Abstract
Human lens epithelial cells (HLE) undergo mesenchymal transition and become fibrotic during posterior capsule opacification (PCO), which is a frequent complication after cataract surgery. TGF-β2 has been implicated in this fibrosis. Previous studies have focused on the role of hypoxia-inducible factor-1α (HIF-1α) in fibrotic diseases, but the role of HIF-1α in the TGF-β2-mediated fibrosis in HLE is not known. TGF-β2 treatment (10 ng/mL, 48 h) increased the HIF-1α levels along with the EMT markers in cultured human lens epithelial cells (FHL124 cells). The increase in HIF-1α corresponded to an increase in VEGF-A in the culture medium. However, exogenous addition of VEGF-A (up to10 ng/mL) did not alter the EMT marker levels in HLE. Addition of a prolyl hydroxylase inhibitor, dimethyloxalylglycine (DMOG, up to 10 μM), enhanced the levels of HIF-1α, and secreted VEGF-A but did not alter the EMT marker levels. However, treatment of cells with a HIF-1α translational inhibitor, KC7F2, significantly reduced the TGF-β2-mediated EMT response. This was accompanied by a reduction in the ERK phosphorylation and nuclear translocation of Snail and Slug. Together, these data suggest that HIF-1α is important for the TGF-β2-mediated EMT of human lens epithelial cells.
Keywords: epithelial-to-mesenchymal transition, HIF-1α, KC7F2, lens epithelial cells, TGF-β2, VEGF-A
1 |. INTRODUCTION
Cataracts are the major blinding disease in the world. The number of people who suffered from cataracts in the US in 2010 was approximately 24 million, and this is expected to increase to nearly 40 million by 2030 (https://nei.nih.gov/eyedata/cataract). Worldwide, visual impairment from cataracts was nearly 95 million in 2014,1 and this is expected to significantly increase in the coming years.
The lens is avascular and abundant in proteins. The single layer of anterior epithelial cells adherent to capsule is the principal metabolic source for the ens. The rest of the lens is formed of terminally differentiated and elongated fiber cells, which are rich in proteins known as crystallins. During cataract surgery, a circular portion of the anterior capsule of the lens is excised (capsulorhexis), which allows surgeons to access the cataractous fiber material, emulsify, and suction it out of the lens, leaving behind a hollow bag of lens capsule.2 An intraocular lens (IOL) is implanted in the capsular bag. This procedure results in restoration of vision to a high degree in most patients. However, cataract surgery is not foolproof; attempts are made during surgery to remove the epithelial cells that adhere to the anterior capsule adjacent to the capsulorhexis region. Despite best efforts (polishing), some epithelial cells remain adherent to the capsule. These cells proliferate and migrate to the cell-free posterior capsule and transdifferentiate into myofibro-blast-like cells in a process known as epithelial-to-mesenchymal transition (EMT).3 During EMT, epithelial cells secrete excessive extracellular matrix proteins such as collagen I and fibronectin and lose their apical-basal polarity through degradation of the transmembrane protein, E-cadherin. This leads to loss of cell-to-cell adhesion, and eventually the cells lose their epithelial cell morphology. During this process, the cells synthesize α-smooth muscle actin (α-SMA), and vimentin along with a host of other proteins that are typically upregulated in cells with a mesenchymal phenotype.3 In addition, integrins that play an important role in cell proliferation and migration4 are upregulated during EMT (reviewed in5). The excessive synthesis of extracellular matrix and α-SMA leads to wrinkling of the posterior capsule along with fibrous tissue formation that results in a blockage of the visual axis. This process is known as posterior capsule opacification (PCO) or secondary cataract formation.6
Improvement in the design and material composition of IOL have reduced the incidence of PCO. However, soft IOLs, which are being used for multifocal vision, generally increase the rate of PCO. In general, PCO occurs in nearly 30–40% of elderly cataract patients within 2–5 years after surgery7,8 and requires clearing of the visual axis by treatment with a ND-Yag laser (posterior capsulotomy). While the posterior capsulotomy restores vision again, it can result in other complications, such as endophthalmitis, retinal detachment, elevation of intraocular pressure, cystoid macular edema, and IOL subluxation.9 Thus, better therapies are required to prevent PCO, which in turn requires a better understanding of mechanisms involved in EMT.
Transforming growth factor beta (TGF-β) is generally considered to be the initiating cytokine for this process. This protein has three isoforms: 1, 2, and 3. Among these, TGF-β2 is the dominant isoform in the eye.10,11 Previous studies have shown that TGF-β2 plays an important role in the EMT of lens epithelial cells.12,13 TGF-β2 induces EMT through the classical canonical (Smad signaling),14 and non-canonical (extracellular signal-regulated kinases [ERK], mitogen-activated protein kinases (MAPK) and phosphoinositide 3-kinase [PI3K]) pathways.15 Smad signaling is accompanied by the activation of transcription factors such as Snail and Slug.16,17 Previous studies have also shown that histone deacetylases, sprouty protein, aldose reductase, and β-catenin/CBP play important roles in TGF-β2-mediated EMT.17–20
HIF is a heterodimer consisting of an α- and a β-subunit, which are highly conserved and expressed at basal levels in cells. Under normoxic conditions, the prolyl hydroxylase (PHD) that is present in the cytosol of cells hydroxylates proline residues (at positions 402 and 564) in HIF-1α, marking it for degradation via the ubiquitin E3 ligase complex.21 However, under hypoxic conditions, PHD is inhibited, which leads to the retention of HIF-1α, and subsequent nuclear translocation. HIF-1α thus translocated binds to the hypoxia-responsive element (HRE) in DNA and initiates transcription of various genes that play roles in angiogenesis, cell proliferation/survival, apoptosis, erythropoiesis, etc.22,23 In addition, HIF-1α has been shown to promote EMT in various types of tumors and fibrotic diseases.24–26 VEGF is one of the target genes of HIF-1α27 and has been implicated in EMT.28,29 VEGF binding to its receptor leads to autophosphorylation of specific tyrosine residues in the cytoplasmic domain and such autophosphorylation plays an important role in cell proliferation and migration.30
The lens is situated in a hypoxic environment in the eye. The oxygen partial pressure (pO2) at the surface of the lens is 2.9 mm Hg, which is lower than 24.1 mm Hg at the surface of the eye.31 However, after cataract surgery the pO2 at the surface of the lens increases to ~11 mm Hg.32 After cataract surgery, because of the higher pO2, HIF-1α should degrade. However, TGF-β2, which is elevated in the aqueous humor after cataract surgery (reviewed in13), has been shown to upregulate HIF-1α in cells.33,34 As, TGF-β2 is the major cytokine for the EMT of lens epithelial cells, we reasoned that HIF-1α might be involved in the EMT of lens epithelial cells. In this study, we tested the role of HIF-1α in the TGF-β2-mediated EMT of lens epithelial cells.
2 |. MATERIALS AND METHODS
2.1 |. Treatment of FHL124 cells with TGF-β2
FHL124 cells were obtained from Dr. Michael Wormstone, University of East Anglia, UK (originally from Prof. John Reddan, Oakland University, MI) and were authenticated by the analysis of short tandem repeats.35 Cells were cultured in MEM containing 5% FBS and gentamycin/L-glutamate (1:100) and treated with human recombinant TGF-β2 (10 ng/mL, Sigma-Aldrich, St. Louis, MO) in serum-free medium for 4 h (for the signaling experiments), or 16 h (PHD-2), 24 h (for PHD-2, qPCR, and immunocytochemistry) or 48 h (for PHD-2 and EMT marker assessments).
2.2 |. Treatment of FHL124 cells with VEGF-A or dimethyloxalylglycine (DMOG) or overexpression of HIF-1α
To study the effect of externally added VEGF-A, human recombinant VEGF-A (PeproTech, Rocky Hill, NJ) was used at selected concentrations (0–10 ng/mL) for 24 h (in pVEGFR2 analyses) or 48 h (in EMT marker analyses) in serum-free medium. The cells were treated with DMOG (Santa Cruz Biotechnology, Dallas, TX, 0–10 μM) for 48 h to increase the levels of HIF-1α in cells. To overexpress HIF-1α, the cells were transfected with either an empty vector (Kind gift from Dr. Niklaus Mueller, Department of Ophthalmology, University of Colorado Denver) or a HIF-1α plasmid (Addgene, Cambridge, MA) using Lipofectamine LTX and Plus Reagent (Life Technologies, Grand Island, NY) according to the manufacturers’ instructions. After the above procedures (4 h), the cells were treated with TGF-β2 (10 ng/mL) for either 4 h (for HIF-1α measurement) or 20 h (EMT markers measurements).
2.3 |. Treatment with a HIF-1α translational inhibitor
To study the effect of TGF-β2 under reduced levels of HIF-1α, the cells were treated with various concentrations (0–200 nM) of a HIF-1α translational inhibitor, KC7F2 (Tocris, Minneapolis, MN) for 3.5 h and then treated with or without TGF-β2, as above.
2.4 |. Measurement of HIF-1α mRNA levels
FHL124 cell were treated with or without TGF-β2 (10 ng/mL) for 24 h and total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) and cDNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen). qPCR was performed with SYBR green using a CFX Connect Real-time PCR System (Bio-Rad laboratories, Hercules, CA) with the following primers, HIF-1α- FP: 5′-CCAGAACCTACTGC -TAATGC-3′and RP:5′-TTATGTATGTGGGTAGGAGATG-3′. GAPDH-FP: 5′-GTCAGTGGTGGACCTGACCT-3′ and RP: 5′-TGCTGTAGCCAAATTCGTTG-3′.
2.5 |. Western blotting
Mammalian Protein Extraction Reagent (M-PER—Thermo Scientific, Waltham, MA) that contained a protease and phosphatase inhibitor cocktail (Sigma-Aldrich) was diluted 1:100 for use in preparing the total cell lysates. The cytosolic and nuclear fractions were prepared using NucBuster protein extraction reagent (Millipore, Billerica, MA). For VEGFR2, cell lysate was prepared using 1× RIPA buffer (Cell Signaling, Danvers, MA). We used lysates from human umbilical vein endothelial cells and human retinal endothelial cells as positive controls for Western blots in Supplementary Figure S2A and S2B. These protein extracts (10–30 μg) were used for SDS-PAGE (12%) or gradient gel (4–20%, Bio-Rad, Hercules, CA) and Western blotting. After electrophoretic transfer, membranes were blocked with 5% blocking-grade non-fat dry milk in TBST, and the membranes were incubated overnight at 4°C with primary antibodies against the following proteins: α-SMA (Sigma-Aldrich, Cat # A5228, diluted 1:5000), β-actin (Cat # 4970L, diluted 1:5000), HIF-1α (Cat # 3716S), PHD-2 (Cat #3293S), VEGFR2 (phosphorylated Y951 Cat # 4991T and total Cat # 9698T), E-cadherin (Cat #3195S), αV Integrin (Cat # 4711S), β1 Integrin (Cat # 4706S), ERK (phosphorylated Cat # 4370S and total Cat # 4694S), Snail (Cat # 3879S), Slug (Cat # 9585S), and histone (H3) (Cat # 9715S, diluted 1:5000) (from Cell Signaling and diluted 1:1000 unless noted otherwise). Appropriate HRP-conjugated secondary antibodies (diluted 1:5000, Cell Signaling) were used, and the protein bands were detected using the SuperSignal West Pico or Femto Kits (Pierce Chemical, Dallas, TX).
2.6 |. Immunocytochemistry
FHL124 cells were cultured on chamber slides (Lab-Tek II, NY) and treated with KC7F2 (200 nM) for 3.5 h, followed by TGF-β2 for 24–48 h. After treatment, the cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and stained for f-actin as described below. The cells were treated with 0.1% Triton X-100 for 5 min at room temperature, blocked with 5% normal goat serum for 1 h, and then incubated with Texas Red-conjugated phalloidin (1:40 dilution, Life Technologies) at 37°C for 1 h. For HIF-1α, the cells were permeabilized with ice-cold 80% methanol for 20 min at −20°C, blocked with 5% normal goat serum for 1 h, and incubated overnight at 4°C with an antibody for HIF-1α (Sigma-Aldrich, Cat # H6536, 1:50 dilution). This was followed by 1 h of incubation with Alexa Fluor 488-goat anti-mouse IgG (1:250 dilution, Life Technologies) at 37°C. Cells that were stained with Alexa Fluor 488-goat anti-mouse IgG alone served as the negative control. The slides were mounted with DAPI/Vectashield for observations of the nuclei. Images were taken with a fluorescence microscope (Nikon Eclipse 80i, Nikon Corporation, Tokyo, Japan) using Nikon NIS software. Mean fluorescence intensity of HIF-1α was calculated using ImageJ software (NIH).
2.7 |. ELISA for VEGF-A
FHL124 cells were treated with TGF-β2 as described above, and the media was collected after culturing cells for 48 h. This was used immediately for analysis of VEGF-A or stored at 4°C for 2–3 days prior to the ELISA assay. The VEGF-A levels were measured using a VEGF Human ELISA Kit (Thermo Fisher Scientific) according to the manufacturer′s instructions.
2.8 |. Statistical analysis
The data are presented as the means ± SD of the specific number of experiments indicated in the figure legends. The data were analyzed with GraphPad Prism software (Version 7) by either one-way ANOVA or Student′s t-test. The differences were considered significant at P < 0.05.
3 |. RESULTS
3.1 |. TGF-β2 upregulates HIF-1α and VEGF-A during EMT of FHL124 cells
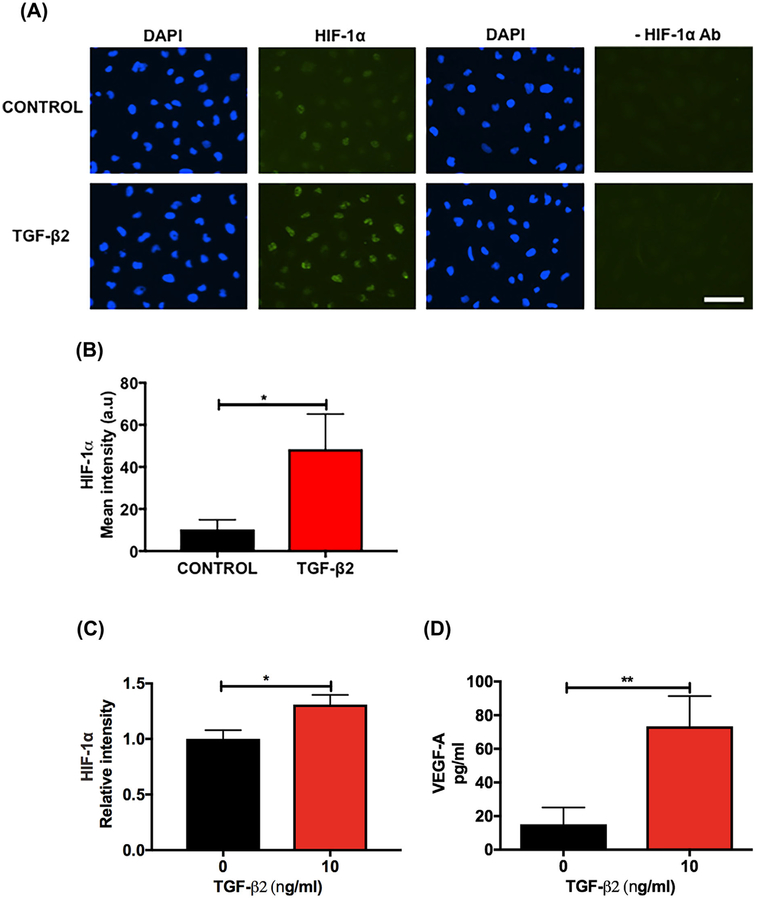
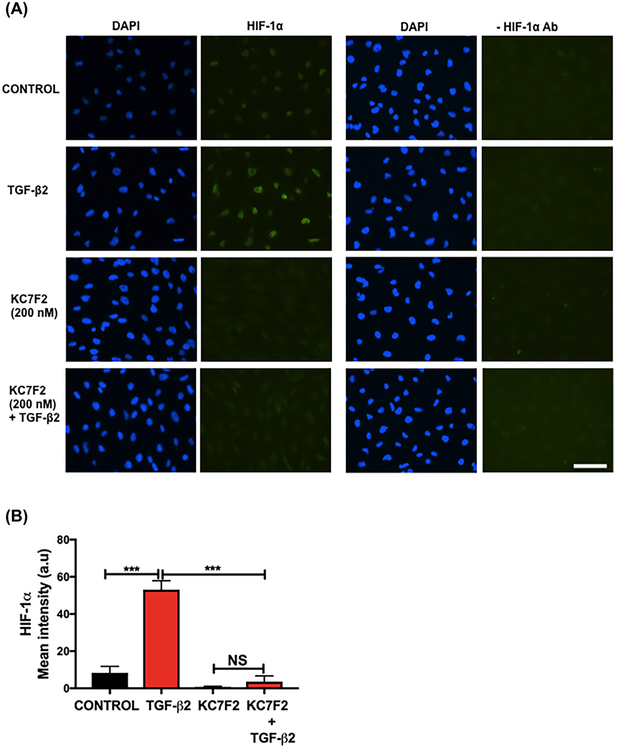
First, we determined the effect of TGF-β2 on HIF-1α in FHL124 cells. Treatment of the cells with TGF-β2 for 24 h caused an increase in the levels of HIF-1α, as evident from the immunocytochemical data (Figure 1A, left four panels), which showed an increase in fluorescence intensity (Figure 1B). The negative control (secondary antibody alone, Figure 1A, right four panels) showed negligible staining. We were not able to show the upregulation of HIF-1α protein by Western blotting because the results were inconsistent, possibly due to extremely low levels of HIF-1α, but we found an increase in HIF-1α mRNA (Figure 1C) upon TGF-β2 treatment. However, TGF-β2 treatment had no effect on the PHD-2 protein content, determined by Western blotting (Supplementary Figure S1A and S1B). TGF-β2 treatment increased the VEGF-A levels in the conditioned media (Figure 1D). Western blotting for p-VEGFR2 (Y951) showed very faint bands in FHL124 cells and the levels were unaltered by the TGF-β2 treatment (Supplementary Figure S2A). We also determined the effect of TGF-β2 on the EMT-associated proteins α-SMA, αV integrin, and β1 integrin by Western blotting and found that they were all significantly upregulated in the TGF-β2-treated cells compared to the untreated control cells (Figure 1E–G).
FIGURE 1.
TGF-β2 upregulates HIF-1α and VEGF-A during EMT in human lens epithelial cells. The cells were treated with human recombinant TGF-β2 (10 ng/mL) for 24–48 h. HIF-1α (A) was detected using an antibody against HIF-1α and Alexa Fluor 488-goat anti-mouse IgG (left four panels). Mean fluorescence intensity of HIF-1α was calculated using ImageJ software (B). In the negative controls, the HIF-1α antibody was omitted (right four panels). The images are shown at 20× magnification. Scale bar = 100 μm. HIF-1α mRNA was measured by qPCR (C). The VEGF-A levels in the conditioned media were measured by an ELISA (D). The levels of the EMT-associated proteins α-SMA, αV integrin, and β1 integrin were measured by Western blotting (E-G). The densitometric analyses from triplicate assays (means ± SD) are shown in the bar graphs. *P < 0.05, **P < 0.005 and ***P < 0.0005
3.2 |. Exogenous VEGF-A does not promote the TGF-β2-mediated EMT of FHL124 cells
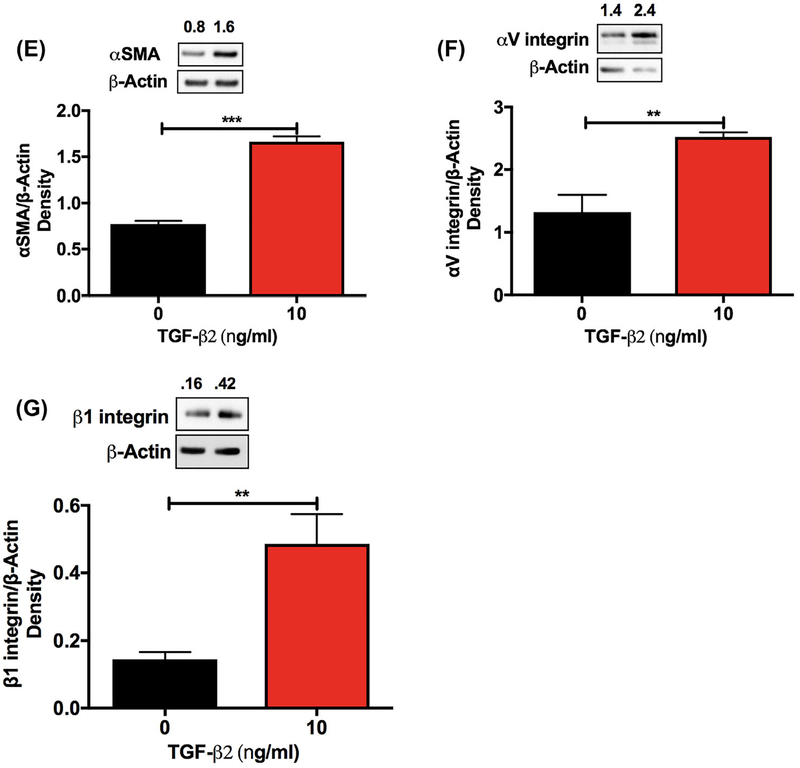
As we found higher levels of VEGF-A in the conditioned media after TGF-β2 treatment, we sought to determine whether there was an association between externally added VEGF-A and the EMT response. Exogenous VEGF-A did not significantly alter the TGF-β2-mediated effects on α-SMA, αV integrin, and β1 integrin compared with untreated cells (Figure 2A–C). Similar to TGF-β2 treatment, VEGF-A treatment had no effect on the phosphorylation of VEGFR2 (Y951) (Supplementary Figure S2B).
FIGURE 2.
Exogenous VEGF-A does not induce EMT in human lens epithelial cells. FHL124 cells were treated with human recombinant VEGF-A for 48 h. The VEGF-A-mediated induction of the α-SMA, αV integrin, and β1 integrin proteins was measured by Western blotting (A-C). The densitometric analyses from triplicate assays (means ± SD) are shown in the bar graphs. NS = not significant
3.3 |. Upregulation of HIF-1α with a PHD inhibitor (DMOG) did not induce an EMT response in FHL124 cells
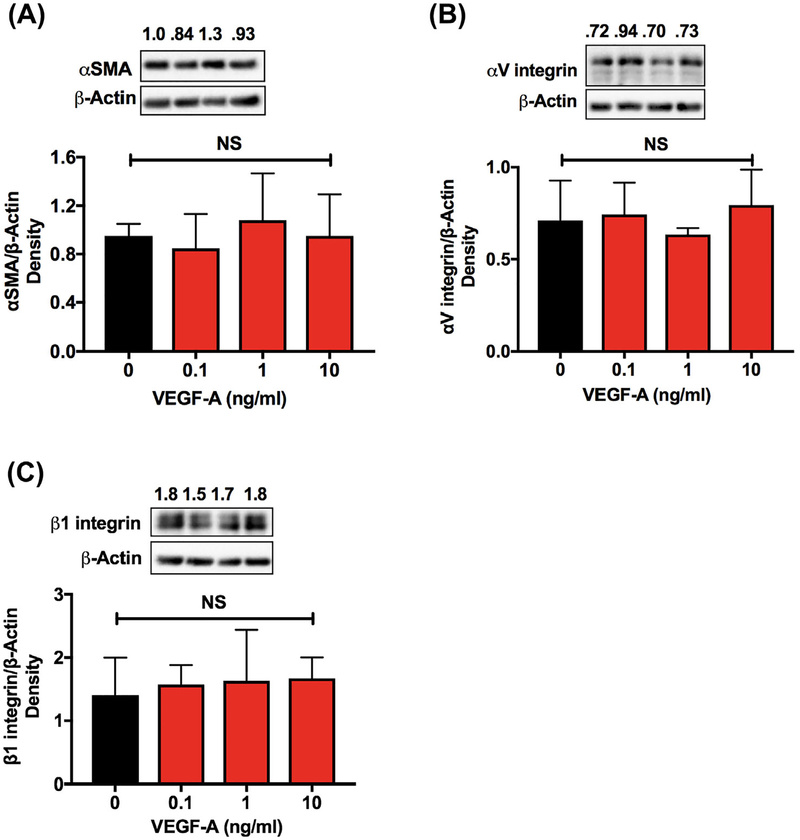
We observed a concentration-dependent increase in HIF-1α (Figure 3A, up to 6 μM) and VEGF-A (Figure 3B, in the media, up to 10 μM) following treatment with DMOG for 48 h. However, this agent had no significant effect on the levels of EMT markers, α-SMA, αV integrin, and β1 integrin, compared with the untreated cells (Figure 3C–E).
FIGURE 3.
The upregulation of HIF-1α by DMOG had no effect on the EMT-associated proteins in human lens epithelial cells. FHL124 cells were treated with DMOG (0–10 μM) β1 integrin were measured by Western blotting (C-E). The densitometric analyses from triplicate assays (means ± SD) are shown in the bar graphs. *P < 0.05, **P < 0.005, ***P < 0.0005 and NS = not significant
3.4 |. A HIF-1α translational inhibitor (KC7F2) inhibited TGF-β2-mediated EMT in FHL124 cells
Next, we investigated whether a reduction in the levels of HIF-1α would negatively affect the TGF-β2-mediated EMT. We used KC7F2, a translational inhibitor of HIF-1α. Cells that had been treated with TGF-β2 for 24 h showed an increase in HIF-1α staining (Figure 4A). KC7F2 treatment reduced the levels of HIF-1α in cells (Figure 4A, left panels). The HIF-1α fluorescence intensity was reduced in KC7F2 + TGF-β2 treated cells compared to the TGF-β2 alone treated cells (Figure 4B). The specificity of the HIF-1α staining was confirmed in negative control experiments in which the HIF-1α antibody was omitted during immunohistochemistry; those experiments showed no significant staining for HIF-1α (Figure 4A, right panels). Our attempts to demonstrate a reduction in HIF-1α at the protein level by Western blotting were not successful due to inconsistent results that were possibly the result of the extremely low levels of HIF-1α. We did not check the mRNA levels of HIF-1α since KC7F2 is a translation inhibitor.
FIGURE 4.
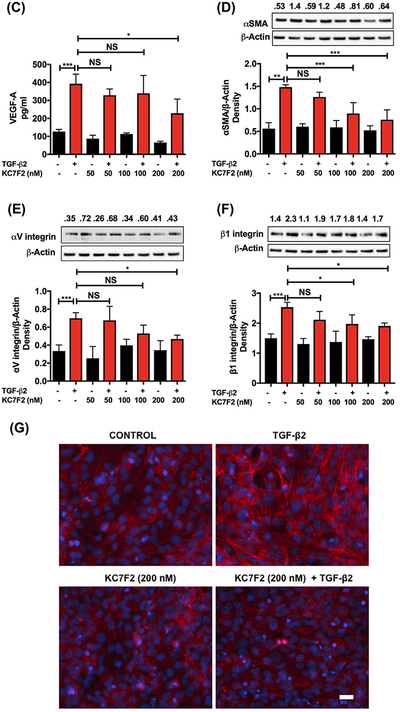
Effect of a HIF-1α translational inhibitor on the TGF-β2-mediated EMT and cytoskeleton remodeling in human lens epithelial cells. FHL124 cells were treated with KC7F2 (0–200 nM) for 3.5 h and then with TGF-β2 (10 ng/mL) for 24–48 h. The HIF-1α levels (A) were measured by immunofluorescence (left panels) and mean fluorescence intensity of HIF-1α (B). The HIF-1α primary antibody was omitted in the negative controls (right panels). The VEGF-A levels were measured by an ELISA (C). The protein levels of α-SMA, αV integrin, and β1 integrin were measured by Western blotting (D-F). Cytoskeletal remodeling (F-actin) was assessed using Texas Red-conjugated phalloidin (G). All images are shown at 20 × magnification. Scale bar = 100 μm. The densitometric analyses from triplicate assays (means ± SD) are shown in the bar graphs. *P < 0.05, **P < 0.005, ***P < 0.0005 and NS = not significant
As expected, TGF-β2 treatment increased the VEGF-A levels in the media, but KC7F2 (at 200 nM) significantly reduced the levels (Figure 4C). We found concentration-dependent decreases in the levels of α-SMA, αV integrin, and β1 integrin with increasing concentrations of KC7F2 compared to the cells treated with TGF-β2 alone (Figure 4D–F). TGF-β2 treatment showed a significant reduction (P < 0.05) in the E-cadherin levels and the treatment with KC7F2 reversed this effect (Supplementary Figure S3). We also investigated the cytoskeletal remodeling (F-actin) by immunofluorescence. We found an increase in the F-actin staining after the cells were treated with TGF-β2 compared to control cells (Figure 4G). Such a staining was reduced by KC7F2 treatment (Figure 4G).
3.5 |. KC7F2 inhibited the TGF-β2-meditated signaling in FHL124 cells
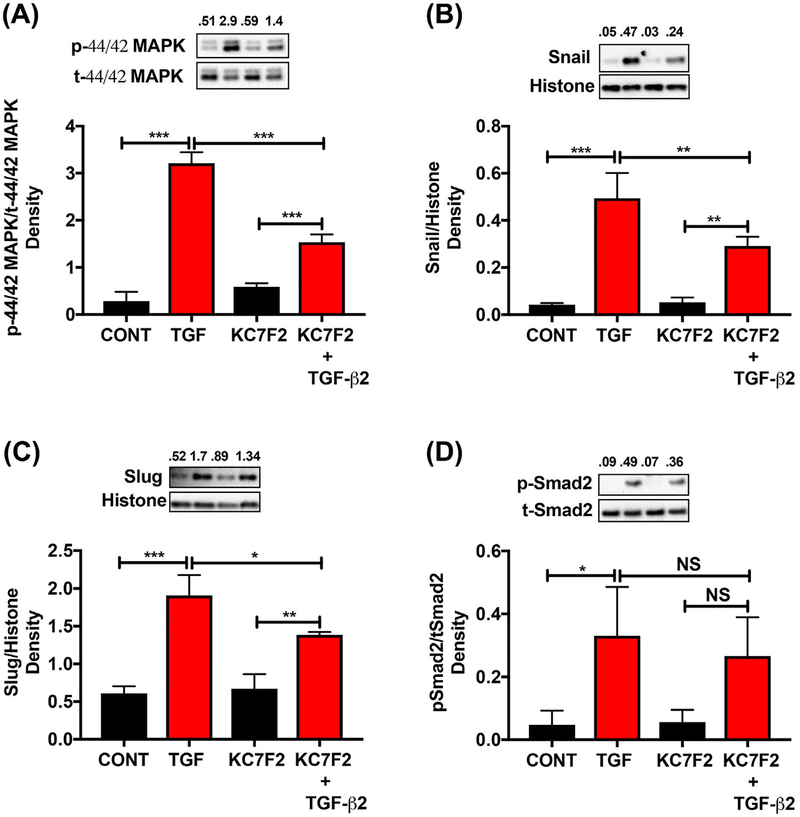
We further verified the effect of KC7F2 on the TGF-β2-mediated phosphorylation and nuclear translocation of signaling molecules. The cells that were treated with TGF-β2 showed increases in p44/42 MAPK (ERK) phosphorylation and the nuclear translocation of the transcription factors Snail and Slug, which were reduced by the KC7F2 treatment (Figure 5A–C). The TGF-β2-treated cells showed increases in the phosphorylation and nuclear translocation of Smad2, but there were no significant differences between the TGF-β2-treated and TGF-β2 + KC7F2-treated groups (Figure 5D).
FIGURE 5.
Effect of a HIF-1α translational inhibitor on TGF-β2-meditated signaling in human lens epithelial cells. FHL124 cells were treated with KC7F2 and TGF-β2 as described in Methods. The levels of p44/42 MAPK (A) in the cytosolic fraction and Snail (B), Slug (C), and pSmad2 (D) in the nuclear fraction were measured by Western blotting. The densitometric analyses from triplicate assays (means ± SD) are shown in the bar graphs. *P < 0.05, **P < 0.005, ***P < 0.0005 and NS = not significant. CONT = Control and TGF = TGF-β2 treated
3.6 |. Overexpression of HIF-1α had no effect on the TGF-β2-mediated EMT of FHL124 cells
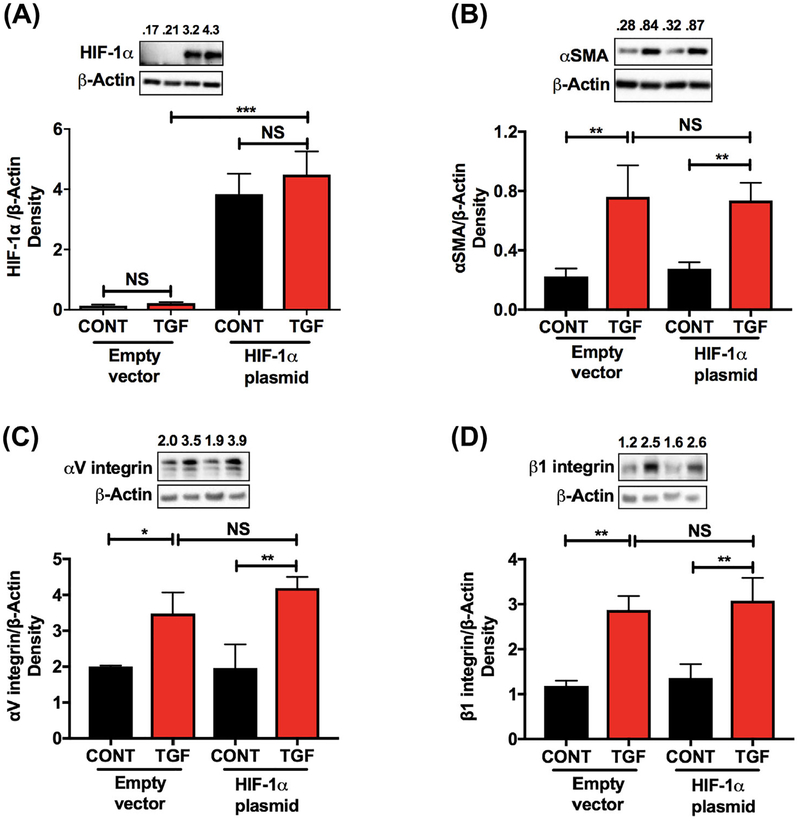
Cells transfected with a HIF-1α plasmid showed significantly higher levels of HIF-1α after 8 h of transfection (Figure 6A). Interestingly, we did not see any significant difference in the TGF-β2-mediated EMT response between the empty vector and HIF-1α transfected cells (Figure 6B–D).
FIGURE 6.
Overexpression of HIF-1α had no effect on the TGF-β2-mediated EMT in human lens epithelial cells. FHL124 cells were transfected with either the empty vector or the HIF-1α plasmid and treated with or without TGF-β2 (10 ng/mL) for 4–20 h as described in the methods. The protein content of HIF-1α was measured by Western blotting (A). The levels of α-SMA, αV integrin, and β1 integrin were measured by Western blotting (B-D). The densitometric analyses from triplicate assays (means ± SD) are shown in the bar graphs. *P < 0.05, **P < 0.005, ***P < 0.0005 and NS = not significant CONT = Control and TGF = TGF-β2 treated
4 |. DISCUSSION
The objective of this study was to determine the role of HIF-1α in the TGF-β2-mediated EMT of lens epithelial cells. This was based on numerous studies on other cell types that have shown that HIF-1α plays an important role in EMT.29,36–38 The eye lens exists in a hypoxic environment31 and therefore should favor HIF-1α activation in its epithelial cells. In fact, a previous study showed such activation in mouse lens epithelial cells.39 Introduction of oxygen during cataract surgery would be expected to decrease the HIF-1α levels in lens epithelial cells and inhibit their EMT. However, TGF-β2 levels are upregulated in the anterior eye after cataract surgery,40 which could upregulate HIF-1α in epithelial cells. Thus, whether TGF-β2 opposes the effects of normoxia and initiates an EMT effect through HIF-1α upregulation (independent of oxygen) was not known.
Our study showed that TGF-β2 upregulates the HIF-1α levels in FHL124 cells. The higher VEGF-A levels in the conditioned media further confirmed that HIF-1α was activated in response to TGF-β2. Previous studies have shown HIF-1α stabilization through PHD inhibition in response to TGF-β1 in the hepatoma cell line HepG2, fibrosarcoma HT1080 cells,33 and renal tubular cells.41 The observed HIF-1α up-regulation in HLE does not appear to be due to changes in PHD-2 levels, as we found the levels to be unaltered by TGF-β2 treatment. However, it could be due the observed increase in HIF-1α mRNA levels.
Higher levels of HIF-1α upon DMOG treatment did not elicit an EMT response. This result suggested that upregulation of HIF-1α alone is not a critical factor for the EMT of FHL124 cells. Similarly, externally added VEGF-A failed to induce the EMT response without TGF-β2. One recent study reported that VEGF-A released from lens epithelial cells, through an autocrine mechanism promotes cell survival, growth and myofibroblast formation,42 and showed that those changes were inhibited by a small molecule inhibitor for the VEGF-A receptor. Our results showed phosphorylation of VEGFR2 at tyrosine residue (Y951) was not altered after TGF-β2 or VEGF-A treatment. This could explain why neither the upregulated VEGF-A (after TGF-β2 treatment) nor exogenous VEGF-A was able to induce/promote EMT in FHL124 cells.
Previous studies have shown that KC7F2 suppresses the key regulators of HIF-1α protein synthesis43 and thereby reduces the HIF-1α protein content.44 Similar to these findings, we observed that KC7F2 reduced the levels of the HIF-1α protein in TGF-β2-treated cells. What was striking in our study was the reduction in the TGF-β2-mediated EMT when the HIF-1α levels were reduced by the treatment with KC7F2. This implied that HIF-1α is important for the TGF-β2-mediated EMT of lens epithelial cells. One previous study suggested that HIF-1α augments the TGF-β-mediated EMT by promoting the activation of latent TGF-β1.45 However, this was unlikely in our study because we used the active form of TGF-β2 to induce EMT.
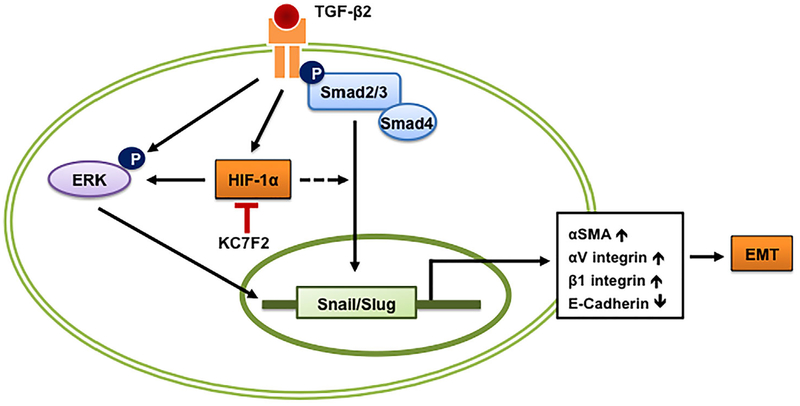
The above observation that HIF-1α is important for the TGF-β2-mediated EMT begs the question as to what signaling pathway of TGF-β2 might be affected by very low levels of HIF-1α. Our results suggested that subnormal HIF-1α levels suppressed the non-canonical signaling arm of TGF-β2. This corroborates several other reports that have shown ERK activation under hypoxic conditions.46–49 Additionally, low levels of HIF-1α also decreased the Slug and Snail levels. These transcription factors are essential for the downregulation of E-cadherin during EMT. Similar observations have been made in other cells. For example, treatment with a HIF-1α siRNA inhibited the epidermal growth factor-mediated upregulation of Snail and Slug in ovarian cancer cell lines.50 When HIF-1α was overexpressed, there was no further increase in the TGF-β2-mediated EMT of FHL124 cells. This indicated that HIF-1α upregulated by TGF-β2 alone was sufficient for an EMT response. Based on these results, we propose a scheme (Figure 7) in which we hypothesize that HIF-1α promotes TGF-β2 mediated EMT through the non-canonical pathway. However, we cannot rule out the participation of the canonical pathway, as Snail and Slug (which are Smad-dependent) are downregulated when the HIF-1α expression was inhibited by KC7F2.
FIGURE 7.
Proposed scheme on the role of HIF-1α in TGF-β2-induced EMT of human lens epithelial cells
In conclusion, our study showed that HIF-1α is important for the TGF-β2-mediated EMT in lens epithelial cells and suggested that HIF-1α could be a target for the prevention of PCO.
Supplementary Material
ACKNOWLEDGMENTS
The author thank Dr Micheal Wormstone for providing FHL124 cells and Drs Mi-hyun Nam, Johanna Rankenberg, and Sandip K. Nandi for critical reading of the manuscript. This work was supported by the National Institutes of Health Grants EY022061 and EY023286 (to RHN) and Research to Prevent Blindness, NY.
Abbreviations:
- PCO
posterior capsule opacification
- HLE
human lens epithelial cells
- EMT
epithelial-to-mesenchymal transition
- α-SMA
alpha smooth muscle actin
- FBS
fetal bovine serum
- MEM
minimum essential medium
- HIF-1α
hypoxia-inducible factor-1α
- VEGF-A
vascular endothelial growth factor-A
- VEGFR2
vascular endothelial growth factor receptor 2
- DMOG
dimethyloxalylglycine
- TGF-β2
transforming growth factor-beta 2
- IOL
intraocular lens
- ERK
extracellular signal-regulated kinases
- MAPK
mitogen-activated protein kinases
- PI3 K
phosphoinositide 3-kinase
- PHD
prolyl hydroxylase
- PHD-2
prolyl hydroxylase-2
- HRE
hypoxia-responsive element
- pO2
oxygen partial pressure
- M-PER
mammalian protein extraction reagent
- KC7F2
N,N′-(dithiodi-2,1-ethanediyl)bis[2,5-dichlorobenzenesulfonamide
- ECM
extracellular matrix
- DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390:600–612. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadpour M, Erfanian R, Karimi N. Capsulorhexis: pearls and pitfalls. Saudi J Ophthalmol. 2012;26:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wormstone IM. Posterior capsule opacification: a cell biological perspective. Exp Eye Res. 2002;74:337–347. [DOI] [PubMed] [Google Scholar]
- 4.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. [DOI] [PubMed] [Google Scholar]
- 5.Mamuya FA, Duncan MK. AV integrins and TGF-beta-induced EMT: a circle of regulation. J Cell Mol Med. 2012;16:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. 2009;88:257–269. [DOI] [PubMed] [Google Scholar]
- 7.Awasthi N, Guo S, Wagner BJ. Posterior capsular opacification: a problem reduced but not yet eradicated. Arch Ophthalmol. 2009;127:555–562. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg PB, Tseng VL, Wu WC, et al. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118:507–514. [DOI] [PubMed] [Google Scholar]
- 9.Billotte C, Berdeaux G. Adverse clinical consequences of neodymium:YAG laser treatment of posterior capsule opacification. J Cataract Refract Surg. 2004;30:2064–2071. [DOI] [PubMed] [Google Scholar]
- 10.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9: 963–969. [DOI] [PubMed] [Google Scholar]
- 11.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- 12.Dawes LJ, Elliott RM, Reddan JR, Wormstone YM, Wormstone IM. Oligonucleotide microarray analysis of human lens epithelial cells: tGFbeta regulated gene expression. Mol Vis. 2007;13: 1181–1197. [PubMed] [Google Scholar]
- 13.Eldred JA, Dawes LJ, Wormstone IM. The lens as a model for fibrotic disease. Philos Trans R Soc Lond B Biol Sci. 2011;366: 1301–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114:4359–4369. [DOI] [PubMed] [Google Scholar]
- 15.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425: 577–584. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie L, Santhoshkumar P, Reneker LW, Sharma KK. Histone deacetylase inhibitors trichostatin A and vorinostat inhibit TGFbeta2-induced lens epithelial-to-mesenchymal cell transition. Invest Ophthalmol Vis Sci. 2014;55:4731–4740. [DOI] [PubMed] [Google Scholar]
- 18.Chang KC, Petrash JM. Aldose reductase mediates transforming growth factor beta2 (TGF-beta2)-Induced migration and epithelial-To-Mesenchymal transition of lens-Derived epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:4198–4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovicu FJ, Shin EH, McAvoy JW. Fibrosis in the lens. Sprouty regulation of TGFbeta-signaling prevents lens EMT leading to cataract. Exp Eye Res. 2016;142:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taiyab A, Korol A, Deschamps PA, West-Mays JA. Beta-Catenin/CBP-Dependent signaling regulates TGF-beta-Induced epithelial to mesenchymal transition of lens epithelial cells. Invest Ophthalmol Vis Sci. 2016;57:5736–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80:51–60. [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. [DOI] [PubMed] [Google Scholar]
- 23.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzouvelekis A, Harokopos V, Paparountas T, et al. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am J Respir Crit Care Med. 2007;176:1108–1119. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Huang G, Li X, et al. Hypoxia induces epithelialmesenchymal transition via activation of SNAI1 by hypoxiainducible factor −1alpha in hepatocellular carcinoma. BMC Cancer. 2013;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Shi X, Peng Y, et al. HIF-1alpha promotes epithelial-Mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS ONE. 2015;10: e0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C, McGough R, Aswad B, Block JA, Terek R. Hypoxia induces HIF-1alpha and VEGF expression in chondrosarcoma cells and chondrocytes. J Orthop Res. 2004;22:1175–1181. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Moreno O, Lecanda J, Green JE, et al. VEGF elicits epithelial-mesenchymal transition (EMT) in prostate intraepithelial neoplasia (PIN)-like cells via an autocrine loop. Exp Cell Res. 2010;316:554–567. [DOI] [PubMed] [Google Scholar]
- 29.Luo M, Hou L, Li J, et al. VEGF/NRP-1axis promotes progression of breast cancer via enhancement of epithelial-mesenchymal transition and activation of NF-kappaB and beta-catenin. Cancer Lett. 2016;373:1–11. [DOI] [PubMed] [Google Scholar]
- 30.Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helbig H, Hinz JP, Kellner U, Foerster MH. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2: 161–164. [PubMed] [Google Scholar]
- 32.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci. 2010; 51:5731–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. [DOI] [PubMed] [Google Scholar]
- 34.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1alpha and TGF-beta/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol. 2011;300: F898–F905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghavan CT, Nagaraj RH. AGE-RAGE interaction in the TGFbeta2-mediated epithelial to mesenchymal transition of human lens epithelial cells. Glycoconj J. 2016;33:631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Wu Y, Yan Q, et al. Deferoxamine enhances cell migration and invasion through promotion of HIF-1alpha expression and epithelial-mesenchymal transition in colorectal cancer. Oncol Rep. 2014;31:111–116. [DOI] [PubMed] [Google Scholar]
- 37.Morishita Y, Ookawara S, Hirahara I, Muto S, Nagata D. HIF-1alpha mediates Hypoxia-induced epithelial-mesenchymal transition in peritoneal mesothelial cells. Ren Fail. 2016;38:282–289. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Tan J, Xie H, Wang J, Meng X, Wang R. HIF-1alpha regulates EMT via the Snail and beta-catenin pathways in paraquat poisoning-induced early pulmonary fibrosis. J Cell Mol Med. 2016;20:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shui YB, Arbeit JM, Johnson RS, Beebe DC. HIF-1: an age-dependent regulator of lens cell proliferation. Invest Ophthalmol Vis Sci. 2008;49:4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallentin N, Wickstrom K, Lundberg C. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1998;39:1410–1418. [PubMed] [Google Scholar]
- 41.Han WQ, Zhu Q, Hu J, Li PL, Zhang F, Li N. Hypoxiainducible factor prolyl-hydroxylase-2 mediates transforming growth factor beta 1-induced epithelial-mesenchymal transition in renal tubular cells. Biochim Biophys Acta. 2013;1833: 1454–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eldred JA, McDonald M, Wilkes HS, Spalton DJ, Wormstone IM. Growth factor restriction impedes progression of wound healing following cataract surgery: identification of VEGF as a putative therapeutic target. Sci Rep. 2016;6:24453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narita T, Yin S, Gelin CF, et al. Identification of a novel small molecule HIF-1alpha translation inhibitor. Clin Cancer Res. 2009;15:6128–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cammarata PR, Neelam S, Brooks MM. Inhibition of hypoxia inducible factor-1alpha downregulates the expression of epithelial to mesenchymal transition early marker proteins without undermining cell survival in hypoxic lens epithelial cells. Mol Vis. 2015;21:1024–1035. [PMC free article] [PubMed] [Google Scholar]
- 45.Copple BL. Hypoxia stimulates hepatocyte epithelial to mesenchymal transition by hypoxia-inducible factor and transforming growth factor-beta-dependent mechanisms. Liver Int. 2010;30: 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minet E, Arnould T, Michel G, et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468:53–58. [DOI] [PubMed] [Google Scholar]
- 47.Sang N, Stiehl DP, Bohensky J, Leshchinsky I, Srinivas V, Caro J. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278: 14013–14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM. Snail is a direct target of hypoxia-inducible factor 1alpha (HIF1alpha) in hypoxia-induced endothelial to mesenchymal transition of human Coronary endothelial cells. J Biol Chem. 2015;290:16653–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu GH, Huang C, Feng ZZ, Lv XH, Qiu ZJ. Hypoxia-induced snail expression through transcriptional regulation by HIF-1alpha in pancreatic cancer cells. Dig Dis Sci. 2013;58:3503–3515. [DOI] [PubMed] [Google Scholar]
- 50.Cheng JC, Klausen C, Leung PC. Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett. 2013;329:197–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.