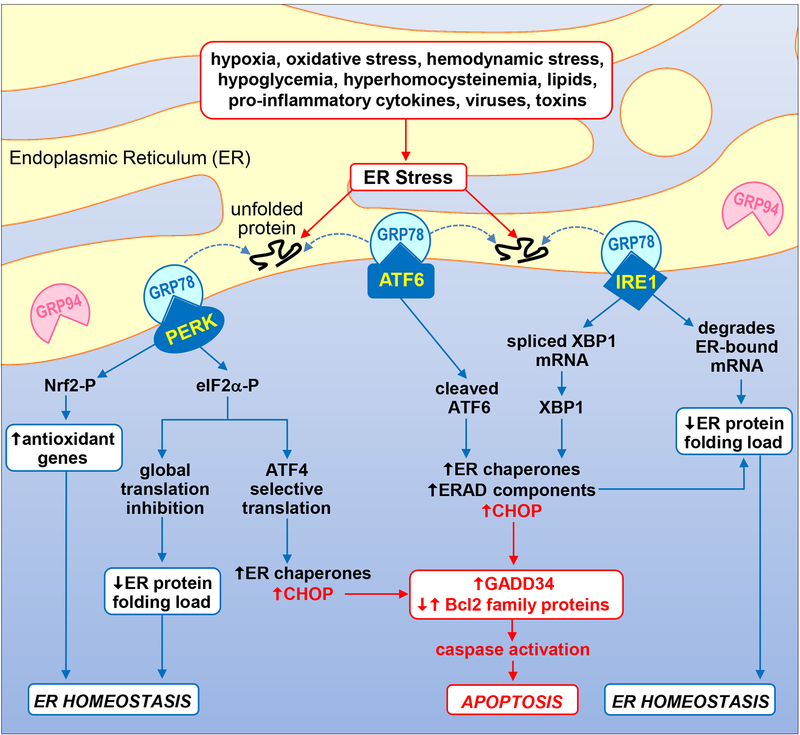
The endoplasmic reticulum (ER) is a centrally localized intracellular organelle that plays a critical role in protein folding, calcium homeostasis, and lipid biosynthesis [1]. Numerous pathophysiologic stimuli such as oxidative stress, hypoxia, hemodynamic stress, hypoglycemia, hyperhomocysteinemia, viral infections, lipids, pro-inflammatory cytokines, and environmental toxins lead to the accumulation of unfolded and misfolded proteins in the ER lumen causing ER stress (Figure 1). This stress triggers a highly conserved signal transduction pathway termed the unfolded protein response (UPR) that initiates a complex translational and transcription program to restore ER homeostasis [1,2]. Three main ER transmembrane stress sensors initiate the UPR; inositol-requiring enzyme-1 (IRE1), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6). All three sensors are maintained in an inactive state through the interactions of their luminal domains with the ER chaperone glucose-regulated protein 78kDa (GRP78). However, in the presence of ER stress, GRP78 is released from these sensors and recruited to improperly folded proteins resulting in the activation of three distinct UPR branches. While all three branches stimulate the transcription of chaperones such as GRP78 and GRP94, each branch also activates unique signaling cascades. For instance, the IRE1 branch possesses endoribonuclease activity that splices X-box-binding protein 1 (XBP1) mRNA to form mature XBP1 mRNA which is then translated to a potent transcription factor that induces the expression of enzymes that target misfolded proteins for ubiquitination and ER-associated degradation (ERAD). IRE1 also degrades certain ER-localized mRNAs through regulated IRE1-dependent decay. The PERK branch phosphorylates the translation initiation factor 2α (eIF2α) and nuclear factor-E2-related factor 2 (Nrf2) to repress global protein synthesis and oxidative stress, respectively. Intriguingly, phosphorylated eIF2α allows for the cap-independent translation of the transcription factor ATF4 which stimulates the expression of ER chaperones. In addition, liberation of ATF6 from GRP78 promotes its translocation to the Golgi where it is cleaved to an active transcription factor that induces the expression of genes encoding ER chaperones and ERAD components.
1.
Schematic diagram illustrating the UPR signal transduction pathway. Numerous pathophysiological stimuli result in the accumulation of unfolded and misfolded proteins in the ER lumen causing ER stress and the activation of the UPR. The presence of improperly folded proteins leads to the dissociation of GRP78 from the UPR sensors PERK, ATF6, and IRE1, and their activation. Subsequently, the kinase PERK phosphorylates eIF2α and Nrf2 to repress global protein synthesis and oxidative stress, respectively. Interestingly, phosphorylated eIF2α favors the cap-independent translation of the transcription factor ATF4 which promotes the expression of ER chaperones such as GRP78 and GRP94. Upon activation, the transcription factor ATF6 induces the expression of genes involved in protein folding activity (ER chaperones) and protein degradation (ERAD components). In addition, IRE1 splices XBP1 mRNA to mature XBP1 mRNA which is then translated to a potent transcription factor that stimulates the expression of ER chaperones and enzymes that target misfolded proteins for ERAD. IRE1 also degrades certain ER-localized mRNAs through regulated IRE1-dependent decay. Collectively, these actions function in an adaptive manner to reduce the ER protein folding load and redox stress in order to restore ER homeostasis. However, if ER stress is too severe or protracted, all three UPR sensors can induce the expression of the transcription factor CHOP which promotes caspase activation and apoptosis by increasing the expression of pro-apoptotic GADD34 and pro-apoptotic Bcl2 family members while suppressing the expression of anti-apoptotic Bcl2 proteins.
While these actions of the UPR function in an adaptive manner to resolve ER stress, if ER stress is too severe or prolonged, the UPR initiates an apoptotic response. Notably, PERK stimulates the expression of the pro-apoptotic transcription factor C/EBP homologous protein (CHOP) via the mobilization of ATF4 [3]. Interestingly, the other two arms of the UPR are also capable of stimulating CHOP expression, underscoring the central role of this protein in ER stress-induced apoptosis. CHOP mediates cell death via the induction of various pro-apoptotic genes, including growth arrest and DNA damage-inducible protein (GADD34). Moreover, CHOP directly interacts with the apoptotic machinery and differentially regulates the expression of Bcl-2 protein family members to favor apoptosis. In most cases, the intrinsic pathway of apoptosis is activated where caspase-9 serves as the proximal caspase in the cascade. However, the recruitment and activation of caspase-12 at the ER has also been implicated in ER stress-induced apoptosis in rodents but a catalytically active form of this enzyme is absent in humans [4]. Thus, the UPR has a dual role, transmitting both adaptive and apoptotic signals.
ER stress has been implicated in numerous pathologies including atherosclerosis, hypertension, cancer, neurodegenerative diseases, diabetes, and obesity [5–7]. In the heart, ER stress has been detected in response to numerous inimical stimuli and has been shown to be involved in the pathogenesis of myocardial ischemia-reperfusion injury, cardiac hypertrophy, ischemic cardiomyopathy, diabetic cardiomyopathy, and cardiac fibrosis [8–12]. In addition, ER stress may contribute to the transition from compensated left ventricular hypertrophy to heart failure [13]. While hypoxia is also known to stimulate apoptosis of cultured cardiomyocytes in an ER stress-dependent manner [10], the in-vivo importance of cardiac ER stress in response to extended periods of hypoxia is not known. Chronic hypoxia affects millions of people around the world who live at high altitudes and is associated with a number of cardiac adaptations [14]. Chronic environmental hypoxia stimulates erythropoiesis and angiogenesis that aids in the preservation of myocardial perfusion in the presence of low oxygen tension. In addition, the heart demonstrates a greater dependency on glucose metabolism for ATP production, which is a more oxygen-efficient fuel relative to fatty acids. These adaptations are driven, in part, by the oxygen-sensitive transcription factor hypoxia-inducible factor-1α (HIF-1α) which stimulates the expression of erythropoietin and vascular endothelial growth factor (VEGF) contributing to polycythemia and angiogenesis, respectively. Furthermore, HIF-1α promotes glucose metabolism by inducing the expression of the glucose transporter 1 (GLUT1) and several glycolytic genes. Chronic hypoxia also results in right ventricular hypertrophy permitting the ventricle to overcome the increased afterload associated with hypoxic pulmonary vasoconstriction. However, excessive vasoconstriction and remodeling of the pulmonary vasculature can precipitate pulmonary hypertension and congestive heart failure.
In this issue of Vascular Pharmacology, Jain and co-workers [15] convincingly demonstrate that chronic hypobaric hypoxia that simulates a high altitude environment stimulates cardiac hypertrophy and injury in rats via the induction of ER stress. They report that right ventricular hypertrophy occurs in a progressive fashion over the course of 14 days and is associated with the activation of the PI3K/Akt/mTOR signaling axis. Engagement of this hypertrophic signaling pathway is most likely due to the rise in right ventricular systolic pressure following exposure to hypoxia. As expected, an increase in HIF-1α and its downstream targets GLUT1 and VEGF is also detected. An increase in heme oxygenase-1 is also revealed, and this may serve an important adaptive role by counteracting ER stress-mediated apoptosis via the generation of carbon monoxide [16]. Myocardial damage, necrosis, inflammation, and oxidative stress are also observed, most prominently on day 14, suggesting a possible switch from physiological to pathological hypertrophy with prolonged hypoxia. In addition, chronic hypoxia stimulates ER stress and activates all three UPR signal transducers leading to increased ATF4 expression, eIF2α phosphorylation, ER chaperone expression and protein degradation. A time-dependent increase in apoptotic markers including CHOP and caspase-12, 9 and 3 is also noted which corresponds with evidence of enhanced cardiac damage. Remarkably, treatment of rats with the chemical chaperone 4-phenylbutyric acid (PBA) reduces chronic hypoxia-mediated activation of the UPR and its downstream effectors, and this is paralleled by a decline in cardiac hypertrophy, damage, inflammation, and apoptosis. Collectively, these novel findings identify ER stress as a critical mediator of cardiac injury in chronic hypoxia and suggest that protracted duration of hypoxia may preferential harness the maladaptive arm of the UPR. These findings also extend recent work showing that ER stress contributes to pulmonary hypertension and cardiac hypertrophy in response to monocrotaline toxicity and normobaric hypoxia in rodents, and that ER stress mediates cardiac injury in response to intermittent chronic hypoxia [17–19].
In the same study, Jain and colleagues [15] also evaluated the role of ER stress on hypoxic tolerance by exposing rats to acute sublethal hypobaric hypoxia. Having recently shown that higher susceptibility to an acute hypoxic episode is associated with the activation of ER stress, they went on to find that inhibition of ER stress by PBA enhances hypoxic tolerance and ameliorates cardiac damage and inflammation while further induction of ER stress by the glycosylation inhibitor tunicamycin reduces hypoxic tolerance and exacerbates cardiac injury due to sublethal hypoxic stress. Since previous work by the group demonstrated that HIF-1α contributes to better hypoxic tolerance and survival, possible interactions between ER stress and HIF-1α were explored. Interestingly, PBA stimulates a marked increase in cardiac HIF-1α levels whereas tunicamycin inhibited the expression of HIF-1α, highlighting an inverse relationship between HIF-1α and ER stress in hypoxia. However, the molecular basis for this reciprocal interaction was not defined.
The study by Jain et al. [15] sets the stage for future work dissecting the specific actions of ER stress on hypoxia-induced cardiac dysfunction. In particular, a more detailed analysis of inflammation is needed. Beyond the stimulation of TNFα, what other pro-inflammatory effects are driven by ER stress in the setting of hypoxia? Studies characterizing the number, type, and polarization of immune cells that infiltrate the heart along with measurement of a panel of inflammatory cytokines may provide valuable information regarding the nature of the inflammatory response instigated by ER stress. Similarly, a more quantitative approach in measuring cardiac necrosis and apoptosis as well as autophagy may give new insight regarding the ability of ER stress to activate distinct cell death pathways in the heart. Given that ER stress has been demonstrated to promote cardiac fibrosis following ischemia [12], examination of the deposition of collagen and other extracellular matrix molecules during chronic hypoxia is also warranted. In addition, a rigorous analysis of cardiac function will be needed to establish the role of ER stress in cardiac dysfunction by hypoxia.
Finally, the report by Jain et al. [15] provides further evidence of the detrimental effect of ER stress on the heart, and identifies ER stress as a therapeutic target in chronic hypoxia-induced cardiac injury. Several strategies may be employed to ameliorate ER stress. One approach involves blockade of upstream signals that trigger ER stress. In particular, the use of antioxidants may be effective in preventing cardiac ER stress by reactive oxygen species that are formed during hypoxia. More promising are the chemical chaperones such as PBA and taurine-conjugated ursodeoxycholic acid (TUDCA) that mimic the actions of ER chaperones. They have proven highly effective in mitigating ER stress in various preclinical animal models and PBA is currently used clinically for the treatment of urea cycle disorders. Nevertheless, high doses of these agents are required to block ER stress and they may also have non-specific effects [20]. Alternatively, specific components of the UPR may be targeted for therapeutic effect. For example, overexpression of GRP78/94 blocks hypoxia-mediated cardiomyocyte death and cardiac damage following ischemia-reperfusion while PERK inhibition or deletion reduces myocardial tissue damage [8,21–23]. However, given that all three branches of the UPR elicit salutary and deleterious effects caution is needed when modifying this multifaceted signaling pathway. Clearly, further studies are needed to better understand the molecular mechanisms underlying the dual function of the UPR so that it may be more precisely targeted and directed towards an adaptive cardiac response.
Acknowledgement
William Durante received funding from the American Heart Association Midwest Affiliate (Award #15GRNT25250015).
References
- 1.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Anuu Rev Biochem 2005;74:739–789. [DOI] [PubMed] [Google Scholar]
- 2.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 2011;334:1081–1085. [DOI] [PubMed] [Google Scholar]
- 3.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 2004;11:381–389. [DOI] [PubMed] [Google Scholar]
- 4.Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ 2004;11:365–368. [DOI] [PubMed] [Google Scholar]
- 5.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 2005;115:2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 2010;107:1071–1082. [DOI] [PubMed] [Google Scholar]
- 7.Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol 2013;75:49–67. [DOI] [PubMed] [Google Scholar]
- 8.Tao I, Zhu W, Li Y, Xin P, Li J, Liu M, et al. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol 2011;301:H1471–H1486. [DOI] [PubMed] [Google Scholar]
- 9.Park CS, Cha H, Kwon EJ, Sreenivasaiah PK, Kim H. The chemical chaperone 4-phenylbutyric acid attenuates pressure overload cardiac hypertrophy by alleviating endoplasmic reticulum stress. Biochem Biophys Res Commun 2012;421:578–584. [DOI] [PubMed] [Google Scholar]
- 10.Luo T, Kim JK, Chen B, Abdel-Latif A, Kitkaze M, Yan L. Attenuation of ER stress prevents post-infarction-induced cardiac rupture and remodeling by modulating both cardiac apoptosis and fibrosis. Chem Biol Int 2015;225:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu ZW, Zhu HT, Chen KL, Dong X, Wei J, Qiu C, et al. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc Diabetol 2013;12:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayala P, Montenegro J, Vivar A, Letelier A, Urroz PA, Capaja M, et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol 2012;92:97–104. [DOI] [PubMed] [Google Scholar]
- 13.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, et al. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 2004;110:705–712. [DOI] [PubMed] [Google Scholar]
- 14.Essop MF. Cardiac metabolic adaptations in response to chronic hypoxia. J Physiol 584:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jain K, Suryakumar G, Ganju L, Singh SB. Amelioration of ER stress by phenylbutyric acid reduces chronic hypoxia induced cardiac damage and improves hypoxic tolerance through upregulation of HIF-1α. Vasc Pharmacol doi: 10.1016/j.vph.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI. Alam J, et al. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle: role in cell survival. J Biol Chem 2005; 280:872–877. [DOI] [PubMed] [Google Scholar]
- 17.Koyama M, Furuhashi M, Ishimura S, Mita T, Fuseya T, Okazaki Y, et al. Reduction of endoplasmic reticulum stress by phenylbutyric acid prevents the development of hypoxia-induced pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol 2014;306:H1314–1323. [DOI] [PubMed] [Google Scholar]
- 18.Dromparis P, Paulin R, Stenson TH, Haromy A, Sutendra G, Michelakis ED. Attenuating endoplasmic reticulum stress as a novel therapeutic strategy in pulmonary hypertension. Circulation 2013;127:115–125. [DOI] [PubMed] [Google Scholar]
- 19.Ding W, Zhang X, Huang H, Ding N, Zhang S, Hutchinson SZ. Adiponectin protects rat myocardium against chronic intermittent hypoxia-induced injury via inhibition of endoplasmic reticulum stress. PLoS One 2014;9:e94545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daosukho C, Chen Y, Noel T, Sompol P, Nithipongvanitch R, Velez JM, et al. Phenylbutyrate, a histone deacetylase inhibitor, protects against Adriamycin-induced cardiac injury. Free Radic Biol Med 2007;42:1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan YX, Lin L, Ren AJ, Pan XJ, Chen H, Tang CS, et al. HSp70 and GRP78 induced by endothelin-1 pretreatment enhances tolerance to hypoxia in cultured neonatal rat cardiomyocytes. J Cardiovasc Pharmacol 2004;44:S117–120. [DOI] [PubMed] [Google Scholar]
- 22.Vitadello M, Penzo D, Petronelli V, Michieli G, Gomirato S, Menabo R, et al. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J 2003;17:923–935. [DOI] [PubMed] [Google Scholar]
- 23.Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 2010;122:361–369. [DOI] [PubMed] [Google Scholar]