Abstract
Current clinically available retinal imaging techniques have limitations, including limited depth of penetration or requirement for the invasive injection of exogenous contrast agents. Here, we developed a novel multimodal imaging system for high-speed, high-resolution retinal imaging of larger animals, such as rabbits. The system integrates three state-of-the-art imaging modalities, including photoacoustic microscopy (PAM), optical coherence tomography (OCT), and fluorescence microscopy (FM). In vivo experimental results of rabbit eyes show that the PAM is able to visualize laser-induced retinal burns and distinguish individual eye blood vessels using a laser exposure dose of ~80 nJ, which is well below the American National Standards Institute (ANSI) safety limit 160 nJ. The OCT can discern different retinal layers and visualize laser burns and choroidal detachments. The novel multi-modal imaging platform holds great promise in ophthalmic imaging.
Keywords: Ophthalmology, photoacoustic microscopy, PAM, optical coherence tomography, OCT, fluorescence, ophthalmic optics and devices, chorioretinal imaging, retinal imaging
1. INTRODUCTION
The field of biomedical photoacoustic imaging has experienced explosive development recently [1–8]. Based on energy conversion of nanosecond pulse duration laser light into ultrasound, the emerging photoacoustic imaging can visualize biological samples at scales from organelles, cells, tissues, organs to small-animal whole body and can reveal its anatomic, functional, molecular, genetic, and metabolic information [1, 2, 9–12]. Photoacoustic imaging has found unique applications in a range of biomedical fields, such as cell biology [12–14], vascular biology [15, 16], neurology [17, 18], oncology [19–22], dermatology [23], pharmacology [24], rheumatology, and hematology [25, 26]. Its application in ophthalmology, that is, photoacoustic ocular imaging, has attracted substantial interests from both scientists and clinicians and is currently under active investigation.
Different from routinely used ocular imaging technologies [27, 28], such as fluorescein angiography (FA) and indocyanine green angiography (ICGA), optical coherence tomography (OCT), and OCT angiography, photoacoustic ocular imaging uses optical absorption as the contrast mechanism. This provides a unique tool for studying optical absorption properties of the eye. To date, significant work has been done in photoacoustic ocular imaging [29–36], but these studies focus on the posterior segment imaging of the eyes of small animals, such as rats and mice. The pioneering studies well demonstrate the feasibility in ophthalmology but there is still a long way to go towards clinical translation since eyeball sizes of rats and mice are much smaller (less than one-third) than that of humans.
Towards this goal, we developed noninvasive, label-free chorioretinal imaging in living rabbits using integrated photoacoustic microscopy (PAM), spectral-domain OCT (SD-OCT), and fluorescence microscopy (FM) [37, 38]. The developed system has excellent performance and could visualize the retina and choroid of the eyes of larger animals.
2. METHODS
2.1. Multi-modality imaging system
The light source of the photoacoustic microscopy (PAM) is an optical parametric oscillator (OPO) laser pumped by a diode-pumped solid-state laser (Ekspla NT-242, Lithuania). Key technical specifications include pulse repetition rate 1 kHz, pulse duration 3 – 6 ns, and tunable wavelength range 405 – 2600 nm. In PAM, the emanating laser beam at 570 nm is focused, filtered, and collimated by a beam collimator [see Fig. 1(a)]. The beam collimator is composed of a focusing lens (focal length 250 mm), a pinhole (diameter 50 μm), and a collimating lens (focal length 30 mm). After being collimated, the laser beam should be circular in shape with a diameter of about 2 mm. The collimated beam is successively deflected by a mirror and a dichroic mirror (DM) and raster-scanned by a two-dimensional galvanometer, which is a shared component with the spectral domain (SD)-OCT system.
Fig. 1.

Integrated photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy for chorioretinal imaging. A: Schematic; B: physical setup. OPO: Optical parametric oscillator; SLD: superluminescent diode; BS: beam splitter; DM: dichroic mirror; DCG: dispersion compensation glass; galvo: galvanometer.
The scanned beam travels through a telescope composed of a scan lens (focal length 36 mm) and an ophthalmic lens (OL, focal length 10 mm) and is finally focused on the fundus by the rabbit eye optics. Endogenous ocular chromophores, mainly hemoglobin in this work, absorb the laser energy and produce photoacoustic signal. The generated photoacoustic signal propagates through the eye and is captured by a custom-built needle-shaped ultrasonic transducer (center frequency 27 MHz, two-way −6 dB bandwidth 60%, Optosonic Inc., Arcadia, CA, USA), which is placed in contact with the conjunctiva off the central visual axis. The signal is then amplified by an ultrasonic amplifier (gain 57 dB), filtered by a low-pass filter (cutoff frequency 32 MHz), and digitized by a high-speed digitizer at a sampling rate of 200 MS/s. Laser pulse energy on the rabbit cornea is monitored by a power meter and is kept below the American National Standards Institute (ANSI) safety limit 160 nJ at 570 nm [37]. No signal averaging is used in data acquisition. The imaging speed is limited by the pulse repetition rate of the OPO laser, which is 1 kHz in this case. The laser, the galvanometer, and the digitizer are synchronized through a data acquisition (DAQ) board. The software for system control and data acquisition is custom-programmed in Matlab.
The spectral-domain optical coherence tomography (SD-OCT) system was adapted from a commercially available system from Thorlabs (Ganymede-II-HR, Thorlabs, Newton, NJ, USA) by adding an ocular lens (OL) after the scan lens and a piece of dispersion compensation glass (DCG) in the reference arm [see Fig. 1(a)]. The light source centered at 905 nm consists of two individual superluminescent diodes (SLD), one centered at 846 nm, the other one at 932 nm. The estimated bandwidth is about 220 nm. A zooming housing tube is used to adjust the reference arm length to ensure its match with the optical path length of the sample arm. An iris is used to control the intensity of retro-reflected reference light to ensure its match with back-scattered light intensity from the rabbit fundus to achieve maximum image contrast. A charge-coupled device (CCD) camera encapsulated in the scan head is used for real-time visualization of rabbit fundus with an illumination light emitting diode (LED) as an external illumination source. The OCT imaging speed is determined by the scanning rate of the galvanometer, which is 36 kHz in this case.
The fluorescence microscopy (FM) shares the same laser source with PAM and uses a DM, a clean-up filter, a collecting lens, and an avalanche photodiode (APD) to receive fluorescence signals.
2.2. Animal preparation
All rabbit experiments were performed in accordance with the ARVO (Association for Research in Vision and Ophthalmology) Statement for the Use of Animals in Ophthalmic and Vision Research, after approval of the laboratory animal protocol by the Institutional Animal Care and Use Committee (IACUC) of the University of Michigan (Protocol PRO00006486, PI Yannis Paulus).
As a general procedure, pre-experiment vitals, including overall animal state, mucous membrane color, heart rate, respiratory rate, and rectal temperature, were first monitored and recorded. Rabbits were then anesthetized with a mixture of Ketamine (40 mg/kg) and Xylazine (4 mg/kg) through intramuscular (IM) injection. Rabbit pupils were dilated using Tropicamide 1% ophthalmic and Phenylephrine Hydrochloride 2.5% ophthalmic. A speculum was used to hold the eyelids out of the way and frequent eye lubricant was then applied to moisten the cornea. A drop of topical Tetracaine 0.5% was instilled in the eye before the imaging procedure. The rabbit body and head were supported on different platforms to minimize motion artifacts during imaging. A water-circulating blanket (TP-700, Stryker Corporation, Kalamazoo, MI) was used to warm the rabbit during the experiment. Vitals, such as mucous membrane color, heart rate, respiratory rate, and rectal temperature, were monitored and recorded every 15 minutes to ensure animal comfort and correct level of anesthesia.
3. RESULTS
3.1. Resolution calibration
The lateral and axial resolution of PAM and OCT were calibrated with chromium gratings (linewidth: 10 μm, pitch: 40 μm) coated on a coverslip. Using data in small rectangular boxes of acquired C-scan images, edge spread functions [ESFs, Figs. 2(a) and 2(c)] of PAM and OCT were fitted with the Gauss error function; line spread functions (LSFs) were thus calculated from the ESFs to give lateral resolutions. The quantified lateral resolutions of PAM and OCT are 4.1 μm and 3.8 μm, respectively, in this case.
Fig. 2.

System resolution calibration using a chromium grating coated on a coverslip. (a) Fitted edge spread function (ESF) and line spread function (LSF) of experimental data shows that PAM has a lateral resolution of 4.1 μm. (b) Summation of two A-line axial spread function shows that PAM has an axial resolution of 37.0 μm. (c) Fitted ESF and LSF of experimental data show that OCT has a lateral resolution of 3.8 μm at −6 dB. (d) A-line spread function shows that OCT has an axial resolution of 4.0 μm at −6 dB in air.
The axial resolution of PAM was calculated by shifting a typical A-line signal (one-dimensional point spread function) and then adding the shifted signal to the original [Fig. 2(b)]. The minimum shift distance that still allows for discrimination of the two peaks according to the full-width half-maximum (FWHM) criterion is regarded as the axial resolution [39]. The axial resolution of OCT was simply estimated using the A-line signal width at FWHM [Fig. 2(d)]. The quantified axial resolutions of PAM and OCT are 37.0 μm and 4.0 μm, respectively, in this case.
3.2. Imaging results
To validate the performance of the multi-modality imaging system, laser burns on the retina were induced to three New Zealand white rabbits (male, ~6 months old, body weight ~3 Kg) using a 532 nm photocoagulator laser (Vitra, Quantel Medical, pulse duration: 100 ms). A clinical endpoint of moderate intensity laser burns was applied. The laser-induced burns were then monitored using the imaging platform.
Figures 3 shows a typical PAM C-scan image 7 days after the application of the laser burns. The imaging wavelength is 570 nm. The PAM image discerns individual choroidal vessels and maps blood vessels status around the laser burn sites. Vessels in the laser burns are much fewer compared with those in untreated retina areas. Figure 4 shows a typical OCT B-scan image immediately following laser photocoagulation, which led to hyper-reflective, full thickness circular regions of retinal thickening as well as retinal and choroidal detachments are clearly visible. Figure 5 shows fluorescence imaging results of mouse footpad tissue labeled with Alexa 488 fluorophore. The tissue could be resolved with fine details.
Fig. 3.

PAM image of laser-induced burns in rabbit retina
Fig. 4.

SD-OCT image of laser-induced burns in rabbit fundus
Fig. 5.
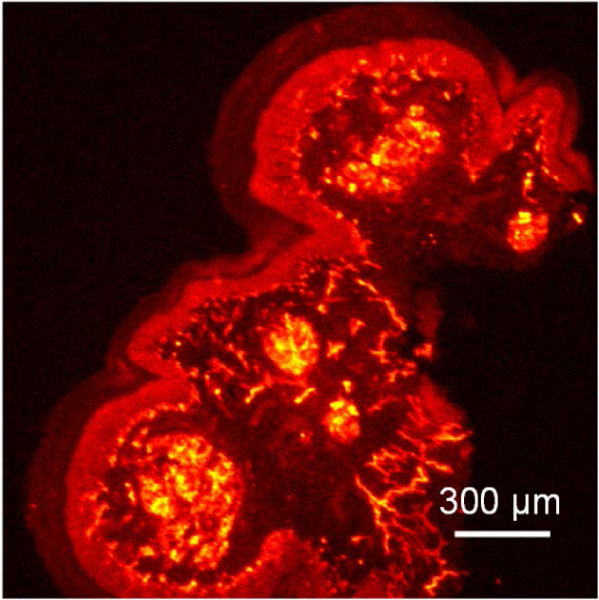
FM image of mouse footpad tissue on a coverslip
4. CONCLUSION
Towards the goal of clinical translation of photoacoustic ocular imaging, we developed a novel, high-speed, high-resolution, multimodal retinal imaging system, including photoacoustic microscopy (PAM), optical coherence tomography (OCT), and fluorescence microscopy (FM). Phantom experiments show that the system has quantified lateral resolutions of 4.1 μm (PAM) and 3.8 μm (OCT), and axial resolutions of 37.0 μm (PAM) and 4.0 μm (OCT) at the focal plane of the objective. In vivo rabbit experiments show that the PAM is able to visualize laser-induced retinal burns and distinguish individual eye blood vessels using a safe laser exposure dose at half of the ANSI safety limit. The OCT can discern different retinal layers and visualize laser burns and choroidal detachments. The novel multimodal system holds great promise in ophthalmic imaging.
ACKNOWLEDGEMENTS
This work was supported by the generous support of the National Eye Institute 4K12EY022299 (YMP), Fight for Sight-International Retinal Research Foundation FFS GIA16002 (YMP), unrestricted departmental support from Research to Prevent Blindness, and the University of Michigan Department of Ophthalmology and Visual Sciences. This work utilized the Core Center for Vision Research funded by P30 EY007003 from the National Eye Institute. We thank Mitchell Gillett for preparing the fluorescence slide, Dr. Cheng Zhang and Qiaochu Li for preparing the chromium grating sample, and Scott Szalay for preparing the rabbit support.
REFERENCES
- [1].Wang LV and Hu S, “Photoacoustic tomography: in vivo imaging from organelles to organs,” Science 335, 1458–1462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beard P, “Biomedical photoacoustic imaging,” Interface Focus, rsfs20110028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taruttis A and Ntziachristos V, “Advances in real-time multispectral optoacoustic imaging and its applications,” Nat. Photon. 9, 219–227 (2015). [Google Scholar]
- [4].Tian C, Xie Z, Fabiilli ML, Liu S, Wang C, Cheng Q, and Wang X, “Dual-pulse nonlinear photoacoustic technique: a practical investigation,” Biomed. Opt. Express 6, 2923–2933 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tian C, Xie Z, Fabiilli ML, and Wang X, “Imaging and sensing based on dual-pulse nonlinear photoacoustic contrast: a preliminary study on fatty liver,” Opt. Lett. 40, 2253–2256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tian C, Feng T, Wang C, Liu S, Cheng Q, Oliver DE, Wang X, and Xu G, “Non-Contact Photoacoustic Imaging Using a Commercial Heterodyne Interferometer,” IEEE Sens. J. 16, 8381–8388 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim KH, Luo W, Zhang C, Tian C, Guo LJ, Wang X, and Fan X, “Air-coupled ultrasound detection using capillary-based optical ring resonators,” Sci. Rep. 7, 1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Feng T, Kozloff KM, Tian C, Perosky JE, Hsiao Y-S, Du S, Yuan J, Deng CX, and Wang X, “Bone assessment via thermal photo-acoustic measurements,” Opt. Lett. 40, 1721–1724 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen S-L, Xie Z, Carson PL, Wang X, and Guo LJ, “In vivo flow speed measurement of capillaries by photoacoustic correlation spectroscopy,” Opt. Lett. 36, 4017–4019 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Galanzha EI, Shashkov EV, Kelly T, Kim J-W, Yang L, and Zharov VP, “In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells,” Nat. Nanotechnol. 4, 855–860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xiang L, Wang B, Ji L, and Jiang H, “4-D photoacoustic tomography,” Sci. Rep. 3(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tian C, Qian W, Shao X, Xie Z, Cheng X, Liu S, Cheng Q, Liu B, and Wang X, “Plasmonic nanoparticles with quantitatively controlled bioconjugation for photoacoustic imaging of live cancer cells,” Adv. Sci. 3(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou F, Wu S, Yuan Y, Chen WR, and Xing D, “Mitochondria‐Targeting Photoacoustic Therapy Using Single‐Walled Carbon Nanotubes,” Small 8, 1543–1550 (2012). [DOI] [PubMed] [Google Scholar]
- [14].Koker T, Tang N, Tian C, Zhang W, Wang X, and Martel R, “Targeted cell imaging by in-situ assembly and activation of hot spot SERS and photoacoustic nanoprobes using split-fluorescent protein scaffolds,” Nat. Commun, in press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maslov K, Zhang HF, Hu S, and Wang LV, “Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries,” Opt. Lett. 33, 929–931 (2008). [DOI] [PubMed] [Google Scholar]
- [16].Hu S, Maslov K, and Wang LV, “Three-dimensional optical-resolution photoacoustic microscopy,” in Biomedical Optical Imaging Technologies (Springer, 2013), pp. 55–77. [Google Scholar]
- [17].Yao J, Wang L, Yang J-M, Maslov KI, Wong TT, Li L, Huang C-H, Zou J, and Wang LV, “High-speed label-free functional photoacoustic microscopy of mouse brain in action,” Nature Methods 12, 407–410 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yang X, Skrabalak SE, Li Z-Y, Xia Y, and Wang LV, “Photoacoustic tomography of a rat cerebral cortex in vivo with au nanocages as an optical contrast agent,” Nano Lett. 7, 3798–3802 (2007). [DOI] [PubMed] [Google Scholar]
- [19].Agarwal A, Huang S, O’donnell M, Day K, Day M, Kotov N, and Ashkenazi S, “Targeted gold nanorod contrast agent for prostate cancer detection by photoacoustic imaging,” J. Appl. Phys. 102, 064701 (2007). [Google Scholar]
- [20].Zackrisson S, van de Ven S, and Gambhir S, “Light in and sound out: emerging translational strategies for photoacoustic imaging,” Cancer Res. 74, 979–1004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ermilov SA, Khamapirad T, Conjusteau A, Leonard MH, Lacewell R, Mehta K, Miller T, and Oraevsky AA, “Laser optoacoustic imaging system for detection of breast cancer,” J. Biomed. Opt. 14, 024007 (2009). [DOI] [PubMed] [Google Scholar]
- [22].Mallidi S, Luke GP, and Emelianov S, “Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance,” Trends Biotechnol 29, 213–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang HF, Maslov K, Stoica G, and Wang LV, “Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging,” Nature Biotechnol. 24, 848–851 (2006). [DOI] [PubMed] [Google Scholar]
- [24].Keswani RK, Tian C, Peryea T, Girish G, Wang X, and Rosania GR, “Repositioning Clofazimine as a Macrophage-Targeting Photoacoustic Contrast Agent,” Sci. Rep. 6, 23528 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Strohm EM, Berndl ES, and Kolios MC, “Probing red blood cell morphology using high-frequency photoacoustics,” Biophys. J. 105, 59–67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nguyen VP, Kim J, Ha K.-l., Oh J, and Kang HW, “Feasibility study on photoacoustic guidance for high-intensity focused ultrasound-induced hemostasis,” J. Biomed. Opt. 19, 105010 (2014). [DOI] [PubMed] [Google Scholar]
- [27].Keane PA and Sadda SR, “Retinal imaging in the twenty-first century: state of the art and future directions,” Ophthalmology 121, 2489–2500 (2014). [DOI] [PubMed] [Google Scholar]
- [28].Li Y, Xia X, and Paulus Y, “Novel Retinal Imaging Technologies,” Int J Ophthalmol Eye Res 5, 1–5 (2017). [Google Scholar]
- [29].de la Zerda A, Paulus YM, Teed R, Bodapati S, Dollberg Y, Khuri-Yakub BT, Blumenkranz MS, Moshfeghi DM, and Gambhir SS, “Photoacoustic ocular imaging,” Opt. Lett. 35, 270–272 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hu S, Rao B, Maslov K, and Wang LV, “Label-free photoacoustic ophthalmic angiography,” Opt. Lett. 35, 1–3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiao S, Jiang M, Hu J, Fawzi A, Zhou Q, Shung KK, Puliafito CA, and Zhang HF, “Photoacoustic ophthalmoscopy for in vivo retinal imaging,” Opt. Express 18, 3967–3972 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Silverman RH, Kong F, Chen Y, Lloyd HO, Kim HH, Cannata JM, Shung KK, and Coleman DJ, “High-resolution photoacoustic imaging of ocular tissues,” Ultrasound Med. Biol. 36, 733–742 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu N, Ye S, Ren Q, and Li C, “High-resolution dual-modality photoacoustic ocular imaging,” Opt. Lett. 39, 2451–2454 (2014). [DOI] [PubMed] [Google Scholar]
- [34].Song W, Wei Q, Liu W, Liu T, Yi J, Sheibani N, Fawzi AA, Linsenmeier RA, Jiao S, and Zhang HF, “A combined method to quantify the retinal metabolic rate of oxygen using photoacoustic ophthalmoscopy and optical coherence tomography,” Sci. Rep. 4, 6525 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hennen SN, Xing W, Shui Y-B, Zhou Y, Kalishman J, Andrews-Kaminsky LB, Kass MA, Beebe DC, Maslov KI, and Wang LV, “Photoacoustic tomography imaging and estimation of oxygen saturation of hemoglobin in ocular tissue of rabbits,” Exp. Eye Res. 138, 153–158 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu T, Wei Q, Song W, Burke JM, Jiao S, and Zhang HF, “Near-infrared light photoacoustic ophthalmoscopy,” Biomed. Opt. Express 3, 792–799 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tian C, Zhang W, Mordovanakis A, Wang X, and Paulus YM, “Noninvasive chorioretinal imaging in living rabbits using integrated photoacoustic microscopy and optical coherence tomography,” Opt. Express 25, 15947–15955 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Tian C, Zhang W, Nguyen VP, Wang X, and Paulus YM, “Novel Photoacoustic Microscopy and Optical Coherence Tomography Dual-modality Chorioretinal Imaging in Living Rabbit Eyes,” J. Vis. Exp, in press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xie Z, Jiao S, Zhang HF, and Puliafito CA, “Laser-scanning optical-resolution photoacoustic microscopy,” Opt. Lett. 34, 1771–1773 (2009). [DOI] [PubMed] [Google Scholar]


