Abstract
Psychopathy is a complex disorder comprised of harmful personality traits and impulsive-lifestyle and antisocial behaviors. Weakened functional connectivity between limbic and prefrontal brain regions is thought to underlie impaired sensitivity to others’ emotions that contribute to the interpersonal and affective personality traits associated with psychopathy. We tested whether weakened functional connectivity between the amygdala and ventromedial prefrontal cortex (vmPFC) during the processing of fearful, angry, and neutral facial expressions, was prospectively related to psychopathic traits in early adulthood. The sample included 167 low-income, racially diverse, urban males who completed an fMRI scan at age 20 and questionnaire measures at ages 20 and 22. Weakened amygdala-vmPFC functional connectivity to fearful, but not neutral or angry, faces at age 20 was related to higher psychopathic traits at age 22.
Keywords: amygdala, fMRI, functional connectivity, psychopathy, ventromedial prefrontal cortex
Psychopathy is a complex construct defined by deficits in four domains: affective personality traits (e.g., absence of remorse and empathy), interpersonal personality traits (e.g., charm and deceitfulness), impulsive-lifestyle behaviors (e.g., irresponsibility and absence of long-term goals), and antisocial behaviors (e.g., criminal versatility and recidivism) (Hare & Neumann, 2008). Psychopathy is present among a quarter of prison inmates, and is related to the highest rates of both violent and non-violent crimes (Hare & Neumann, 2008). Research has established a relationship between the affective and interpersonal facets of psychopathy with impairments in responding to emotional cues of distress in others. For example, individuals high on psychopathy show deficits in recognizing and responding to fearful facial or verbal expressions of others (Marsh & Blair, 2008). Such deficits increase risk for psychopathy because they make it challenging for individuals to associate acts of harm, aggression, or transgressions with unpleasant negative arousal or reactivity to others’ fear/distress, increasing the likelihood of continued harm against others or crime (Blair, 2001; Lykken, 1957).
The deficient sensitivity to fearful expressions of emotion that characterizes psychopathy is thought to stem from impairments in neural reactivity, primarily in the limbic and paralimbic structures that regulate social and emotional behavior (Kiehl et al., 2001). In support of this hypothesis, adults high on psychopathy exhibit reduced amygdala reactivity in response to others’ cues of fear (Kiehl et al., 2001; Rilling et al., 2007). Adolescents with callous-unemotional (CU) traits (i.e., overlapping with psychopathic traits) also show reduced amygdala reactivity specifically to fearful expressions of emotion (Jones, Laurens, Herba, Barker, & Viding, 2009). However, as the amygdala is densely and reciprocally connected with the ventromedial prefrontal cortex (vmPFC) (Greicius, Krasnow, Reiss, & Menon, 2003), the processing of fear is thought to simultaneously trigger a bottom-up representation of the emotion (amygdala) and top-down regulatory control (vmPFC) that guides behavior, moral decision-making, and socialization. Psychopathy is thus proposed to arise from impairments in correlated activity of the amygdala and vmPFC in response to others’ fearful emotions (Blair, 2007; Blair, Peschardt, Budhani, Mitchell, & Pine, 2006).
Functional connectivity analysis can be used to examine whether the correlated activity of the amygdala and vmPFC is related to psychopathic traits. Among adult samples, a handful of studies have reported that psychopathy is related to weakened functional connectivity between the amygdala and vmPFC. However, these studies have only used resting-state functional connectivity (Motzkin, Newman, Kiehl, & Koenigs, 2011) or task-based functional connectivity employing paradigms that assess responses to emotive versus neutral words (Kiehl et al., 2001), moral scenarios (Yoder, Harenski, Kiehl, & Decety, 2015), or imagining others’ pain (Decety, Chen, Harenski, & Kiehl, 2013). Based on the centrality of emotional expressions to interpersonal interactions and the striking deficits found among individuals high on psychopathy in responding to others’ distress, an exploration of amygdala-vmPFC functional connectivity during the processing of fearful faces is needed.
We sought to address several important gaps in the literature exploring amygdalavmPFC functional connectivity to emotional faces and psychopathic traits. First, while studies have examined amygdala-vmPFC functional connectivity during the processing of facial expressions among youth with CU traits (Finger et al., 2012; Marsh et al., 2011), no studies have used this paradigm in adult samples. Second, existing functional connectivity studies of psychopathy in adults have largely focused on incarcerated populations that are limited in accessibility, render small sample sizes, and present with comorbid problems, including substance abuse (Fazel, Yoon, & Hayes, 2017), that could impact the brain. Thus, the study of neural risk factors for psychopathy can be advanced by examining larger community samples with variability in the full range of psychopathy. Finally, no prior studies have explored the specificity of response deficits to fearful faces versus deficits in responding more generally to threatening facial emotional expressions. That is, while the literature has consistently highlighted psychopathy to be linked to deficits in responding to fearful faces, no studies have simultaneously explored amygdala-vmPFC functional connectivity to fearful faces in a model with other emotions that could be perceived as threatening or ambiguous, including angry (Coccaro, McCloskey, Fitzgerald, & Phan, 2007) and neutral (Cooney, Atlas, Joormann, Eugène, & Gotlib, 2006) facial expressions to test unique relationships.
In the current paper, we thus investigated amygdala-vmPFC functional connectivity during an emotional faces task comparing specificity in the deficits for the processing of fearful faces, neutral faces, and angry faces. We utilized a low-income, male community sample, considered to be at heightened risk for antisocial behavior based on gender, socioeconomic status, and urbanicity (Shaw, Hyde, & Brennan, 2012). In line with the literature that has established psychopathy to be underpinned by deficits in responding to others’ fear and distress, we hypothesized that weakened amygdala-vmPFC functional connectivity to fearful, but not neutral or angry, faces at age 20 would be related to psychopathic traits at age 22 (and additionally, when controlling for earlier CU traits and antisocial behavior). We also examined separate factor scores for psychopathy and hypothesized that weakened amygdala-vmPFC functional connectivity to fearful faces would be uniquely related to Factor 1 psychopathy scores (i.e., interpersonal/affective deficits of psychopathy), controlling for overlap with Factor 2 psychopathy scores (i.e., impulsive-lifestyle/antisocial behaviors).
Methods
Participants
167 participants were drawn from the Pitt Mother & Child Project, an ongoing longitudinal study of 310 racially diverse boys and their families (52% European-American, 38% African-American, 7% biracial, 3% other; Shaw et al., 2012). The sample was recruited in infancy based on well-established risk factors for antisocial behavior, including male gender and growing up in a low-income, urban setting (Shaw et al., 2012). Target children and their mothers were seen almost yearly from age 1.5–22. Assessments included questionnaires and an fMRI scan at age 20, and questionnaires at age 22. Attrition to the age 20 and 22 visits was low for such a long-term study (256 men participated at age 20; 258 participants at age 22, 83% retention across 20 years) (Gard et al., 2017; Hyde et al., 2016; Shaw et al., 2012). However, the fMRI component at age 20 introduced several sources of data loss resulting in a sample size of 167 (Supplemental Table 1).
fMRI task
Participants performed an implicit emotional faces processing task, consisting of four blocks of perceptual face processing interleaved with five blocks of sensorimotor control (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002). During the task, participants viewed three faces of the same emotional expression and selected one of two faces (bottom of screen) that was identical to a target face (top of screen). Each face processing block consisted of six images, balanced for sex, derived from a standard set of pictures of facial affect (Ekman & Friesen, 1976). Each block consisted of a different emotional facial expression (anger, fear, surprise, neutral), and participants were randomly assigned to one of four different orders of block presentation. During the sensorimotor control blocks, participants viewed three simple geometric shapes and selected the shape that matched the target shape. As the task involves matching the person not the emotional expression, it is not considered an emotional recognition task. Accordingly, accuracy on the task was very high. For more details about the task and fMRI data acquisition and data pre-processing, see the Supplemental Methods and Supplemental Figure 1 (also see Gard et al., 2017; Gard et al., 2018; Hyde et al., 2016).
Functional connectivity analysis
To examine amygdala-vmPFC connectivity during the emotional faces processing task, we defined the left and right amygdala as the seed regions (AAL definition using WFU PickAtlas, version 1.04; Maldjian, Laurienti, Kraft, & Burdette, 2003) using the same definitions as prior publications in this sample (Gard et al., 2017). The vmPFC was defined as the target region (vmPFC centered on x, y, z, = −2, 44, −4; 30; Acikalin, Gorgolewski, & Poldrack, 2017, see Supplemental Figure 2). We established functionally connectivity of the amygdala and vmPFC during the face processing task in a prior study that adopted a whole-brain approach (Gard et al., 2018). The vmPFC mask was created using MarsBaR in SPM8 (Brett, Anton, Valabregue, & Poline, 2002). Psychological-physiological interaction (PPI) analyses from the generalized PPI (gPPI) toolbox (McLaren, Ries, Xu, & Johnson, 2012) were used to measure functional connectivity. Two general linear models at the individual level were constructed (i.e., one for each of the left and right amygdala seeds). For each model, the time series for the amygdala seed was entered as the physiological variable in the design matrix, the onset times for all task conditions were entered as psychological variables, and all product terms between amygdala seeds and conditions were entered as interaction terms.
Previous task-based PPI analyses of amygdala-vmPFC functional connectivity consistently show that activity in the amygdala and vmPFC is negatively correlated while participants view faces versus a non-face baseline (Hariri, Bookheimer, & Mazziotta, 2000). Thus, to examine whether amygdala-vmPFC activation was more negatively correlated (indicated by a negative regression weight) while participants looked at fearful, angry, and neutral faces than when they looked at shapes, we specified three primary contrasts at the individual level: fearful faces interaction < shapes interaction, angry faces interaction < shapes interaction and neutral faces interaction < shapes interaction (Gard et al., 2018). Group level models were constructed to examine contrasts across all participants. We report the extent thresholds (i.e., peaks) and cluster size within the vmPFC that met a Family-Wise-Error (FWE) correction of p<.05 for multiple comparisons (Supplemental Table 2).
Behavioral Measures
Psychopathic traits (age 22).
Psychopathic traits were assessed using the 28-item version of the Self-Report Psychopathy Short-Form (SRP-SF; Neumann, Hare, & Pardini, 2015), via a 5-point Likert-type scale for all items: affective (e.g., “never feel guilty over hurting others”), interpersonal (e.g., “think I can beat a lie detector”), impulsive-lifestyle (e.g., “done dangerous things just for the thrill), and antisocial (e.g., “tried to hit someone with a vehicle”) behaviors. The SRP-SF shows measurement invariance across sex and race, and its construct validity has been established by studies that have demonstrated theoretically meaningful associations with relevant measures of personality, cognitive, social, and neural processes (for more details, see Dotterer et al., 2017; Neumann et al., 2015; Neumann, Schmitt, Carter, Embley, & Hare, 2012). We examined links between amygdala-vmPFC functional connectivity with total psychopathy scores (α=.93) and separate Factor 1 (α=.90) and Factor 2 (α=.84) scores.
Covariates.
To identify specificity in the associations between amygdala-vmPFC functional connectivity at age 20 and psychopathy at age 22, we accounted for the effects of the following covariates: (1) Race (European-American vs. non-European-American) and (2) Income (age 20). Our presentation of the results focuses on models including race and income as covariates. However, we also examined longitudinal models that accounted for earlier antisocial behavior and callous-unemotional traits at age 20. These models assessed whether weaker amygdala-vmPFC functional connectivity was related to psychopathy over and above expected continuity in these traits over time thus reflecting brain effects on behavior, rather than the reverse (Supplemental Materials).
Analytic Strategy
We examined structural equation models (SEM) in Mplus vs. 7.2 using extracted estimates for amygdala-vmPFC functional connectivity to fearful faces < shapes, angry faces < shapes, and neutral faces < shapes, based on the findings of a prior paper using this sample that explored whole-brain functional connectivity of the amygdala during the emotional faces task (for more details, see Gard et al., 2018). Using an SEM approach allowed us to account for the overlap of variables, including between different psychopathy factors and neural responses to different emotional faces. First, we examined a regression model to test whether weaker amygdala-vmPFC negative functional connectivity to fearful, angry, or neutral faces versus shapes at age 20 was related to total psychopathic traits at age 22, accounting for income and race (Supplemental Figure 2A). Second, we examined a path model testing pathways from amygdala-vmPFC negative connectivity during fearful, angry, and neutral face processing to the Factor 1 and Factor 2 scores as correlated outcomes (Supplemental Figure 2B). Note that the Supplemental Material also presents findings for individual facet scores (Supplemental Figure 2C). To account for small amounts of missing data, analyses were conducted using full information maximum likelihood estimation with robust standard errors, which has been shown to be more efficient than listwise deletion and produce unbiased results with up to 50% missing at random (Enders & Bandalos, 2001).
Results
Main effects of amygdala-vmPFC functional connectivity
During face processing, the amygdala was significantly negatively functionally connected to the vmPFC (Supplemental Table 2; Supplemental Figure 3). That is, activity in the left and right amygdala was more negatively correlated with activity in the vmPFC when participants viewed fearful, angry, and neutral expressions (relative to shapes). We extracted these main effect estimates to examine links with psychopathy in Mplus. As we had no a priori hypotheses about laterality and because they were moderately correlated (fearful, r=.51, p<.001; angry, r=.56, p<.001; neutral, r=.68, p<.001), we combined estimates for the left and right amygdala into mean estimates. Hereinafter, the phrase “functional connectivity” refers to negative functional connectivity (i.e., main effect whereby the amygdala was significantly negatively correlated with the vmPFC during the processing of emotional faces versus shapes).
Amygdala-vmPFC functional connectivity at 20 and psychopathic traits at age 22
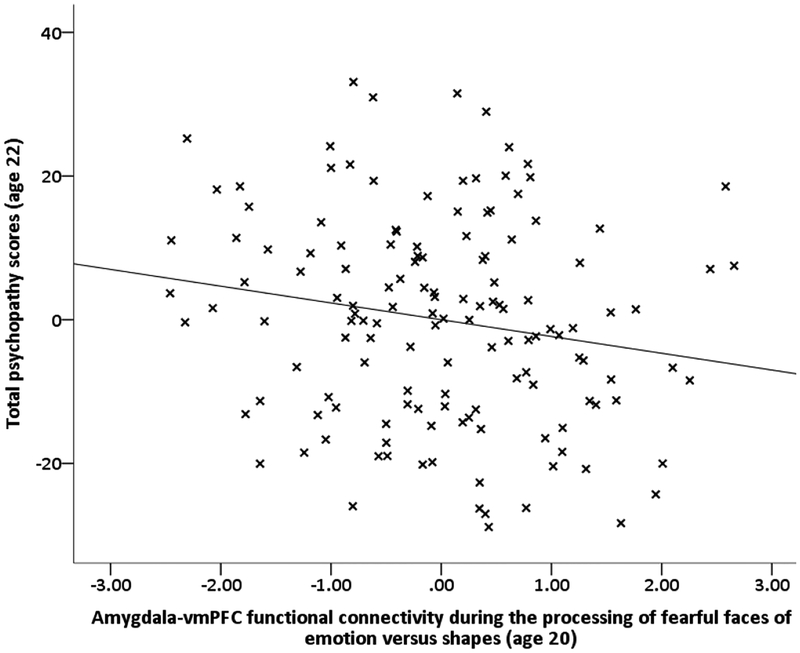
Descriptive statistics and bivariate correlations are presented in Supplemental Table 3. To test our hypotheses, we first examined relationships between amygdala-vmPFC functional connectivity to emotional faces and total psychopathy scores within a multivariate SEM that accounted for the covariance of functional connectivity estimates for all three face versus shape contrasts and race and income. Consistent with our hypothesis, higher total psychopathy scores at age 22 were related to weaker amygdala-vmPFC functional connectivity to fearful faces relative to shapes (β=−.16, p<.05), but not to neutral or angry faces versus shapes (Supplemental Table 4; Figure 1). Results were similar when earlier antisocial behavior and callous-unemotional traits at age 20 were included in the model (Supplemental Table 4).
Figure 1.
Psychopathic traits are related to weakened negative amygdala-vmPFC functional connectivity during the processing of fearful faces versus shapes
Note. Scatterplot of relationship between amygdala-vmPFC functional connectivity during processing of fearful faces versus shapes age 20 and later psychopathic traits at age 20. The scatterplot is a partial regression plot of the relationship, thus plotting the residualized/adjusted effects taking into account race and monthly income, as well as the estimates for amygdala-vmPFC functional connectivity during processing of angry faces < shapes and neutral faces < shapes (see Supplemental Table 4, Model 1 for estimates). Results were similar after accounting for earlier antisocial behavior and CU traits at age 20 Supplemental Table 4, Model 2)
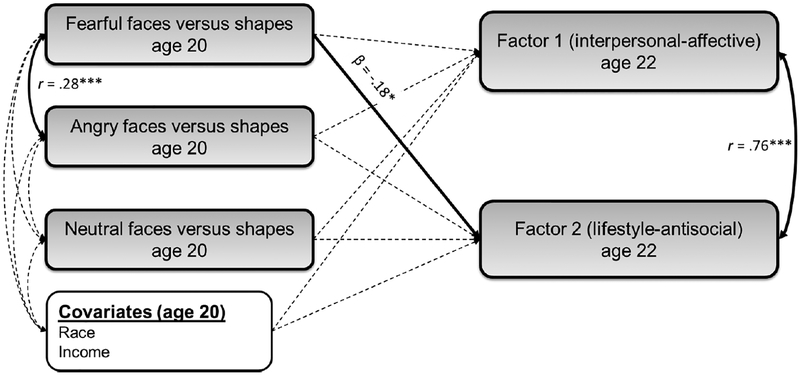
Next, we examined associations for factor scores of psychopathy. In contrast to our hypothesis, we found that weaker amygdala-vmPFC functional connectivity to fearful faces was significantly related to higher Factor 2 scores (i.e., impulsive-lifestyle and antisocial behaviors; β=−.18, p<.05), but not Factor 1 scores (i.e., interpersonal and affective traits; Figure 2, Supplemental Table 5). A similar pattern was evident for individual facet scores, with weaker amygdala-vmPFC functional connectivity being significantly related to impulsive-lifestyle (β=−.17, p<.05) and antisocial (β=−.14, p<.05), but not interpersonal or affective facets (Supplemental Table 6). However, the estimate for the association between weaker amygdalavmPFC functional connectivity to fearful faces and Factor 2 scores was not significantly different to that for Factor 1 scores (Δβ=.07, p>.05; Figure 2).
Figure 2.
Weakened negative amygdala-vmPFC functional connectivity during the processing of fearful faces versus shapes at age 20 is specifically related to Factor 2 psychopathy scores at age 22
Note. The processing of fearful faces < shapes (but not neutral faces < shapes or angry faces < shapes) at age 20 was significantly related to higher Factor 2 scores (i.e., irresponsible-lifestyle and antisocial behaviors) at age 22, accounting for race and income, as well as the covariance between predictors and outcomes within the model (see Supplemental Table 5, Model 1). Pathways that were modeled in the SEM, but that were non-significant, are shown as dotted grey lines. Model fit statistics: χ2=4.16, df=4, CFI=.999, TLI=.997, RMSEA=.02, SRMR=.03. Findings were unchanged when earlier antisocial behavior and callous-unemotional traits at age 20 were included as covariates in the model (Supplemental Table 5, Model 2). Notably, the difference in the path estimates from fearful faces versus shapes for Factor 1 and Factor 2 scores was not significantly different, based on calculating whether the confidence intervals overlapped by more or less than 50% (Cumming, 2009) (i.e., Δβ=.07, p>.05; see Supplemental Table 5).
Discussion
We found that that disrupted amygdala-vmPFC functional connectivity during the processing of fearful faces at age 20 was related to higher psychopathic traits at age 22. Our findings support the notion that weakened amygdala-vmPFC connectivity during emotional processing of others’ distress and fear represents a neurobiological correlate of psychopathic traits. Prior studies have focused on impaired functioning of the amygdala and vmPFC at rest and during the processing of emotion words and moral scenarios. This is the first study of young adults to establish a link between weakened amygdala-vmPFC functional connectivity and psychopathic traits focused on neural responses to fearful faces, which helps to better understand the interpersonal deficits associated with psychopathy. Moreover, by focusing on low-income urban males, we established the relationship between impaired amygdala-vmPFC connectivity and psychopathy across a full dimensional spectrum of psychopathic traits. Importantly, while this was not a forensic sample, a significant proportion of the sample (n=29, 17%) had “elevated” scores of ≥70 on the SRP (Paulhus, Neumann, Hare, Williams, & Hemphill, 2015). Moreover, six of those 29 men were diagnosed with Antisocial Personality Disorder at age 22. Thus, our community sample included individuals who were high on psychopathy and exhibited severe antisocial behavior.
These findings provide neuroimaging support for well-established theory that psychopathy is associated with dysfunction in amygdala-vmPFC connectivity in response to social cues of distress, in this case, fearful emotional expressions (Blair, 2007; Blair et al., 2006). This conclusion is bolstered by the inclusion of functional connectivity estimates for neutral and angry faces in models: our findings were specific to pathways between psychopathic traits and the processing of fearful faces relative to shapes. These results highlight a neurobiological mechanism whereby impairments in the neural representation of salient, negative emotional information by the amygdala and vmPFC might underpin psychopathic features.
In contrast to our hypothesis, we found that it was Factor 2 scores (i.e., lifestyle-antisocial behaviors), rather than Factor 1 scores (i.e., interpersonal-affective traits) that were driving the association between weakened amygdala-vmPFC functional connectivity to fear and total psychopathic traits. Previous studies that have examined links between amygdala-vmPFC functional connectivity and psychopathic traits in adults have focused on comparisons between healthy individuals and incarcerated individuals with versus without psychopathy using person-centered analyses and interviewer assessments of psychopathy (Kiehl et al., 2001; Motzkin et al., 2011). Thus, in the current high-risk urban sample, weakened amygdala-vmPFC functional connectivity may underlie greater overall severity of disinhibited externalizing psychopathology, especially when measured by self-reported items that capture harmful lifestyle and impulsive behaviors. Indeed, some of the foundational evidence implicating the vmPFC in emotion regulation came from studies of patients with prefrontal lesions, who manifested impulsive aggression that overlaps conceptually with the antisocial and impulsive-lifestyle facets of psychopathy (Davidson, Putnam, & Larson, 2000). Recent work has also emphasized emotion regulation deficits as a critical target of future etiologic and treatment research in psychopathy (Garofalo, Neumann, & Velotti, 2018).
At the same time, it is important to note that the SRP scales were strongly correlated, supporting the existence of a superordinate psychopathy construct underpinned by the four facets. Thus the current study also helps tie the pattern of neurobiological findings to the larger syndrome of psychopathic personality. Moreover, the magnitude of estimates for Factor 1 and Factor 2 did not differ significantly, further emphasizing that impairments in fear processing underlie a broader phenotype of psychopathy. At the same time, future studies that compare categorical versus dimensional measures of psychopathy in samples with a wide range of these traits, could help to clarify the nature of the relationship between amygdala-vmPFC functional connectivity to emotional stimuli and whether the relationships are contingent on psychopathic traits or explained by severity of antisocial behavior (which is typically correlated with psychopathy regardless of sample type).
The current study had several strengths, including a well-established task for eliciting amygdala reactivity and relatively large community sample. However, several limitations are worth noting. First, our sample consisted of males raised in low-income, urban homes. Thus, the findings may not generalize to women, individuals not living in low-income, urban environments, and/or those incarcerated in prisons. Second, PPI analyses do not establish directionality and we do not have information about temporal ordering for connectivity estimates, although previous research using dynamic functional connectivity methods suggests that amygdala activation precedes activation in prefrontal regions (Sato, Kochiyama, Uono, Yoshikawa, & Toichi, 2017). Third, our assessment of amygdala-vmPFC functional connectivity was two years prior to our assessment of psychopathy, making it harder to situate our findings alongside studies that have explored psychopathy and brain functional connectivity within traditional cross-sectional designs (Motzkin et al., 2011). Nevertheless, we also tested models that accounted for earlier antisocial behavior and callous-unemotional traits with similar findings, providing a stronger test of the unique relationship between brain and behavior. Finally, despite the inclusion of longitudinal data, our results cannot speak to the developmental origins of psychopathy, which are traceable to earlier in development (Waller, Shaw, Forbes, & Hyde, 2015).
The current study is the first to use a community sample of low-income, racially diverse males to examine how individual differences in amygdala-vmPFC functional connectivity during emotional face processing are related to psychopathic traits. Weakened amygdala-vmPFC functional connectivity to fearful faces at age 20 was related to higher psychopathic traits at age 22. Results provide evidence to support the hypothesis that severely antisocial and psychopathic behaviors are underpinned by poor sensitivity to the distress and fear of others, particularly in how these emotions are represented and regulated by fronto-limbic neural circuitry. Thus, weakened amygdala-vmPFC functional connectivity during the processing of emotional expressions represents a neurobiological marker for psychopathic traits across community, clinic, and forensic samples and could be targeted in the future within novel treatment strategies for antisocial behavior and psychopathy.
Supplementary Material
Acknowledgements
This research was supported by Grant R01 MH050907 from the National Institutes of Health to D.S.S., Grant R01 DA026222 to D.S.S. and E.E.F.. R.W. was supported by Grant T32 AA007477 and A.M.G. was supported by Grant T32 HD00710936. The authors thank the staff and study families of the Pitt Mother and Child Project.
Footnotes
Financial Disclosures
The author(s) declared the following potential financial conflicts of interest: C.S.N. is a coauthor for the Self-Report Psychopathy Scale—III and the Self-Report Psychopathy Scale—III Short Form.
References
- Acikalin MY, Gorgolewski KJ, & Poldrack RA (2017). A Coordinate-Based Meta-Analysis of Overlaps in Regional Specialization and Functional Connectivity across Subjective Value and Default Mode Networks. Frontiers in neuroscience, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2001). Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery & Psychiatry, 71(6), 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2007). The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in cognitive sciences, 11(9), 387–392. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Peschardt K, Budhani S, Mitchell D, & Pine D (2006). The development of psychopathy. Journal of Child Psychology and Psychiatry, 47(3–4), 262–276. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, & Poline J-B (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage, 16(2), S497. [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, & Phan KL (2007). Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological psychiatry, 62(2), 168–178. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Atlas LY, Joormann J, Eugène F, & Gotlib IH (2006). Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Research: Neuroimaging, 148(1), 55–59. [DOI] [PubMed] [Google Scholar]
- Cumming G (2009). Inference by eye: reading the overlap of independent confidence intervals. Statistics in medicine, 28(2), 205–220. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, & Larson CL (2000). Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science, 289(5479), 591–594. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski C, & Kiehl KA (2013). An fMRI study of affective perspective taking in individuals with psychopathy: imagining another in pain does not evoke empathy. Frontiers in human neuroscience, 7, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer HL, Waller R, Neumann CS, Shaw DS, Forbes EE, Hariri AR, & Hyde LW (2017). Examining the Factor Structure of the Self-Report of Psychopathy Short-Form Across Four Young Adult Samples. Assessment, 24, 1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, & Friesen WV (1976). Measuring facial movement. Environmental psychology and nonverbal behavior, 1(1), 56–75. [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural equation modeling, 8(3), 430–457. [PubMed] [Google Scholar]
- Fazel S, Yoon IA, & Hayes AJ (2017). Substance use disorders in prisoners: an updated systematic review and meta - regression analysis in recently incarcerated men and women. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, … Fowler K (2012). Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research: Neuroimaging, 202(3), 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A, Waller R, Shaw DS, Forbes EE, Hariri AR, & Hyde LW (2017). The long reach of early adversity: Parenting, stress, and neural pathways to antisocial behavior in adulthood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 10.1016/j.bpsc.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A, Waller R, Swartz JR, Shaw DS, Forbes EE, & Hyde LW (2018). Amygdala functional connectivity during socioemotional processing prospectively predicts increases in internalizing symptoms in a sample of low-income, urban, young men. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo C, Neumann CS, & Velotti P (2018). Difficulties in emotion regulation and psychopathic traits in violent offenders. Journal of Criminal Justice, 57, 116–125. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD, & Neumann CS (2008). Psychopathy as a clinical and empirical construct. Annu. Rev. Clin. Psychol, 4, 217–246. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, & Mazziotta JC (2000). Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport, 11(1), 43–48. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, & Forbes EE (2016). Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clinical psychological science, 4(3), 527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, & Viding E (2009). Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry, 166, 95–102. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, & Liddle PF (2001). Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological psychiatry, 50(9), 677–684. [DOI] [PubMed] [Google Scholar]
- Lykken DT (1957). A study of anxiety in the sociopathic personality. The Journal of Abnormal and Social Psychology, 55(1), 6–10. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–1239. [DOI] [PubMed] [Google Scholar]
- Marsh AA, & Blair RJR (2008). Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience & Biobehavioral Reviews, 32(3), 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Henry HY, … Blair R (2011). Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research: Neuroimaging, 194(3), 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, & Koenigs M (2011). Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience, 31(48), 17348–17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CS, Hare RD, & Pardini DA (2015). Antisociality and the construct of psychopathy: Data from across the globe. Journal of personality, 83(6), 678–692. [DOI] [PubMed] [Google Scholar]
- Neumann CS, Schmitt DS, Carter R, Embley I, & Hare RD (2012). Psychopathic traits in females and males across the globe. Behavioral sciences & the law, 30(5), 557–574. [DOI] [PubMed] [Google Scholar]
- Paulhus D, Neumann C, Hare R, Williams K, & Hemphill J (2015). The SRP-4: Self-Report Psychopathy Scale. In: Toronto, ON, Canada: Multi-Health Systems. [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, & Lilienfeld SO (2007). Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological psychiatry, 61(11), 1260–1271. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Yoshikawa S, & Toichi M (2017). Direction of amygdalaneocortex interaction during dynamic facial expression processing. Cerebral Cortex, 27(3), 1878–1890. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, & Brennan LM (2012). Early predictors of boys’ antisocial trajectories. Development and psychopathology, 24(3), 871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Shaw DS, Forbes EE, & Hyde LW (2015). Understanding early contextual and parental risk factors for the development of limited prosocial emotions. Journal of Abnormal Child Psychology, 43(6), 1025–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder K, Harenski C, Kiehl K, & Decety J (2015). Neural networks underlying implicit and explicit moral evaluations in psychopathy. Translational psychiatry, 5(8), e625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.