigated CCR9+ IL-17+ Treg cells to CCR9+ CD4+ T ratios (Fig. 7e) and the total number of CCR9+ IL-17+ Treg cells (Fig. 7f) in peripheral blood against gestational age (GA), age, and postmenstrual age. No association was found between either total numbers or the ratios with GA, age, or postmenstrual age (Fig. 7, e and f). Together, these results demonstrate that a marked negative correlation is found between the circulating CCR9+ IL-17+ Treg cells and clinical severity of NEC patients.
4. Discussion
Our study shows that CCR9+ IL-17+ Treg cells are significantly increased in the peripheral blood of both patients and mice with NEC. These elevated CCR9+ IL-17+ Treg cells exhibit the phenotypic characteristics of conventional Treg cells, but the suppressive function is seriously impaired in NEC patients. In vitro studies demonstrate that IL-6 promotes the conversion of CCR9+ Treg cells to CCR9+ IL-17+ Treg cells and that blocking IL-6 signalling could inhibit the above conversion. More importantly, treatment with antibodies targeting IL-6R could ameliorate NEC severity in vivo. We also demonstrate a strong negative correlation between circulating CCR9+ IL-17+ Treg cells and clinical severity including Bell stages, and intestinal barrier integrity biomarkers in patients and histological scoring in mice.
CCR9+ CD4+ T cells are markedly enhanced in the peripheral blood of patients with several gastrointestinal diseases [[8], [9], [10]]. Recent studies have shown that the infiltration of CCR9+ CD4+ T cells to the intestinal tract and their conversion into Th17 cells plays a key role in the progression of NEC [3]. However, it was unclear whether CCR9+ CD4+ T cells were increased in the peripheral blood of patients with NEC. Utilizing polychromatic flow cytometric analysis, we have demonstrated for the first time that the frequency of CCR9+ CD4+ T cells is significantly increased in the peripheral blood of both patients and mice with NEC. In addition, CCR9+ IL-17+ Treg cells are the major elevated component of CCR9+ CD4+ T cells. Interestingly, the enhanced CCR9+ IL-17+ Treg cells negatively correlate with clinical severity in patients and histological scoring in mice. These observations suggest that peripheral blood CCR9+ IL-17+ Treg cells and the severity of intestinal tissue injury are intertwined: on the one hand, it has been shown that CCR9+ IL-17+ Treg cells in peripheral blood are the main source of effector Th17 cells of inflammatory intestinal tissue [35,36], on the other hand, intestinal inflammation could promote the circulating CCR9+ IL-17+ Treg cells infiltrating into the inflamed intestine resulting in a reduced population in peripheral blood [3].
The functions of IL-17+ Treg cells remain controversial [37]. A previous study showed that suppressive activity of IL-17+ Treg cells was impaired [18]. In contrast, recent investigations in peripheral blood of RA indicated that IL-17+ Treg cells retained their suppressive ability [13]. Here, we find that IL-17+ Treg cells exhibit features of conventional Treg cells, but the suppressive functions of Treg cells are markedly impaired in patients with NEC. Through investigations of the distribution of IL-17+ Treg cells in Foxp3+ CD4+ Treg subpopulations based on whether they expressed CD45RA and Foxp3 [18], we find that the major source (>75%) of CCR9+ IL-17+ Treg cells is from CD45RA− Foxp3low fraction, which is defined as transiently and unstably expressing Foxp3 and is exhibited to impaired suppressive activity [18], while only 25% of CCR9+ IL-17+ Treg cells are distributed across the CD45RA+ Foxp3low fraction (~20%) and CD45RA− Foxp3hi fraction (<5%) in patients with NEC. Taken together, these data from patients with NEC suggest that CCR9+ IL-17+ Treg cells might derive from different Treg lineages, but were shown to lack suppressive activity.
IL-6 is a key factor in Th17 cell differentiation from naive CD4+ T cell precursors; it also controls the conversion of Treg cells to IL-17+ Treg cells [15]. In the present study, we find that IL-6 alone or IL-6 and IL-1β potently promoted the polarization of CCR9+ Treg cells to CCR9+ IL-17+ Treg cells from NEC and controls patients in vitro. Previous studies in various autoimmune and inflammatory diseases have shown that IL-6 blockade is a novel treatment strategy [38,39]. Similarly, we also find that blocking IL-6 signalling would ameliorate NEC through increased the ratio of Treg/Th17 cells in an experimental NEC model, suggesting that some of the beneficial effects of these therapies may derive from regulating the plasticity of Treg cell fate. IL-6 signalling, through the JAK-mediated phosphorylation of STAT3, is required for Th17 cell production [40]. Importantly, in our experimental NEC model, we observe a decrease in the levels of STAT3 phosphorylation and the frequency of Th17 cells in inflammatory tissues when mice were treated with anti-IL6R antibodies. As a key positive regulator of Foxp3, we also observe an increase in the levels of STAT5 phosphorylation and the frequency of Treg cells. These data suggested that elevated polarizing cytokines, especially IL-6, might promote the conversion of Treg cells to IL-17+ Treg cells in peripheral blood and NEC tissues; blocking the signalling pathway might be a novel approach for the prevention or treatment of NEC.
Since blood volume is limited in preterm infants, we extrapolated from the suppressive activity of CCR9+ CD4+ CD25+ Treg cells to CCR9+ IL-17+ Treg cells. Although we added the suppression assay of CCR9+ Treg and CCR9+ IL-17+ Treg cells using cultured cord blood CCR9+ Treg cells under Th17-polarizing conditions, these data need to be viewed cautiously. Additionally, our data highlight the negative correlation between circulating CCR9+ IL-17+ Treg cells and the severity of intestinal tissue injury, the data must be considered within the context of mouse model. Since this work was lacking in human surgical tissue specimens, we offer an indirect explanation of the correlation between enhanced circulating CCR9+ IL-17+ Treg cells and clinical severity, which should also to be viewed cautiously.
In conclusion, our observations of increased circulating CCR9+ IL-17+ Treg cells and its inverse correlation with the severity of intestinal tissue injury in NEC are novel. Simultaneously, our studies not only provide a potential explanation for these phenomena but also open a new avenue towards early interventions for NEC aiming to restore the balance of Treg/Th17 cells.
Funding
This work was supported by the Science and Technology Planning Project of Guangdong Province, China (2017A020215100), the Science and Technology Foundation of Guangzhou, China (201704020086 and 201604020154), the Medical Scientific Research Foundation of Guangdong Province, China (A2017304 and A2014704), and the Social Science and Technology Development Foundation of Dongguan, China (2016108101037).
Declaration of interests
The authors have no potential conflicts of interest to disclose.
Author contributions
F.M. conceived and designed the study, performed flow cytometry and animal studies, analysed and interpreted the data, and drafted the manuscript. S.T.L. performed cell sorting and immunoblot assays. X.Y.G. performed some of the animal studies. J.L.Z. performed ELISA. D.S.W., Y.C., F.L., Q.P.Y., X.G., W.P.G., and X.X.H. collected and processed the specimens. H.L.L., X.X., and H.H. conceived the study and revised the manuscript. X.C.Z., X.X., and H.H. supervised the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.05.042.
Contributor Information
Huanliang Liu, Email: liuhuanl@mail.sysu.edu.cn.
Xin Xiao, Email: xiaoxin2@mail.sysu.edu.cn.
Hu Hao, Email: haohu@mail.sysu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Nino D.F., Sodhi C.P., Hackam D.J. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. 2016;13:590–600. doi: 10.1038/nrgastro.2016.119. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nino DF, Sodhi CP, Hackam DJ. Necrotizing enterocolitis: new insights into pathogenesis and mechanisms. Nat Rev Gastroenterol Hepatol. 2016; 13: 590-600. [DOI] [PMC free article] [PubMed]
- 2.Neu J., Walker W.A. Necrotizing enterocolitis. N Engl J Med. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]; Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. 2011; 364: 255-64. [DOI] [PMC free article] [PubMed]
- 3.Egan C.E., Sodhi C.P., Good M., Lin J., Jia H., Yamaguchi Y. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016;126:495–508. doi: 10.1172/JCI83356. [DOI] [PMC free article] [PubMed] [Google Scholar]; Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. 2016; 126: 495-508. [DOI] [PMC free article] [PubMed]
- 4.Liu Y., Tran D.Q., Fatheree N.Y., Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014;307:G177–G186. doi: 10.1152/ajpgi.00038.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu Y, Tran DQ, Fatheree NY, Marc Rhoads J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2014; 307: G177-86. [DOI] [PMC free article] [PubMed]
- 5.Weitkamp J.-H., Koyama T., Rock M.T., Correa H., Goettel J.A., Matta P. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013;62:73–82. doi: 10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed] [Google Scholar]; Weitkamp J-H, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, et al. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2013; 62: 73-82. [DOI] [PMC free article] [PubMed]
- 6.Hosoe N., Miura S., Watanabe C., Tsuzuki Y., Hokari R., Oyama T. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2004;286:G458–G466. doi: 10.1152/ajpgi.00167.2003. [DOI] [PubMed] [Google Scholar]; Hosoe N, Miura S, Watanabe C, Tsuzuki Y, Hokari R, Oyama T, et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am J Physiol Gastrointest Liver Physiol. 2004; 286: G458-66. [DOI] [PubMed]
- 7.Onai N., Kitabatake M., Zhang Y.Y., Ishikawa H., Ishikawa S., Matsushima K. Pivotal role of CCL25 (TECK)-CCR9 in the formation of gut cryptopatches and consequent appearance of intestinal intraepithelial T lymphocytes. Int Immunol. 2002;14:687–694. doi: 10.1093/intimm/dxf035. [DOI] [PubMed] [Google Scholar]; Onai N, Kitabatake M, Zhang YY, Ishikawa H, Ishikawa S, Matsushima K. Pivotal role of CCL25 (TECK)-CCR9 in the formation of gut cryptopatches and consequent appearance of intestinal intraepithelial T lymphocytes. International immunology. 2002; 14: 687-94. [DOI] [PubMed]
- 8.Papadakis K.A., Prehn J., Moreno S.T., Cheng L., Kouroumalis E.A., Deem R. CCR9–Positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology. 2001;121:246–254. doi: 10.1053/gast.2001.27154. [DOI] [PubMed] [Google Scholar]; Papadakis KA, Prehn J, Moreno ST, Cheng L, Kouroumalis EA, Deem R, et al. CCR9–Positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology. 2001; 121: 246-54. [DOI] [PubMed]
- 9.Liebregts T., Adam B., Bredack C., Gururatsakul M., Pilkington K.R., Brierley S.M. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106:1089–1098. doi: 10.1038/ajg.2010.512. [DOI] [PubMed] [Google Scholar]; Liebregts T, Adam B, Bredack C, Gururatsakul M, Pilkington KR, Brierley SM, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011; 106: 1089-98. [DOI] [PubMed]
- 10.Greis C., Rasuly Z., Janosi R.A., Kordelas L., Beelen D.W., Liebregts T. Intestinal T lymphocyte homing is associated with gastric emptying and epithelial barrier function in critically ill: a prospective observational study. Crit Care. 2017;21:70. doi: 10.1186/s13054-017-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; .Greis C, Rasuly Z, Janosi RA, Kordelas L, Beelen DW, Liebregts T. Intestinal T lymphocyte homing is associated with gastric emptying and epithelial barrier function in critically ill: a prospective observational study. Critical care (London, England). 2017; 21: 70. [DOI] [PMC free article] [PubMed]
- 11.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]; Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441: 235-8. [DOI] [PubMed]
- 12.DuPage M., Bluestone J.A. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]; DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016; 16: 149-63. [DOI] [PubMed]
- 13.Wang T., Sun X., Zhao J., Zhang J., Zhu H., Li C. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 2015;74:1293–1301. doi: 10.1136/annrheumdis-2013-204228. [DOI] [PubMed] [Google Scholar]; Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C, et al. Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Annals of the rheumatic diseases. 2015; 74: 1293-301. [DOI] [PubMed]
- 14.Gagliani N., Amezcua Vesely M.C., Iseppon A., Brockmann L., Xu H., Palm N.W. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015; 523: 221-5. [DOI] [PMC free article] [PubMed]
- 15.Komatsu N., Okamoto K., Sawa S., Nakashima T., Oh-hora M., Kodama T. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]; Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014; 20: 62-8. [DOI] [PubMed]
- 16.Hovhannisyan Z., Treatman J., Littman D.R., Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011; 140: 957-65. [DOI] [PMC free article] [PubMed]
- 17.Valmori D., Raffin C., Raimbaud I., Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010;107:19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Valmori D, Raffin C, Raimbaud I, Ayyoub M. Human RORgammat+ TH17 cells preferentially differentiate from naive FOXP3+Treg in the presence of lineage-specific polarizing factors. Proc Natl Acad Sci U S A. 2010; 107: 19402-7. [DOI] [PMC free article] [PubMed]
- 18.Miyara M., Yoshioka Y., Kitoh A., Shima T., Wing K., Niwa A. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]; Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009; 30: 899-911. [DOI] [PubMed]
- 19.Ueno A., Jijon H., Chan R., Ford K., Hirota C., Kaplan G.G. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522–2534. doi: 10.1097/MIB.0b013e3182a85709. [DOI] [PubMed] [Google Scholar]; Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, et al. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflammatory bowel diseases. 2013; 19: 2522-34. [DOI] [PubMed]
- 20.Sugiyama H., Gyulai R., Toichi E., Garaczi E., Shimada S., Stevens S.R. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, et al. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. Journal of immunology (Baltimore, Md: 1950). 2005; 174: 164-73. [DOI] [PMC free article] [PubMed]
- 21.Maheshwari A., Schelonka R.L., Dimmitt R.A., Carlo W.A., Munoz-Hernandez B., Das A. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76:100–108. doi: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]; Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014; 76: 100-8. [DOI] [PMC free article] [PubMed]
- 22.Walsh M.C., Kliegman R.M. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986; 33: 179-201. [DOI] [PMC free article] [PubMed]
- 23.Pumberger W., Mayr M., Kohlhauser C., Weninger M. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. 2002;195:796–803. doi: 10.1016/s1072-7515(02)01344-3. [DOI] [PubMed] [Google Scholar]; Pumberger W, Mayr M, Kohlhauser C, Weninger M. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. Journal of the American College of Surgeons. 2002; 195: 796-803. [DOI] [PubMed]
- 24.Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]; Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006; 203: 1693-700. [DOI] [PMC free article] [PubMed]
- 25.Liu W., Putnam A.L., Xu-Yu Z., Szot G.L., Lee M.R., Zhu S. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]; Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006; 203: 1701-11. [DOI] [PMC free article] [PubMed]
- 26.Yates J., Rovis F., Mitchell P., Afzali B., Tsang J., Garin M. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]; Yates J, Rovis F, Mitchell P, Afzali B, Tsang J, Garin M, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. International immunology. 2007; 19: 785-99. [DOI] [PubMed]
- 27.Venken K., Thewissen M., Hellings N., Somers V., Hensen K., Rummens J.L. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]; Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods. 2007; 322: 1-11. [DOI] [PubMed]
- 28.Good M., Sodhi C.P., Ozolek J.A., Buck R.H., Goehring K.C., Thomas D.L. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol. 2014;306:G1021–G1032. doi: 10.1152/ajpgi.00452.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol. 2014; 306: G1021-32. [DOI] [PMC free article] [PubMed]
- 29.Good M., Siggers R.H., Sodhi C.P., Afrazi A., Alkhudari F., Egan C.E. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc Natl Acad Sci U S A. 2012;109:11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2012; 109: 11330-5. [DOI] [PMC free article] [PubMed]
- 30.Nadler E.P., Dickinson E., Knisely A., Zhang X.R., Boyle P., Beer-Stolz D. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]; Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. The Journal of surgical research. 2000; 92: 71-7. [DOI] [PubMed]
- 31.Sakaguchi S., Miyara M., Costantino C.M., Hafler D.A. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]; Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010; 10: 490-500. [DOI] [PubMed]
- 32.Kleinschek M.A., Boniface K., Sadekova S., Grein J., Murphy E.E., Turner S.P. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kleinschek MA, Boniface K, Sadekova S, Grein J, Murphy EE, Turner SP, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J Exp Med. 2009; 206: 525-34. [DOI] [PMC free article] [PubMed]
- 33.Afzali B., Mitchell P.J., Edozie F.C., Povoleri G.A., Dowson S.E., Demandt L. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. Eur J Immunol. 2013;43:2043–2054. doi: 10.1002/eji.201243296. [DOI] [PMC free article] [PubMed] [Google Scholar]; Afzali B, Mitchell PJ, Edozie FC, Povoleri GA, Dowson SE, Demandt L, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. Eur J Immunol. 2013; 43: 2043-54. [DOI] [PMC free article] [PubMed]
- 34.Yang X.P., Ghoreschi K., Steward-Tharp S.M., Rodriguez-Canales J., Zhu J., Grainger J.R. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011; 12: 247-54. [DOI] [PMC free article] [PubMed]
- 35.Kawabe T., Suzuki N., Yamaki S., Sun S.L., Asao A., Okuyama Y. Mesenteric lymph nodes contribute to proinflammatory Th17-cell generation during inflammation of the small intestine in mice. Eur J Immunol. 2016;46:1119–1131. doi: 10.1002/eji.201545907. [DOI] [PubMed] [Google Scholar]; Kawabe T, Suzuki N, Yamaki S, Sun SL, Asao A, Okuyama Y, et al. Mesenteric lymph nodes contribute to proinflammatory Th17-cell generation during inflammation of the small intestine in mice. Eur J Immunol. 2016; 46: 1119-31. [DOI] [PubMed]
- 36.Esplugues E., Huber S., Gagliani N., Hauser A.E., Town T., Wan Y.Y. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]; Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011; 475: 514-8. [DOI] [PMC free article] [PubMed]
- 37.Pandiyan P., Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine. 2015;76:13–24. doi: 10.1016/j.cyto.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine. 2015; 76: 13-24. [DOI] [PMC free article] [PubMed]
- 38.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]; Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nature immunology. 2015; 16: 448-57. [DOI] [PubMed]
- 39.Tanaka T., Narazaki M., Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]; Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annual review of pharmacology and toxicology. 2012; 52: 199-219. [DOI] [PubMed]
- 40.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]; Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009; 27: 485-517. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material