Abstract
The high Andean plateau of Peru is known to suffer harsh environmental conditions. Acidic soils containing high amount of heavy metals due to mining activities and withstanding very low temperatures affect agricultural activities by diminishing crop quality and yield. In this context, plant growth promoting rhizobacteria (PGPR) adapted to low temperatures and tolerant to heavy metals can be considered as an environment-friendly biological alternative for andean crop management. The aim of this work was to select and characterize psychrotrophic PGPR isolated from the rhizosphere of maca (Lepidium meyenii Walp.) a traditional andean food crop. A total of 44 psychrotrophic strains isolated from 3 areas located in the Bombon plateu of Junin-Peru were tested for their PGPR characteristics like indole acetic acid (IAA) production, phosphate solubilization and for their ability to improve seed germination. In addition, their capacity to grow in the presence of heavy metals like cadmium (Cd), lead (Pb), cobalt (Co) and mercury (Hg) was tested. Of the total number of strains tested, 12 were positive for IAA production at 22 °C, 8 at 12 °C and 16 at 6 °C. Phosphate solubilization activities were higher at 12 °C and 6 °C than at 22 °C. Red clover plant assays showed that 16 strains were capable to improve seed germination at 22 °C and 4 at 12 °C. Moreover, 11 strains showed tolerance to Cd and Pb at varying concentrations. This study highlight the importance of obtaining PGPRs to be used in high andean plateu crops that are exposed to low temperatures and presence of heavy metals on soil.
Keywords: andean, psychrotrophs, heavy metals, PGPR, germination
1. Introduction
The Bombon plateu is located on the high Andes of Junin, Peru. It is known for its dry climate, extremely low temperatures and acidic soils (pH < 5) with relatively high concentration of toxic heavy metals. Among these, low temperatures and soil contamination are common problems which affect farmers of this region, together, these two factors diminish the yield of economically important crops such as maca (Lepidium meyenii Walp.) a Brassicaceae whose roots are used as food. Quality of the crop is also affected reducing its demand in international markets.
The soil is a habitat for a wide variety of microorganisms. The rhizosphere, an area surrounding plant roots, is a special ecological niche that supports a vast group of metabolically versatile microorganisms [1]. Among these, plant growth promoting rhizobacteria (PGPR) are considered of great importance due to their ability to solubilize inorganic phosphates, transport iron, produce phytohormones like indole acetic acid (IAA) [2] and fix atmospheric nitrogen [3]. These attributes allow those microbes to induce plant growth and development. In this study, PGPRs can be considered as a biological alternative to improve the yield and growth of crops growing in the high Andes under harsh environmental conditions. In this context, the aims of this research were to select and characterize psychrotrophic bacteria isolated from maca rhizosphere by testing their plant growth promoting ability and tolerance to heavy metals. There are only few studies that explore the application of biofertilizers based on psychotrophic bacteria with PGPR characteristics, adapted to the harsh climatic conditions of the high Andean region and with potential to bioremediate soils contaminated with heavy metals.
2. Materials and Methods
2.1. Sampling and isolation of bacteria
Soil samples were collected from fields in the Comunidad Campesina San Pedro de Cajas, Province of Tarma-Junin (located at 4100 meters above sea level). Maximum and minimum soil temperatures were 22 °C and 6 °C, respectively, with a relative humidity of 55.2%. Three locations were sampled: Galpón Condorín (Longitude W 75°53′49.54″ Latitude S 11°15′12.18″), Acomachay (Longitude W 75°53′38.64″ Latitude S 11°14′15.24″) and Condorín (Longitude W 81°23′42″ Latitude S 82°7′32.01″) which were either at the stage of first harvest, second harvest and 10 years of land fallow (without planting any crop), respectively. Rhizosphere and soil samples were collected from different plants. Each plant was taken completely along with their associated soil. Moreover, 500 g of soil was also collected from the area lacking any plant material. Samples were processed later in accordance with the methodologies established by APHA (1998) [4] for isolation of viable aerobic bacteria. Temperature and time of incubation was modified to 6 °C for 7 days to obtain isolates with the ability to grow at low temperatures. Rhizosphere and soil samples were also characterized for their physico-chemical properties like pH, conductivity, texture, organic matter, nutrients, among others.
2.2. Bacterial growth at different temperatures
Isolates were grown in Nutrient Agar (NA) medium and incubated at 6 °C, 12 °C, 22 °C and 28 °C [5] in order to determine their nature as psychrotrophic or psychrophilic bacteria. Growth was evaluated after 7 days of incubation and results were expressed on a scale of 1 to 4 (where 4 represent the maximum colony growth).
2.3. Characterization of IAA production
IAA production was estimated using the colorimetric technique based on the Salkowski reagent [6]. Strains were grown in 5 ml of Yeast Extract Mannitol (YEM) broth suplemented with 5 mM L-tryptophan and were incubated at 6 °C, 12 °C and 22 °C for 28, 19 and 11 days, respectively. Salkowski reagent (1:4) [7] was added to each culture media and the mix was kept in the dark for 30 min. Positive samples turned from shades of pink to intense rose. Optical density (OD) was registrated at 530 nm to measure IAA production. The amount of IAA in µg/ml was calculated by plotting a standard curve [8].
2.4. Phosphate solubilization capacity of bacteria
Isolates were grown in YEM broth and incubated at 6 °C, 12 °C and, 22 °C for 28, 19 and 11 days, respectively. Five µl of each culture at a concentration of 108 CFU/ml [9] was inoculated in National Botanical Research Institute's Phosphate (NBRIP) basal medium plates supplemented with di-calcium (CaHPO4) or tri-calcium (Ca3(PO4)2) phosphate [10]. Plates were incubated at 6 °C and 12 °C for 36 days and at 22 °C for 8 days. Strains with a translucent halo around the colony were considered positive for phosphate solubilization.
2.5. Seed germination assay
Strains for this assay were selected according to positive results obtained on at least one of the in vitro PGPR traits. Red clover (Trifolium pratense) seeds were used as a model plant to evaluate the efficacy of the isolates on germination promotion. Disinfection and inoculation of seeds (106 UFC/ml) was carried out following the methodology described by Ogata and Zúñiga [12]. Seeds were incubated at 12 °C or 22 °C after inoculation. Inoculation with water was used as control. Each experimental unit consisted of 25 inoculated seeds in a water agar (0.75%) plate. Four replicates were used for each treatment. The percentage of germination was evaluated with the following formula:
2.6. Heavy metal resistance
All strains tested in the germination assay were also evaluated for their ability to grow in the presence of four heavy metals: Pb (lead trihydrate), Cd (cadmium chloride), Co (cobalt chloride) and Hg (mercury chloride II). The assay was conducted following the method described by Lalitha [13] using 0.5 mM Cd, Pb or Co and 0.01 mM Hg [14]. In addition, a mixture of all metals at 0.5 mM was also used. Strains resistant to the different metals tested were further evaluated for their minimum inhibitory concentration (MIC) using Pb and Cd separately. The assay was conducted using 8 concentrations (0.005, 0.01, 0.05, 0.1, 0.5, 1, 2.5 and 5 mM) of each heavy metal on NA medium adjusted to pH 5 [14] and incubated at 6 °C for 30 days.
2.7. Molecular identification and phylogenetic analysis
Molecular identification was carried out using 16S rDNA gene polymerase chain reaction (PCR) amplification and sequencing by using primer pair fD1 (5′-CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG-3′) and rD1 (5′-CCCGGGATCCAAGCTTAAGGAGGTGATCCAGCC-3′) [15]. The ClustalX2 software [16] was used for alignment of sequences generated in this study along with sequences obtained from databases. Phylogenetic relationships were established with the neighbor-joining (NJ) method and distances calculated according to the Kimura-2 parameter model using the MEGA6 software [17].
3. Results and Discussion
3.1. Isolation of PGPR
Physicochemical characterization of the soils samples revealed that they were non-saline, moderately thick with sandy loam consistency and acidic (pH values ranging from 4 to 5). Concentrations of the metals such as B, Fe, Mn, Cu, Zn and Cr were in the range of 13.86–18.87 ppm. The concentration of lead (Pb) was in the range of 33.92–56.14 ppm while that of cadmium (Cd) was between 1.43–1.98 ppm. Based on the Environmental Quality Standards of agricultural soils, the samples slightly exceded the maximum permissible values of Cd but not of Pb. According to the Peruvian normative, concentrations under 70 ppm and 1.4 ppm of Pb and Cd, respectively, are allowed for agricultural soil.
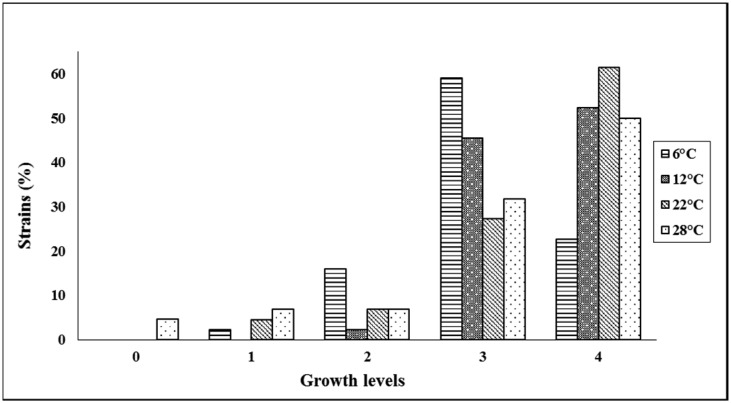
A total of 44 bacterial strains were isolated: 8 from Galpón Condorín (1st year of harvest), 30 from Acomachay (2nd year of harvest) and 6 from Condorin (10 years of rest). Twenty three were isolated from the rhizosphere and 21 from non-rhizospheric soil. Growth at different temperatures, showed that more than 59% of the strains have a growth level of 4 when they were incubated at 12 °C, 22 °C and 28 °C (Figure 1). All isolates grew at 12 °C and 6 °C. These results may be related to their origin in the high andean zone, where soils reach very low temperatures (close to 0 °C) during the freezing season. Their versatility to grow at different temperatures indicated that the strains belong to the psychrotrophic group (Przemieniecki et al., 2014). It is worth noting that strains obtained in this study took 7 days to grow under low temperatures while Calvo and Zúñiga (2010) reported that strains belonging to the genus Bacillus took only 4 days to grow under the same conditions.
Figure 1. Percentage of strains showing different growth levels (1–4) at different temperatures (6 °C, 12 °C, 22 °C, 28 °C).
3.2. IAA production assays
Strains were tested for their ability to produce IAA at three temperatures i.e. 22 °C, 12 °C and 6 °C (Table 1). Among the tested strains, LMTK39 was able to produce the maximum amount of the phytohormone at 22 °C and 12 °C, 60.6 µg/ml and 31.1 µg/ml, respectively. Whereas strain LMTK11 showed the highest ability to produce IAA at 6 °C (14.1 µg/ml). It was observed that the number of strains having the ability to produce IAA at 6 °C were higher than that of those at 22 and 12 °C. The above finding was not unexpected due to the fact that the amount of IAA biosynthesis varies as per the nature of the strain and environmental factors like altitude, temperature, drought conditions etc. In addition, a number of other factors related to stress conditions affecting these microbes are also known to influence with IAA biosynthesis [2],[20]. Subramanian et al. (2016) [21] reported that bacterial strains isolated from soils collected in regions with an average temperature of about 2 °C, have the ability to produce IAA at 5 °C. IAA production by these strains was found to be in the range of 0.3 to 17 µg/ml, values which are similar to those found in this study at 6 °C. A similar test was also performed by Zúñiga et al. (2011) [22] with strains isolated from maca rhizosphere at 28 °C showing less IAA production ability (0.3–11.9 µg/ml) at 5 °C than those reported in this study.
Table 1. PGPR traits of the strains used in this study. IAA (µg/ml) production and halo of phosphate solubilization (mm).
| STRAINS | IAA (µg/ml) |
CaHPO4 (mm) |
Ca3(PO4)2 (mm) |
||||||
| 22 °C | 12 °C | 6 °C | 22 °C | 12 °C | 6 °C | 22 °C | 12 °C | 6 °C | |
| LMTK1 | – | – | 3.6 | – | – | – | – | – | – |
| LMTK4 | – | – | 3.3 | – | – | – | – | – | – |
| LMTK5 | – | – | 4.0 | – | – | – | – | – | – |
| LMTK6 | 2.9 | – | 3.2 | 1.25 | 5.25 | – | – | 2 | – |
| LMTK7 | 7.0 | 3.0 | 3.2 | – | – | – | – | – | – |
| LMTK10 | 1.8 | 4.8 | 3.2 | – | – | – | – | – | – |
| LMTK11 | 5.4 | 15.1 | 14.1 | 11.5 | 23.5 | 18 | 2 | 2.5 | 2.75 |
| LMTK15 | – | – | 4.5 | – | – | – | – | – | – |
| LMTK25 | 1.8 | 0.9 | 6.9 | – | – | – | – | – | – |
| LMTK28 | 3.7 | – | 0.9 | 8 | 20.5 | 11.25 | – | 2.5 | – |
| LMTK32 | 2.0 | – | 1.1 | 8 | 19.25 | 11 | – | 2.5 | 2.5 |
| LMTK34 | 12.3 | – | 4.7 | 7 | 20.5 | 10 | – | 2 | 4.5 |
| LMTK36 | – | – | – | 9 | 17 | 12.5 | – | 2 | 1 |
| LMTK37 | 12.8 | 6.0 | 12.1 | – | – | – | – | – | – |
| LMTK39 | 60.6 | 31.1 | 12.5 | – | – | – | – | – | – |
| LMTK42 | 0.4 | 1.5 | 12.6 | 8 | 16.25 | 12.25 | – | 1.25 | 1 |
| LMTK43 | 3.0 | 9.8 | 9.5 | 3 | 6.25 | 3.25 | – | 1.25 | 1.5 |
3.3. Phosphate solubilization ability
Eight strains were able to solubilize di-calcium and tri-calcium phosphate. Strain LMTK11 was the only one which had the ability to solubilize tri-calcium phosphate at 22 °C. This strain showed a solubilization halo of 2 mm, while in a di-calcium phosphate media it was capable to produce a halo size of 11.5 mm. Solubilization halo of the strains from this study varied from 5.25 mm to 23.5 mm at 12 °C. Strains LMTK11, LMTK28 and LMTK34 showed the maximum ability to solubilize di-calcium phosphate at this temperature. The ability of the strains to solubilize di-calcium at 6 °C was lower than at 12 °C and higher than at 22 °C. At this temperature (6 °C), strains LMTK11, LMTK28 and LMTK34 displayed solubilization halos of 18, 11.3 and 10 mm respectively (Table 1). In a similar study, strains isolated from the Himalayas and living at harsh conditions of low temperature and high altitude, showed a range of solubilization efficiency between 25.8–375.4 and 31.7–456.5 at incubation temperatures of 4 and 10 °C, respectively [23]. From all the strains tested, Pseudomonas sp. showed high ability to solubilize di-calcium and tri-calcium phosphates. Mishra et al. (2015) [24] also reported Pseudomonas sp. as the bacterial genus with the highest solubilization activity. In contrast, Zúñiga et al. (2011) [22] found that free-living diazotrophs showed the best solubilization halos in di-calcium and tri-calcium media at temperatures of 5, 14 and 28 °C.
3.4. Promotion of seed germination
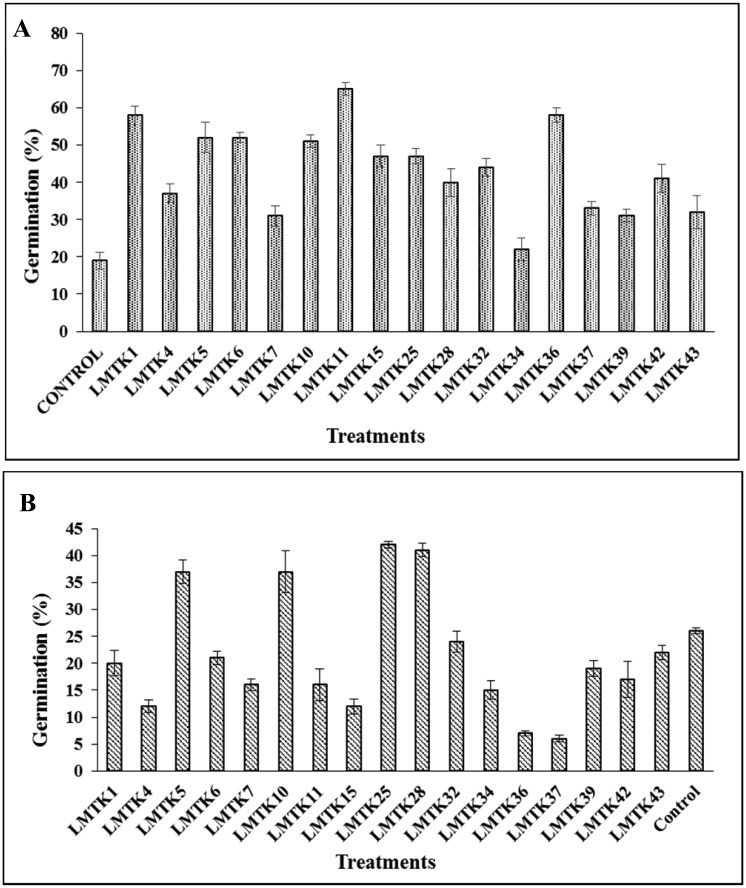
Sixteen from the seventeen strains tested in the red clover germination assay at 22 °C significantly increased the percentage of germination compared to the non-inoculated control (Figure 2A). LMTK11 strain showed the best performance in the germination assay at this temperature. These results are consistent with those reported by Kloepper et al. (1991) [25], which emphasize the role of PGPR in improving seed germination rates through the production of plant growth regulating substances such as auxins, cytokinins or gibberellins [26]. The majority of the strains able to increase the germination percentage were also positive for both IAA production and phosphate solubilization. Although, some of the strains did not show high values in all PGPR traits, they were able to improve seed germination in comparison to the non-inoculated control. At 12 °C, strains LMTK25 and LMTK28 showed better ability to increase the germination rate (42 and 41%, respectively) than the control (26%) (Figure 2b). A study conducted by Acosta et al. (2012) [27] also higlighted the importance of incubation temperature for the induction of seed germination.
Figure 2. Effect of inoculation with different bacterial strains on red clover seed germination. A. 22 °C; B. 12 °C.
3.5. Bacterial growth at different heavy metal concentrations
Eleven out of the seventeen strains tested were able to grow in the presence of different heavy metals (0.5 mM Pb, Cd and Co; 0.01 mM Hg). It was shown that the strains were able to better tolerate the heavy metals when they were cultivated at pH 5 rather than 4 (Table 2). Other studies show a variable effect of heavy metals such as lead and cadmium in different organisms, demonstrating that the toxicity depends not only on pH but also on the concentration and organism evaluated. [28],[29],[30]. Strain LMTK11 was the only one able to grow at both pH 4 and 5 in a culture media supplemented with Hg; while in media supplemented with Co at pH 5, the growth of strains LMTK11, LMTK32 and LMTK36 was the only observed. Interestingly, strain LMTK11 was able to grow in NA plates supplemented with a mixture of heavy metals (Pb, Cd, Co and Hg). Mechanisms reported for heavy metal resistance in bacteria are: (1) exclusion of metal by a permeable barrier, (2) exclusion by active transport of the metal from the inside of the cell outwards, (3) intracellular physical sequestration of the metal using a polymer to prevent metal cellular damage, (4) extracellular sequestration, (5) enzymatic detoxification of the metal to a less toxic form, and (6) cell reduction of the sensitivity to metals [31].
Table 2. Growth ability of eleven strains in the presence of different heavy metals at pH 4 and 5.
| STRAINS | pH 4 |
pH 5 |
||||||||
| Cd | Pb | Co | Hg | MIX | Cd | Pb | Co | Hg | MIX | |
| LMTK5 | x | x | x | |||||||
| LMTK11 | x | x | x | x | x | x | x | x | ||
| LMTK28 | x | x | x | |||||||
| LMTK32 | x | x | x | x | ||||||
| LMTK33 | x | x | x | |||||||
| LMTK34 | x | x | x | |||||||
| LMTK36 | x | x | x | x | ||||||
| LMTK37 | x | |||||||||
| LMTK39 | x | |||||||||
| LMTK42 | x | x | x | |||||||
| LMTK43 | x | x | x | x | ||||||
x, Growth ability; MIX, mixture of different heavy metals.
3.6. Minimum inhibitory concentration assay
Minimum inhibitory concentration (MIC) assays was performed using Pb and Cd. Based on the results obtained, MIC values of the 11 selected strains were calculated to be 0.5 mM and 0.05 mM for Pb and Cd, respectively (Table 3). Kumar et al. (2015) [32] reported two Bacillus strains with MIC values of 250 and 1000 ppm for Pb. On this kind of assay, reduction in colony size is common. This reduction is attributed to the toxic effects that both metals exert on bacterial cell affecting its metabolism, causing a blockage of enzymes and polynucleotides activities [33],[34]. These heavy metals are also known to interfere with the transport of essential nutrients and ions. They also affect organisms by either substitution or displacement of essential ions in the cell or through denaturation and inactivation of key enzymes. In addition, heavy metals are able to affect bacterial membrane integrity [31]. Although high concentrations of heavy metals inhibit bacterial growth, there are some species which are resistant and could grow up to 5000 ppm of Pb [35]. In course of the assays, it was noticed that some bacterial colony sizes increased as the concentration of the metals were increased. Strain LMTK43 showed an increase in colony size in comparison to the control devoid of heavy metals. Some studies have described the ability of certain strains to use these metals as growth enhancers. For instance, in a study conducted by Azario et al. 2010 [36], significant increase in growth of E. coli ATCC 35218 was observed when it was cultivated in a media supplemented with Cr, Cd and Pb separately or together, as a cocktail.
Table 3. Strain tolerance percentages to different concentrations (mM) of lead and cadmium.
| Heavy metals | lead and cadmium concentrations (mM) |
|||||||
| 0.005 | 0.01 | 0.05 | 0.1 | 0.5 | 1 | 2.5 | 5 | |
| Pb | 100 | 100 | 100 | 100 | 72.7 | 0 | 0 | 0 |
| Cd | 100 | 100 | 72.7 | 63.6 | 63.6 | 54.5 | 0 | 0 |
3.7. Phylogenetic analysis of the strains
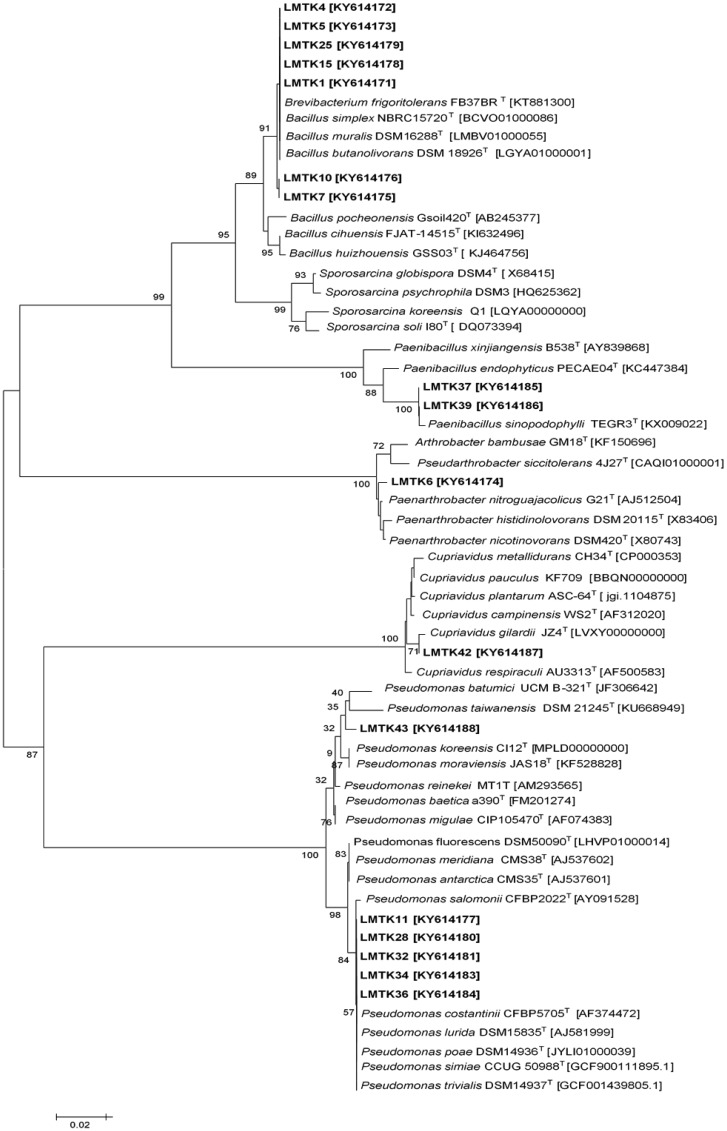
The NJ phylogenetic tree (Figure 4) showed that the studied strains were clustered in 8 groups distributed in the Pseudomonas, Bacillus, Paenibacillus, Sporosarcina, Cupriavidus and Paenarthrobacter genera. The majority of strains belonged to Bacillus and Pseudomonas. Bacillus and Pseudomonas genera are usually reported as PGPR and are found to be dominant in bacterial-plant studies [37]. Among the Firmicutes, five strains were clustered within the Bacillus simplex group and two strains were grouped with Paenibacillus sinodophylli. One strain of Actinobacteria grouped with Paenathrobacter nitroguajacolicus. Proteobacteria were represented by one strain related to Cupriavidus gilardii, one strain to Pseudomonas taiwanensis while five strains were grouped with Pseudomonas constaninii. The comparison of the 16S rDNA gene sequence of all the isolates against type strains of bacterial species recorded in the EzCloud database is shown in Table 4.
Table 4. Comparison of 16S rDNA sequences obtained from strains isolated in this study and related type strains.
| Strains | Molecular identification using 16S rDNA gen | Accession number | Similarity (%) |
| LMTK1 | Bacillus simplex NBRC 15720T | BCVO01000086 | 99.8 |
| LMTK4 | Bacillus simplex NBRC 15720T | BCVO01000086 | 99.8 |
| LMTK5 | Bacillus simplex NBRC 15720T | BCVO01000086 | 99.8 |
| LMTK6 | Paenarthrobacter nitroguajacolicus G2-1T | AJ512504 | 99.8 |
| LMTK7 | Bacillus butanolivorans DSM 18926T | LGYA01000001 | 99.8 |
| LMTK10 | Bacillus butanolivorans DSM 18926T | LGYA01000001 | 99.8 |
| LMTK11 | Pseudomonas fluorescens DSM 50090T | LHVP01000014 | 99.5 |
| LMTK15 | Bacillus simplex NBRC 15720T | BCVO01000086 | 99.8 |
| LMTK25 | Bacillus simplex NBRC 15720T | BCVO01000086 | 99.8 |
| LMTK28 | Pseudomonas trivialis DSM 14937T | JYLK01000002 | 99.2 |
| LMTK32 | Pseudomonas simiae OLiT | AJ936933 | 99 |
| LMTK34 | Pseudomonas simiae OLiT | AJ936933 | 99 |
| LMTK36 | Pseudomonas simiae OLiT | AJ936933 | 99.5 |
| LMTK37 | Paenibacillus sinopodophylli TEGR-3T | KX009022 | 98.2 |
| LMTK39 | Paenibacillus sinopodophylli TEGR-3T | KX009022 | 96 |
| LMTK42 | Cupriavidus gilardii LMG 5886T | AF076645 | 99.5 |
| LMTK43 | Pseudomonas baetica a390T | FM201274 | 99.5 |
Figure 3. Phylogenetic tree based on 16S rDNA sequences. Strains obtained in this study are indicated in bold. Only bootstrap values greater than 60% are shown (1,000 pseudoreplicates). Type strains of related species were obtained from Ez-biocloud.
4. Conclusions
The results obtained in this study demonstrate the PGPR potential of these strains; as well as their ability to grow in the presence of heavy metals such as lead and cadmium under in vitro conditions. This study provides first experimental evidence highlighting the potential of these strains as biofertilizers and bioremediators for the high Andean soils, due to their ability promote plant growth, ability to grow at low temperatures and to tolerate heavy metal toxicity.
Acknowledgments
This research was funded by PROCYT 309-2009-CONCYTEC, Peru Biodiverso-2009-GTZ-CONCYTEC and ECOANDINO S.A.C. We thank Dr. Ernesto Ormeño Orrillo for his suggestions and critical editing of the manuscript.
Abbreviations
- Cd
Cadmium
- Co
Cobalt
- CFU
Colony Formation Unity
- Hg
Mercury
- IAA
Indole Acetic Acid
- NA
Nutrient agar
- OD
Optical density
- Pb
Lead
- SE
Solubilization Effiency
- YEM
Yeast Extract Mannitol
- PGPR
Plant Growth Promotion Rhizobacter
- NBRIP
National Botanical Research Institute's Phosphate
Footnotes
Conflict of Interest: The authors declare no conflict of interest in this research.
Reference
- 1.Upadhyay A, Srivastava S. Evaluation of multiple plant growth promoting traits of an isolate of Pseudomonas fluorescens strain Psd. Indian J Exp Biol. 2010;48:601–609. [PubMed] [Google Scholar]
- 2.Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002;68:3795–3801. doi: 10.1128/AEM.68.8.3795-3801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Döbereiner J. History and new perspectives of diazotrophs in association with non-leguminous plants. Symbiosis. 1992;13:1–13. [Google Scholar]
- 4.American Public Health Association. Standard methods for examination of water and waste water. Washington DC, USA: 1998. [Google Scholar]
- 5.Calvo P, Zúñiga D. Caracterización fisiológica de cepas de Bacillus spp. aisladas de la rizósfera de papa (Solanum tuberosum) Ecol Apl. 2010;9:31–39. [Google Scholar]
- 6.Ehmann A. The Van Urk-Salkowski reagent a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977;132:267–276. doi: 10.1016/s0021-9673(00)89300-0. [DOI] [PubMed] [Google Scholar]
- 7.Gordon SA, Weber RP. Colorimetric estimation of indolacetic acid. Plant Physiol. 1951;26:192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glick BR. The enhancement of plant growth by free-living bacteria. Can J Microbiol. 1995;41:109–117. [Google Scholar]
- 9.Alikhani H, Saleh-Rastin N, Antoun H. Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil. 2006;287:35–41. [Google Scholar]
- 10.Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen C, Yan W, Tacon F, et al. Genetic variability of phosphate solubilizing activity by monocaryotic and dicaryotic mycelia of the ectomycorrhizal fungus Laccaria bicolor (Maire) PD Orton. Plant Soil. 1992;143:193–199. [Google Scholar]
- 12.Ogata K, Zúñiga D. Estudio de la microflora de la rizósfera de Caesalpinia spinosa en la provincia de Huánuco. Zonas Áridas. 2008;12:191–208. [Google Scholar]
- 13.Lalitha MK. Manual on antimicrobial susceptibility testing. Indian Assoc of Med Microbiol. 2005:14–16. [Google Scholar]
- 14.Sabry SA, Ghozlan HA, Abou-Zeid DM. Metal tolerance and antibiotic resistance patterns of a bacterial population isolated from sea water. J Appl Microbiol. 1997;82:245–252. doi: 10.1111/j.1365-2672.1997.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 15.Weisburg WG, Barns SM, Pelletier DA, et al. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larkin MA, Blackshields G, Brown NP, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 17.Tamura K, Stecher G, Peterson D, et al. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MINAM, Decreto supremo 002-2013 Environmental Quality Standards of agricultural soils, El Peruano, 2012. Available from: http://www.minam.gob.pe/calidadambiental/wp-content/uploads/sites/22/2013/10/D-S-N-002-2013-MINAM.pdf.
- 19.Bric JM, Bostock RM, Silvestone SE. Rapid in situ assay for indolacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbio. 1991;57:535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31:425–448. doi: 10.1111/j.1574-6976.2007.00072.x. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian P, Kim K, Krishnamoorthy R, et al. Cold stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. Plos One. 2016;11:1–17. doi: 10.1371/journal.pone.0161592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zúñiga-Dávila D, Tolentino J, García M Pérez W, et al. Characterization of rhizospheric bacteria isolated from maca (Lepidium meyenii Walp.) in the highlands of Junin-Peru. In: Mendez-Vilas A, editor. Microorganisms in Industry and Environment: From scientific and industrial research to consumer products. Singapore: World Scientific Pub Co; 2011. pp. 21–25. [Google Scholar]
- 23.Trivedi P, Pandey A. Low temperature phosphate solubilization and plant growth promotion by psychrotrophic bacteria, isolated from the Himalayan region. Res J Microbiol. 2007;2:452–461. [Google Scholar]
- 24.Mishra V, Gupta A, Kaur P, et al. Synergistic effects of Arbuscular mycorrhizal fungi and plant growth promoting rhizobacterial. Int J Phytoremediation. 2016;18:697–703. doi: 10.1080/15226514.2015.1131231. [DOI] [PubMed] [Google Scholar]
- 25.Kloepper JW, Zablotowicz RM, Tipping EM, et al. Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister DL, Cregan PB, editors. The rhizosphere and plant growth. Netherlands: Kluwer Academic Publishers; 1991. pp. 315–326. [Google Scholar]
- 26.Gul B, Hameed A, Weber DJ, et al. Sabkha Ecosystems Volume V: The Americas Tasks for Vegetation Science. Switzerland: Springer International Publishing; 2016. Assessing seed germination responses of great basin halophytes to various exogenous chemical treatments under saline conditions; pp. 85–104. [Google Scholar]
- 27.Acosta JM, Bentivegna DJ, Panigo ES, et al. Factors affecting seed germination and emergence of Gomphrena perennis. Weed Res. 2012;53:69–75. [Google Scholar]
- 28.Babich H, Stotizky G. Sensitivity of various bacteria, including actinomycetes, and fungi to cadmium and the influence of pH on sensitivity. Appl Environ Microbiol. 1976;33:681–695. doi: 10.1128/aem.33.3.681-695.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franklin NM, Stauber JL, Markich SJ, et al. pH-dependent toxicity of copper and uranium to a tropical freshwater alga (Chlorella sp.) Aquat Toxicol. 2000;48:275–289. doi: 10.1016/s0166-445x(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 30.Sandrin TR, Maier RM. Effect of pH on cadmium toxicity, speciation, and accumulation during naphthalene biodegradation. Environ Toxicol Chem. 2002;21:2075–2079. [PubMed] [Google Scholar]
- 31.Rouch DA, Lee TOB, Morby AP. Understanding cellular responses to toxic agents: a model for mechanisms—choice in bacterial resistance. J Ind Microbiol. 1995;14:132–141. doi: 10.1007/BF01569895. [DOI] [PubMed] [Google Scholar]
- 32.Kumar V, Singh S, Bhadrecha P, et al. Bioremediation of heavy metals by employing resistant microbial isolates from agricultural soil irrigated with industrial waste water. Orient J Chem. 2015;31:357–361. [Google Scholar]
- 33.Ochiai EI. General principles of biochemistry of the elements. New York: Plenum Press; 1987. pp. 381–382. [Google Scholar]
- 34.Gadd GM. Metals and microorganism: a problem of definition. FEMS Microbiol Lett. 1992;100:197–204. doi: 10.1111/j.1574-6968.1992.tb14040.x. [DOI] [PubMed] [Google Scholar]
- 35.Kumar V, Singh S, Singh J, et al. Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol. 2015;94:807–814. doi: 10.1007/s00128-015-1523-7. [DOI] [PubMed] [Google Scholar]
- 36.Azario R, Salvarezza S, Ibarra A, et al. Efecto del cromo hexavalente y trivalente sobre el crecimiento de Escherichia coli ATCC 35218. Información Tecnológica. 2010;21:51–56. [Google Scholar]
- 37.Zahid M, Abbasi MK, Hameed S, et al. Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.) Front Microbiol. 2015;6:1–10. doi: 10.3389/fmicb.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]