Abstract
Objective: During acupuncture stimulation, heart rate (HR) transiently decreases and autonomic nervous system (ANS) function becomes parasympathetic-dominant. To clarify the effect of acupuncture sensations (pain, De Qi), the effects of deep acupuncture sensations on HR and ANS functions were determined.
Materials and Methods: In this comparative study at Teikyo Heisei University, Tokyo, Japan, 40 healthy, male student volunteers rested for 20 minutes before undergoing manual acupuncture to the Shousanli (LI 10) acupoint on the left forearm for 2 minutes at a frequency of 1 Hz, with concurrent electrocardiography. Depth of stimulation was 15–20 mm. These subjects described their subjective acupuncture sensations. Calculations were performed, using HR variability analysis to find HR and low-frequency (LF) normalized units (nu), the ratio of LF components to total components (as an index of sympathetic nervous system function), high-frequency (HF)nu, the ratio of HF components to total components (as an index of parasympathetic nervous system function), and LF/HF (as an index of sympathetic and parasympathetic balance).
Results: For the final analysis, data were available for 32 subjects. Compared to before acupuncture, HR decreased during acupuncture. HR decreased when no acupuncture sensations or when weak De Qi sensations were perceived, and remained unchanged when acupuncture sensations without De Qi or strong De Qi were perceived. LFnu decreased, HFnu increased, and LF/HF decreased, regardless of pain or De Qi.
Conclusions: Acupuncture stimulation reduced HR even without De Qi sensations and caused ANS function to be parasympathetic-dominant, irrespective of the perception of acupuncture sensations.
Keywords: acupuncture, autonomic nervous system function, De Qi, heart rate, human, noxious stimulus
Introduction
Transient decreases in heart rate (HR) occur during acupuncture stimulation.1–4 Pharmacologic studies have revealed that these changes are mediated by inhibition of sympathetic nervous system (SNS) function,1 enhancement of parasympathetic nervous system function (PNS),2,3 or both.4 By analyzing heart rate (HR) variability (HRV), several of the current authors previously confirmed the shift toward parasympathetic dominance during acupuncture, while affirming a lack of correlation between HR changes (ΔHR) and HRV indices among subjects.5
Acupuncture induces various sensations, including pain and De Qi. Considering their different effects,6–9 the influences of both De Qi and pain on HR and HRV were examined. Pain induced by acupuncture is communicated to the sensory cortex via the lateral spinothalamic tract by Aδ-fibers (sharp pricking or fast pain afferent signals) and C-fibers (dull burning or slow pain afferent signals). Some of the C-fibers project to the brainstem or hypothalamus via paths that circumvent the sensory cortex (e.g., spinobulbar projections, the spinomesencephalic tract, or the spinohypothalamic pathway).10
De Qi is a key acupuncture sensation8,11 and is usually described using the words “soreness,” “aching,” “deep pressure,” “heaviness,” “fullness/distention,” “tingling,” “numbness,” “dull pain,” “warmth,” “cold,” and “throbbing”;8 “sharp pain” is usually not considered part of this sensation.11 The De Qi sensation can be mainly explained by C-fiber sensations (“pressing,” “numb,” “dull,” “cold,” and “throbbing”), whereas “aching” is the only descriptor that is more related to Aδ-fibers.12 On electroencephalography, the latency of De Qi, without pain, exceeds that of painful stimulation,13 which is reminiscent of pain transmitted by C-fibers. Deep pain, transmitted by C-fibers, tends to radiate like De Qi14 because of the wide distribution of C-fibers within the spinal cord.15 Kawakita et al. suggested that there were close relationships among De Qi, deep pain, and polymodal C-fiber receptors that induce afferent nerve impulses, even without sensation, and regulate autonomic nervous system (ANS) functioning.16,17
Yu et al. reported passive correlations between De Qi intensity and changes to the low-frequency (LF) and high-frequency (HF) ratio (LF/HF), as a sympathetic index, and HR.18 Sakai et al. observed significant correlations between the number of acupuncture sensations and changes in LF/HF or HF.19 Zhu et al. suggested that acupuncture-induced De Qi sensation influenced ANS functions.9 Streitberger and Kleinhenz reported that De Qi due to acupuncture seemingly leads to specific changes in HRV.20 In contrast, Kurono et al. reported that acupuncture without De Qi reduced HR and decreased HF (parasympathetic) function.21 Thus, the influence of De Qi on HR and ANS functions remains unclear.
Some studies have suggested that acupuncture-related responses are only reflections of the ordinary brain process of somatosensory stimulation6,22 and that acupuncture-induced HR changes are autonomic concomitants of deep pain stimulation.23 Furthermore, a significant correlation between hypothalamic and brainstem-nuclei activity and LF/HF during acupuncture has been demonstrated previously.22 In animal studies, impulses from C-nociceptors to the hypothalamus or brainstem mediate reduction of HR and the onset of parasympathetic dominance.24–29 Muscle stimulation activates proportionately more C-nociceptors than Aβ-nociceptors, due to their respective distributions.26,28 In contrast, transient cutaneous stimulation activates more Aδ-nociceptors, increases HR, and causes a shift toward sympathetic dominance, whereas persistent cutaneous stimulation generally results in parasympathetic dominance.28,29 Such responses have been observed in both nondecerebrate and decerebrate animals.26 Beisssner suggested that acupuncture-related cortical activation might simply reflect the brain's somatosensory processing of pain stimuli.30
Therefore, the aim of this study was to clarify the relationships between acupuncture sensations and both transient reductions in HR and changes in the sympathetic–parasympathetic ANS dominance during acupuncture.
Materials and Methods
Participants
All of the subjects in this study were healthy student volunteers in the department of acupuncture and moxibustion at Teikyo Heisei University, Tokyo, Japan. These subjects were recruited by word-of-mouth and the use of posters. Before participating in this study, the subjects were provided with a written explanation about the study and written informed consents were obtained. The Teikyo Heisei University ethics committee approved this study (Approval Number 27-093-2).
Sample Size
There were 40 healthy male subjects.
Study Protocol
HR measurements commenced after electrodes were affixed to the subjects. After a 20-minute rest, acupuncture was applied for 2 minutes, and measurements continued for 15 minutes after acupuncture cessation. Afterward, the subjects were asked to describe their acupuncture sensations.
Acupuncture Rationale
Each subject underwent acupuncture at the LI 10 (Shousanli) acupoint (located proximal to the radial nerve in the radial side of the forearm, between the extensor carpi radialis brevis and the extensor carpi radialis longus) on the left forearm. Although it was already reported that HR-decrease responses during acupuncture were not particular to specific acupoints and nonacupoints,2 selected LI 10 was chosen based on a previous study about HR reduction during acupuncture.1
Needling Details
Acupuncture was applied on the left forearm using the sparrow-pecking method wherein the needle is inserted and moved vertically, at a frequency of 1 Hz—not twirled—to a depth of 15–20 mm, for 2 minutes. Sterile acupuncture needles that were 40 mm, No. 18, (Seirin Corporation, Shizuoka, Japan) were used. The study was performed at Teikyo Heisei University between 11:00 am and 4:00 pm, in a quiet, dark room with a temperature between 18°C–23°C while the subjects were in a semiseated position, angled at ∼120°.
Practitioner's Background
One acupuncturist, with 6 years of experience after acquiring a license, performed all the acupuncture stimuli.
Electrocardiography Measurements and Analysis
Disposable silver–silver chloride electrodes were placed under each subject's right collarbone (–ve electrode), left chest (+ve electrode), and abdomen (earth electrode). During the measurement, subjects were instructed to breathe normally. Signals were low-pass filtered at 60 Hz, with a time constant of 0.3. After amplification via a bio-amplifier (Model ML132, AD Instruments, Australia), the data were transmitted to an alternating/direct (AD) converter (Power Lab, AD Instruments) at a sampling rate of 1 k/s. Instantaneous HR was calculated from R-wave intervals using Lab ChartTM software (version 5.4.2, AD Instruments).
Outcomes
All analysis duration was 2 minutes, starting from 4 and 2 minutes before acupuncture, going to the start of acupuncture, or immediately afterward, and ending 2 minutes after the completion of acupuncture. Values before and after acupuncture were averaged to determine pre- and post-acupuncture values. The instantaneous average HR per minute was also calculated. Fast Fourier transform was performed using HRV analysis software (Lab Chart,TM version 8.04) and the spectrum was visualized. The analysis frequency was 0.04–0.50 Hz, of which 0.04–0.15 Hz power was LF and 0.15–0.45 Hz power was HF. The respective percentages in the overall power included LFnu (LF normalized unit, sympathetic) and HFnu (HF normalized unit, parasympathetic). LFnu, HFnu, and LF/HF (sympathetic–parasympathetic balance) were calculated. For each index, first, the values of all subjects obtained during acupuncture were compared with the pre- and post-acupuncture values. The values of the subjects in each group related to differences in acupuncture sensation were then compared with the pre-acupuncture values. The specific dependent variables included ΔHR, ΔLFnu, ΔHFnu, and ΔLF/HF.
Pain intensity was measured using a visual analogue scale (VAS), with a score of 100 indicating the “most intense” pain and 0 indicating “no pain.” De Qi was evaluated, using a categorical scale based on 5 levels; namely the following responses: (1) “I perceived nothing” (i.e., acupuncture performed “without any sensation”); (2) “I perceived the stimulation, but not De Qi” (i.e., acupuncture performed “with no De Qi, but with some sensation”); (3) “Although weak, I perceived the De Qi sensation” (i.e., acupuncture performed “with weak De Qi”); (4) “I perceived the De Qi sensation very strongly” (i.e., acupuncture performed “with strong De Qi”); and (5) “The De Qi sensation I perceived was unbearably strong” (i.e., acupuncture performed “with unbearably strong De Qi”).
Comparisons
The first comparison was made among the average HRs per minute of pre-, during-, and post-acupuncture stimulation. The second comparison was made between HR and the HRV indices of pre-, during-, and post-acupuncture stimulation. After that, between-groups were defined by weaker and stronger pain (Weak pain group: subjects whose pain VAS value was included in the lower half of the VAS values of all subjects; Stronger pain group: subjects whose pain VAS values were included in the upper half of the VAS values of all subjects). Between-groups were also defined by the absence or presence of De Qi sensation (without De Qi: “without any sensation” group; “with no De Qi but with some sensation” group; with De Qi: “with weak De Qi” group; “with strong De Qi” group; and “with unbearably strong De Qi” group). Finally, comparisons were made among the groups of perceived De Qi intensity.
Statistical Analysis
Data are shown as median (first quartile, third quartile). Friedman's and Tukey's tests were used to analyze changes in HR and HRV indices. Correlations were determined via the Spearman test. Pre- and during-acupuncture comparisons were performed using Wilcoxon or Mann–Whitney-U tests. A χ2 test was used to determine goodness of fit. JSTAT™ software (Japan)*, was used to perform all statistical analyses. P < 0.05 was considered to be statistically significant.
Results
Of the initially recruited 40 subjects, 2 became agitated after acupuncture was started, and unexpected background noises from surrounding construction sites hampered the measurement in 6 subjects; therefore, final analyses were performed on the data obtained from 32 subjects (mean age: 20.9 ± 0.84 years).
Changes in HR and HRV Indices During Acupuncture
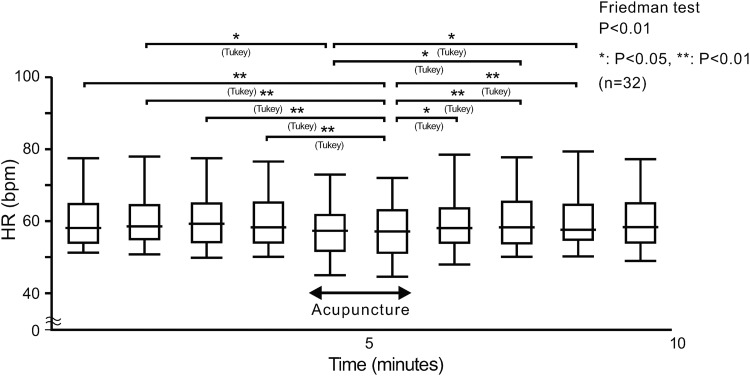
Figure 1 depicts the average HR per minute.
FIG. 1.
Measurement of the average HR per minute in 32 healthy volunteers for 4 minutes pre-, 2 minutes during, and 4 minutes post-acupuncture at LI 10. The average HRs per minute in pre- and post-acupuncture were stable. HR decreased during acupuncture and completely recovered after acupuncture was completed. The median (minimum value, first quartile, third quartile, maximum value) are indicated. HR, heart rate, bpm, beats per minute.
Compared with the pre- (P < 0.01) and post-acupuncture (P < 0.05) HRs, HRs obtained during acupuncture were lower. There were significant changes in LFnu (decreased: P < 0.01), HFnu (increased: P < 0.01), and LF/HF (decreased: P < 0.01) during acupuncture, compared with levels obtained during pre-acupuncture stimulation. There were also significant changes in LFnu (decreased: P < 0.01), HFnu (increased: P < 0.05), and LF/HF (decreased: P < 0.05) during acupuncture, compared with post-acupuncture stimulation levels. There were no significant changes in LFnu, HFnu, and LF/HF when pre- and post-acupuncture data were compared. Both HR reductions and HRV indices shifted toward parasympathetic dominance.
Correlation Between Subjective Pain Intensity and Changes in HR and HRV Indices
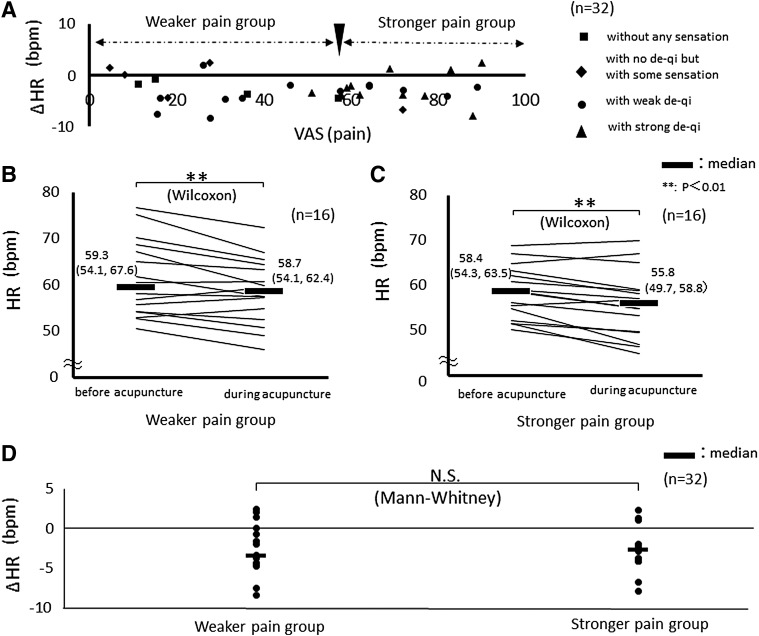
Figure 2A depicts the relationships among pain intensity, De Qi intensity, and ΔHR. There was no significant correlation between pain intensity and ΔHR (rs = 0.0848; P = 0.626) in the total cohort.
FIG. 2.
(A) Correlation between De Qi and ΔHR for 4 minutes before and 2 minutes during acupuncture at LI 10. No significant correlations were found among pain intensity, De Qi intensity, and ΔHR (rs = 0.0848; P = 0.626). (B) HR in subjects who perceived weaker pain (n = 16). HR decreased during acupuncture, compared with before acupuncture. (C) HR in subjects who perceived stronger pain (n = 16). HR decreased during acupuncture, compared with before acupuncture. (D) Comparison of ΔHR between the subjects with weaker or stronger pain. There were no significant between-group differences in ΔHR. Data represent the median (first quartile; third quartile). HR, heart rate; bpm, beats per minute; VAS, visual analogue scale; N.S., not significant; Δ, delta (changes of the index during acupuncture compared to pre-acupuncture levels).
Therefore, the subjects were divided into 2 groups based on ratings of perceived pain intensity (Fig. 2A) and then group-specific ΔHR was examined. Within both the weaker-pain (n = 16) and stronger-pain groups (n = 16), HR decreased significantly during acupuncture (weaker pain: P = 0.0063; stronger pain: P = 0.0013), compared with pre-acupuncture HRs (Fig. 2B and C). <F2B&2C> There was no significant between-group difference in ΔHR (Fig. 2D).
In the weaker-pain group, LFnu decreased significantly (P = 0.011), HFnu increased significantly (P = 0.011), and LF/HF decreased significantly (P = 0.0076) during acupuncture, compared with pre-acupuncture values. In the stronger-pain group, similar and significant changes were observed (LFnu: P = 0.0010; HFnu: P = 0.0052; LF/HF: P = 0.0013). There were no significant between-group differences for ΔLFnu, ΔHFnu, and ΔLF/HF (P = 0.547, P = 0.547, and P = 0.407, respectively). HRV indices showed that the observed changes shifted the ANS toward parasympathetic dominance, irrespective of the perceived pain intensity.
Correlation Between the Existence of De Qi and Changes in HR and HRV Indices
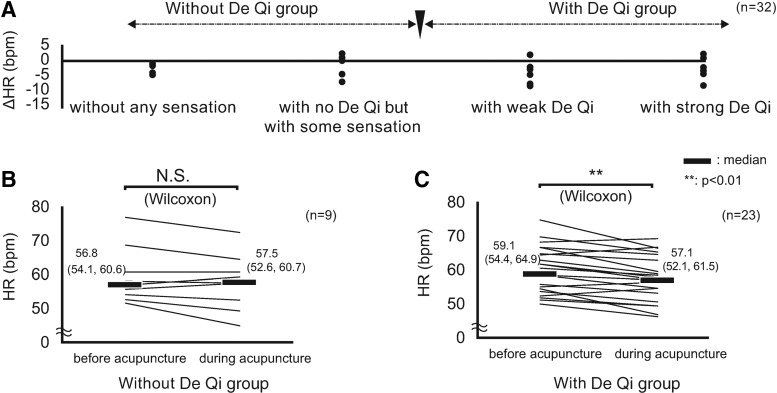
Figure 3A shows the relationship between De Qi intensity and ΔHR. Parts B and C of Figure 3 show <F3B&3C> ΔHR of the group without De Qi and of the group with De Qi. In the group without De Qi, pre-acupuncture HR averages did not change significantly during acupuncture (Fig. 3B). In the group with De Qi, pre-acupuncture HR averages were reduced significantly during acupuncture (Fig. 3C). Notably, HR decreased only in the presence of the De Qi sensation.
FIG. 3.
(A) Correlation between De Qi and ΔHR for 4 minutes before and for 2 minutes during acupuncture at LI 10. (B) ΔHR in subjects without De Qi sensation (n = 9). Pre-acupuncture HR values did not change significantly during acupuncture. (C) ΔHR in subjects with De Qi sensation (n = 23). Pre-acupuncture HR values were reduced significantly during acupuncture. Data represent the median (first quartile; third quartile). HR, heart rate; bpm, beats per minute; N.S., not significant; Δ, delta (changes of the index during acupuncture compared to pre-acupuncture levels).
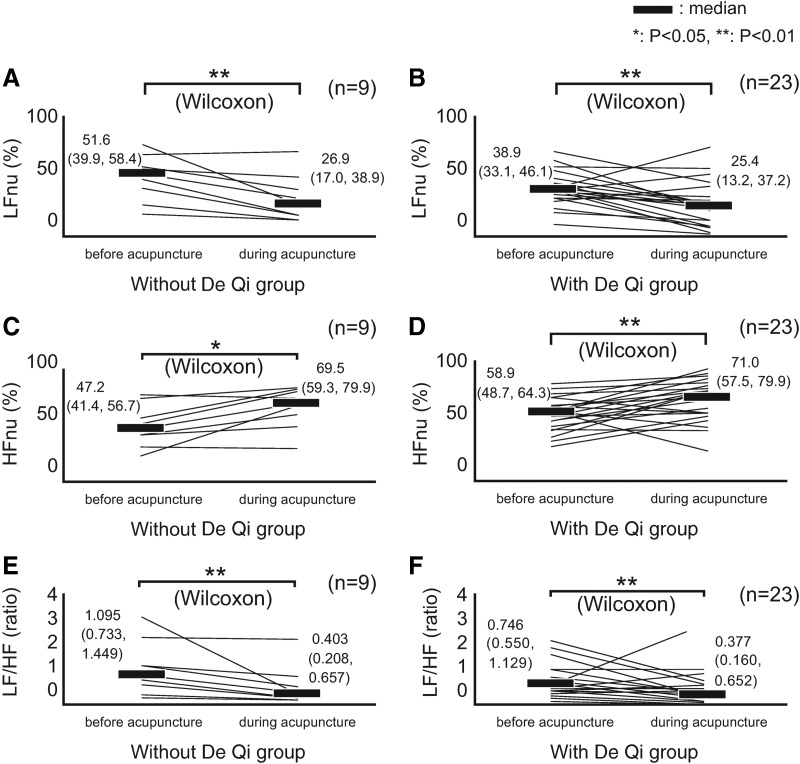
Figure 4 shows the changes of HRV indices, between pre-acupuncture and during acupuncture, for the 2 De Qi groups. LFnu decreased significantly in the group without (Fig. 4A) and in the group with De Qi (Fig. 4B). HFnu increased significantly in the group without (Fig. 4C) and in the group with De Qi (Fig. 4D). LF/HF significantly decreased in the group without (Fig. 4E) and with De Qi (Fig. 4F). For ΔLFnu (P = 0.711), ΔHFnu (P = 0.458), and ΔLF/HF (P = 0.592) there were no significant between-group differences. HRV indices showed a shift toward parasympathetic dominance, regardless of De Qi sensation.
FIG. 4.
Changes in heart rate variability (HRV) indices for 4 min before, and for 2 minutes during acupuncture at LI 10. (A) LFnu in subjects without De Qi sensation. LFnu significantly decreased. (B) LFnu in subjects with De Qi sensation. LFnu significantly decreased. (C) HFnu in subjects without De Qi sensation. HFnu increased significantly. (D) HFnu in subjects with De Qi sensation. HFnu increased significantly. (E) LF/HF in subjects without De Qi sensation. LF/HF decreased significantly. (F) LF/HF, in subjects with De Qi sensation. LF/HF decreased significantly. Data represent the median (first quartile, third quartile). HR, heart rate; HF, high-frequency (components); LF, low-frequency (components); nu, normalized unit; Δ, delta (changes of the index during acupuncture compared with pre-acupuncture levels).
Further analyses were then performed with a goodness-of-fit test with subjects grouped according to the perceived intensity of their De Qi sensations. No subjects reported “unbearably strong De Qi.” Table 1 shows ΔHR for each of the De Qi groups. The proportion of subjects with decreased HR was significantly larger in the groups “without any sensation” and “with weak De Qi.” In contrast, there were no significant changes in HR observed in subjects belonging to the “with no De Qi but with some sensation” and “with strong De Qi” groups.
Table 1.
Ratio of Subjects with Significant Changes in HR for 2 Minutes During Acupuncture at LI 10, Compared with 4 Minutes Before Acupuncture, According to Detailed Subjective Ratings of Di Qi Sensation
| Acupuncture sensation during stimulation | Subjects with HR reduction | P-value |
|---|---|---|
| Without De Qi | ||
| Without any sensation | 4/4 (100%) | P = 0.0046* |
| With no De Qi but with some sensation | 2/5 (40%) | P = 0.65 (N.S.) |
| With De Qi | ||
| With weak De Qi | 11/12 (91.7%) | P = 0.0039* |
| With strong De Qi | 8/11 (72.7%) | P = 0.13 (N.S.) |
P < 0.01.
HR, heart rate; N.S., not significant.
Table 2 shows changes in HRV indices for each of the De Qi groups. Although the proportion of subjects with decreased LFnu or increased HFnu was not statistically significant in the groups, the proportion of subjects with decreased LF/HF ratios was significantly large in all groups.
Table 2.
Ratio of Subjects with Significant Changes in HRV Indicesa for 2 Minutes During Acupuncture at LI 10, Compared with Average Values of the Indices for 2 Minutes from and 4 Minutes Before Acupuncture, and for 2 Minutes from and 2 Minutes Before Acupuncture, According to Detailed Subjective Ratings of De Q sensations.
| Acupuncture sensation | Subjects with LFnu reduction | Subjects with HFnu increase | Subjects with LF/HF reduction |
|---|---|---|---|
| Without Di Qi | |||
| Without any sensation | 3/4 (75%); P = 0.32 (N.S.) | 3/4 (75%); P = 0.32 (N.S.) | 4/4 (100%); P = 0.046* |
| With no de-qi, but | 5/5 (100%); P = 0.025* | 4/5 (80%); P = 0.18 (N.S.) | 5/5 (100%); P = 0.025* |
| with some sensation | |||
| With De Qi | |||
| With weak De Qi | 10/12 (83.3%); P = 0.021* | 9/12 (75.0%); P = 0.083 (N.S.) | 10/12 (83.3%); P = 0.021* |
| With strong De Qi | 10/11 (90.9%); P = 0.0067** | 9/11 (81.8%); P = 0.035* | 10/11 (90.9%); P = 0.0067** |
Normalized low-frequency (LF) component, normalized high-frequency (HF) component, LF/HF.
p < 0.05; **p < 0.01.
HRV, heart rate variability; LFnu, low-frequency normalized units; HFnu, high-frequency normalizd units; N.S., not significant.
Discussion
The average HR per minute (Fig. 1) showed that, during the second half of the acupuncture period, there was a significant decrease in HR, compared with almost all other time points, pre- and post-acupuncture. This was not the case in the first half of the acupuncture period. Just as acute pain increased HR transiently,31 acupuncture increased LF/HF significantly (albeit briefly), indicating a transient shift toward sympathetic dominance, accompanied by a corresponding increase in HR.20 Animal studies have shown that transient cutaneous nociceptive stimulation increases HR.27,28 Acupuncture comprises two main phases: (1) needle skin puncture and (2) subsequent needle manipulation.32 Acupuncture might thus increase HR transiently because HR is likely influenced by the acute cutaneous stimulation generated upon needle insertion. Therefore, based on the result of the second half of the acupuncture period, the current authors suggest that deep acupuncture, in fact, decreases HR.
Next, the data, which were obtained at 4 minutes pre-, 2 minutes during, and 4 minutes post acupuncture confirmed that, when HR decreased, ANS function shifted transiently toward parasympathetic dominance. To obtain sufficient reliability during HRV analyses, it might be beneficial to analyze HR over a 3-minute or longer period. In a previous study, Streitberger and Kleinhenz collected 1-minute, short-term HRV measures to obtain a higher temporal resolution.20 Referring to that study by Streitberger and Kleinhenz, the current authors collected data every 2 minutes to produce a higher temporal resolution while obtaining reliability.
HR decreased during acupuncture in the presence—but not in the absence—of a De Qi sensation. Thus, De Qi was associated with the shift toward parasympathetic dominance. In contrast, HRV analysis showed a shift toward parasympathetic dominance, regardless of the presence or absence of De Qi. Thus, these results were contradictory. To elucidate this apparent contradiction, ΔHR and HRV indices were analyzed qualitatively, focusing on more-detailed sensory perception. In the “weak De Qi” group, a significantly greater number of subjects had HR reductions, indicating a shift toward parasympathetic dominance. In this group, HRV indices also supported a shift toward parasympathetic dominance. In the “I perceived nothing” group, changes in HR and HRV indices also supported a shift toward parasympathetic dominance. Therefore, even in the absence of any sensation, acupuncture caused a shift toward parasympathetic dominance and reduced HR. The reason for this result may be explained because the change to parasympathetic dominance observed in this study was caused by the impulses of acupuncture stimulation but was caused by the impulses that did not project on the sensory cortex among those impulses.
In the “without De Qi but with some sensation” group, ΔHR was nonsignificant, indicating no shift toward parasympathetic dominance; however, HRV indices did show a change toward parasympathetic dominance. It is possible that acupuncture caused the HRV-related shift toward parasympathetic dominance, but that the balance of parasympathetic nerve dominance failed to exceed some threshold value and, therefore, was not sufficient to decrease HR. In addition, impulses that caused sensation but no De Qi might have inhibited the shift toward parasympathetic dominance. This hypothesis could account for the observed contradiction between ΔHR and HRV indices seen in the “strong De Qi” group. Although most acupuncture impulses induced a shift toward parasympathetic dominance, some produced sharp pain and not De Qi; these impulses might inhibit the shift toward parasympathetic dominance and HR reduction. Therefore, further detailed studies that analyze ratios of De Qi and sharp pain quantitatively are needed.
Previous studies revealed that noxious stimuli can change ANS function; impulses from C-nociceptors incite parasympathetic dominance, whereas impulses from Aδ- nociceptors incite sympathetic dominance.28 Beissner et al. concluded that acupuncture might produce a special pain stimulus that activates C-nociceptors.22 In the current study, the current authors propose that the observed changes in ANS function during acupuncture were mostly produced by impulses transmitted by C-fibers projecting to the hypothalamus and the brainstem, rather than via the sensory cortex. Furthermore, impulses transmitted by Aδ-fibers might inhibit ΔHR. The current authors suppose that acupuncture stimulation can alter ANS function by supraspinal reflection, regardless of the acupuncture-related sensations.
A previous study found that changes in parasympathetic nervous activity correlated with the number of De Qi sensations.19 Although De Qi is caused only by C-fiber impulses projected to the sensory cortex, De Qi might be an indicator of the presence of C-fiber impulses generated by acupuncture stimulation. This hypothesis justifies why De Qi has been regarded as an indicator of acupuncture-related ANS effects.
The current study investigated the effects of acupuncture-related pain and De Qi sensations on HR and HRV indices as a measure of ANS function. The results suggested that deep acupuncture stimulation reduced HR even without De Qi, and caused a shift in ANS toward parasympathetic dominance, irrespective of the acupuncture sensation that was perceived.
Limitations
This study had several limitations. The principal limitation was the relatively small sample size. Therefore, additional well-powered studies are needed. In addition, this study only demonstrated the effect of “deep” acupuncture sensation on ANS function. The effects of acupuncture sensation on post-stimulation ANS function and the effects of cutaneous acupuncture sensation on ANS function were not identified in this study. Hence, additional studies are needed.
Conclusions
In this study, the aim was to clarify the relationship between subjectively perceived acupuncture sensations and ΔHR and ANS functions during acupuncture. Deep acupuncture reduced HR significantly, even in the absence of sensation, and shifted ANS function toward parasympathetic dominance, regardless of perceived levels of pain and De Qi.
Acknowledgments
The authors would like to thank Editage for the English-language review.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Imai K, Kitakoji H. Comparison of transient heart rate reduction associated with acupuncture stimulation in supine and sitting subjects. Acupunct Med. 2003;21(4):133–137 [DOI] [PubMed] [Google Scholar]
- 2. Imai K. Effects of acupuncture on electrogastrogram, instantaneous heart rate, and sympathetic skin response with pharmacological analysis to elucidate the neural mechanisms in humans [in Japanese]. Bull Meiji Coll Oriental Med. 1996;19:45–55 [Google Scholar]
- 3. Sugiyama Y, Xue YX, Mano T. Transient increase in human muscle sympathetic nerve activity during manual acupuncture [in Japanese]. Jpn J Physiol. 1995;45:337–345 [DOI] [PubMed] [Google Scholar]
- 4. Nishijo K, Mori H, Yoshikawa K, Yazawa K. Decreased heart rate by acupuncture stimulation in humans via facilitation of vagal cardiac activity and suppression of cardiac sympathetic nerve. Neurosci Lett. 1997;227(3):165–168 [DOI] [PubMed] [Google Scholar]
- 5. Uchida C, Waki H, Minakawa Y, Tamai H, Hisajima T, Imai K. Evaluation of autonomic nervous system function using heart rate variability analysis during transient heart rate reduction caused by acupuncture. Med Acupunct. 2018;30(2):89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun J, Zhu Y, Jin L, et al. Partly separated activations in the spatial distribution between de-qi and sharp pain during acupuncture stimulation: An fMRI-based study. Evid Based Complement Alternat Med. 2012;2012:934085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacPherson H, Asgher A. Acupuncture needle sensations associated with De Qi: A classification based on experts' ratings. J Altern Complement Med. 2006;12(7):633–637 [DOI] [PubMed] [Google Scholar]
- 8. Kong J, Gollub R, Huang T, et al. Acupuncture de qi, from qualitative history to quantitative measurement. J Altern Complement Med. 2007;13(10):1059–1070 [DOI] [PubMed] [Google Scholar]
- 9. Zhu SP, Luo L, Ling Zhang L, et al. Acupuncture De-qi: From characterization to underlying mechanism. Evid Based Complement Alternat Med. 2013;2013:518784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watanabe M, Hayasaki H, Yurugi Y. Mechanisms of pain and pain relief [in Japanese]. JAHS. 2017;8(1):50–63 [Google Scholar]
- 11. Hui KK, Nixon EE, Vangel MG, et al. Characterization of the “deqi” response in acupuncture. BMC Complement Altern Med. 2007;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beissner F, Brandau A, Henke C, et al. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PLoS One. 2010;5(9):e12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watanabe I, Yano T, Mori K, Tanzawa S. Physiological significance of de-qi sensation induced by acupuncture stimulation from the viewpoint of event related potential (ERP). J Jpn Soc Balneol Climatol Phys Med. 1994;57(3):1993–1994 [Google Scholar]
- 14. Burton AR, Birznieks I, Spaak J, Henderson LA, Macefield VG. Effects of deep and superficial experimentally induced acute pain on skin sympathetic nerve activity in human subjects. Exp Brain Res. 2009;195(2):317–324 [DOI] [PubMed] [Google Scholar]
- 15. Sugiura Y, Terui N, Hosoya Y, Tonosaki Y, Nishiyama K, Honda T. Quantitative analysis of central terminal projections of visceral and somatic unmyelinated (C) primary afferent fibers in the guinea pig. J Comp Neurol. 1993;332(3):315–325 [DOI] [PubMed] [Google Scholar]
- 16. Kawakita K, Gotoh K. Role of polymodal receptors in the acupuncture-mediated endogenous pain inhibitory systems. Prog Brain Res. 1996;113:507–523 [DOI] [PubMed] [Google Scholar]
- 17. Kawakita K, Shinbara H, Imai K, Fukuda F, Yano T, Kuriyama K. How do acupuncture and moxibustion act? Focusing on the progress in Japanese acupuncture research J Pharmacol Sci. 2006;100(5):443–459 [DOI] [PubMed] [Google Scholar]
- 18. Yu DT, Jones AY. Physiological changes associated with de qi during electroacupuncture to LI4 and LI11: A randomised, placebo-controlled trial. Acupunct Med. 2013;31(2):143–150 [DOI] [PubMed] [Google Scholar]
- 19. Sakai S, Hori E, Umeno K, Kitabayashi N, Ono T, Nishijo H. Specific acupuncture sensation correlates with EEGs and autonomic changes in human subjects. Auton Neurosci. 2007;133(2):158–169 [DOI] [PubMed] [Google Scholar]
- 20. Streitberger K, Kleinhenz J. Introducing a placebo needle into acupuncture research. Lancet. 1998;352(9125):364–365 [DOI] [PubMed] [Google Scholar]
- 21. Kurono Y, Minagawa M, Ishigami T, Yamada A, Kakamu T, Hayano J. Acupuncture to Danzhong but not to Zhongting increases the cardiac vagal component of heart rate variability. Auton Neurosci. 2011;161(1–2):116–120 [DOI] [PubMed] [Google Scholar]
- 22. Napadow V, Dhond RP, Purdon P, Kettner N, Makris N, Kwong KK, Hui KK. Correlating acupuncture FMRI in the human brainstem with heart rate variability. Conf Proc IEEE Eng Med Biol Soc. 2005;5:4496–4499 [DOI] [PubMed] [Google Scholar]
- 23. Beissner F, Deichmann R, Henke C, Bār KJ. Acupuncture—deep pain with an autonomic dimension? Neuroimage. 2012;60(1):653–660 [DOI] [PubMed] [Google Scholar]
- 24. Bandler R, Shipley MT. Columnar organization in the midbrain periaquductal gray: Modules for emotional expression? Trends Neurosci. 1994;17(9):379–389 [DOI] [PubMed] [Google Scholar]
- 25. Bernard JF, Bandler R. Parallel circuits for emotional coping behavior: New pieces in the puzzle. J Comp Neurol. 1998;401(4):429–436 [PubMed] [Google Scholar]
- 26. Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53(1):95–104 [DOI] [PubMed] [Google Scholar]
- 27. Lumb BM, Parry DM, Semenenko FM, McMullan S, Simpson DA. C-nociceptor activation of hypothalamic neurones and the columnar organisation of their projections to the periaqueductal grey in the rat. Exp Physiol. 2002;87(2):123–128 [DOI] [PubMed] [Google Scholar]
- 28. Lumb BM. Hypothalamic and midbrain circuitry that distinguishes between escapable and inescapable pain. News Physiol Sci. 2004;19:22–26 [DOI] [PubMed] [Google Scholar]
- 29. Keay KA, Bandler R. Distinct central representations of inescapable and escapable pain: Observations and speculation. Exp Physiol. 2002;87(2):275–279 [DOI] [PubMed] [Google Scholar]
- 30. Beissner F. Functional magnetic resonance imaging studies of acupuncture mechanisms: A critique. Focus Altern Complement Ther. 2011;16(1):3–11 [Google Scholar]
- 31. Terkelsen AJ, lgaard M, Hansen HJ, Andersen OK, Jensen TS. Acute pain increases heart rate: Differential mechanisms during rest and mental stress. Auton Neurosci. 2005;121(1–2):101–109 [DOI] [PubMed] [Google Scholar]
- 32. Hori E, Takamoto K, Urakawa S, Ono T, Nishijo H. Effects of acupuncture on the brain hemodynamics. Auton Neurosci. 2010;157(1–2):74–80 [DOI] [PubMed] [Google Scholar]