Abstract
Purpose of review:
In this manuscript, the recent advancements and novel approaches for regeneration of the ocular surface are summarized.
Recent findings:
Following severe injuries, persistent inflammation can alter the rehabilitative capability of the ocular surface environment. Limbal stem cell deficiency (LSCD) is one of the most characterized ocular surface disorders mediated by deficiency and/or dysfunction of the limbal epithelial stem cells (LESCs) located in the limbal niche. Currently, the most advanced approach for revitalizing the ocular surface and limbal niche is based on transplantation of limbal tissues harboring LESCs. Emerging approaches have focused on restoring the ocular surface microenvironment using (1) cell-based therapies including cells with capabilities to support the LESCs and modulate the inflammation, e.g., mesenchymal stem cells (MSCs), (2) bio-active extracellular matrices from decellularized tissues, and/or purified/synthetic molecules to regenerate the microenvironment structure, and (3) soluble cytokine/growth factor cocktails to revive the signaling pathways.
Summary:
Ocular surface/limbal environment revitalization provide promising approaches for regeneration of the ocular surface.
Keywords: Ocular Surface Regeneration, Corneal Epithelium, Limbal Stem Cell Niche, Limbal Epithelial Stem Cell Deficiency, Extracellular Matrix, Mesenchymal Stem Cells
1. Introduction: Ocular Surface Homeostasis, Pathologies, and Regeneration
The ocular surface is the outermost layer of the eye including the tear film. It is constructed and protected by structural and functional modules which have highly-regulated cross-talks. These components include the tear film, cornea, conjunctiva, lacrimal glands, meibomian glands, eyelids and nerves [1]. Many of the interactions on the ocular surface are modulated by the immune system. Many ocular surface disorders occur following alteration of the balance in immune system regulation [2]. The resulting persistent inflammation due to immune dysregulation limits the regenerative potential of the ocular surface micro-environment. Thus, local stem cells lose their capacity for proliferation, migration and differentiation, which are vital for rehabilitation of the ocular surface.
The limbal niche is the microenvironment supporting the function of LESCs [3]. It consists of a specialized extracellular matrix, signaling molecules and cells including immune cells, mesenchymal cells, melanocytes, nerve and vascular cells [4]. Injuries to the limbal niche or LESCs may lead to a condition known as limbal stem cell deficiency (LSCD) where the corneal epithelium can no longer be regenerated properly. This can have many consequences including non-healing epithelial defects, corneal neovascularization and opacification [5, 6]. Significant limbal injuries are almost always accompanied by the migration of immune cells in the area and subsequent inflammation [3]. The characteristics of an injured limbal niche include up-regulation of inflammatory and angiogenic factors such as IL-1α, IL-1β, IL-1 RA, IL-6, VEGF, ICAM-1, and VCAM-1. The persistent inflammatory environment in LSCD leads to additional recruitment of immune cells to the area and secretion of more inflammatory cytokines as well as impaired function of immune cells. The recruited immune cells secrete soluble pro-inflammatory factors, and the macrophages lose their ability to phagocytose [6, 7]. Additionally, macrophages stimulate adaptive T-lymphocytes, which worsens pathological inflammation [8]. Persistent inflammation in turn alters the expression of LESC stem cell markers, remodels the extracellular matrix, and disturbs the density and morphology of supporting cells in the limbal niche [5, 6]. Based on these observations, it can be concluded that the ocular surface microenvironment is disturbed in the setting of severe diseases, which in turn impairs regenerative mechanisms [9–13]. Therefore, in addition to repopulation of stem cells, emerging strategies are focusing on restoration of the limbal stem cell niche.
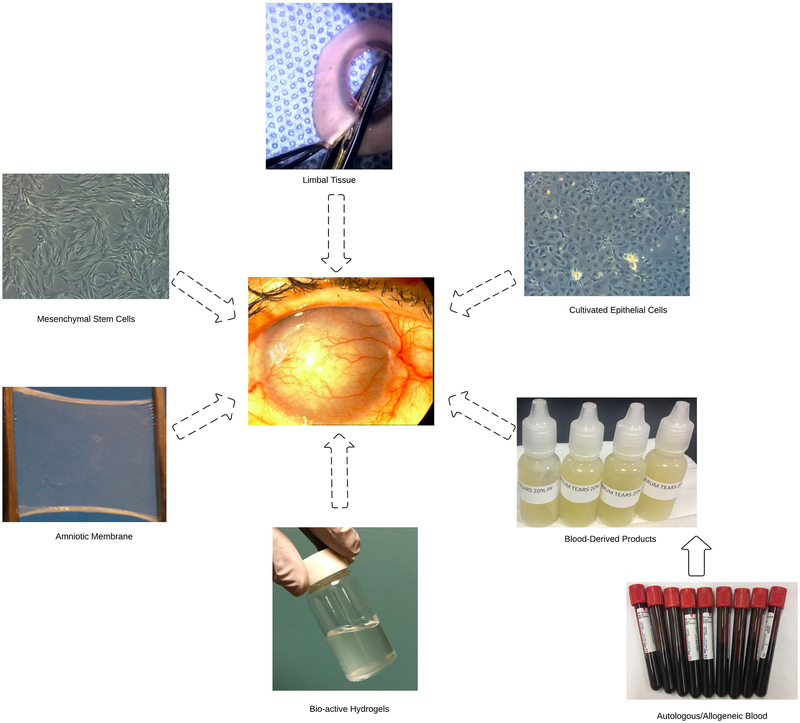
In this review, novel approaches for regeneration of the ocular surface based on repopulation of LESCs and restoration of the ocular surface/limbal microenvironment are summarized (Figure 1).
Figure 1:
Summary of emerging approaches for regeneration of the ocular surface.
2. Cell-based Therapeutic Approaches
2.1. Epithelial Cells
Replacing deficient corneal epithelial cells is currently the most advanced approach available for regenerating the ocular surface. Several protocols have been studied including limbal tissue transplantation, cultivated limbal epithelial transplantation (CLET) and non-limbal epithelial cell transplantation. Limbal tissue transplantation is a surgical method that does not require cell-culture laboratory facilities. Current limbal tissue transplantation techniques consist of conjunctival limbal autograft (CLAU) [14], kerato-limbal allograft (KLAL) [15, 16], conjunctival limbal allograft (CLAL) [15], and more recently, simple limbal epithelial transplantation (SLET) [9, 10]. The limbal tissue can be harvested from donors including the fellow-healthy eye, cadavers, and living individuals. The dissected limbal tissue is placed on the damaged limbal area and is fixed by sutures or glue. The transplanted LESCs help restore corneal epithelial cell phenotype [17].
In cultivated limbal epithelial transplantation (CLET), the LESCs are collected from donors and expanded in the laboratory with or without feeder layers such as 3T3-fibroblasts and with or without a substrate carrier such as amniotic membrane. The resulting epithelial sheet is then transplanted to the diseased eye [11, 18]. Recent novel feeder layers for cultivation of LESCs include mesenchymal stem cells (MSCs), and limbal melanocytes [19, 20]. Xeno-free and serum-free protocols have also developed to decrease the chance of animal infectious transmission [21].
On the other hand, newer approaches have focused on the administration of epithelial cells derived from non-limbal sources. Cultivated oral mucosal epithelial transplantation (COMET) is one of the proposed treatments for reconstruction of the corneal surface in LSCD patients [22–24]. However, the phenotype of epithelial cells after COMET remains oral mucosal which results in thicker and opaquer epithelium with sub-optimal visual outcomes [24, 25]. Conjunctival epithelial cells [22, 23], amniotic epithelial cells [26] and differentiated epithelial cells from stem cells [27] are other potential sources which are under investigation for regeneration of ocular surface. For instance, Oyuyang et al. showed that limbal stem-cell like cells could be generated by transduction of PAX6 transcription factor in skin epithelial stem cells. Transplantation of these differentiated limbal stem-cell like cells to rabbit model of corneal epithelial stem cells deficiency led to regeneration of ocular surface [28]. Moreover, Hayashi et al. generated self-formed ectodermal autonomous multi-zones (SEAMs) of ocular cells from human induced pluripotent stem (iPS) cells using a differentiation culture medium and then induced differentiation of corneal epithelial cells by treating SEAMs with corneal epithelial differentiation medium. The produced human iPS cell-derived corneal epithelial cells repaired corneal epithelial defects in an animal model following transplantation [29].
Successful clinical translation of these approaches might improve the issues with allogeneic limbal tissue shortage and systemic immunosuppression; however, the lack of long-term clinical results and lack of corneal epithelial phenotype restoration in COMET, as well as cost, safety, and technical difficulties for administration of epithelial cells differentiated from iPS cells, may limit their promising wide application.
2.2. Mesenchymal Stromal/Stem Cells
Mesenchymal stromal/stem cells (MSCs) are multipotent stem cells present in almost all tissues of the body, in part providing support to regional stem cells that are responsible for replacing damaged cells. MSCs also have immune-modulatory effects by secreting anti-inflammatory cytokines/growth factors, and thus have been studied for cell-therapy for their immune-modulating and regenerative properties. MSCs derived from bone marrow (BM-MSCs) are the most characterized and administrated stem cells for clinical applications. The immunomodulatory effects of the MSCs have shown to involve various cell-surface molecules such as CXCR4, CD44, integrins, ICAM, CD90, and CD105 or secreted factors including TGF-β, IL-10, Leukemia inhibitory factor, semaphoring-3A and TSG-6 [30]. Several studies have shown the healing effects of MSCs in ocular surface disease models, e.g., chemical-burns [31], dry eye syndrome [32], and corneal transplantation [33]. For instance, periorbital injection of bone marrow-MSCs in a murine model of dry eye syndrome resulted in reduction in infiltration, proliferation and differentiation of CD4+ T cells, attenuation of Il-2 and IFN-γ-inflammatory factors-, increase in aqueous tear production and conjunctival goblet cells [32]. Furthermore, administration of bone marrow-MSCs incorporated in a polysaccharide hydrogel on the ocular surface in a rat alkali-burn model led to healing of the corneal epithelium with less opacity and neovascularization than controls. Also, anti-inflammatory and anti-angiogenic cytokines were up-regulated, whereas, chemotactic and angiogenic factors were attenuated [34]. Therefore, the primary underlying mechanism of BM-MSCs healing effects in corneal damages is modulating the function of immune cells in turn promoting a more regenerative environment.
Recent studies have shown the presence of MSCs in the limbus. Observations made by in vivo confocal microscopy (IVCM) revealed that L-MSCs (a.k.a, limbal/corneal stromal stem cells) are located in clusters at anterior limbal stroma adjacent to LESCs [35] where they make direct physical contact with LESCs in the limbal niche. The interaction of L-MSCs and LESCs are mediated by various signaling molecules e.g. aquaporin-1 and vimentin [36], chondroitin sulfate (6C3 motif) [37], SDF-1/CXCR4 [38], BMP/Wnt [39], and IL-6/STAT3 [40]. Paracrine factors also facilitate the interactions between L-MSCs and LESCs [19]. L-MSCs have been extracted using digestion or explant based methods and found to fulfill the minimum criteria of human MSC characterization [41], defined by International Society of Cell Therapy, including plastic adherence, differentiation to adipocytes, chondrocytes and osteocytes and also the expression of CD105, CD73 and CD90 in addition to lack of CD14, CD34, CD 45 and HLA-DR expression [42]. L-MSCs have similar characteristics to BM-MSCs in terms of immunomodulatory and anti-inflammatory effects [41, 42]. Although the majority of healing effects by MSCs obtained from various sources are mediated by modulating innate and adaptive immune system, there are minor functional differences related to MSCs’ tissue-of-origin [43, 44]. For instance, our team showed that L-MSCs produce soluble fms-like tyrosine kinase-1 (sFLT-1), a well-known antiangiogenic factor. BM-MSCs do not produce sFLT-1, however they still demonstrate anti-angiogenic effects when applied to the cornea due given their anti-inflammatory properties [41].
Administration of L-MSCs in corneal injury models led to improvement in corneal epithelial wound healing. For instance, sub-conjunctivital injection of human L-MSCs in rabbit models of alkali-burn improved corneal epithelial wound healing and attenuated corneal neovascularization, number of goblet cells, and corneal opacity [45]. A pilot clinical study showed that administration of L-MSCs using fibrin sealant in five patients with acute corneal burns, non-healing ulcers and post-keratitis scars, improved visual acuity, enhanced corneal clarity and reduced corneal vascularization up to one year follow-up in comparison with matched controls [46, 47]. Administration of MSCs incorporated in fabricated extracellular matrix is a promising approach for regenerating the ocular surface in moderate to severe disease.
Given the promising results of local MSC administration for ocular surface regeneration in pre-clinical large animal models, clinical applications are being pursued for patients with severe surface disease. Bone marrow-MSCs have the advantage of being more advanced in clinical development as they have been infused intravenously in numerous patients for non-ocular indications. Thus, their clinical administration for ocular surface regeneration may be considered as a natural extension of these studies.
2.3. Melanocytes
Melanocytes are responsible for pigmentation of the PVs and protect the limbal stem cells against UV radiations by producing melanin. Recent studies have shown that limbal melanocytes may have other supportive functions in the limbus [48–50]. Limbal melanocytes locate in the compact clusters of limbal epithelial cells in limbus and have direct contact with limbal epithelial stem cells mediated by N-, P- and E-cadherins and L1-CAM. These cells have been extracted successfully from human cadaveric corneal tissues. Limbal melanocytes provide efficient support of limbal epithelial stem cells in vitro. The epithelial cells co-cultured with human limbal melanocytes retain their stemness proved by high expression of stem cell markers, e.g., CK15, Bmi-1, and p63 and deficient expression of CK3 differentiation marker. Limbal melanocytes have shown comparable effects regarding supporting the limbal epithelial cells as 3T3 fibroblasts and limbal mesenchymal stem cells. They also inhibit activated T-cells and vascular endothelial cells which is useful in regenerating the ocular surface and limbal niche. Moreover, a three-dimensional culture of human melanocytes with limbal epithelial cells enhanced the development of multi-layered epithelial sheets, whereas the basal layer remained undifferentiated [48–50]. The potential therapeutic effects of limbal melanocytes have just emerged into the field and further in vitro and in vivo studies are still needed to elucidate their regenerative capacity.
3. Extracellular Matrix-based Approaches
The extracellular matrix (ECM) is fundamental for physiological renewal of the ocular surface/limbal microenvironment. The limbal ECM is composed of structural proteins and adhesive elements [49]. The physical organization of limbal niche crypts and its basement membrane consists of various collagens, laminins, fibronectin, and chondroitin sulfate [51]. It has been reported that the limbal niche stromal ECM regulates epithelial differentiation, proliferation, and apoptosis of LESCs [52]. Therefore, regenerative approaches to revitalize the extracellular matrix have the potential of restoring the proper function of the limbal niche.
3.1. Amniotic Membrane
Amniotic membrane (AM) is the conventional ECM used for the management of ocular surface disorders. AM is obtained by peeling off the fetal membranes. It has no innervation and vascularization and has appropriate transparency [53]. The healing effects of AM are attributed to its rich extracellular matrix. The AM-ECM contains collagen, fibronectin, and laminin as well as growth factors such as epidermal growth factor (EGF) and hepatocyte growth factor [54]. Besides the innate healing capability of the AM for regeneration of the ocular surface, it is a proper carrier for delivery of different cells on the ocular surface. The collagen-rich structure of the AM provides the potential of fabricating scaffolds for tissue engineering purposes [55].
3.2. Bio-active Hydrogels
An attractive emerging approach to regenerate the ocular surface is fabrication of bio-active hydrogels which could be applied with/without cells. These hydrogels mimic the proper ECM for cell proliferation, migration, and differentiation. Protocols for production of these hydrogels are mainly based on decellularization and digestion of animal tissues or application of commercially available purified structural proteins [56]. Porcine corneas have been decellularized and digested to produce a bio-active hydrogel for regeneration of ocular surface after injuries. This hydrogel is compatible with epithelial and stromal cells; thus, it is a proper candidate for ocular surface cell delivery approaches [57, 58]. The mixture of collagen, hyaluronic acid, laminin, and elastin also provide an appropriate hydrogel for ocular surface regeneration [59]. These combinations are also proper sources for application in three-dimensional bioprinting of structures applicable in ocular surface regeneration [60, 61]. Other biological materials have also been used for production of bio-active ECMs supporting the stability of the ocular surface, such as fibrin [62], silk fibroin [63], and biocompatible polymers [64]. For instance, it has been shown that silk-derived protein enhances migration, adhesion and proliferation of corneal epithelial cells [65]. Moreover, the silk film could be patterned to navigate the corneal epithelial cells behavior by changing their gene expressions, so it could be customized for ocular surface repair [66]. A novel approach is to apply modified collagen hydrogels on diseased ocular surface which can be cross-linked in situ. The idea of this approach is to apply hydrogels containing keratocytes to replenish the damaged corneal stroma to provide proper support for epithelial growth [67].
3.3. Fabricated Extracellular Matrices
Another strategy for regeneration of ocular surface/limbal microenvironment ECM is to fabricate a proper ECM for epithelial (stem) cell homing, proliferation, migration and differentiation. Many approaches have been proposed for the production of the desired ECM. Efficient decellularization of human and/or animal corneas with preserving the integrity and functionality of the native ECM have been taken into consideration recent years. Several protocols have proposed decellularization of human and animal corneas including freeze-thawing, freeze-drying, detergent treatment, and acid/base treatment [68, 69]. Our group previously showed that treatment of human cadaveric corneal tissues with hypertonic Sodium Chloride solution followed by nuclease treatment results in efficient cell content removal while preserving an intact ECM. The decellularized human corneas supported the growth of corneal epithelial and fibroblast cells [70]. Furthermore, transplantation of decellularized human limbal ECM promoted epithelialization and inhibited haze formation in a rat partial limbal injury model [71].
4. Therapeutic Factors
Cellular components and signaling pathways are essential modalities in preserving the homeostasis of the ocular surface. Revitalizing the function of disrupted ocular surface micro-environment by local delivery of exogenous growth factors provides another novel approach for ocular surface regeneration [3, 72]. There are a number of potential sources for obtaining therapeutic factors for the ocular surface: hemoderivatives [73–75], soluble recombinant growth factors [76], derivatives from amniotic membrane [77], and secretions from cells with healing characteristics [41, 42, 78]. The emerging therapeutic factors for ocular surface regeneration are discussed in the following sections.
4.1. Hemoderivatives
Blood-derived eye drops have become increasingly popular in the setting of ocular surface diseases. Platelet-derived preparations and autologous/allogeneic serum eye-drops (ASE) are used therapeutically for restoring the disturbed micro-environment of the ocular surface. They are rich in growth factors, cytokines, vitamins and minerals which are required for normal corneal epithelial homeostasis and can stimulate proliferation, differentiation, and migration on the ocular surface [79]. Autologous serum eye-drops are similar to human tears regarding components such as EGF, TGF-β, fibronectin, vitamin A and other common cytokines [73–75]. The clinical applications of ASE to the ocular surface range from dry eye and Sjögren disease to severe conditions including, graft-versus-host disease and keratoconjunctivitis [73, 80, 81]. [82].
Although successful rehabilitation of corneal pathologies after administration of ASE has been reported [80, 81], they have their limiting drawbacks. Imperfect stability, increased risk of infections during prolonged use, no standardized manufacturing and application protocols are some of the issues with the use of these products [83].
Platelet releasate (PR), plasma rich in growth factors (PRGF), and platelet-rich plasma (PRP) are produced from the supernatant of anti-coagulated whole blood. They are rich in some growth factors (e.g., EGF, TGF, PDEF, bFGF, and IGF-1) and are prepared by different protocols. Although different outcomes regarding various preparation protocols have been reported, in vivo and clinical experiments have shown the potential regenerative and reconstructive capability of platelet-derived products to be comparable to ASE [84, 85]. Moreover, a recent randomized clinical trial evaluated the effects of PRP injection in patients with severe dry eye disease. The results indicate improvements in corneal staining, mean Schirmer value and tear break-up time after PRP injection after 90 days of follow-up [86].
4.2. Soluble Growth Factors
Soluble growth factors with neuroprotective, anti-inflammatory and antiangiogenic properties have been used for ocular surface regeneration. These can be extracted from cell secretomes [87, 41, 42], and/or produced by recombinant techniques [88, 89].
Pigment epithelial-derived factor (PEDF) is extracted from human plasma and can be used in the ocular surface regeneration. The effect of PEDF and PEDF-derived factors in supporting stem cell survival and maintaining their multi-potency has been described [76]. Moreover, ciliary neurotrophic factor and IL-1 receptor antagonist peptide could also be considered as potential regenerative factors in ocular surface diseases [88, 89].
Nerve growth factor (NGF) is a soluble growth factor, which plays a crucial role in the developing and maintaining the visual system as well as controlling pathologic conditions [90]. Under physiological conditions, NGF is secreted in the aqueous humor and expressed in the anterior segment [91]. The positive effects of topical NGF in modulating the corneal and conjunctival healing, in animal models and patients with severe ocular surface diseases such as neurotrophic and autoimmune corneal ulcers, have been reported [92]. The potential application of topical NGF in dry eye, rejected corneal transplantation and herpetic keratitis is also demonstrated [93–95]. In a phase II double blinded, multicenter randomized trial, 156 patients with stage 2 or stage 3 NK in one eye were recuritied and randomized in 3 equal-number groups treated with recombinant human NGF (rhNGF) 10 μg/ml, 20 μg/ml, or vehicle. The 4- and 8-weeks follow-up results showed that rhNGF had led to significant corneal wound healing compared to vehicle. Moreover, 96% of patients remained corneal wound-free after 48- and/or 56-weeks follow-ups with tolerable adverse effects [96]. rhNGF was recently approved by FDA for ocular surface treatment in patients with moderate to severe NK. This achievement in clinical translation of a therapeutic growth factor, sets the stage for other ocular surface regenerative therapies.
4.3. Amniotic Membrane Derivatives
Derivatives from Human Amniotic Membrane (HAM) have been used in ocular surface restoration [97]. Amniotic membrane extract eye-drop (AMEED) is a cocktail of HAM factors which has beneficial effects for in vivo cultivation of limbal stem cells in LSCD patients [77]. Clinical trials assessing the safety and efficacy of AMEED are currently underway [98].
Likewise, HC-HA/PTX3 is a potential factor useful for revitalization of ocular surface homeostasis. It is derived from HAM and contains heavy chain 1 (HC1) of inter-α-trypsin inhibitor which bonds to hyaluronan (HA) [99]. The beneficial effect of HC-HA/PTX3 complex for maintaining the LESCs self-renewal via modulating Wnt/BMP signaling in 3D-culture system has been demonstrated [100].
4.4. Cell Secretions (Secretomes/Conditioned Media, Exosomes)
Secretomes/conditioned media is the cocktail of secreted factors from in vitro cultivated cells. Mesenchymal stem cell conditioned media contains a combination of growth factors that can help regenerate the ocular surface [41, 42]. Both bone-marrow and limbal derived MSCs-secretomes have healing effects on corneal epithelial cells and modulate the immune system. The conditioned media of human bone-marrow MSCs contain high amount of the IL-1 Receptor Antagonist (IL-1RA) and also significantly reduce the expression of IL-1α and IL-1β in human corneal epithelial cells [101]. Moreover, lyophilized secretome of human bone marrow MSCs have synergistic effects with hyaluronic acid and chondroitin sulfate in facilitating corneal wound healing following mechanical epithelial injury and also ocular surface regeneration following alkali-burn in animal models. It is suggested that human BM-MSCs secretome upregulate the expression of CD44 receptors and enhance the binding of hyaluronic acid to CD44 [78].
Similarly, limbal mesenchymal stem cell conditioned media was shown to accelerate epithelial wound healing and decrease angiogenesis via soluble fms-like tyrosine kinase-1 (sFLT-1) and PEDF [41]. It diminishes and modulates the pathologic role of macrophages in developing corneal neovascularization and immunophenotype function, respectively [42].
Exosomes are nano-vesicles containing bioactive molecules such as microRNAs, mRNAs, and transcription and growth factors responsible for cell-cell communications [102, 103]. The size of exosomes is 40 to 100 nm, and while they fuse to the target cell membrane, their internal contents would be released and lead to cell phenotype change. The critical role of corneal epithelial cell exosomes in wound healing and angiogenesis have been already reported [103]. The potential therapeutic effects of L-MSCs-derived exosomes in both in vitro and in vivo models have been demonstrated by our team [104]. We showed that nanovesicles extracted from cultivated L-MSCs express CD9, CD63, and CD81 as the characterizing markers of exosomes. The isolated exosomes were uptaken by corneal epithelial cells both in vitro and in vivo and released their contents inside the cells. In vitro wound healing assays reveled that L-MSCs exosomes promote migration and proliferation of cultivated human corneal epithelial cells. Moreover, the corneal epithelial wounds in rodent models healed faster by treating with L-MSCs exosomes [104].
5. Summary and Conclusions
Major injuries or insults to the cornea can lead to significant loss of function on the ocular surface, which is primarily due to the disruption of its “regenerative” environment. Emerging strategies for regeneration of the ocular surface have focused on restoring a healthy and “regenerative” environment by replacing the deficient cells (e.g. limbal stem cells), applying immunomodulatory and regenerative cells and/or secretomes (e.g. MSCs) and reconstructing the ECM (e.g. hydrogels).
Acknowledgments
Sources of Support
This research was supported by R01 EY024349 (ARD), and Core grant EY01792 from NEI/NIH; unrestricted grant to the Department of Ophthalmology from Research to Prevent Blindness.
Footnotes
Conflict of Interest GY, SJ, and ARD declare no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance,
•• Of major importance
- 1.Ambroziak AM, Szaflik J, Szaflik JP, Ambroziak M, Witkiewicz J, Skopinski P. Immunomodulation on the ocular surface: a review. Cent Eur J Immunol. 2016;41(2):195–208. doi: 10.5114/ceji.2016.60995.• A review summarizing the role of immune system in ocular surface physiopathology and current available treatments for impaired ocular surface immune function.
- 2.Galletti JG, Guzman M, Giordano MN. Mucosal immune tolerance at the ocular surface in health and disease. Immunology. 2017;150(4):397–407. doi: 10.1111/imm.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yazdanpanah G, Jabbehdari S, Djalilian AR. Limbal and corneal epithelial homeostasis. Current opinion in ophthalmology. 2017;28(4):348–54. doi: 10.1097/ICU.0000000000000378.•• A leading review manuscript from our group illustrating the underlying mechanisms of ocular surface epithelial homeostasis with emphases on the vital role of limbal niche, the alterations in limbal niche following injuries and potential therapeutic approaches to restore limbal niche.
- 4.Eslani M, Haq Z, Movahedan A, Moss A, Baradaran-Rafii A, Mogilishetty G et al. Late Acute Rejection After Allograft Limbal Stem Cell Transplantation: Evidence for Long-Term Donor Survival. Cornea. 2017;36(1):26–31. doi: 10.1097/ICO.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nubile M, Curcio C, Dua HS, Calienno R, Lanzini M, Iezzi M et al. Pathological changes of the anatomical structure and markers of the limbal stem cell niche due to inflammation. Molecular vision. 2013;19:516–25.• An original study elucidating the three-dimensional structure of limbal niche using full-field optical coherence microscopy. It has been shown here that mesenchymal/stromal cells have projections through basement membrane leading to phycsical contact with limbal epithelial stem cells.
- 6.Notara M, Refaian N, Braun G, Steven P, Bock F, Cursiefen C. Short-term uvb-irradiation leads to putative limbal stem cell damage and niche cell-mediated upregulation of macrophage recruiting cytokines. Stem cell research. 2015;15(3):643–54. doi: 10.1016/j.scr.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Chang JH, Putra I, Huang YH, Chang M, Han K, Zhong W et al. Limited versus total epithelial debridement ocular surface injury: Live fluorescence imaging of hemangiogenesis and lymphangiogenesis in Prox1-GFP/Flk1::Myr-mCherry mice. Biochim Biophys Acta. 2016;1860(10):2148–56. doi: 10.1016/j.bbagen.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng SC, He H, Zhang S, Chen SY. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. The ocular surface. 2016;14(2):100–12. doi: 10.1016/j.jtos.2015.12.002.• A comprehensive review summarizing the role of inflammation in ocular surface disorders and potential therapeutic approaches especially administration of amniotic membrane and its derivatives.
- 9.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple Limbal Epithelial Transplantation: Long-Term Clinical Outcomes in 125 Cases of Unilateral Chronic Ocular Surface Burns. Ophthalmology. 2016;123(5):1000–10. doi: 10.1016/j.ophtha.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 10.Vazirani J, Ali MH, Sharma N, Gupta N, Mittal V, Atallah M et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. The British journal of ophthalmology. 2016;100(10):1416–20. doi: 10.1136/bjophthalmol-2015-307348. [DOI] [PubMed] [Google Scholar]
- 11.Ganger A, Vanathi M, Mohanty S, Tandon R. Long-Term Outcomes of Cultivated Limbal Epithelial Transplantation: Evaluation and Comparison of Results in Children and Adults. Biomed Res Int. 2015;2015:480983. doi: 10.1155/2015/480983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen C, Chan CC, Holland EJ. Limbal Stem Cell Transplantation for Soft Contact Lens Wear-Related Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015;160(6):1142–9 e1. doi: 10.1016/j.ajo.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 13.Zakaria N, Possemiers T, Dhubhghaill SN, Leysen I, Rozema J, Koppen C et al. Results of a phase I/II clinical trial: standardized, non-xenogenic, cultivated limbal stem cell transplantation. Journal of translational medicine. 2014;12:58. doi: 10.1186/1479-5876-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daya SM. Conjunctival-limbal autograft. Current opinion in ophthalmology. 2017;28(4):370–6. doi: 10.1097/ICU.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 15.Titiyal JS, Sharma N, Agarwal AK, Prakash G, Tandon R, Vajpayee R. Live Related versus Cadaveric Limbal Allograft in Limbal Stem Cell Deficiency. Ocul Immunol Inflamm. 2015;23(3):232–9. doi: 10.3109/09273948.2014.902076. [DOI] [PubMed] [Google Scholar]
- 16.Cheung AY, Holland EJ. Keratolimbal allograft. Current opinion in ophthalmology. 2017;28(4):377–81. doi: 10.1097/ICU.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 17.Yin J, Jurkunas U. Limbal Stem Cell Transplantation and Complications. Semin Ophthalmol. 2018;33(1):134–41. doi: 10.1080/08820538.2017.1353834. [DOI] [PubMed] [Google Scholar]
- 18.Rama P, Ferrari G, Pellegrini G. Cultivated limbal epithelial transplantation. Current opinion in ophthalmology. 2017;28(4):387–9. doi: 10.1097/ICU.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez S, Deng SX. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Experimental eye research. 2013;116:169–76. doi: 10.1016/j.exer.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kureshi AK, Dziasko M, Funderburgh JL, Daniels JT. Human corneal stromal stem cells support limbal epithelial cells cultured on RAFT tissue equivalents. Scientific reports. 2015;5:16186. doi: 10.1038/srep16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez BE, Sanchez A, Herreras JM, Fernandez I, Garcia-Sancho J, Nieto-Miguel T et al. Stem Cell Therapy for Corneal Epithelium Regeneration following Good Manufacturing and Clinical Procedures. Biomed Res Int. 2015;2015:408495. doi: 10.1155/2015/408495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silber PC, Ricardo JR, Cristovam PC, Hazarbassanov RM, Dreyfuss JL, Gomes JA. Conjunctival epithelial cells cultivated ex vivo from patients with total limbal stem cell deficiency. Eur J Ophthalmol. 2014:0. doi: 10.5301/ejo.5000511. [DOI] [PubMed] [Google Scholar]
- 23.Jeon S, Choi SH, Wolosin JM, Chung SH, Joo CK. Regeneration of the corneal epithelium with conjunctival epithelial equivalents generated in serum- and feeder-cell-free media. Molecular vision. 2013;19:2542–50. [PMC free article] [PubMed] [Google Scholar]
- 24.Kolli S, Ahmad S, Mudhar HS, Meeny A, Lako M, Figueiredo FC. Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem cells. 2014;32(8):2135–46. doi: 10.1002/stem.1694. [DOI] [PubMed] [Google Scholar]
- 25.Ilmarinen T, Laine J, Juuti-Uusitalo K, Numminen J, Seppanen-Suuronen R, Uusitalo H et al. Towards a defined, serum- and feeder-free culture of stratified human oral mucosal epithelium for ocular surface reconstruction. Acta ophthalmologica. 2013;91(8):744–50. doi: 10.1111/j.1755-3768.2012.02523.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Q, Liu XY, Ruan YX, Wang L, Jiang MM, Wu J et al. Construction of corneal epithelium with human amniotic epithelial cells and repair of limbal deficiency in rabbit models. Human cell. 2015;28(1):22–36. doi: 10.1007/s13577-014-0099-6. [DOI] [PubMed] [Google Scholar]
- 27.Brzeszczynska J, Samuel K, Greenhough S, Ramaesh K, Dhillon B, Hay DC et al. Differentiation and molecular profiling of human embryonic stem cell-derived corneal epithelial cells. Int J Mol Med. 2014;33(6):1597–606. doi: 10.3892/ijmm.2014.1714. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S et al. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature. 2014;511(7509):358–61. doi: 10.1038/nature13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi R, Ishikawa Y, Sasamoto Y, Katori R, Nomura N, Ichikawa T et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. 2016;531(7594):376–80. doi: 10.1038/nature17000.• An interesting study reporting generation of corneal and conjunctival epithelial cells from human iPS-cells with ocular surface regeneration capabilities.
- 30.Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Kao WW. Extrinsic and Intrinsic Mechanisms by Which Mesenchymal Stem Cells Suppress the Immune System. The ocular surface. 2016;14(2):121–34. doi: 10.1016/j.jtos.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cejkova J, Trosan P, Cejka C, Lencova A, Zajicova A, Javorkova E et al. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Experimental eye research. 2013;116:312–23. doi: 10.1016/j.exer.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Lee MJ, Ko AY, Ko JH, Lee HJ, Kim MK, Wee WR et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23(1):139–46. doi: 10.1038/mt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omoto M, Katikireddy KR, Rezazadeh A, Dohlman TH, Chauhan SK. Mesenchymal stem cells home to inflamed ocular surface and suppress allosensitization in corneal transplantation. Investigative ophthalmology & visual science. 2014;55(10):6631–8. doi: 10.1167/iovs.14-15413. [DOI] [PubMed] [Google Scholar]
- 34.Ke Y, Wu Y, Cui X, Liu X, Yu M, Yang C et al. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PloS one. 2015;10(3):e0119725. doi: 10.1371/journal.pone.0119725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathews S, Chidambaram JD, Lanjewar S, Mascarenhas J, Prajna NV, Muthukkaruppan V et al. In vivo confocal microscopic analysis of normal human anterior limbal stroma. Cornea. 2015;34(4):464–70. doi: 10.1097/ICO.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higa K, Kato N, Yoshida S, Ogawa Y, Shimazaki J, Tsubota K et al. Aquaporin 1-positive stromal niche-like cells directly interact with N-cadherin-positive clusters in the basal limbal epithelium. Stem cell research. 2013;10(2):147–55. doi: 10.1016/j.scr.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Young RD, Lewis PN, Shinomiya K, Meek KM, Kinoshita S et al. Mesenchymal-epithelial cell interactions and proteoglycan matrix composition in the presumptive stem cell niche of the rabbit corneal limbus. Molecular vision. 2015;21:1328–39. [PMC free article] [PubMed] [Google Scholar]
- 38.Xie HT, Chen SY, Li GG, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem cells. 2011;29(11):1874–85. doi: 10.1002/stem.743. [DOI] [PubMed] [Google Scholar]
- 39.Han B, Chen SY, Zhu YT, Tseng SC. Integration of BMP/Wnt signaling to control clonal growth of limbal epithelial progenitor cells by niche cells. Stem cell research. 2014;12(2):562–73. doi: 10.1016/j.scr.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Notara M, Shortt AJ, Galatowicz G, Calder V, Daniels JT. IL6 and the human limbal stem cell niche: a mediator of epithelial-stromal interaction. Stem cell research. 2010;5(3):188–200. doi: 10.1016/j.scr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S et al. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investigative ophthalmology & visual science. 2017;58(12):5507–17. doi: 10.1167/iovs.17-22680.• A report of therapeutic effects of human cornea derived mesenchymal stem cells in ocular surface wounds providing data about underlying mechanisms.
- 42.Eslani M, Putra I, Shen X, Hamouie J, Tadepalli A, Anwar KN et al. Cornea-Derived Mesenchymal Stromal Cells Therapeutically Modulate Macrophage Immunophenotype and Angiogenic Function. Stem cells. 2018;36(5):775–84. doi: 10.1002/stem.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37(1):115–25. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wegmeyer H, Broske AM, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem cells and development. 2013;22(19):2606–18. doi: 10.1089/scd.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li G, Zhang Y, Cai S, Sun M, Wang J, Li S et al. Human limbal niche cells are a powerful regenerative source for the prevention of limbal stem cell deficiency in a rabbit model. Scientific reports. 2018;8(1):6566. doi: 10.1038/s41598-018-24862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funderburgh J, Basu S, Damala M, Tavakkoli F, Sangwan V, Singh V. Limbal Stromal Stem Cell Therapy for Acute and Chronic Superficial Corneal Pathologies: One-Year Outcomes. Investigative ophthalmology & visual science. 2018;59(9):3455–3455. [Google Scholar]
- 47.Basu S, Damala M, Singh V. Limbal Stromal Stem Cell Therapy for Acute and Chronic Superficial Corneal Pathologies: Early Clinical Outcomes of The Funderburgh Technique. Investigative ophthalmology & visual science. 2017;58(8):3371–3371. [Google Scholar]
- 48.Dziasko MA, Tuft SJ, Daniels JT. Limbal melanocytes support limbal epithelial stem cells in 2D and 3D microenvironments. Experimental eye research. 2015;138:70–9. doi: 10.1016/j.exer.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Polisetti N, Zenkel M, Menzel-Severing J, Kruse FE, Schlotzer-Schrehardt U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem cells. 2016;34(1):203–19. doi: 10.1002/stem.2191.• In this study the adhesion molecules mediating the cell-cell and cell-extracellular matrix interaction/cross-talk in limbal niche were identified.
- 50.Schlotzer-Schrehardt U, Polisetti N, Zenkel M, Naschberger E, Heger L, Dudziak D et al. Melanocytes as an emerging key player in niche regulation of limbal stem cells. Investigative ophthalmology & visual science. 2018;59(9):3453–3453. [Google Scholar]
- 51.Grieve K, Ghoubay D, Georgeon C, Thouvenin O, Bouheraoua N, Paques M et al. Three-dimensional structure of the mammalian limbal stem cell niche. Experimental eye research. 2015;140:75–84. doi: 10.1016/j.exer.2015.08.003.• An original study elucidating the three-dimensional structure of limbal niche using full-field optical coherence microscopy. It has been shown here that mesenchymal/stromal cells have projections through basement membrane leading to phycsical contact with limbal epithelial stem cells.
- 52.Notara M, Lentzsch A, Coroneo M, Cursiefen C. The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int. 2018;2018:8620172. doi: 10.1155/2018/8620172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deihim T, Yazdanpanah G, Niknejad H. Different Light Transmittance of Placental and Reflected Regions of Human Amniotic Membrane That Could Be Crucial for Corneal Tissue Engineering. Cornea. 2016;35(7):997–1003. doi: 10.1097/ICO.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 54.Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell and tissue research. 2016;363(3):599–608. doi: 10.1007/s00441-016-2364-3. [DOI] [PubMed] [Google Scholar]
- 55.Ma DH, Chen HC, Ma KS, Lai JY, Yang U, Yeh LK et al. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta biomaterialia. 2016;31:144–55. doi: 10.1016/j.actbio.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 56.Levis HJ, Daniels JT. Recreating the Human Limbal Epithelial Stem Cell Niche with Bioengineered Limbal Crypts. Current eye research. 2016;41(9):1153–60. doi: 10.3109/02713683.2015.1095932. [DOI] [PubMed] [Google Scholar]
- 57.Ahearne M, Lynch AP. Early Observation of Extracellular Matrix-Derived Hydrogels for Corneal Stroma Regeneration. Tissue engineering Part C, Methods. 2015;21(10):1059–69. doi: 10.1089/ten.TEC.2015.0008.• The protocol of producing solubilized extracellular matrix from porcine corneas applicable in ocular surface regeneration.
- 58.Lu Y, Yao QK, Feng B, Yan CX, Zhu MY, Chen JZ et al. Characterization of a hydrogel derived from decellularized corneal extracellular matrix. 2015;5(12):951–60. [Google Scholar]
- 59.Tidu A, Ghoubay-Benallaoua D, Lynch B, Haye B, Illoul C, Allain JM et al. Development of human corneal epithelium on organized fibrillated transparent collagen matrices synthesized at high concentration. Acta biomaterialia. 2015;22:50–8. doi: 10.1016/j.actbio.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Sorkio A, Koch L, Koivusalo L, Deiwick A, Miettinen S, Chichkov B et al. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials. 2018;171:57–71. doi: 10.1016/j.biomaterials.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 61.Dehghani S, Rasoulianboroujeni M, Ghasemi H, Keshel SH, Nozarian Z, Hashemian MN et al. 3D-Printed membrane as an alternative to amniotic membrane for ocular surface/conjunctival defect reconstruction: An in vitro & in vivo study. Biomaterials. 2018;174:95–112. doi: 10.1016/j.biomaterials.2018.05.013.• In this study, development of a bio-ink for three-dimensional printing of structures for ocular surface applications with suturing potential and proper degradation properties, has reported.
- 62.Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72(9):1478–85. [DOI] [PubMed] [Google Scholar]
- 63.Bray LJ, George KA, Hutmacher DW, Chirila TV, Harkin DG. A dual-layer silk fibroin scaffold for reconstructing the human corneal limbus. Biomaterials. 2012;33(13):3529–38. doi: 10.1016/j.biomaterials.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 64.Palchesko RN, Carrasquilla SD, Feinberg AW. Natural Biomaterials for Corneal Tissue Engineering, Repair, and Regeneration. Adv Healthc Mater. 2018;7(16):e1701434. doi: 10.1002/adhm.201701434. [DOI] [PubMed] [Google Scholar]
- 65.Abdel-Naby W, Cole B, Liu A, Liu J, Wan P, Guaiquil VH et al. Silk-Derived Protein Enhances Corneal Epithelial Migration, Adhesion, and Proliferation. Investigative ophthalmology & visual science. 2017;58(3):1425–33. doi: 10.1167/iovs.16-19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang KB, Lawrence BD, Gao XR, Luo Y, Zhou Q, Liu A et al. Micro- and Nanoscale Topographies on Silk Regulate Gene Expression of Human Corneal Epithelial Cells. Investigative ophthalmology & visual science. 2017;58(14):6388–98. doi: 10.1167/iovs.17-22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee HJ, Fernandes-Cunha GM, Na KS, Hull SM, Myung D. Bio-Orthogonally Crosslinked, In Situ Forming Corneal Stromal Tissue Substitute. Adv Healthc Mater. 2018;7(19):e1800560. doi: 10.1002/adhm.201800560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lynch AP, Ahearne M. Strategies for developing decellularized corneal scaffolds. Experimental eye research. 2013;108:42–7. doi: 10.1016/j.exer.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 69.Oh JY, Kim MK, Lee HJ, Ko JH, Wee WR, Lee JH. Comparative observation of freeze-thaw-induced damage in pig, rabbit, and human corneal stroma. Veterinary ophthalmology. 2009;12 Suppl 1:50–6. doi: 10.1111/j.1463-5224.2009.00723.x. [DOI] [PubMed] [Google Scholar]
- 70.Shafiq MA, Gemeinhart RA, Yue BY, Djalilian AR. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue engineering Part C, Methods. 2012;18(5):340–8. doi: 10.1089/ten.TEC.2011.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shafiq MA, Milani BY, Djalilian ARJIJoTE. In Vivo Evaluation of a Decellularized Limbal Graft for Limbal Reconstruction. 2014;2014. [Google Scholar]
- 72.Zhang X, VJ M, Qu Y, He X, Ou S, Bu J et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. International journal of molecular sciences. 2017;18(7). doi: 10.3390/ijms18071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azari AA, Rapuano CJ. Autologous serum eye drops for the treatment of ocular surface disease. Eye & contact lens. 2015;41(3):133–40. doi: 10.1097/ICL.0000000000000104.• A systematic review based on PubMed, the ISI Web of Knowledge database, and the Cochrane library, evaluating the superiority of autologous serum tears over conventional eye lubricants for treatment of ocular surface diseases.
- 74.Giannaccare G, Versura P, Buzzi M, Primavera L, Pellegrini M, Campos EC. Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci. 2017;56(4):595–604. doi: 10.1016/j.transci.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 75.Tseng CL, Chen ZY, Renn TY, Hsiao SH, Burnouf T. Solvent/Detergent Virally Inactivated Serum Eye Drops Restore Healthy Ocular Epithelium in a Rabbit Model of Dry-Eye Syndrome. PloS one. 2016;11(4):e0153573. doi: 10.1371/journal.pone.0153573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeh SI, Ho TC, Chen SL, Chen CP, Cheng HC, Lan YW et al. Pigment Epithelial-Derived Factor Peptide Regenerated Limbus Serves as Regeneration Source for Limbal Regeneration in Rabbit Limbal Deficiency. Investigative ophthalmology & visual science. 2016;57(6):2629–36. doi: 10.1167/iovs.15-17171. [DOI] [PubMed] [Google Scholar]
- 77.Baradaran-Rafii A, Asl NS, Ebrahimi M, Jabbehdari S, Bamdad S, Roshandel D et al. The role of amniotic membrane extract eye drop (AMEED) in in vivo cultivation of limbal stem cells. The ocular surface. 2018;16(1):146–53. doi: 10.1016/j.jtos.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Gabriella Fernandes-Cunha, Kyung-Sun Na, Ilham Putra, Hyun Jong Lee, Sarah Hull, Yu-Chia Cheng et al. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered within a Viscoelastic Gel Carrier. Stem cells translational medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Akcam HT, Unlu M, Karaca EE, Yazici H, Aydin B, Hondur AM. Autologous serum eye-drops and enhanced epithelial healing time after photorefractive keratectomy. Clinical & experimental optometry. 2018;101(1):34–7. doi: 10.1111/cxo.12574. [DOI] [PubMed] [Google Scholar]
- 80.Harritshoj LH, Nielsen C, Ullum H, Hansen MB, Julian HO. Ready-made allogeneic ABO-specific serum eye drops: production from regular male blood donors, clinical routine, safety and efficacy. Acta ophthalmologica. 2014;92(8):783–6. doi: 10.1111/aos.12386. [DOI] [PubMed] [Google Scholar]
- 81.Semeraro F, Forbice E, Braga O, Bova A, Di Salvatore A, Azzolini C. Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. Biomed Res Int. 2014;2014:826970. doi: 10.1155/2014/826970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lekhanont K, Jongkhajornpong P, Anothaisintawee T, Chuckpaiwong V. Undiluted Serum Eye Drops for the Treatment of Persistent Corneal Epitheilal Defects. Scientific reports. 2016;6:38143. doi: 10.1038/srep38143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anitua E, de la Fuente M, Muruzabal F, Riestra A, Merayo-Lloves J, Orive G. Plasma rich in growth factors (PRGF) eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Experimental eye research. 2015;135:118–26. doi: 10.1016/j.exer.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 84.Freire V, Andollo N, Etxebarria J, Hernaez-Moya R, Duran JA, Morales MC. Corneal wound healing promoted by 3 blood derivatives: an in vitro and in vivo comparative study. Cornea. 2014;33(6):614–20. doi: 10.1097/ICO.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 85.Lee JH, Kim MJ, Ha SW, Kim HK. Autologous Platelet-rich Plasma Eye Drops in the Treatment of Recurrent Corneal Erosions. Korean J Ophthalmol. 2016;30(2):101–7. doi: 10.3341/kjo.2016.30.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Avila MY, Igua AM, Mora AM. Randomised, prospective clinical trial of platelet-rich plasma injection in the management of severe dry eye. The British journal of ophthalmology. 2018. doi: 10.1136/bjophthalmol-2018-312072. [DOI] [PubMed] [Google Scholar]
- 87.Eslani M, Putra I, Shen X, Hamouie J, Tadepalli A, Movahedan A et al. Corneal-Limbal Mesenchymal Stromal Cell Secretome is Antiangiogenic in Vitro. Investigative ophthalmology & visual science. 2017;58(8):997–997.28535271 [Google Scholar]
- 88.Zhou Q, Chen P, Di G, Zhang Y, Wang Y, Qi X et al. Ciliary neurotrophic factor promotes the activation of corneal epithelial stem/progenitor cells and accelerates corneal epithelial wound healing. Stem cells. 2015;33(5):1566–76. doi: 10.1002/stem.1942. [DOI] [PubMed] [Google Scholar]
- 89.Fok E, Sandeman SR, Guildford AL, Martin YH. The use of an IL-1 receptor antagonist peptide to control inflammation in the treatment of corneal limbal epithelial stem cell deficiency. Biomed Res Int. 2015;2015:516318. doi: 10.1155/2015/516318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tirassa P, Rosso P, Iannitelli A. Ocular Nerve Growth Factor (NGF) and NGF Eye Drop Application as Paradigms to Investigate NGF Neuroprotective and Reparative Actions. Methods in molecular biology. 2018;1727:19–38. doi: 10.1007/978-1-4939-7571-6_2. [DOI] [PubMed] [Google Scholar]
- 91.Lambiase A, Bonini S, Manni L, Ghinelli E, Tirassa P, Rama P et al. Intraocular production and release of nerve growth factor after iridectomy. Investigative ophthalmology & visual science. 2002;43(7):2334–40. [PubMed] [Google Scholar]
- 92.Lambiase A, Sacchetti M, Bonini S. Nerve growth factor therapy for corneal disease. Current opinion in ophthalmology. 2012;23(4):296–302. doi: 10.1097/ICU.0b013e3283543b61. [DOI] [PubMed] [Google Scholar]
- 93.Qi H, Li DQ, Shine HD, Chen Z, Yoon KC, Jones DB et al. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Experimental eye research. 2008;86(1):34–40. doi: 10.1016/j.exer.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambiase A, Coassin M, Costa N, Lauretti P, Micera A, Ghinelli E et al. Topical treatment with nerve growth factor in an animal model of herpetic keratitis. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246(1):121–7. doi: 10.1007/s00417-007-0593-6. [DOI] [PubMed] [Google Scholar]
- 95.Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011;129(8):981–6. doi: 10.1001/archophthalmol.2011.200. [DOI] [PubMed] [Google Scholar]
- 96.Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, Chao W et al. Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology. 2018;125(9):1332–43. doi: 10.1016/j.ophtha.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 97.Dudok DV, Nagdee I, Cheung K, Liu H, Vedovelli L, Ghinelli E et al. Effects of amniotic membrane extract on primary human corneal epithelial and limbal cells. Clin Exp Ophthalmol. 2015;43(5):443–8. doi: 10.1111/ceo.12480. [DOI] [PubMed] [Google Scholar]
- 98.Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clinical ophthalmology. 2018;12:1105–12. doi: 10.2147/OPTH.S165553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng SC. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: Insight Into Relationship Between Inflammation and Regeneration. Investigative ophthalmology & visual science. 2016;57(5):ORSFh1–8. doi: 10.1167/iovs.15-17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen SY, Han B, Zhu YT, Mahabole M, Huang J, Beebe DC et al. HC-HA/PTX3 Purified From Amniotic Membrane Promotes BMP Signaling in Limbal Niche Cells to Maintain Quiescence of Limbal Epithelial Progenitor/Stem Cells. Stem cells. 2015;33(11):3341–55. doi: 10.1002/stem.2091. [DOI] [PubMed] [Google Scholar]
- 101.Jeong WY, Kim JH, Kim CW. Co-culture of human bone marrow mesenchymal stem cells and macrophages attenuates lipopolysaccharide-induced inflammation in human corneal epithelial cells. Biosci Biotechnol Biochem. 2018;82(5):800–9. doi: 10.1080/09168451.2018.1438167. [DOI] [PubMed] [Google Scholar]
- 102.Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L et al. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Scientific reports. 2017;7(1):4323. doi: 10.1038/s41598-017-04559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han KY, Tran JA, Chang JH, Azar DT, Zieske JD. Potential role of corneal epithelial cell-derived exosomes in corneal wound healing and neovascularization. Scientific reports. 2017;7:40548. doi: 10.1038/srep40548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P et al. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investigative ophthalmology & visual science. 2018;59(12):5194–200. doi: 10.1167/iovs.18-24803.• The report of potential corneal epithelial wound healing effects of corneal mesenchymal stem cell-derived exosomes by our group.