Abstract
Hemolysis is an unintended sequel of temporary or permanent intracardiac devices. However, limited data exist on the characteristics and treatment of hemolysis in patients with cardiac prostheses. This entity, albeit uncommon, often poses significant diagnostic and management challenges to the clinical cardiologist. In this article, we aim to provide a contemporary overview of the incidence, mechanisms, diagnosis, and management of cardiac prosthesis‐related hemolysis.
Keywords: anemia, cardiac prosthesis, hemolysis, left ventricular assist device, paravalvular leak
1. INTRODUCTION
Cardiac prosthesis‐related hemolytic anemia (CPHA) is a well described but likely an under‐recognized phenomenon. This potentially life‐threatening complication was first described in the 1950s to 1960s in patients undergoing valve replacement with early generation surgical prostheses.1, 2 The incidence of clinically evident hemolysis after surgical valve replacement has since declined due to the improved valve design and surgical implantation techniques.3, 4 However, interest in CPHA has been recently renewed given the increasing number of studies reporting various rates of clinical and subclinical CPHA with mechanical circulatory support devices and transcatheter valvular interventions.5, 6, 7 Nonetheless, the management of CPHA is often challenging due to its atypical presentation, lack of standardized definitions/classifications, and due to the dearth of outcomes data on its various treatment strategies. We sought to provide a contemporary overview of the current literature on the incidence, mechanisms, and management strategies of hemolytic anemia associated with various cardiac prostheses.
2. DEFINITION OF HEMOLYTIC ANEMIA
There is no single specific definition of hemolytic anemia. However, the diagnosis of hemolytic anemia is usually established if three major criteria are present: (a) unexplained anemia, and (b) signs of accelerated right blood cells (RBCs) production in the bone marrow (eg, high reticulocyte count), and (c) signs of RBCs destruction (eg, elevated unconjugated bilirubin, lactate dehydrogenase [LDH], low haptoglobin). The term “sub‐clinical hemolysis” is used to describe patients who meet the latter two criteria but do not have anemia. In these patients, the bone marrow adequately compensate for the hemolysis, maintaining normal hemoglobin. Prosthesis‐related hemolytic anemia can then be assumed if new hemolysis is diagnosed in patients with cardiac prostheses, and/or mechanical assist devices in the absence of other causes of hemolysis.
3. INCIDENCE AND ETIOLOGY OF CARDIAC PROSTHESIS‐RELATED HEMOLYSIS
The incidence of hemolysis in patients with cardiac prostheses varies widely according to the device type and its indwelling time. Mechanical damage to the RBCs due to increased shear stress is the most widely accepted etiology of CPHA. However, causes of this increased shear stress are device‐ and disease‐specific.
3.1. Hemolysis after open valve surgery
Hemolytic anemia was a common complication of old generation valves, occurring in up to 15% of surgical valves in the 1960s to 1970s.8, 9 However, this incidence decreased to <1% with modern valve designs. In a study of 301 patients who underwent On‐X mechanical valve replacement, clinical hemolysis at long‐term occurred in 0% and 0.2% of patients who had aortic and mitral valve replacement, respectively.3 Several other studies confirmed the rarity of clinical hemolysis after valve replacement with contemporary prostheses.4, 10, 11 Nonetheless, subclinical hemolysis is not uncommon, occurring in 18% to 51% and in 5% to 10% of contemporary mechanical tissue prostheses, respectively.12, 13 The main mechanism of hemolysis after surgical valve replacement is paravalvular leak (PVL), which may result from suture dehiscence due to heavy annular calcifications, endocarditis, chronic steroids, or suboptimal surgical techniques.14, 15, 16, 17 Other less common etiologies of hemolysis related to surgical prostheses are listed in Table 1.18, 19, 20, 21
Table 1.
Mechanisms of cardiac prosthesis‐related hemolysis
| Cardiac device | Mechanisms of hemolysis |
|---|---|
| Surgical aortic and mitral valve replacement | PVL, SVD, PPM, endocarditis, leaflet thrombosis |
| Surgical mitral valve repair | Ring dehiscence, residual eccentric or para‐ring regurgitation, protrusion of suture material, free‐floating chordae in hyperdynamic left ventricle |
| Transcatheter aortic valve replacement | PVL, PPM, increased red cell shear stress in the sinuses due to residual native valve fissuring and balloon‐induced endothelial denudation |
| Transcatheter mitral valve replacement | PVL |
| Surgical left ventricular assist devices | Pump thrombosis, transfusion‐associated hemolysis, cannula kinks or malposition, dehydration → LV under filling → increased inlet velocity |
| Percutaneous left ventricular assist devices | Pump‐related shear stress, device malpositioning, device malfunction |
| Intracardiac shunt closure | Incomplete closure (peri‐device leak) |
Abbreviations: LV; left ventricle; PVL, paravalvular leak; PPM; patient‐prosthesis mismatch; SVD, structural heart deterioration.
Hemolysis also complicates a small percentage (<1%) of mitral valve repair and annular ring placement surgeries.22, 23, 24, 25 Although ring dehiscence appears to be the main mechanism of CPAH in this group, other reported mechanisms include: protruding of the paravalvular suture material, “whiplash motion” of residual free‐floating chordae in hyperkinetic ventricles, and small but turbulent eccentric residual regurgitation jet (Table 1). In this large series, valve replacement led to the resolution of hemolysis in the vast majority of cases.26, 27
3.2. Hemolysis after transcatheter valve replacement
The incidence of hemolysis after transcatheter aortic valve replacement (TAVR) is unknown because routine surveys are not performed in these patients. Although clinical hemolysis is not commonly seen, subclinical hemolysis following TAVR may not be uncommon.17, 28 In a study of 122 patients who had TAVR with balloon‐expandable valves, subclinical hemolysis occurred in 15%. The strongest predictor of hemolysis was patient‐prosthesis mismatch, rather than the degree of PVL.7 This intriguing finding, albeit requires confirmation in additional studies, suggests that transcatheter (vs surgical) prostheses may be associated with less hemolysis given their lower reported incidence of patient‐prosthesis mismatch.29 In another study of 64 TAVR patients, 37.5% had evidence of subclinical hemolysis 6 months after the procedure.30 Moderate to severe PVL and bicuspid valve morphology independently predicted hemolysis (Figure 1A,B), and hemolysis was associated with a 4‐fold increase in hospital readmissions at 1 year. Of note, 21% of patients had evidence of sub‐clinical hemolysis before TAVR; supporting the notion that severe native aortic stenosis can lead to hemolysis due to flow acceleration across the stenotic valve.31, 32 Other TAVR‐specific mechanisms of CPAH are related to the remaining native leaflets and their potential impact on red cell shear stress (Table 1).7
Figure 1.
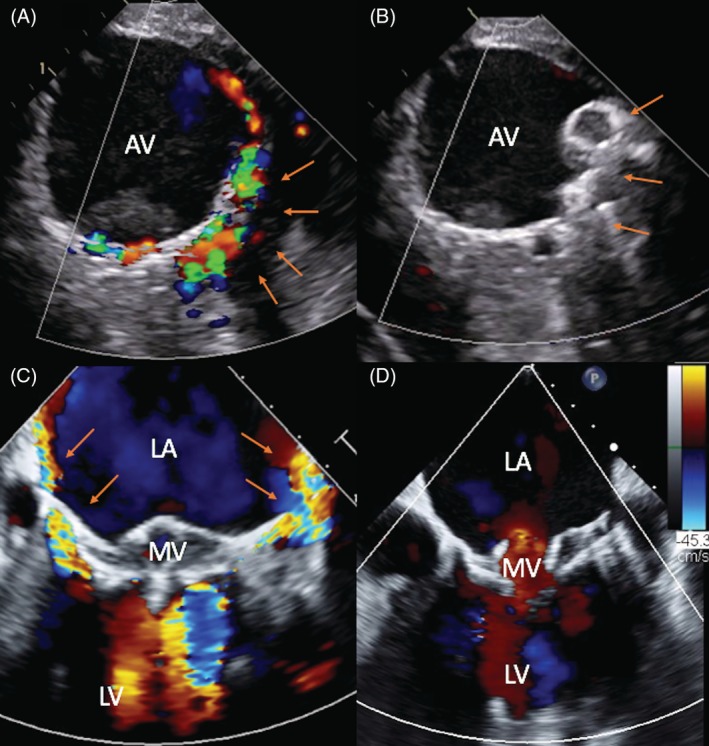
Severe hemolysis due to paravalvular leak after transcatheter aortic and mitral valve replacement. A,B, Paravalvular leak after transcatheter aortic valve replacement before and after percutaneous closure. C,D, Paravalvular leak after TMVR before and after a second procedure to reposition the mitral prosthesis. AV, aortic valve; LA, left atrium; LV, left ventricle; MV, mitral valve; arrows annotate the location of the paravalvular leak
The field of transcatheter mitral valve replacement (TMVR) is rapidly evolving.33 However, given the small number of TMVRs performed worldwide, data on TMVR associated hemolysis are limited. In the early feasibly trial of the Tendyne valve (Abbott, Roseville, Minnesota), only 1 of 30 patient (3.3%) developed severe hemolysis.34 The main mechanism of hemolysis after TMVR is PVL due to incomplete sealing, device undersizing, or progressive left ventricular remodeling (Figure 1C,D). Cases of severe clinical hemolysis have also been reported following transcatheter mitral valve in valve/ring implantation.35
3.3. Hemolysis with left ventricular assist devices
The reported incidence of hemolysis with the HeartMate II (HMII; Thoratec, Pleasanton, California) is approximately 13% to 18%.5, 36, 37 However, early experience with the third generation magnetically levitated left ventricular assist devices (LVAD) (HeartMate III) revealed very low (<1%) rates of hemolysis.38, 39 Similarly, the novel TORVAD toroidal‐flow LVAD has shown negligible rates of hemolysis in pre‐clinical testing.40 A unique aspect of hemolysis in LVAD patients is its strong relationship with thrombotic complications. Local thrombosis increases shear stress and leads to local destruction of the red cells.41 However, other mechanisms may be implicated such as increased inlet velocities due to dehydration and under‐filling of the left ventricle, transfusion‐associated hemolysis, and cannula kinks or malposition (Table 1).41, 42, 43 In current practice, an increase in LDH and plasma free hemoglobin levels in LVAD patients is viewed as a possible early cannula thrombosis.5 Clinical hemolysis in surgical LVAD patients is associated with significant morbidity and mortality.44, 45, 46
Mild hemolysis occurs in 10% to 30% of patients who receive short‐term percutaneous LVAD support with the Impella device (Abiomed Inc., Danvers, Massachusetts).47, 48, 49 However, the incidence increases to 60% with device indwelling times >6 hours.6 In a study of patients undergoing veno‐arterial extracorporeal membrane oxygenation, concomitant Impella use was associated with higher incidence of hemolysis (76% vs 33%, P = .004).50 Clinically significant hemolysis may also occur unexpectedly due to device malfunction or improper placement.51, 52, 53 To the best of our knowledge, no cases of clinical hemolysis due to intra‐aortic balloon pumps have been reported.
3.4. Hemolysis after transcatheter shunt closure
New hemolysis requiring blood transfusion occurred in 1% to 2% of patients undergoing percutaneous PVL closure in two large registries in the United Kingdom and the United States, likely due to incomplete obliteration of the PVL (Figure 2).28, 54 Higher profile devices (eg, ventricular septal occluders) are more associated with more hemolysis than the lower profile Amplatzer vascular plugs.55, 56 Severe hemolysis has also been reported following percutaneous closure of septal defects and peri‐MitraClip regurgitation mostly due to residual peri‐device shunt.57, 58, 59, 60, 61, 62, 63
Figure 2.
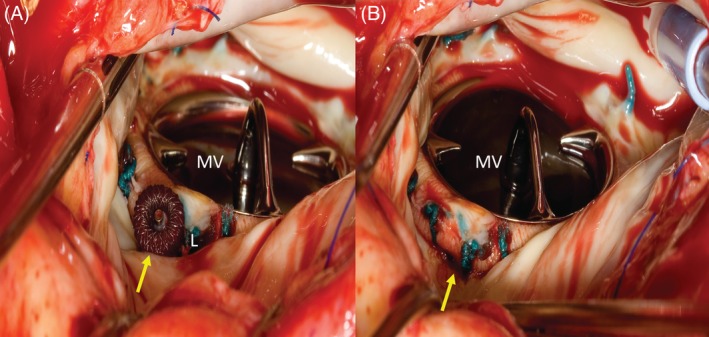
Redo surgery for severe hemolysis following failed percutaneous paravalvular leak closure attempt. A, Amplatzer vascular plug in place, but residual leak is present (L). B, Removal of the Amplatzer vascular plug followed by patch repair of the leak. MV, mitral valve, arrows annotate the location of the paravalvular leak
4. CLINICAL PRESENTATION AND DIAGNOSTIC APPROACHES
The recognition of hemolytic anemia in patients with classic presentations is straightforward. However, CPHA commonly presents with ambiguous symptoms, and insidious onset posing highlighting the need for a high index of suspicion. In a study of 381 patients who were referred for treatment of mitral PVL (of whom 40% had hemolysis), the mean time from index valve replacement to referral was 85.1 ± 115.6 months.64 Hence, in patients with cardiac prostheses who have unexplained anemia, a systematic step‐wise approach to exclude/diagnose CPHA is warranted (Figure 3):
Figure 3.
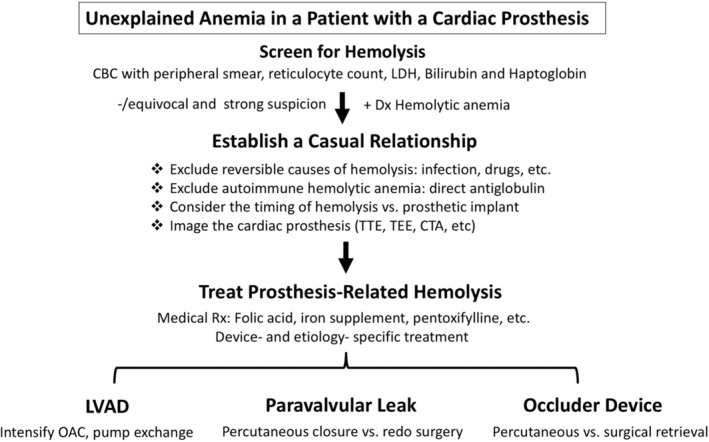
An algorithmic approach to a patient with suspected cardiac prosthesis‐related hemolysis. CTA; computed tomography angiogram; LDH, lactate dehydrogenase; LVAD, left ventricular assist device; OAC, oral anticoagulation; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography
4.1. Laboratory confirmation of hemolysis
Initial testing should include: complete blood count with peripheral blood smear examination, reticulocyte count, LDH, bilirubin, and haptoglobin levels. The normal values, accuracy, and pitfalls of these tests are summarized in Table 1.
(a) Blood smear examination: erythrocyte fragments (eg, Schistocytes) are common in “mechanical” hemolytic anemia. These fragments, however, are not specific to CPHA (Table 1). (b) Reticulocytes: an elevated reticulocyte count is a typical feature of hemolysis. However, reticulocytes can be elevated in other causes of accelerated red cells production (Table 1). In addition, normal or low reticulocyte counts do not preclude hemolysis. A blunted bone marrow response can be seen with myelodysplasia, alcoholism, or iron and folate deficiency. (c) LDH: this enzyme catalyzes the conversion of lactate into pyruvic acid, and its iso‐enzymes LDH‐1 and 2 are therefore increased in hemolysis. Although non‐specific, an elevated LDH >2.5‐folds strongly suggests hemolysis. Temporal trends in LDH are also useful in assessing treatment success in patients with PVL and LVAD dysfunction. (d) Haptoglobin: this scavenger binds free circulating hemoglobin released with RBC turnover, and it hence becomes diminished or undetectable in significant hemolysis. A haptoglobin level < 25 mg/dL provides an 87% probability of having hemolysis.65 A combination of haptoglobin <25 mg/dL and elevated LDH increases the predictive value to >90%. However, haptoglobin is an acute phase reactant and its level can hence be normal or elevated in systemic inflammation or acute infection. (e) Bilirubin: indirect bilirubin is a product of hemoglobin catabolism, and its levels are thus increased in hemolysis. Although severely elevated unconjugated bilirubin can occur with acute massive hemolysis, levels >4 mg/dL in non‐acute hemolysis typically indicate a concomitant liver pathology impairing the conjugation of bilirubin or its hepatic uptake.
Additional markers of hemolysis can be used in certain populations. For example, plasma free hemoglobin (pFH) is usually indicative of intra‐vascular hemolysis. This marker is useful in early detection of hemolysis in patients with LVADs. In the Interagency for Mechanically Assisted Circulatory Support registry, hemolysis is diagnosed when pFH exceeds 40 mg/dL.46 The utility of pFH in diagnosing and monitoring hemolysis in patients with pLVAD and in those with PVL has also been suggested in several studies.49, 66 Indeed, pFH was superior to LDH in detecting hemolysis in patients with cardiogenic shock treated with the Impella micro‐axial pump.49 However, pFH is not widely used in this setting due to the dearth of supportive data and the frequent need to send out to a reference lab. Other tests indirect/less specific markers of hemolysis (mean cell volume, hemosiderinuria, hemoglobin A1C, aspartate aminotransferase, etc.) may aid in establishing or excluding the diagnosis in equivocal cases (Table 2).
Table 2.
Markers of hemolysis
| Type | Test | Findings in hemolysis | Normal values | Characteristics/pitfalls |
|---|---|---|---|---|
| Direct | Haptoglobin | <25 mg/dL | 0.5‐3.2 g/L | Most specific |
| ↑ = Acute phase reactant, nephrotic syndrome, wide range of normal values | ||||
| ↓Trauma, congenital ahaptoglobinemia, cirrhosis | ||||
| Lactate dehydrogenase | >460 μ/L | 230‐460 μ/L | Non‐specific but LDH + ↓ Haptoglobin>90% specific for hemolysis | |
| Other sources of LDH increase liver, myocardial, or muscle injury | ||||
| Indirect bilirubin | >2 mg/dL | 0.3‐1.6 mg/dL | Non‐specific | |
| Aspartate aminotransferase | >40 μ/L | 10‐40 μ/L | Non‐specific | |
| Cell deformities on peripheral smear, “eg, schistocytes” | >0.5% | Absent | Non‐specific, also seen in DIC, thrombotic microangiopathies, spherocytes, elliptocytes, and sickle cells can be seen | |
| Indirect | Reticulocyte count | >2% | <2% | ↑ With bleeding and erythropoietin use |
| ↓ with myelodysplasia, alcohol, B12/folate/iron deficiency | ||||
| Mean cell volume | >96 femtoliters | 80‐96 femtoliters | ↑ Due to reticulocytosis | |
| Hemosiderinuria | Brown‐color urine | Absent | More characteristics of acute and marked hemolysis, which is uncommon with cardiac etiologies of hemolysis | |
| Hemoglobin A1C | Unexpectedly low | ≤5.6 | Limited time for red blood cell glycation | |
| Plasma free Hgb | >40 mg/dL | <5 mg/dL | Not widely available (reference labs) |
Abbreviations: Hgb, hemoglobin; DIC, disseminated intravascular coagulation; LDH, lactate dehydrogenase.
4.2. Establishing the relationship between cardiac prostheses and hemolysis
Once the hemolysis diagnosis is confirmed, establishing its relationship with cardiac prostheses is essential to guide therapy. Although this can be challenging due to the absence of a specific test for CPHA, the following steps may be helpful in elucidating the etiology of hemolysis.
1. Excluding common causes of hemolysis: infection and drugs are frequent causes of hemolysis. Discontinuation of the potential offenders (eg, new antibiotics), and treating underlying infections may resolve the hemolysis. Autoimmune hemolytic anemia should also always be excluded with a direct antiglobulin test before confirming the diagnosis of CPHA.
2. Identifying specific laboratory clues: certain laboratory findings may suggest a specific etiology for the hemolysis. For example, the presence of spherocytes and/or a positive direct antiglobulin test suggest an immune‐mediated mechanism, while the abundance of schistocytes indicates mechanical disruption of the red cells. Hence, the latter pattern is more likely to be observed in patients with CPHA, although it can also be seen with thrombotic thrombocytopenic purpura or hemolytic uremic syndrome.
3. Imaging the cardiac prosthesis: echocardiography allows detailed evaluation of prosthetic valve (transvalvular velocity, leaflet function, PVL, etc.), LVADs (position and integrity/kinks of the cannula/pump, function of aortic valve, etc.), and intra‐cardiac shunt occluder devices (eg, residual leak). Although transthoracic echocardiography can often identify the site and mechanism of prosthesis dysfunction, transesophageal echo is usually necessary. Cardiac computed tomography might also be useful in confirming the diagnosis and guiding treatment of PVL, peri‐occluder residual shunting, and LVAD outflow graft kinks.17, 42
4. Timing of presentation: Severe anemia after LVAD insertion or valve replacement should raise suspicion of a causal relationship between the cardiac prosthesis and hemolysis in the absence of bleeding or infection. Nonetheless, CPHA can often be insidious and may not be detected clinically until later stages.64
5. MANAGEMENT OF CARDIAC PROSTHESIS‐RELATED HEMOLYSIS
The optimal treatment strategy of cardiac prosthesis‐related hemolysis is determined by the degree of hemolysis, clinical symptoms, severity of prosthetic dysfunction, and the predicted risk and success of surgical or percutaneous interventions.
5.1. Medical therapy
Medical therapy with close follow‐up is appropriate for patients with mild hemolysis that is not significantly interfering with the quality of life.
Folic acid: Folate deficiency is common in chronic hemolysis due to the increased consumption from accelerated erythropoiesis.67 In persistent hemolysis, prophylactic oral folic acid supplementation is recommended to avoid substantial folate deficiency.
Iron supplementation and blood transfusion: Oral ± intravenous iron supplements may be sufficient to treat stable degrees of hemolysis. However, blood transfusion is often needed is severe hemolysis until mechanical corrective measures are undertaken.
Beta‐blocker: Beta‐blockers can reduce shear forces in patients with PVL‐related hemolysis reducing blood pressure and heart rate. Oral beta‐blockers led to significant improvement in hemolytic anemia in several retrospective series.68, 69, 70
Pentoxifylline: Pentoxifylline improves blood viscosity and erythrocyte deformability. The use of pentoxifylline may subside mild hemolysis in patients with LVAD or mechanical valves.71, 72 In a small randomized trial of 40 patients with CPHA, hemolysis indices improved in 60% of patients on pentoxifylline compared to 5% of patients in the placebo group.73
Erythropoietin: This recombinant hormone has been shown to eliminate the need for transfusion in selected patients with prosthetic valve‐related hemolysis.74, 75 However, erythropoietin administration to treat LVAD‐related hemolysis was associated with higher odds of pump thrombosis (hazard ratio 2.35; 95% confidence interval: 1.38‐4.00; P = .002), and mortality (hazard ratio 1.62; 95% confidence interval: 1.12‐2.33; P = .01).76
Anticoagulation: Intensification of antithrombotic therapy is the first recommended step in the management of LVAD associated hemolysis that is believed to be due to thrombosis.41, 77
5.2. Invasive management
Invasive treatment is reserved for patients with severe symptomatic hemolysis despite maximal medical therapy. The specific invasive treatment differs according to the implicated cardiac prosthesis and the underlying mechanism of hemolysis.
Paravalvular leak repair: The efficacy of transcatheter PVL repair in reducing heart failure symptoms and long‐term mortality has been demonstrated in multiple studies.28, 64, 78 However, the literature on the role of transcatheter PVL correction in resolving hemolysis is scarce, and conflicting. For example, Ruiz et al reported a substantial decrease in the percentage of patients who required blood transfusion or erythropoietin injections after percutaneous PVL repair from 56% to 5%.55 On the contrary, in another study of 168 patients with PVL, blood transfusion requirements decreased only modestly after percutaneous repair from 34% to 21%.79 These inconsistencies may be related to the variable definitions of hemolysis and the degree of PVL reduction achieved. Several studies have demonstrated that effective correction of hemolysis in these patients requires complete amelioration of the PVL.16, 17, 55, 64, 66, 80 Nonetheless, this can be challenging due to various patients' and device‐specific reasons. Indeed, up to 30% of patients undergoing percutaneous PVL repair have >mild residual PVL following intervention even at centers of excellent.17, 28, 64, 80 Among patients with significant residual leaks, a small percentage will experience worsening of hemolysis often requiring percutaneous or surgical device retrieval.81 Surgical PVL correction has been shown to be a more effective method in treating severe hemolysis than percutaneous repair.82 In one study, persistence of or worsening hemolysis was responsible for 50% of crossovers to surgery in patients initially treated with transcatheter techniques.64 However, redo surgery is associated with significant morbidity and mortality, and the choice of transcatheter or surgical intervention requires a collaborative interdisciplinary approach weighting the risks and potential success of each procedure.78
Refractory LVAD‐related hemolysis: Intensification of antithrombotic therapy is able to improve or resolve hemolysis in the majority of LVAD patients. However, persistent hemolysis despite maximally tolerated anticoagulation is associated with a substantial increase in the risk of stroke and death.5, 77 Hence, pump exchange through various surgical techniques (subxiphoid ± thoracotomy or redo sternotomy) should be considered early in these patients.41
Occluder devices‐induced hemolysis: Severe hemolysis that developed or worsened after transcathter shunt closure is often due to the residual peri‐device shunt. Those residual shunts can be often ameliorated with additional occluder devices, and/or intra‐device coil deployment within the Nitinol cage of the occluder.79, 83 However, replacement of the involved prosthesis with a different device or conversion to surgical repair is often required.
6. SUMMARY
CPHA is an uncommon but important source of morbidity and mortality in patients undergoing valve surgery, transcatheter structural heart interventions, and mechanical circulatory support device implantations. Knowledge of the incidence, etiologies, and the various treatment strategies is key for effective management of this rare but potentially life‐threatening entity.
CONFLICT OF INTEREST
The authors declare no potential conflict of interests.
Alkhouli M, Farooq A, Go RS, Balla S, Berzingi C. Cardiac prostheses‐related hemolytic anemia. Clin Cardiol. 2019;42:692–700. 10.1002/clc.23191
REFERENCES
- 1. Decesare W, Rath C, Hufnagel C. Hemolytic anemia of mechanical origin with aortic‐valve prosthesis. N Engl J Med 1965;272:1045‐1050. [DOI] [PubMed] [Google Scholar]
- 2. Rose JC, Hufnagel CA, Freis ED, Harvey WP, Partenope EA. The hemodynamic alterations produced by a plastic valvular prosthesis for severe aortic insufficiency in man. J Clin Invest 1954;33(6):891‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palatianos GM, Laczkovics AM, Simon P, Pomar JL, Birnbaum DE, Greve HH, Haverich A Multicentered European study on safety and effectiveness of the on‐X prosthetic heart valve: intermediate follow‐up. Ann Thorac Surg 2007;83(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 4. Bavaria JE, Desai ND, Cheung A, Petracek MR, Groh MA, Borger MA, Schaff HV The St Jude medical trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg 2014;147(2):590‐597. [DOI] [PubMed] [Google Scholar]
- 5. Levin AP, Saeed O, Willey JZ, Levin CJ, Fried JA, Patel SR, Sims DB, Nguyen JD, Shin JJ, Topkara VK, Colombo PC, Goldstein DJ, Naka Y, Takayama H, Uriel N, Jorde UP Watchful waiting in continuous‐flow left ventricular assist device patients with ongoing hemolysis is associated with an increased risk for cerebrovascular accident or death. Circ Heart Fail 2016;9(5). [DOI] [PubMed] [Google Scholar]
- 6. Lauten A, Engstrom AE, Jung C, et al. Percutaneous left‐ventricular support with the Impella‐2.5‐assist device in acute cardiogenic shock: results of the Impella‐EUROSHOCK‐registry. Circ Heart Fail 2013;6(1):23‐30. [DOI] [PubMed] [Google Scholar]
- 7. Laflamme J, Puri R, Urena M, Laflamme L, DeLarochellière H, Abdul‐Jawad Altisent O, del Trigo M, Campelo‐Parada F, DeLarochellière R, Paradis JM, Dumont E, Doyle D, Mohammadi S, Côté M, Pibarot P, Laroche V, Rodés‐Cabau J Incidence and risk factors of hemolysis after transcatheter aortic valve implantation with a balloon‐expandable valve. Am J Cardiol 2015;115(11):1574‐1579. [DOI] [PubMed] [Google Scholar]
- 8. Iguro Y, Moriyama Y, Yamaoka A, et al. Clinical experience of 473 patients with the omnicarbon prosthetic heart valve. J Heart Valve Dis 1999;8(6):674‐679. [PubMed] [Google Scholar]
- 9. Skoularigis J, Essop MR, Skudicky D, Middlemost SJ, Sareli P. Frequency and severity of intravascular hemolysis after left‐sided cardiac valve replacement with Medtronic hall and St. Jude Medical prostheses, and influence of prosthetic type, position, size and number. Am J Cardiol 1993;71(7):587‐591. [DOI] [PubMed] [Google Scholar]
- 10. Concistre G, Chiaramonti F, Bianchi G, et al. Aortic valve replacement with perceval bioprosthesis: single‐center experience with 617 implants. Ann Thorac Surg 2018;105(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 11. Hwang HY, Choi JW, Kim HK, Kim KH, Kim KB, Ahn H. Paravalvular leak after mitral valve replacement: 20‐year follow‐up. Ann Thorac Surg 2015;100(4):1347‐1352. [DOI] [PubMed] [Google Scholar]
- 12. Mecozzi G, Milano AD, De Carlo M, et al. Intravascular hemolysis in patients with new‐generation prosthetic heart valves: a prospective study. J Thorac Cardiovasc Surg 2002;123(3):550‐556. [DOI] [PubMed] [Google Scholar]
- 13. Shapira Y, Vaturi M, Sagie A. Hemolysis associated with prosthetic heart valves: a review. Cardiol Rev 2009;17(3):121‐124. [DOI] [PubMed] [Google Scholar]
- 14. Alonso‐Lej F. Straight suture plane to avoid periprosthetic leak in aortic valve replacement. Ann Thorac Surg 1975;19(5):571‐573. [DOI] [PubMed] [Google Scholar]
- 15. Dhasmana JP, Blackstone EH, Kirklin JW, Kouchoukos NT. Factors associated with periprosthetic leakage following primary mitral valve replacement: with special consideration of the suture technique. Ann Thorac Surg 1983;35(2):170‐178. [DOI] [PubMed] [Google Scholar]
- 16. Garcia MJ, Vandervoort P, Stewart WJ, Lytle BW, Cosgrove DM III, Thomas JD, Griffin BP Mechanisms of hemolysis with mitral prosthetic regurgitation. Study using transesophageal echocardiography and fluid dynamic simulation. J am Coll Cardiol 1996;27(2):399‐406. [DOI] [PubMed] [Google Scholar]
- 17. Alkhouli M, Sarraf M, Maor E, Sanon S, Cabalka A, Eleid MF, Hagler DJ, Pollak P, Reeder G, Rihal CS Techniques and outcomes of percutaneous aortic paravalvular leak closure. JACC Cardiovasc Interv 2016;9(23):2416‐2426. [DOI] [PubMed] [Google Scholar]
- 18. Conti VR, Nishimura A, Coughlin TR, Farrell RW. Indications for replacement of the Beall 103 and 104 disc valves. Ann Thorac Surg 1986;42(3):315‐320. [DOI] [PubMed] [Google Scholar]
- 19. Silver MD, Wilson GJ. The pathology of wear in the Beall model 104 heart valve prosthesis. Circulation 1977;56(4 Pt 1):617‐622. [DOI] [PubMed] [Google Scholar]
- 20. Kaymaz C, Ozkan M, Ozdemir N, Kirma C, Deligonul U. Spontaneous echocardiographic microbubbles associated with prosthetic mitral valves: mechanistic insights from thrombolytic treatment results. J am Soc Echocardiogr 2002;15(4):323‐327. [DOI] [PubMed] [Google Scholar]
- 21. Okumiya T, Ishikawa‐Nishi M, Doi T, Kamioka M, Takeuchi H, Doi Y, Sugiura T Evaluation of intravascular hemolysis with erythrocyte creatine in patients with cardiac valve prostheses. Chest 2004;125(6):2115‐2120. [DOI] [PubMed] [Google Scholar]
- 22. Inoue M, Kaku B, Kanaya H, Ohka T, Ueda M, Masahiro S, Shimizu M, Mabuchi H Reduction of hemolysis without reoperation following mitral valve repair. Circulation 2003;67(9):799‐801. [DOI] [PubMed] [Google Scholar]
- 23. Choi JH, Park YH, Yun KW, Lee SH, Kim JS, Kim J, Kim JH, Je HG, Lee SK, Chun KJ Intractable hemolytic anemia after mitral valve repair: a report of three cases. Echocardiography 2013;30(9):E281‐284. [DOI] [PubMed] [Google Scholar]
- 24. Cerfolio RJ, Orszulak TA, Daly RC, Schaff HV. Reoperation for hemolytic, anaemia complicating mitral valve repair, Eur J Cardiothorac Surg 1997;11(3):479‐484. [DOI] [PubMed] [Google Scholar]
- 25. Ward RP, Sugeng L, Weinert L, Korcarz C, Verdino RJ, Spencer KT, Lang RM Images in cardiovascular medicine. Hemolysis after mitral valve repair. Circulation 2000;101(6):695‐696. [DOI] [PubMed] [Google Scholar]
- 26. Shingu Y, Aoki H, Ebuoka N, et al. A surgical case for severe hemolytic anemia after mitral valve repair. Ann Thorac Cardiovasc Surg 2005;11(3):198‐200. [PubMed] [Google Scholar]
- 27. Abourjaili G, Torbey E, Alsaghir T, Olkovski Y, Costantino T. Hemolytic anemia following mitral valve repair: a case presentation and literature review. Exp Clin Cardiol 2012;17(4):248‐250. [PMC free article] [PubMed] [Google Scholar]
- 28. Calvert PA, Northridge DB, Malik IS, Shapiro L, Ludman P, Qureshi SA, Mullen M, Henderson R, Turner M, Been M, Walsh KP, Casserly I, Morrison L, Walker NL, Thomson J, Spence MS, Mahadevan VS, Hoye A, MacCarthy PA, Daniels MJ, Clift P, Davies WR, Adamson PD, Morgan G, Aggarwal SK, Ismail Y, Ormerod JOM, Khan HR, Chandran SS, de Giovanni J, Rana BS, Ormerod O, Hildick‐Smith D Percutaneous device closure of paravalvular leak: combined experience from the United Kingdom and Ireland. Circulation 2016;134(13):934‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zorn GL, 3rd , Little SH, Tadros P, et al. Prosthesis‐patient mismatch in high‐risk patients with severe aortic stenosis: a randomized trial of a self‐expanding prosthesis. J Thorac Cardiovasc Surg. 2016;151(4):1014‐1022, 1023 e1011‐1013. [DOI] [PubMed] [Google Scholar]
- 30. Ko TY, Lin MS, Lin LC, Liu YJ, Yeh CF, Huang CC, Chen YH, Chen YS, Kao HL Frequency and significance of intravascular hemolysis before and after Transcatheter aortic valve implantation in patients with severe aortic stenosis. Am J Cardiol 2018;121(1):69‐72. [DOI] [PubMed] [Google Scholar]
- 31. Blackshear JL, McRee CW, Safford RE, et al. von Willebrand factor abnormalities and Heyde syndrome in dysfunctional heart valve prostheses. JAMA Cardiol 2016;1(2):198‐204. [DOI] [PubMed] [Google Scholar]
- 32. Kawase I, Matsuo T, Sasayama K, Suzuki H, Nishikawa H. Hemolytic anemia with aortic stenosis resolved by urgent aortic valve replacement. Ann Thorac Surg 2008;86(2):645‐646. [DOI] [PubMed] [Google Scholar]
- 33. Alkhouli M, Alqahtani F, Aljohani S. Transcatheter mitral valve replacement: an evolution of a revolution. J Thoracic Disease 2017;9(Suppl 7):S668‐S672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Muller DWM, Farivar RS, Jansz P, Bae R, Walters D, Clarke A, Grayburn PA, Stoler RC, Dahle G, Rein KA, Shaw M, Scalia GM, Guerrero M, Pearson P, Kapadia S, Gillinov M, Pichard A, Corso P, Popma J, Chuang M, Blanke P, Leipsic J, Sorajja P, Muller D, Jansz P, Shaw M, Conellan M, Spina R, Pedersen W, Sorajja P, Farivar RS, Bae R, Sun B, Walters D, Clarke A, Scalia G, Grayburn P, Stoler R, Hebeler R, Dahle G, Rein KA, Fiane A, Guerrero M, Pearson P, Feldman T, Salinger M, Smart S, Kapadia S, Gillinov M, Mick S, Krishnaswamy A, Pichard A, Corso P, Chuang M, Popma J, Leipsic J, Blanke P, Carroll J, George I, Missov E, Kiser A Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J am Coll Cardiol 2017;69(4):381‐391. [DOI] [PubMed] [Google Scholar]
- 35. Urena M, Brochet E, Lecomte M, Kerneis C, Carrasco JL, Ghodbane W, Abtan J, Alkhoder S, Raffoul R, Iung B, Nataf P, Vahanian A, Himbert D Clinical and haemodynamic outcomes of balloon‐expandable transcatheter mitral valve implantation: a 7‐year experience. Eur Heart J 2018;39(28):2679‐2689. [DOI] [PubMed] [Google Scholar]
- 36. John R, Holley CT, Eckman P, Roy SS, Cogswell R, Harvey L, Shumway S, Liao K A decade of experience with continuous‐flow left ventricular assist devices. Semin Thorac Cardiovasc Surg 2016;28(2):363‐375. [DOI] [PubMed] [Google Scholar]
- 37. Trejo R, Sierra I, Ferez S, Cardenas M. [Predictive value of ventricular extrasystole in the exertion test and its relation to the magnitude of coronary damage]. Archivos del Instituto de Cardiologia de Mexico 1986;56(3):255‐258. [PubMed] [Google Scholar]
- 38. Krabatsch T, Netuka I, Schmitto JD, Zimpfer D, Garbade J, Rao V, Morshuis M, Beyersdorf F, Marasco S, Damme L, Pya Y Heartmate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure −1 year results from the Ce mark trial. J Cardiothorac Surg 2017;12(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, Patel CB, Ewald GA, Tatooles AJ, Silvestry SC, John R, Caldeira C, Jeevanandam V, Boyle AJ, Sundareswaran KS, Sood P, Mehra MR Hemocompatibility‐related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation 2017;135(21):2003‐2012. [DOI] [PubMed] [Google Scholar]
- 40. Bartoli CR, Hennessy‐Strahs S, Gohean J, Villeda M, Larson E, Longoria R, Kurusz M, Acker M, Smalling R A novel Toroidal‐flow left ventricular assist device minimizes blood trauma: implications of improved ventricular assist device Hemocompatibility. Ann Thorac Surg 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tchantchaleishvili V, Sagebin F, Ross RE, Hallinan W, Schwarz KQ, Massey HT. Evaluation and treatment of pump thrombosis and hemolysis. Ann Cardiothorac Surg 2014;3(5):490‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El Sabbagh A, Al‐Hijji M, Gulati R, Rihal CS, Pollak PM, Behfar A. Percutaneous stenting of a left ventricular assist device outflow kink. JACC Cardiovasc Interv 2016;9(24):e229‐e231. [DOI] [PubMed] [Google Scholar]
- 43. Carr SM, Lubbe DF, Huber PR. Percutaneous transcatheter balloon dilatation and stenting to the inflow cannula stenosis of a left ventricular assist device. Catheter Cardiovasc Interv 2017;89(7):1219‐1223. [DOI] [PubMed] [Google Scholar]
- 44. Ravichandran AK, Parker J, Novak E, Joseph SM, Schilling JD, Ewald GA, Silvestry S Hemolysis in left ventricular assist device: a retrospective analysis of outcomes. J Heart Lung Transplant 2014;33(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 45. Cowger JA, Romano MA, Shah P, Shah N, Mehta V, Haft JW, Aaronson KD, Pagani FD Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant 2014;33(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 46. Katz JN, Jensen BC, Chang PP, Myers SL, Pagani FD, Kirklin JK. A multicenter analysis of clinical hemolysis in patients supported with durable, long‐term left ventricular assist device therapy. J Heart Lung Transplant 2015;34(5):701‐709. [DOI] [PubMed] [Google Scholar]
- 47. Badiye AP, Hernandez GA, Novoa I, Chaparro SV. Incidence of hemolysis in patients with cardiogenic shock treated with Impella percutaneous left ventricular assist device. ASAIO J 2016;62(1):11‐14. [DOI] [PubMed] [Google Scholar]
- 48. Dixon SR, Henriques JPS, Mauri L, Sjauw K, Civitello A, Kar B, Loyalka P, Resnic FS, Teirstein P, Makkar R, Palacios IF, Collins M, Moses J, Benali K, O'Neill WW A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high‐risk percutaneous coronary intervention (the PROTECT I trial): JACC Cardiovasc Interv 2009;2(2):91‐96. [DOI] [PubMed] [Google Scholar]
- 49. Esposito ML, Morine KJ, Annamalai SK, et al. Increased plasma‐free hemoglobin levels identify hemolysis in patients with cardiogenic shock and a trans valvular micro‐axial flow pump. Artif Organs 2018. [DOI] [PubMed] [Google Scholar]
- 50. Pappalardo F, Schulte C, Pieri M, Schrage B, Contri R, Soeffker G, Greco T, Lembo R, Müllerleile K, Colombo A, Sydow K, de Bonis M, Wagner F, Reichenspurner H, Blankenberg S, Zangrillo A, Westermann D Concomitant implantation of Impella([R]) on top of veno‐arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur J Heart Fail 2017;19(3):404‐412. [DOI] [PubMed] [Google Scholar]
- 51. Tanawuttiwat T, Chaparro SV. An unexpected cause of massive hemolysis in percutaneous left ventricular assist device. Cardiovasc Revasc Med 2013;14(1):66‐67. [DOI] [PubMed] [Google Scholar]
- 52. Cardozo S, Ahmed T, Belgrave K. Impella induced massive hemolysis: reemphasizing echocardiographic guidance for correct placement. Case Rep Cardiol 2015;2015:464135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sibbald M, Dzavik V. Severe hemolysis associated with use of the Impella LP 2.5 mechanical assist device. Catheter Cardiovasc Interv 2012;80(5):840‐844. [DOI] [PubMed] [Google Scholar]
- 54. Sorajja P, Cabalka AK, Hagler DJ, Rihal CS. Long‐term follow‐up of percutaneous repair of paravalvular prosthetic regurgitation. J am Coll Cardiol 2011;58(21):2218‐2224. [DOI] [PubMed] [Google Scholar]
- 55. Ruiz CE, Jelnin V, Kronzon I, Dudiy Y, del Valle‐Fernandez R, Einhorn BN, Chiam PTL, Martinez C, Eiros R, Roubin G, Cohen HA Clinical outcomes in patients undergoing percutaneous closure of periprosthetic paravalvular leaks. J am Coll Cardiol 2011;58(21):2210‐2217. [DOI] [PubMed] [Google Scholar]
- 56. Altarabsheh SE, Deo SV, Rihal CS, Park SJ. Mitral paravalvular leak: caution in percutaneous occluder device deployment. Heart Surg Forum 2013;16(1):E21‐23. [DOI] [PubMed] [Google Scholar]
- 57. Bulut MO, Kucuk M, Balli S, Celebi A. Treatment of severe hemolysis following nit‐Occlud Le VSD coil implantation with Amplatzer duct Occluder II. Turk Kardiyoloji Dernegi Arsivi Derneginin Yayin Organidir 2016;44(7):593‐596. [DOI] [PubMed] [Google Scholar]
- 58. Tang L, Tang JJ, Fang ZF, Hu XQ, Shen XQ, Zhou SH. Severe mechanical hemolysis after Transcatheter closure of a traumatic ventricular Septal defect using the Amplatzer atrial Septal Occluder. Int Heart J 2016;57(4):519‐521. [DOI] [PubMed] [Google Scholar]
- 59. McCaul ME, Turkkan JS, Stitzer ML. Psychophysiological effects of alcohol‐related stimuli: I. the role of stimulus intensity. Alcohol Clin Exp Res 1989;13(3):386‐391. [DOI] [PubMed] [Google Scholar]
- 60. Reiss N, Schuett U, Maleszka A, Kleikamp G, Schenk S, Gummert J. Surgical removal of occluder devices: complications and pitfalls. Heart Surg Forum 2009;12(3):E143‐146. [DOI] [PubMed] [Google Scholar]
- 61. Spence MS, Thomson JD, Weber N, Qureshi SA. Transient renal failure due to hemolysis following transcatheter closure of a muscular VSD using an Amplatzer muscular VSD occluder. Catheter Cardiovasc Interv 2006;67(5):663‐667. [DOI] [PubMed] [Google Scholar]
- 62. Lambert V, Belli E, Piot JD, Planche C, Losay J. [Hemolysis, a rare complication after percutaneous closure of an atrial septal defect]. Arch mal Coeur Vaiss 2000;93(5):623‐625. [PubMed] [Google Scholar]
- 63. Raphael CE, Malouf JF, Maor E, et al. A hybrid technique for treatment of commissural primary mitral regurgitation. Catheter Cardiovasc Interv 2018. [DOI] [PubMed] [Google Scholar]
- 64. Alkhouli M, Rihal CS, Zack CJ, Eleid MF, Maor E, Sarraf M, Cabalka AK, Reeder GS, Hagler DJ, Maalouf JF, Nkomo VT, Schaff HV, Said SM Transcatheter and surgical management of mitral paravalvular leak: long‐term outcomes, JACC Cardiovasc Interv 2017;10(19):1946‐1956. [DOI] [PubMed] [Google Scholar]
- 65. Marchand A, Galen RS, Van Lente F. The predictive value of serum haptoglobin in hemolytic disease. JAMA 1980;243(19):1909‐1911. [PubMed] [Google Scholar]
- 66. Kliger C, Eiros R, Isasti G, Einhorn B, Jelnin V, Cohen H, Kronzon I, Perk G, Fontana GP, Ruiz CE Review of surgical prosthetic paravalvular leaks: diagnosis and catheter‐based closure. Eur Heart J 2013;34(9):638‐649. [DOI] [PubMed] [Google Scholar]
- 67. Shojania AM, Gross S. Hemolytic Anemias and folic acid deficiency in children. Am J Dis Child 1964;108:53‐61. [DOI] [PubMed] [Google Scholar]
- 68. Santinga JT, Flora JD, Rush JB, Penner JA, Willis PW. The effect of propranolol on hemolysis in patients with an aortic prosthetic valve. Am Heart J 1977;93(2):197‐201. [DOI] [PubMed] [Google Scholar]
- 69. Aoyagi S, Fukunaga S, Tayama E, Nakamura E, Egawa N, Hosokawa Y. Benefits of a beta‐blocker for intractable hemolysis due to paraprosthetic leakage. Asian Cardiovasc Thorac Ann 2007;15(5):441‐443. [DOI] [PubMed] [Google Scholar]
- 70. Okita Y, Miki S, Kusuhara K, Ueda Y, Tahata T, Yamanaka K. Propranolol for intractable hemolysis after open heart operation. Ann Thorac Surg 1991;52(5):1158‐1160. [DOI] [PubMed] [Google Scholar]
- 71. Geller S, Gelber R. Pentoxifylline treatment for microangiopathic hemolytic anemia caused by mechanical heart valves. Md Med J 1999;48(4):173. [PubMed] [Google Scholar]
- 72. Jennings DL, Williams CT, Morgan JA. Pentoxifylline for the treatment of hemolytic anemia in a patient who developed recurrent gastrointestinal bleeding while on continuous‐flow left ventricular assist device support. ASAIO J 2013;59(5):526‐527. [DOI] [PubMed] [Google Scholar]
- 73. Golbasi I, Turkay C, Timuragaoglu A, et al. The effect of pentoxifylline on haemolysis in patients with double cardiac prosthetic valves. Acta Cardiol 2003;58(5):379‐383. [DOI] [PubMed] [Google Scholar]
- 74. Hirawat S, Lichtman SM, Allen SL. Recombinant human erythropoietin use in hemolytic anemia due to prosthetic heart valves: a promising treatment. Am J Hematol 2001;66(3):224‐226. [DOI] [PubMed] [Google Scholar]
- 75. Kornowski R, Schwartz D, Joffe A, Pines A, Aderka D, Levo Y. Erythropoietin therapy obviates the need for recurrent transfusions in a patient with severe hemolysis due to prosthetic valves. Chest 1992;102(1):315‐316. [DOI] [PubMed] [Google Scholar]
- 76. Nassif ME, Patel JS, Shuster JE, Raymer DS, Jackups R Jr, Novak E, Gage BF, Prasad S, Silvestry SC, Ewald GA, LaRue SJ Clinical outcomes with use of erythropoiesis stimulating agents in patients with the HeartMate II left ventricular assist device. JACC Heart Fail 2015;3(2):146‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goldstein DJ, John R, Salerno C, Silvestry S, Moazami N, Horstmanshof D, Adamson R, Boyle A, Zucker M, Rogers J, Russell S, Long J, Pagani F, Jorde U Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant 2013;32(7):667‐670. [DOI] [PubMed] [Google Scholar]
- 78. Busu T, Alqahtani F, Badhwar V, Cook CC, Rihal CS, Alkhouli M. Meta‐analysis comparing Transcatheter and surgical treatments of paravalvular leaks. Am J Cardiol 2018;122(2):302‐309. [DOI] [PubMed] [Google Scholar]
- 79. Panaich SS, Maor E, Reddy G, et al. Effect of percutaneous paravalvular leak closure on hemolysis. Catheter Cardiovasc Interv 2018. [DOI] [PubMed] [Google Scholar]
- 80. Cruz‐Gonzalez I, Rama‐Merchan JC, Arribas‐Jimenez A, Rodriguez‐Collado J, Martin‐Moreiras J, Cascon‐Bueno M, Luengo CM Paravalvular leak closure with the Amplatzer vascular plug III device: immediate and short‐term results. Rev Esp Cardiol (Engl Ed) 2014;67(8):608‐614. [DOI] [PubMed] [Google Scholar]
- 81. Smolka G, Pysz P, Ochala A, et al. Transcatheter paravalvular leak closure and hemolysis ‐ a prospective registry. Arch Med Sci 2017;13(3):575‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Millan X, Bouhout I, Nozza A, et al. Surgery versus Transcatheter interventions for significant paravalvular prosthetic leaks. JACC Cardiovasc Interv 2017;10(19):1959‐1969. [DOI] [PubMed] [Google Scholar]
- 83. Joseph G, Mandalay A, Zacharias TU, George B. Severe intravascular hemolysis after transcatheter closure of a large patent ductus arteriosus using the Amplatzer duct occluder: successful resolution by intradevice coil deployment. Catheter Cardiovasc Interv 2002;55(2):245‐249. [DOI] [PubMed] [Google Scholar]


