Abstract
Purpose
To perform a comprehensive review and to investigate the presence and role of autoimmune antibodies in 25 cases of acute zonal occult outer retinopathy (AZOOR) identified using the classification originally proposed by J.Donald Gass.
Design
Observational case series
Methods
Setting
Institutional
Study Population
Twenty-five patients were identified by characteristic symptoms (abrupt onset of photopsias, followed by large scotomata at or connected to the blind spot), ocular findings (paucity of pigmentary changes with no sign of vitreous inflammation and abnormal electroretinogram in at least one eye,) and a negative family history for retinitis pigmentosa.
Observation Procedures
Patients underwent a full comprehensive ophthalmologic exam fundus retinography, Goldmann kinetic visual field (GVF), and full-field electroretinogram (ffERG). Blood samples were also obtained to verify for the presence of anti-retinal antibodies by Western blot analysis.
Main outcome measures
Clinical presentation, best-corrected visual acuity (BCVA), fundus abnormalities, visual field defects, ffERG changes and presence of anti-retinal antibodies.
Results
Sixteen patients (64%) presented with photopsias, 56% (14/25) with night blindness, and 56% (14/25) with loss of peripheral vision. 64% (16/25) of cases were bilateral. All patients demonstrated retinal vascular attenuation, optic nerve head pallor, and mottling of RPE. The most common visual field changes included enlargement and expansion of the blind spot extending into large pericentral or other types of scotomata (64%). Both scotopic and photopic ffERG values were abnormal and affected to a similar degree in our patients. Nine patients (36%) had a greater than 20% asymmetry in ERG values between the two eyes. All patients had anti-retinal antibodies on Western blot with an average of 6.6 bands.
Conclusion
Evidence suggests that AZOOR is a unique form of autoimmune retinopathy and retinal manifestation suggests possible anti-retinal antibody leakage from the disc margin with spread of immune products under the retina resulting in large scotomata that connect to the optic nerve head.
Keywords: autoimmune, AZOOR, autoimmune retinopathy, enlarged blind spots, retinal degeneration
Introduction
Acute zonal occult outer retinopathy (AZOOR) was first described in 1993 by J. Donald Gass as a syndrome with rapid loss of one or more extensive zones of outer retinal function. 1 The initial 13 patients he reported were mostly young females who presented with photopsias associated with progressive scotomata, profound electroretinographic abnormalities in one or both eyes and minimal funduscopic changes. 1–3
Subsequently in 2002, Dr. Gass, Agarwal, and Scott further characterized 51 AZOOR patients who were followed for at least three years. 4 In this study, the patients presented with photopsias and visual field defects with highly variable patterns and sizes of field defects. The most common defects were enlarged blind spots and large scotomata connecting to blind spots. (Figure 1) In many cases, there were visible zones of pigment epithelial atrophy and retinal vessel narrowing.
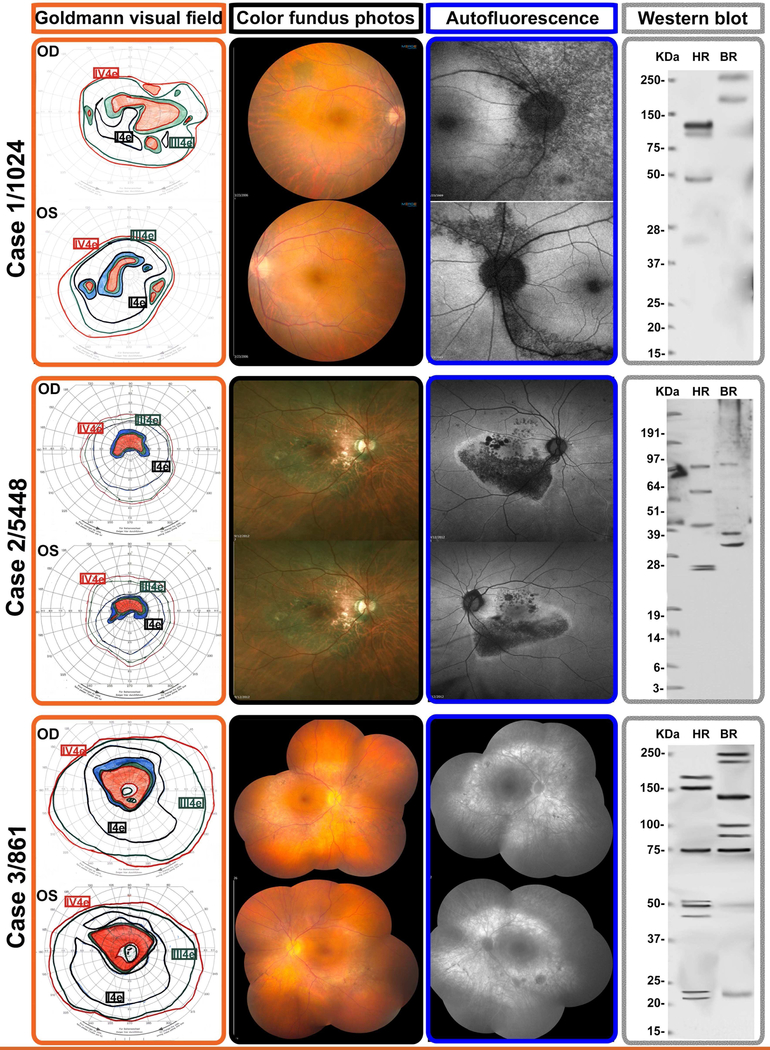
Figure 1-. Representative multimodal imaging of 3 patients presenting with Acute zonal occult outer retinopathy.
Selected cases to illustrate the testing and imaging findings of 3 patients presenting with typical AZOOR findings as defined by the criteria originally defined by Gass et al. These correspond to patients listed in Table 1 and identified with their laboratory identification number
Top row: Case 1 is patient 1024, a 42-year-old woman who presented with progressive two-and-a-half year history of decreased visual acuity and distortion of fine print, OD worse than OS. The Goldmann visual fields showed enlarged blind spots and peripheral scotomata OS>OD. Fundus examination showed no overt lesions and mild vascular attenuation; the autofluorescence (AF) demonstrated RPE damage emanating from the discs OU. Western blot showed five anti-retinal antibody bands in the human test lane.
Middle row: Case 2 is patient 5448, a 58-year-old man who presented with a two-year history of bilateral peripheral visual field loss and central vision blurring. He had a family history of diabetes and connective tissue disease. His autofluoresence was remarkable for RPE damage emanating from focal points of the optic disc, which correspond exquisitely well to the color fundus photographs and the outline of his GVF. He had four anti-retinal antibody bands on Western blot.
Bottom row: Case 3 is patient 861, a 63-year-old lady with a one-year history of visual distortion OS>OD. Goldmann visual field testing showed full isopters, but with pericentral scotomatous regions OU, consistent with the areas of atrophy in her fundus. Fundus color photographs and autofluorescence show areas of diffuse RPE loss corresponding to the visual field scotomata. The pattern of RPE damage OU was suggestive that toxic immune products leaked from the disc margins and spread into the subretinal space. There were minimal pigmentary deposits in the areas of RPE atrophy. She had eight anti-retinal immunoreactive bands on Western blot
The improvement in imaging techniques over time has allowed for a new era in defining and describing this condition. Loss or irregularity of the inner segment-outer segment (IS/OS) boundary can be found in scotomatous areas and can be demonstrated by optical coherence tomography (OCT). 5–7 Retinal red free and fundus autofluorescence (FAF) image may also reveal retinal pigment epithelium (RPE) alterations. (Figure 1) 8,9 Mrejen et al. recently performed a comprehensive review of AZOOR emphasizing the value of a multimodal imaging approach. 9 They described in detail the outer retinal demarcation line of lesion progression and the trizonal appearance of subacute or chronic lesions on FAF, OCT and indocyanine green (ICG) imaging. 9
Despite being first described more than 20 years ago, the etiology of AZOOR remains elusive and is undergoing constant redefinition. Gass proposed an infectious viral process that first involves the peripapillary area with progressive advancement. 1 Jampol and Becker postulated a genetics-driven autoimmune and inflammatory basis for a spectrum of separate disease entities including AZOOR and other white dot diseases such as multiple evanescent white dot syndrome (MEWDS), multifocal choroiditis and panuveitis (MFC-PU), and punctate inner choroidopathy (PIC). 10,11 Other mechanisms such as toxic retinopathy 10,12,13 or fungal infiltration 14–16 have also been considered. In this report, we investigated 25 patients with AZOOR in order to better establish natural history and to further elucidate the role of autoimmunity in their disease.
Methods
The University of Michigan institutional review board, following the tenets of the Declaration of Helsinki, approved this study.
A retrospective chart review of all consecutive patients with AZOOR seen between January 2004 and June 2013 was conducted at the Retinal Dystrophy Clinic of the Kellogg Eye Center (University of Michigan).
Patients were selected based on the strict criteria for AZOOR as reported by Gass. 1,4 These selection criteria included: sudden onset of visual symptoms, enlarged blind spots or large scotomatous areas connected to the blind spot, asymmetry of findings, and abnormal ffERG in the presence of a normal fundus examination. Family history was obtained by a genetic counselor looking for a history of retinal degeneration and autoimmune disorders in the family pedigree. Cases with family history of retinitis pigmentosa were excluded.
The patients underwent a full comprehensive ophthalmic exam, including: best-corrected visual acuity (BCVA) using a Snellen chart, slit-lamp biomicroscopic examination, dilated fundus examination with a 78-diopter (Volk Optical Inc, Mentor, OH) lens, and indirect ophthalmoscopy with a 20-diopter (Volk Optical Inc, Mentor, OH) lens. Patients were also evaluated using standardized GVF with I-4-e, III-4-e, IV-4-e targets (Goldmann, Haag Streit, Koeniz, Switzerland), ffERG (UTAS-3000, LKC Technologies, Gaithersburg, MD) following the ISCEV standard 17 and fundus retinography. Kinetic visual field testing was preferred over static perimetry because the latter typically measures central field, and often misses the larger scotomata seen in AZOOR patients. The electroretinographic a- and b-wave amplitudes were reported in each eye in microvolts and as a percentage of mean normal to put any loss in perspective. (Table 1) Fundus autofluorescence (FF450, Carl Zeiss, Germany) and spectral-domain OCT (Heidelberg Engineering, Heidelberg, Germany) were analyzed when available.
Table 1-.
Clinical information on all 25 patients with acute zonal occult outer retinopathy
| Lab ID |
Age/ Sex |
Onset | Initial VA OD/OS |
Laterality | Family Hx AI Disease | Goldmann visual field | Dilated Fundus Examination | Bands WB |
ERG (in μV, % normal mean) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photopic | Scotopic | Mixed | |||||||||||
| 480 | 14/F | 12 yrs | 20/70 20/400 |
Bilateral | Astdma (GM) Tdyroid (Mt) |
PCS, EBS, contraction OU | Vasc atten, diffuse retinal atrophy OU, no pg | 10 | A-WAVE | B-WAVE | B-WAVE | A-WAVE | B-WAVE |
| 13 (33%) OD, 9 (23%) OS |
58 (36%) OD, 51 (31%) OS |
<5 (<2%) OD, <5 (<2%) OS |
32 (15%) OD, 23 (11%) OS |
57 (15%) OD, 56 (15%) OS |
|||||||||
| 848 | 49/F | 29 yrs | 20/20 20/20 |
Unilateral | None | PCS OS, EBS OS, mild contraction OS | Perivascular pg OS, patchy RPE thinning OS | 10 | 24 (62%) OD, 12 (31%) OS* |
105 (64%) OD, 38 (23%) OS* |
159 (57%) OD, 47 (17%) OS* |
105 (50%) OD, 40 (19%) OS* |
208 (52%) OD, 59 (15%) OS* |
| 861 | 42/F | 39 yrs | 20/20 20/20 |
Bilateral | Thyroid (Pt, Mt, S) | EBS OU, CS OU, temporal scotoma OS | Vasc atten, mild temporal optic atrophy OU | 8 | 11 (28%) OD, 16 (41%) OS |
31 (19%) OD, 55 (34%)OS |
36 (13%) OD, 91 (33%) OS* |
18 (9%) OD, 86 (41%) OS* |
62 (16%) OD, 2 (1%) OS |
| 906 | 51/F | 50 yrs | 20/60 20/40 |
Bilateral | RA (Mt), Asthma (S), Thyroid (S) |
EBS OU, CS OD, PS OU, mild contraction OD>OS | Blonde fundus, patches of atrophy superiorly OU | 10 | 21 (54%) OD, 22 (56%) OS |
18 (11%) OD, 87 (53%) OS* |
55 (20%) OD, 26 (9%) OS |
97 (46%) OD, 93 (44%) OS |
127 (37%) OD, 133 (24%) OS |
| 910 | 42/F | 32 yrs | 20/15 20/15 |
Unilateral | None | PCS OS | Vasc atten OS, equatorial pg OS | 10 | 42(107%) OD, 26 (67%) OS* |
152 (93%) OD, 108 (66%) OS* |
334 (120%) OD, 178 (64%) OS* |
243 (115%) OD, 135 (64%) OS* |
469 (117%) OD, 283 (70%) OS* |
| 1024 | 63/F | 63 yrs | 20/25 20/200 |
Bilateral | None | EBS OU, multiple scotomata OD, PCS OS | Pale optic nerve, diffuse retinal loss OU, RPE pg OS | 6 | 15 (38%) OD, 23 (59%) OS* |
53 (33%) OD, 71 (44%) OS |
86 (31%) OD, 76 (27%) OS |
79 (37%) OD, 82 (39%) OS |
120 (30%) OD, 119 (30%) OS |
| 2040 | 58/F | 49 yrs | 20/400 20/20 |
Bilateral | None | CS OD, mild contraction OU | No pg, peripapillary atrophy, flat fovea OU | 10 | 19 (49%) OD, 39 (100%) OS* |
43 (26%) OD, 120 (74%) OS* |
103 (37%) OD, 216 (78%) OS* |
76 (36%) OD, 173 (82%) OS* |
189 (47%) OD, 355 (89%) OS* |
| 3035 | 47/F | 46 yrs | 20/30 20/20 |
Bilateral | None | Temporal scotomata OU | RPE thinning, fine pg, focal atrophy OU | 8 | 19 (49%) OD, 19 (49%) OS |
110 (67%) OD, 99 (61%) OS |
99 (36%) OD, 105 (38%) OS |
58 (27%) OD, 91 (43%) OS |
157 (40%) OD, 209 (53%) OS |
| 3076 | 50/F | 33 yrs | 20/20 20/20 |
Bilateral | Thyroid (Mt) | PCS OD, contraction ring OS | RPE depigmentation and equatorial atrophy OU, no pg | 6 | 30 (56%) OD, 23 (59%) OS |
124 (76%) OD, 116 (71%) OS |
218 (78%) OD, 160 (58%) OS* |
125 (59%) OD, 141 (67%) OS |
353 (90%) OD, 339 (86%) OS |
| 3179 | 35/F | 27 yrs | 20/15 20/20 |
Bilateral | Thyroid (S) Raynaud (Mt) |
EBS OU, scattered scotomata OD, PCS OS | Scattered white dots, sheathed vessels OU, no pg | 10 | 30 (77%) OD, 26 (67%) OS |
105 (64%) OD, 101 (62%) OS |
135 (49%) OD, 108 (39%) OS |
111 (53%) OD, 98 (46%) OS |
248 (63%) OD 253 (64%) OS |
| 3180 | 48/F | 46 yrs | 20/20 20/30 |
Unilateral | Fib (Pt), Poly (Ft) | CS OS | Sheathing retinal arterioles OS, diminished foveal reflex OS, no pg | 6 | 32 (82%) OD, 17 (44%) OS* |
175(107%) OD, 31 (19%) OS* |
331 (119%) OD, 215 (77%) OS* |
22 (10%) OD, 17 (8%) OS |
418 (106%) OD, 265 (67%) OS* |
| 3439 | 55/F | 53 yrs | 20/20 20/30 |
Unilateral | Thyroid (Pt, Mt) | Moderate contraction OU | Peripheral retinal/RPE atrophy OU, CME OS, no pg | 6 | 10 (26%) OD, <5 (13%) OS |
20 (12%) OD, 5 (3%) OS |
38 (14%) OD, 11 (4%) OS |
47 (22%) OD, NR (0%) OS* |
60 (15%) OD, NR (0%) OS |
| 3556 | 73/F | 71 yrs | 20/25 20/30 |
Bilateral | Psoriasis (B) | EBS OU, contraction OU | Choroidal loss, bare sclera, subretinal pg OU | 2 | 5 (13%) OD, 10 (26%) OS |
15 (9%) OD, 40 (25%) OS |
41 (15%) OD, 125 (45%) OS* |
54 (26%) OD, 128 (61%) OS* |
80 (20%) OD, 183 (47%) OS* |
| 3626 | 29/F | 28 yrs | 20/40 20/30 |
Bilateral | None | Multiple scotomata OD, mild contraction OU | None | 10 | 35 (90%) OD, 29 (74%) OS |
163(100%) OD, 139 (85%) OS |
239 (83%) OD, 232 (83%) OS |
201 (95%) OD, 155 (73%) OS* |
378 (96%) OD, 308 (78%) OS |
| 3634 | 55/F | 55 yrs | 20/15 20/25 |
Bilateral | None | Scattered scotomata OU | Blonde fundus, mild atrophy OU, no pg | 2 | 17 (44%) OD, 12 (31%) OS |
84 (52%) OD, 54 (34%) OS |
203 (73%) OD, 245 (88%) OS |
137 (65%) OD, 158 (75%) OS |
277 (70%) OD, 303 (77%) OS |
| 3712 | 18/F | 17 yrs | 20/20 20/15 |
Bilateral | Asthma (Pt, Mt, S), RA (Mt), Fib (Mt) | EBS OD, scotomata, contraction OD | Retinal/RPE atrophy OU, scars, central atrophy OD | 10 | 11 (28%) OD, 27 (69%) OS* |
17 (10%) OD, 128 (79%) OS* |
13 (5%) OD, 191 (69%) OS* |
47 (22%) OD, 139 (66%) OS* |
28 (7%) OD, 264 (67%) OS* |
| 3776 | 57/F | 47 yrs | 20/25 20/40 |
Bilateral | Fib (Pt) | EBS OU, PCS OD, ring OS | Paracentral atrophy OU, no pg | 2 | 15 (38%) OD, 33 (85%) OS* |
57 (35%) OD, 84 (52%) OS |
143 (51%) OD, 227 (82%) OS * |
115 (55%) OD, 164 (78%) OS* |
194 (49%) OD, 262 (67%) |
| 3816 | 60/M | 59 yrs | 20/200 20/100 |
Bilateral | Fib (S) | EBS OU, contraction OS>OD | Cryo scars OU, RD scars OS, no pg | 6 | 14 (36%) OD, 16 (41%) OS |
34 (21%) OD, 38 (23%) OS |
140 (50%) OD, 63 (23%) OS* |
82 (39%) OD, 54 (26%) OS |
206 (52%) OD, 107 (27%) OS* |
| 3952 | 36/F | 34 yrs | 20/25 20/20 |
Bilateral | SLE (GM) | EBS, contraction OU, scattered scotomata OD | Diffuse retinal atrophy OU; minor bone spicule-type pg OD | 4 | 7 (18%) OD, 6 (15%) OS |
9 (6%) OD, 7 (4%) OS |
<5 (<2%) OU | 17 (8%) OD, 22 (10%) OS |
20 (5%) OD, 15 (4%) OS |
| 3954 | 52/F | 40 yrs | 20/30 20/40 |
Unilateral | Thyroid (Pt) | EBS OS, mild contraction OS | Mottled RPE OD, posterior pole scar OS, no pg | 2 | 32 (82%) OD, 20 (51%) OS* |
109 (67%) OD, 77 (47%) OS |
190 (68%) OD, 108 (39%) OS* |
175 (83%) OD, 118 (56%) OS* |
372 (95%) OD, 248 (63%) OS* |
| 4454 | 38/F | 26 yrs | 20/25 20/160 |
Unilateral | None | PCS OS, severe contraction | Depigmented spots OU, mild pigmentary changes, CME OS | 4 | 43 (110%) OD, 4 (10%) OS* |
188(115%) OD, 43 (26%) OS* |
390 (140%) OD, NR (0%) OS* |
329 (156%) OD, NR (0%) OS* |
594 (151%) OD, NR (0%) OS* |
| 4486 | 51/M | 49 yrs | 20/400 20/20 |
Unilateral | RA (S) | EBS OD, CS OD scattered PCS/PS, contraction OD | Optic disc pallor OD, no pg | 4 | 9 (23%) OD, 40 (102%) OS* |
19 (12%) OD, 226(138%)OS* |
150 (54%) OD, 288 (104%) OS* |
83 (39%) OD, 252 (119%) OS* |
260 (66%) OD, 504 (128%) OS* |
| 4488 | 51/F | 47 yrs | 20/40 20/40 |
Bilateral | Asthma (Pt), Raynaud (Pt), Thyroid (Pt) | contraction, multiple scotomata OU, CS OS. | Diffuse retinal atrophy, lack of pigment OU. CWS OS. | 6 | 10 (26%) OD, 11 (28%) OS |
21 (13%) OD, 28 (17%) OS |
59 (21%) OD, 19 (7%) OS |
72 (34%) OD, 46 (22%) OS |
110 (28%) OD, 75 (19%) OS |
| 4505 | 81/M | 71 yrs | 20/50 20/250 |
Bilateral | None | EBS OD, severe contraction OS | Patches of pg OU | 2 | <5 (<13%) OD, NR (0%) OS |
11 (7%) OD, NR (0%) OS |
28 (10%) OD, NR (0%) OS |
47 (22%) OD, NR (0%) OS* |
95 (24%) OD, NR (0%) OS* |
| 5448 | 58/M | 56 yrs | 20/40 20/40 |
Bilateral | CTD (S), DM2 (Mt,Ft) | Moderate contraction OU, EBS OU | Retinal atrophy, exudates, peripapillary subretinal pg OU | 4 | 9 (23%) OD, 11 (28%) OS |
26 (16%) OS, 35 (22%) OS |
77 (28%) OD, 103 (37%) OS |
79 (37%) OD, 111 (53%) OS |
131 (33%) OD, 165 (42%) OS |
Normal ERG range for a-wave and b-wave; Photopic conditions a-wave normal range: 23–54 μV; b-wave normal range 95–230 μV. Scotopic b-wave normal range is 180 to 375 μV. Combined response a-wave normal range: 120–301 μV; bwave normal range: 230–555 μV. Normal mean ERG values were calculated by averaging the upper and lower limits of the normal range. The percentage of the normal mean for each patient was recorded in parentheses.
= μV asymmetry between eyes ≥ 20%.
Immunoreactive bands refers to anti-retinal antibody activity on Western blot.
Abbreviations:
AI: Autoimmune; B: Brother; CME: Cystoid macular edema; Cryo: Cryotherapy; CS: Central Scotoma; CTD: Connective Tissue Disease; DA: Dark Adaptation; EBS: Enlarged blind spot; ERG: Electroretinogram; Ft: Father; F: Female; Fib: Fibromyalgia; GVF: Goldmann Visual Field, GM=Grandmother; GF=Grandfather; Hx: History; M: Male; Mt: Mother; PCS: paracentral scotoma, Pg: Pigment deposits; Poly: Polymyositis; PS: Peripheral scotoma; Pt: Patient; RA: Rheumatoid arthritis; Raynaud: Raynaud’s disease; Ring: Ring scotoma; RPE: Retinal pigment epithelium; S: Sister; SLE: Systemic lupus erythematosus; Thyroid: Thyroid disease, VA: Visual acuity; Vasc atten: vascular attenuation; WB: Western blot.
Outcome measures included investigation of scotoma size and pattern of visual field loss. Unilateral versus bilateral visual field loss was also noted. The severity and types of electroretinographic changes, presence of fundus lesions or changes, time of onset of symptoms as best recollected by the patient to first visit were recorded.
Western blot analysis
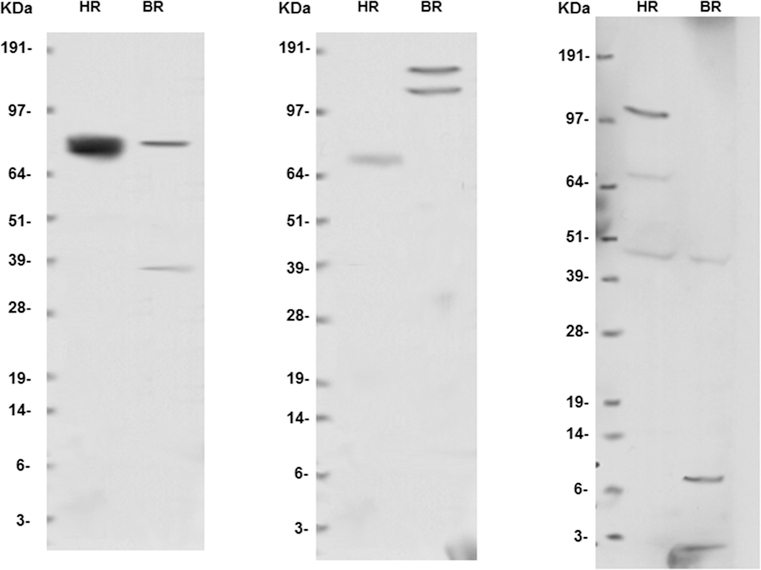
Most AZOOR patients have a number of clinical findings overlapping with autoimmune retinopathy presentation. These include a sudden onset of symptoms in otherwise healthy patients, abnormal ERGs, and no abnormalities on fundus examination except in long standing cases. We therefore considered it prudent to check patients for anti-retinal antibodies by Western blot analysis. (Figure 2) After informed consent, serum and blood samples were obtained, processed, and stored at −80°C until studied. As c ontrol subjects, 10 healthy volunteers with normal ERGs and no family history of autoimmune diseases were recruited. Normal donor bovine retinal tissue was used. The latter retinal extracts were used as a means for reproducibility validation. Samples were suspended in phosphate-buffered saline, 1mM phenylmethylsulfonyl fluoride (PMSF), protease inhibiter cocktail and sonicated. Thirty micrograms of each lysate was heated for 10 minutes at 80°C in sample buffer with dithiothre itol, followed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membrane and were incubated overnight with patients’ serum at 1:100 dilution. The membrane was washed and incubated with anti–human IgG–tagged horseradish peroxidase, and proteins were detected using a chemiluminescent detection kit (SuperSignal West Pico; Thermo Scientific Pierce, Rockford, Illinois) and were then exposed to film. 18
Figure 2-. Examples of normal western blot results on peripheral blood samples of normal subjects.
Left: HR: human retina; BR: bovine retina, KDa: kilodaltons
Results
We evaluated 25 patients that fit the criteria for AZOOR as outlined above. Patients presented with similar histories, but the history of disease activity varied from occurring many years previously to recent onset. Patient findings are detailed in Table 1. The mean age was 46.3±11 years old (range 14 to 81 years) and 84% of patients (2½5) were female. Age of symptom onset ranged from age12 to age 71 (mean 51.1±19.7 years). Time of onset of symptoms to examination in our clinic varied from 4 months to 20 years. 64% of patients had personal or family histories of autoimmune disorders. None of the patients or their families had a history of retinitis pigmentosa. Macular function better than or equal 20/40 in at least one eye was present in 84% of patients. All patients had retinal vascular attenuation, optic nerve head pallor, and mottling of RPE. While diffuse retinal atrophy could be seen in affected areas, only two cases with longer duration had bone-spicule like pigment patches in the areas of scotomata.
All 25 (100%) AZOOR patients were positive for presence of anti-retinal antibodies (average 6.6 bands) on Western blot, compared to an average of 4.2 bands when compared to 25 consecutive autoimmune retinopathy patients, and 0–2 bands for normal controls.
Clinically, the AZOOR patients presented with similar patterns of findings with a rapid onset that included photopsias (64%), night blindness (56%), and loss of peripheral vision (56%). Nine (36%) patients had visual changes in one eye, while 64% had visual changes in both. (Table 1)
Characteristic visual field changes included enlarged blind spots and expansion of blind spots extending into large pericentral or other scotomata in 64% of patients. (Figure 1) Visual field defects often corresponded to changes seen in red free or autofluorescence photos. (Figure 1B) Asymmetric generalized constriction of fields was seen in nine patients (36%). (Table 1)
All patients manifested retinal dysfunction on electroretinography, either monocularly or binocularly. Nine patients (36%) had a greater than 20% asymmetry in ERG values between the two eyes. Under scotopic conditions, the mean a-wave value was 47.6±22.8% that of normal controls and the b-wave value was 43.3±27.4% of normal controls. Average photopic b-wave value amongst patients was 44±28.9% of normal database while combined response was decreased to a mean of 45.1±24.6% and 49.1±29.5% for a- and b-waves respectively. (Table 1)
Discussion
AZOOR has been a difficult disease to understand due to its unclear etiology, the lack of diagnostic biomarkers, and the paucity of initial retinal changes to help confirm that AZOOR. This has led to confusing the diagnostic delineation between AZOOR and a multitude of other conditions that possess similar findings. The diagnosis is made by recognizing a common disease pattern including the abrupt onset of photopsias, scotomata, nyctalopia and vision loss in previously normal patients without history of retinitis pigmentosa. Retinal abnormalities are confirmed by abnormal full field electroretinogram, all the more remarkable in the face of a lack of discernible changes on funduscopy.
Specialized equipment, i.e. an electroretinogram and kinetic perimetry greatly assist in establishing the diagnosis, but these instruments are not found in many clinics. The most helpful diagnostic sign is an enlargement of the blind spot. (Figure 1) A minority of cases (10%) have large peripheral confluent scotomata, which Gass hypothesized was because the anterior retina was thinner giving easier access to a serum-borne damaging agent. 4
Several studies, including this paper, show that abnormal photopic and scotopic ERGs are common in AZOOR. 3 The photoreceptor functional loss in addition to the pattern of damage emanating from the optic nerve rim, seen on visual field and in autofluorescence studies raise the possibility that anti-retinal antibodies may be breaking through the blood-retinal barrier at the optic canalretina junction, and may be a key component in causing enlargement of the blind spot. Autoantibodies spreading across the subretinal space in many patients likely result in the scotomata connecting to the blind spot. The subretinal damage can be seen as hypofluorescent patches on autofluorescence or with hyperfluorescent RPE staining that corresponds to scotomata noted on visual fields. (Figure 1C) The presumed leakage of immune products into the subretinal space may be one of the main events initiating large blind spots, a distinguishing feature of AZOOR.
Until this study, there has been no clear underlying etiology; however, anti-retinal antibodies were abundantly present in all our cases. (Table 1) Each of our patients demonstrated a varying number of bands and mixture of different anti-retinal antibodies on Western blots, implying that different retinal cell types were likely targeted, giving each individual a unique set of anti-retinal antibody composition.
A question that commonly arises is: given the perception that the retina is an immune privileged site where tight junctions of the endothelium and the RPE basement membrane are purported to inhibit antibody penetration, how do the antibodies reach the retina? It is known that inflammation disrupts the tight junctions of the RPE. 19–21 In the case of AZOOR, it is highly likely that the blood retinal barrier may be breeched at the optic nerve rim, allowing the anti-retinal antibodies to gain access to the subretinal space where they are toxic to the photoreceptors and the RPE. Since autofluorescence studies often show damaged RPE emanating from the disc rim matched to similarly shaped scotomata on visual field, it can be hypothesized that this subretinal leakage of anti-retinal antibodies originates at the disc rim and is a unique feature in AZOOR (Figure 1). Some patients also have a normal zonal withdrawal of the peripapillary RPE, which might open up subretinal access to circulating antibodies. It is possible that the macula may present with tight junctions and zonula occludens particular to its location and more impermeable to this effect and hence may also be relatively spared in comparison.
The mechanisms by which anti-retinal autoantibodies are present is unknown. A minority of patients have other autoimmune diseases while others give a history of head trauma or a prodromal viral infection. There are also reports of immune-mediated retinal diseases such as MEWDs or multifocal choroiditis preceding onset of symptoms. There may have been release of retinal antigens that may result in an autoimmune response. 10
In many patients, AZOOR is a self-limiting disease and becomes quiescent, typically after six months. 4 The history of onset and the current immediate history of progression are important in understanding if the disease is active. We found that most patients who undergo progression give clear histories that their vision is deteriorating. The presence of anti-retinal antibodies is helpful diagnostically, although they do not distinguish active from inactive cases. If immunosuppressive treatment is undertaken, it is important to evaluate the patients for active disease with repeat functional tests such as GVF and ffERG. This approach allows treatment effects to be validated, since the clinical signs of AZOOR remain even after the disorder becomes inactive. Currently there are no biomarkers for autoimmune retinopathy that are known to help in tailoring the treatment regimen.
In summary, the current study suggest that AZOOR is a form of autoimmune retinopathy in which the pattern of visual field damage may be strongly influenced by peripapillary leakage of anti-retinal antibodies into the subretinal space. This information is useful in designing future biomarkers to survey the level of autoimmune activity in the condition, which in turn may help tailor and gauge the effect of an immunologic treatment regimen for AZOOR.
Supplementary Material
Acknowledgement/Disclosure
This work was supported by: Core Center for Vision Research and RPB support.
Footnotes
None of the authors have any financial conflict of interest regarding any material discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gass JDM. Acute zonal occult outer retinopathy. Donders Lecture: The Netherlands Ophthalmological Society, Maastricht, Holland, June 19, 1992. J Neuroophthalmol. 1993;13(2):79–97. [PubMed] [Google Scholar]
- 2.Francis P, Marinescu A, Fitzke F, Bird A, Holder G. Acute zonal occult outer retinopathy: towards a set of diagnostic criteria. Br J Ophthalmol. 2005;89(1):70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai M, Nao-i N, Sawada A, Hayashida T. Multifocal electroretinogram indicates visual field loss in acute zonal occult outer retinopathy. Am J Ophthalmol. 1998;126(3):466–469. [DOI] [PubMed] [Google Scholar]
- 4.Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study1. Am J Ophthalmol. 2002;134(3):329–339. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Koizumi H, Freund KB. Photoreceptor Outer Segment Abnormalities as a Cause of Blind Spot Enlargement in Acute Zonal Occult Outer Retinopathy–Complex Diseases. Am J Ophthalmol. 2008;146(1):111–120. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Kishi S. Loss of photoreceptor outer segment in acute zonal occult outer retinopathy. Archives of Ophthalmology. 2007;125(9):1194–1200. [DOI] [PubMed] [Google Scholar]
- 7.Zibrandtsen N, Munch IC, Klemp K, Jørgensen TM, Sander B, Larsen M. Photoreceptor atrophy in acute zonal occult outer retinopathy. Acta Ophthalmol. 2008;86(8):913–916. [DOI] [PubMed] [Google Scholar]
- 8.Monson DM, Smith JR. Acute Zonal Occult Outer Retinopathy. Surv Ophthalmol. 2011;56(1):23–35. [DOI] [PubMed] [Google Scholar]
- 9.Mrejen S, Khan S, Gallego-Pinazo R, Jampol LM, Yannuzzi LA. Acute zonal occult outer retinopathy: A classification based on multimodal imaging. JAMA Ophthalmol. 2014;132(9):1089–1098. [DOI] [PubMed] [Google Scholar]
- 10.Jampol LM, Becker KG. White spot syndromes of the retina: a hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am J Ophthalmol. 2003;135(3):376–379. [DOI] [PubMed] [Google Scholar]
- 11.Jampol LM, Wiredu A. MEWDS, MFC, PIC, AMN, AIBSE, and AZOOR: one disease or many? Retina. 1994;15(5):373–378. [PubMed] [Google Scholar]
- 12.Cheung MC, Nune GC, Hwang DG, Sutter EE, Duncan JL. Acute zonal occult outer retinopathy in a patient with graft-versus-host disease. Am J Ophthalmol. 2004; 138(6):1058–1060. [DOI] [PubMed] [Google Scholar]
- 13.Mahajan VB, Stone EM. Patients with an acute zonal occult outer retinopathy–like illness rapidly improve with valacyclovir treatment. Am J Ophthalmol. 2010;150(4):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahendradas P, Shetty R, Avadhani K, Ross C, Gupta A, Shetty BK . Polycythemia vera and increased hemophilic factor VIII causing acute zonal occult outer retinopathy: a case report. Ocul Immunol Inflamm. 2010;18(4):319–321. [DOI] [PubMed] [Google Scholar]
- 15.Carrasco L, Ramos M, Galisteo R, Pisa D, Fresno M, González ME. Isolation of Candida famata from a patient with acute zonal occult outer retinopathy. J Clin Microbiol. 2005;43(2):635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisa D, Ramos M, García P, et al. Fungal Infection in Patients with Serpiginous Choroiditis or Acute Zonal Occult Outer Retinopathy. J Clin Microbiol. 2008;46(1):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmor M, Fulton A, Holder G, et al. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118(1):69–77. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Jia L, He S, et al. Melanoma-associated retinopathy: A paraneoplastic autoimmune complication. Arch Ophthalmol. 2009; 127(12):1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zech J-C, Pouvreau I, Cotinet A, Goureau O, Le Varlet B, De Kozak Y. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998;39(9):1600–1608. [PubMed] [Google Scholar]
- 20.Narimatsu T, Ozawa Y, Miyake S, et al. Disruption of Cell-Cell Junctions and Induction of Pathological Cytokines in the Retinal Pigment Epithelium of Light-Exposed MiceAMD-Associated Photo-Damage in the RPE. Invest Ophthalmol Vis Sci. 2013;54(7):4555–4562. [DOI] [PubMed] [Google Scholar]
- 21.Peng S, Gan G, Rao VS, Adelman RA, Rizzolo LJ. Effects of Proinflammatory Cytokines on the Claudin-19 Rich Tight Junctions of Human Retinal Pigment EpitheliumCytokine Effects on RPE Tight Junctions. Invest Ophthalmol Vis Sci. 2012;53(8):5016–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.