Abstract
Significant research has been conducted on copper corrosion and solubility in drinking water, including the establishment of the “cupric hydroxide model”. The model describes the temporal aging and associated solubility changes of copper minerals beginning with the most soluble solid, cupric hydroxide. Although the model explains copper levels in field observations well, there are aspects of the model that are not well understood, including a lack of evidence of the presence of cupric hydroxide in drinking water distribution systems. This study aimed to understand the effect of water chemistry on the solubility and properties of newly precipitated cupric solids, including mineral identification. Bench-scale copper precipitation tests were performed in water under a matrix of pH and dissolved inorganic carbon conditions. Copper solids were analyzed using a combination of materials analysis tools including XRD, FT-IR, TGA, and inorganic carbon analyses. Copper solids were X-ray amorphous, isotropic, and were light blue to blue. Based on repeated analysis, georgeite (Cu2(CO3)(OH)2·6H2O) was conclusively identified as the solid at all test conditions. Georgeite is an extremely rare, amorphous malachite analog, and because of its rarity, very little has been reported on its presence in any environment.
Keywords: Georgeite, copper, drinking water
1. INTRODUCTION
Elevated copper levels in consumers’ tap water can cause acute health problems at high concentrations, blue water, staining of sinks and tubs, and clogging of filters. Because of potential health concerns, the U.S. Environmental Protection Agency (EPA) Lead and Copper Rule (LCR) established a copper action level (AL) of 1.3 mg/L in a 1 liter first draw sample collected from the consumers’ tap (Federal Register, 1992; Federal Register, 1991a; Federal Register, 1991b). The AL is health-based associated with stomach and intestinal distress, liver or kidney damage, and complications of Wilson’s disease in genetically predisposed people, and considers total copper ingestion (i.e., soluble and particulate copper). Since the implementation of the LCR, a huge amount of research has been conducted on copper corrosion, solubility, and leaching from materials in drinking water distribution systems. Many of these research findings (Lytle & Liggett, 2016; Vargas et al., 2010a; Vargas et al. 2010b; Lytle & Schock, 2000; Schock, 1999; Edwards et al., 1999) have improved the water industry’s understanding of how water chemistry affects the solubility of copper minerals found in drinking water distribution systems, the role of stagnation time on copper leaching from copper pipes, and the use of corrosion inhibitors to reduce copper levels at the consumer’s tap. Additionally, new information on copper solubility in water has relevance to the drinking water premise plumbing (including buildings) treatment field. For example, the use of copper-silver ionization systems to control Legionella in the drinking water of large buildings (e.g., hospitals) has grown in interest (Kusnetsov et al., 2001; Walraven et al., 2016; Traintafyllidou et al., 2016; Barbosa & Thompson, 2016), and by water chemistry and associated copper solubility control the applied copper dose.
A particularly important development was the establishment of the “cupric hydroxide model” (Schock et al., 1995a). As pipes age and passivating films build and become more thermodynamically stable, the associated copper solubility changes. For example, new copper piping is more cuprosolvent at equilibrium than older piping, though not necessarily corrosive toward copper (Turek et al., 2011; Edwards et al., 2002; Lagos et al., 2001; Schock et al., 1995a). As a result, some researchers (Edwards et al., 2002; Lagos et al., 2001; Schock et al., 1995a) have noted that older methods for predicting Cu(II) solubility fail when applied to newer plumbing systems because these methods assume the formation of tenorite or malachite minerals that may or may not be present on the pipe surface. The cupric hydroxide model provides an important understanding of cuprosolvency, particularly the impact of solids-aging and water chemistry on soluble copper levels in water (Schock et al., 1995a). The model essentially proposes that copper solubility-controlling solids on the surface of copper plumbing change as they “age,” from a relatively soluble copper solid to an insoluble one. The reliability of this model has been substantiated in many ways, including bench-scale and pilot-scale studies, field observations, and chemical equilibrium modeling simulations. Although cupric hydroxide solubility and model explain copper levels in field observations reasonably well (Schock et al., 1995a), there are aspects of the model that are not well understood. For example, although the aging process is assumed, to begin with cupric hydroxide, it is not readily formed in drinking water and rarely identified in drinking water distribution systems where its presence has been questioned (Broo et al., 1997).
There are many significant Cu(II) mineral corrosion by-products that have been identified on drinking water distribution materials, including tenorite (CuO), langite (Cu4(SO4)(OH)6∙2H2O), posnjakite (Cu4(SO4)(OH)6∙H2O), malachite (Cu2CO3(OH)2), azurite (Cu3(CO3)2(OH)2), atacamite (Cu2(OH)3Cl), cupric chloride (CuCl2), and brochantite (Cu4(SO4)(OH)6) (Vargas et al., 2010b; Merkel et al., 1999; Schock et al.; 1995a, Singley et al., 1984). In addition to these solids, georgeite represents an extremely rare, amorphous copper hydroxy-carbonate hydrate mineral that exists in nature (Bridge et al., 1979; Pollard et al., 1989) and has been synthesized in a limited number of environments (Pollard et al., 1991; Pollard et al., 1992; Kondrat et al., 2016). First reported in 1975, georgeite (properties shown in Table 1) was discovered in the Carr Boyd Mine in Western Australia (Bridge et al., 1979) and later reported to be found at the Britannia Mine in Snowdonia, Wales (Pollard et al., 1989).
Table 1.
Reported properties of Georgeite.
| Formula | Carbon Composition (%) | Crystal System | Color | Streak | Density (g/cm3) | Optical Data | Reference |
|---|---|---|---|---|---|---|---|
| CU2(CO3)(OH)2 | 5.43 | Amorphous | Sky blue | Pale blue | 2.55(10) | Isotropic, n=1.593(2), δ=0.000 | Mindat.org |
| Cu5(CO3)3(OH)2*6H2O | 5.63 | Amorphous | Light blue | Light blue | 2.55 | Isotropic, n=1.593 | Webmineral.com |
| CU2(CO3)(OH2)*6H2O | 3.65 | Amorphous | Pale blue | Mineralogy of Wales Database | |||
| Cu5(CO3)3(OH)4*6H2O | 5.34 | Amorphous | Pale Cerulean blue | Very pale blue | Isotropic, n=1.593 |
Georgeite is an X-ray amorphous analog of malachite having several reported chemical formulas: Cu2CO3(OH)2∙6H2O (Mineralogy of Wales Database, 2017; Pollard et al., 1989; Bevins, 1994), Cu2(CO3)(OH)2 (Mindat.org, 2017) and Cu5(CO3)3(OH)4∙6H2O (Bridge et al., 1979). Georgeite is light, pale, or sky blue; non-crystalline or amorphous; and isotropic. Georgeite, as well as other copper hydroxy-carbonates, are of great interest to the chemical industry as they are widely used catalyst precursors (Spencer 1999; Hartic et al. 2014; Pollard et al. 1991; Kondrat et al. 2016). Pollard et al. (1991) synthesized georgeite in the laboratory by mixing 0.8 g (47 mmol) of CuCl2∙2H2O to 100 mL of a solution containing 5.3 g (50 mmol) Na2CO3 at 25 °C. In later work, Pollard et al. (1992) produced georgeite by mixing a 0.1 M aqueous solution of CuSO4 with 1 M aqueous Na2CO3 solution at 60°C. More recently, Kondrat et al. (2016) synthesized georgeite using a supercritical anti-solvent precipitation process that uses carbon dioxide rather than sodium carbonate as the carbonate source. Catalysts developed from a georgeite precursor were superior to those formed in Na2CO3 (Pollard et al., 1991) because the sodium, a potential catalyst poison, was eliminated and reduced from the catalyst precursors (Kondrat et al., 2016; Lehigh University 2016). Due to its rarity, very little has been reported on its properties in any environment, including water where its solubility has yet to be determined. Georgeite has never been recognized by the drinking water industry and has never been reported in drinking water suspensions or in drinking water distribution systems.
Although many significant developments have been made on controlling copper corrosion and reducing copper solubility in water, many questions remain. There remains a great need to better understand copper geochemistry in aqueous environments, including drinking water systems. Specifically, although the conceptual understanding of copper aging in water described by the cupric hydroxide model, cupric hydroxide is not identified in drinking water systems nor is it easily formed in aqueous environments. Increasing our understanding of copper behavior in drinking water will improve corrosion control, protection of public health, and Legionella treatment in buildings. This study aimed to examine the effect of water chemistry on the solubility and properties of newly precipitated copper solids. Most importantly, the primary objective of this work was to conclusively identify the copper mineral (s) that freshly form in water. Understanding the impact of water chemistry on the solubility and properties of newly precipitated copper solids is necessary for controlling copper in consumer’s drinking water and protecting public health.
2. EXPERIMENTAL
2.1. Chemicals and reference standards.
Unless otherwise specified, all chemicals used in this study were Analytical Reagent (AR) grade. Dilute 0.6 M HCl and 0.5 N NaOH (Fisher Scientific; Fairlawn, NJ) were used to adjust pH. Sodium bicarbonate (Fisher Scientific; Fair Lawn, NJ) was added to adjust DIC concentration. Copper was added as cupric perchlorate (Cu(ClO4)2•H2O) (GFS Chemicals; Columbus, OH).
Copper reference materials were obtained to compare against the experimentally synthesized precipitates. Cupric hydroxide (Cu(OH)2) was purchased from Alfa Aesar (Haverhill, MA). Azurite (Cu3(CO3)2(OH)2) was a geological mineral specimen from the Morenci Mine in Morenci, Arizona. Copper (II) carbonate basic (CuCO3 · Cu (OH)2) was obtained from Sigma-Aldrich (St. Louis, MO). Malachite (Cu2(CO3)(OH)2) was obtained from a precipitate formed in copper corrosion research experiments (Lytle & Schock, 2008). Lastly, georgeite was obtained as a geological mineral specimen and reference material from the Carr Boyd Nickel mine in Kalgoorlie, W.A. Australia.
2.2. General experimental design.
Cu(II) solubility and particle formation experiments were conducted in a 3 L glass beaker. The beaker top contained ports for acid and base injection, sample collection, pH electrode, dissolved oxygen/temperature probe, mechanical stirrer, and a gas feed tube.
Experiments were initiated by adding 2 liters of double deionized (DDI) water to the beaker located on a stir plate set at 200 rpm. DDI water was prepared by passing distilled water through a deionized water system (Milli-Q Plus, Millipore Corporation; Belford, MA) having a resistivity ≥ 18.2 MΩ·cm. An appropriate amount of sodium bicarbonate was then added to the water to achieve target DIC concentrations of 10, 50, and 100 mg C/L.
After DIC addition to the reaction vessel, the treatment water was titrated to the desired pH and thereafter maintained during the experiment using the Jensen Systems Multi-T 2.2™ software (Jenson Systems; Hamburg, Germany) and Schott Titronic Universal dual auto-titrators (Schott Gerate; Germany). The pH was maintained by rapidly adding small increments of 0.6 N hydrochloric acid or 0.6 N sodium hydroxide to compensate for pH changes (±0.05) caused by chemical additions and subsequent reactions. The software recorded pH values and titrant volumes that were stored in a data file.
Before cupric perchlorate being added to the treatment water, a polypropylene syringe was used to collect a 40 mL headspace free sample for DIC analysis (refrigerated at 4 °C until analysis). After pH stabilization, cupric perchlorate (Cu(ClO4)2•H2O) was added slowly to avoid significant pH changes. For solubility studies, cupric perchlorate was added to give initial target copper concentrations of 4, 8, 16, 20, 24, or 32 mg/L as Cu(II), and for solids generation studies, 32 mg/L was the necessary initial target copper concentration to produce enough material for solids analysis.
2.3. Solubility studies.
Three different experimental approaches were considered: (i) incremental precipitation, (ii) incremental dissolution, and (iii) single pH. Incremental precipitation experiments were conducted by initially programming the titrator to reach a low pH (~6.5). The pH was incrementally increased by 0.3 to 0.6 pH units to approximately pH 8.5. Incremental dissolution experiments were initiated by adding copper to the reaction vessel at a pH of approximately 8.5. The titrator was programmed to incrementally decrease the pH by 0.3 to 0.6 pH units to pH 6.5. Experiments were also conducted with the titrator programmed at a single pH. Copper was measured at the programmed pH, the water was then wasted, and fresh water was prepared to repeat the test at a new pH.
In all approaches, copper samples were drawn out of the beaker with a polypropylene syringe approximately 10 minutes after the cupric perchlorate had time to mix at the desired pH. Subsequently, copper and DIC samples were drawn after an additional 10 minutes (20 minutes total), and the solution was filtered using a 0.20 μm filter paper (Whatman Inc.; Clifton, NJ). Samples for copper and other metals to be analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) and DIC analysis were taken from the filtered solution, and the filter paper with the copper solids was placed in a vial and allowed to dry under atmospheric conditions. Particulate copper was separated using 0.20 μm polypropylene disk syringe filters (Whatman Inc.; Clifton, NJ)
2.4. Solids generation studies.
The aqueous copper suspension was stirred at the desired pH for 20 minutes. After mixing, a 60 mL water sample was collected for ICP-AES copper analysis. The remaining solution was then filtered through 0.20 μm Whatman filter paper using vacuum filtration. After filtration, liquid samples were taken for copper and DIC analysis following the previously described procedure. Copper solids formed during the experiment were separated from the solution by filter paper at the same time as the collection of water samples (20 min of mixing) and placed in a vial to dry under atmospheric conditions. Once dried, the copper carbonate solids were submitted for solids analysis, including TIC, XRD, HRTEM, TGA, and FT-IR.
2.5. Analytical water quality methods.
The pH was measured with a benchtop pH/ISE meter (Hach Company, EC 40 Benchtop pH/ISE meter, Model 50125; Loveland, CO) and a combination pH electrode with temperature correction (Hach Company, Combination pH electrode, Model 48600; Loveland, CO). The instrument was standardized daily using a two-point calibration with pH 7 and 10 standard solutions (Thermo Fisher Scientific; Chelmsford, MA). Dissolved oxygen was measured with a dissolved oxygen meter (Hach Company, Dissolved oxygen meter, Model DO175; Loveland, CO) and a dissolved oxygen probe (Hach Company, Dissolved oxygen probe, Model 50180; Loveland, CO). Copper concentrations were measured with a HACH spectrometer (Hach Company, Spectrometer. Model DR/2010. Loveland, CO) and by ICP-AES using EPA Method 200.7, 1990).
2.6. Inorganic carbon analysis of water and solids.
Aqueous samples were analyzed for inorganic carbon on a Shimadzu TOC-VCPH Total Organic Carbon Analyzer (Kyoto, Japan) using the Inorganic Carbon (IC) method in the TOC-Control V software. Inorganic carbon analysis of solids was determined by a coulometric procedure on a UIC Model 5011 CO2 coulometer (Joliet, IL.) with a Model 50 acidification module operated under computer control.
2.7. X-ray diffraction (XRD).
A theta-theta diffractometer (PANalytical X’Pert Pro, PANalytical B.V., The Netherlands) with a copper x-ray tube was used to identify crystalline phases of the copper precipitates. Filters were mounted on plastic back plates using spray adhesive in such a way that the focus plane was at the filter surface. The XRD tube was operated at 45 keV and 40 mA for the analyses. Scans were performed over a 2-theta range between 5° to 90° with a step of 0.02° and a one-second count time at each step. Pattern analysis was performed generally following ASTM procedures (ASTM, 1996) using computer software (Materials Data, Incorporated. Jade+ v.5–8 XRD Processing Software. Livermore, California) with reference to the 2002 ICDD PDF-2 data files (International Center for Diffraction Data, Incorporated; Newtown Square, PA). XRD d-spacing results were compensated for sample displacement due to filter thickness by the way the filter media were mounted and inserted into the sample holder. XRD d-spacing results were not corrected for displacement due to deposit thickness on top of the filter surface.
2.8. Isotropic/anisotropic properties.
A Nikon LABOPHOT-POL polarizing light microscope (Nikon, Inc.; Melville, NY) was used to examine precipitate optical properties. Precipitates were crushed using a mortar and pestle and placed dry on a clear glass slide for observation.
2.9. Thermogravimetric analysis (TGA).
TGA measurements were performed to compare precipitate decomposition rates to known standards. A TA Q500 (TA Instruments; New Castle, DE) was employed with a 4 to10 mg sample size and thermal program of 10 °C/min from 28 °C to 900 °C under nitrogen at a 60 mL/min nitrogen flow rate.
2.10. Fourier transform-infrared (FT-IR) spectroscopy.
Infrared analysis was executed using a Cary 600 Series FT-IR Spectrometer (Agilent Technologies, Inc,, Santa Clara, CA) over a range of 400 to 4000 cm−1. Samples were run under a nitrogen atmosphere to avoid signature of air adsorbed CO2.
2.11. High-resolution transmission electron microscopy (TEM).
The amorphous morphology and crystallinity of experimentally developed solid samples were investigated using A JEOL (Jem-2010F) high resolution-transmittance electron microscope (HR-TEM) with field transmission of 200 kV. To prepare the samples for HR-TEM, the copper solid samples were suspended in water and sonicated for 30 min, then fixed on a Lacy Carbon-Copper grid (LC300-Cu, EMS) by the drop-casting method.
2.12. Solubility modelling.
The method of Schock et al. (1995a) was used to simulate copper solubility for the 50 mg C/L DIC experiments. Simulations were generated by assuming control by one of three different copper containing solids: cupric hydroxide; azurite; or malachite. Reaction and associated equilibrium constants were taken from the literature (Schock et al. 1995a; Powell et al. 2005; Powell et al. 2007) and are summarized in Table 2. For the solubility simulations, chloride and sulfate concentrations were set to 100 mg/L each and DIC was assumed constant at 50 mg C/L. A 0.01 M ionic strength was used and equilibrium constants were adjusted for ionic strength using the Davies equation (Benjamin, 2002).
Table 2.
Thermodynamic chemical input constants included in copper solubility simulations.
| Reaction | log Constant Value (I = 0 M, 25°C) | Source |
|---|---|---|
| Cu(OH)2(s) + 2H+ ⇌ Cu2+ + 2H2O | 8.89 | Schock et al. (1995a) |
| Cu3(CO3)2(OH)2(s) ⇌ 3Cu2+ + 2CO32− + 2OH− | −44.9 | Powell et al. (2007) |
| CU2(CO3)3(OH)2(s) ⇌ 2Cu2+ + CO32− + 2OH− | −33.16 | Powell et al. (2007) |
|
| ||
| Cu2+ + H2O ⇌ CuOH+ + H+ | −7.95 | Powell et al. (2007) |
| Cu2+ + 2H2O ⇌ Cu(OH)2(aq) + 2H+ | −16.2 | Powell et al. (2007) |
| Cu2+ + 3H2O ⇌ Cu(OH)3− + 3H+ | −26.60 | Powell et al. (2007) |
| Cu2+ + 4H2O ⇌ Cu(OH)42− + 4H+ | −39.74 | Powell et al. (2007) |
| 2Cu2+ + H2O ⇌ Cu2OH3+ + H+ | −6.40 | Powell et al. (2007) |
| 2Cu2+ + 2H2O ⇌ Cu2(OH)22+ + 2H+ | −10.43 | Powell et al. (2007) |
| 3Cu2+ + 4H2O ⇌ Cu3(OH)42+ + 4H+ | −21.1 | Powell et al. (2007) |
| Cu2+ + Cl− ⇌ CuCl+ | 0.83 | Powell et al. (2007) |
| Cu2+ + 2Cl− ⇌ CuCl2(aq) | 0.6 | Powell et al. (2007) |
| Cu2+ + SO42− ⇌ CuSO4(aq) | 2.35 | Powell et al. (2007) |
| HCO3− + H+ ⇌ H2CO3* | 6.355 | Powell et al. (2005) |
| HCO3− ⇌ H+ + CO32− | −10.336 | Powell et al. (2005) |
| Cu2+ + HCO3− ⇌ CuHCO3+ | 1.84 | Powell et al. (2007) |
| Cu2+ + HCO3− ⇌ CuCO3(aq) + H+ | −3.56 | Powell et al. (2007) |
| Cu2+ + 2HCO3− ⇌ Cu(CO3)22− + 2H+ | −10.3 | Powell et al. (2007) |
| Cu2+ + CO32− + H2O ⇌ Cu(CO3)OH− + H+ | −2.79 | Powell et al. (2007) |
| Cu2+ + CO32− + 2H2O ⇌ Cu(CO3)OH22− + 2H+ | −13.14 | Schock et al. (1995a) |
2.13. Other.
Glassware used for standard and solution preparation was cleaned using a 5% solution of Contrad 70 soak cleaner (Catalog no. 18417–5, Polysciences incorporated; Warrington, Pennsylvania) followed by thorough rinsing with deionized water. Reused glassware was immediately cleaned by soaking in 10% (v/v) concentrated HNO3 and rinsing with DDI water. Air displacement micropipettes with disposable tips were used for handling and transferring solutions.
3. RESULTS AND DISCUSSION
3.1. Solubility Studies.
Detailed analysis of the solubility studies will be the focus of a future manuscript given the additional in-depth analysis necessary to develop reliable thermodynamic chemistry-based predictions, but a brief discussion on selected results is included herein as the solubility analysis supported the investigation of formed solids.
3.1.1. Copper solubility.
The experimental approach by which copper solids were formed had no impact on copper solubility when only pH and DIC effects were considered; therefore, the discussion considers all data (i.e., from incremental precipitation, incremental dissolution, or single pH experiments). Cu(II) levels decreased dramatically with increasing pH from approximately 6.5 to 9 at all DIC levels evaluated. For example, soluble Cu(II) levels dropped from nearly 4 mg/L at pH 6.6 to 0.3 mg/L at pH 8.6 in 10 mg C/L DIC water. As DIC increased, copper solubility trends shifted upward (increased) over the entire pH range. Over the pH range of 6.5 to 9, copper levels ranged from 8.2 to 0.85 mg/L and >10 to 1.3 mg/L in waters containing 50 (Figure 1) and 100 mg C/L waters, respectively.
FIGURE 1.
Soluble copper levels associated with precipitated georgeite in 50 mg C/L DIC water compared to theoretical copper solubility simulations (25°C).
3.1.2. Theoretical solubility modelling.
The experimental data from the solubility experiments were simulated with a theoretical cupric hydroxide solubility model previously described (Table 2). Figure 1 displays the results for the 50 mg C/L DIC experiments and is representative of the results seen for 10 and 100 mg C/L DIC. Even for cupric hydroxide solid control, measured copper solubility was greater than theoretical model simulations, except for pH values less than approximately 7 where the simulated solubility was similar to the experimental data. There is wide variation in cupric hydroxide solubility constants in the literature (Gulens et al., 1984, Hidmi & Edwards, 1999; Paulson & Kester, 1980: Schindler et al., 1965; Schindler, 1967) that has been attributed to mineral age and crystal size. Theoretical cupric hydroxide plots were simulated using a range of reported solubility constants (Schock et al. 1995a; Powell et al. 2005; Powell et al. 2007) (Table 2 and Figure 1). Although the experimental data fell within the range of predicted cupric hydroxide solubility curves, the theoretical plot shapes did not reflect the impact of carbonate in the mid- to high pH range on the experimental data.
Attempts were made to simulate the experimental data by assuming various copper solids and revised solubilities (data shown for azurite and malachite in Fig. 1). Ultimately, the experimental data were represented by a hypothetical solid with the same chemical formula as malachite but with a greater solubility. Based on the solubility analysis, it was hypothesized that an amorphous solid was formed with the chemical formula identical of malachite but with a much greater solubility. Upon a literature search, georgeite was identified as an amorphous analog of malachite (Bridge et al., 1979; Pollard et al., 1989) but with an unknown solubility. Subsequently, an in-depth solid analysis study was conducted to investigate the hypothesis that georgeite was the solid formed in the solubility experiments. If verified, future analysis could be conducted to provide the first estimates of georgeite’s solubility in water.
3.2. Solids Analysis.
Based on the preliminary solubility modeling and theoretical simulations, a wide range of solids analyses was conducted to identify the copper solids formed in the solubility experiments across a relatively wide range of pH and DIC conditions. A multi-analysis approach was necessary to confirm the identity that included physical appearance, and crystallographic and compositional characteristics. A comparison of results with the findings of past researchers reported properties and direct analysis of reference material was critical to conclusive solids identification.
3.2.1. Color.
The visual color of precipitated copper solids ranged from light blue to blue (Figure 2). The shade of blue appeared relatively darker in copper solids formed in 10 mg C/L DIC water where carbonate may have been limiting. Many copper minerals are identified by shades of blue to green in color. Malachite is light to dark green and did not match the precipitated solids which contained no hint of green (Figure 2). The reference materials, cupric hydroxide, and azurite, are pale blue and deep blue to violet blue, respectively (Figure 2) and they did resemble the color of precipitated copper solids. The color of georgeite has been described as pale blue (Pollard et al., 1989) and sky blue (Mindat,org, 2017) in color. Bridge et al. (1979) described the color of georgeite as Ridgeway Light Cerulean Blue 45-BG-Bb, Pale Cerulean Blue 45-BG-Bd to Calamine Blue 45-G-Bd. The range of blue shades in experimental solids (Figure 2) was consistent with the color range reported for georgeite and extended to Cerulean Blue 45-BG-B.
FIGURE 2.
Color of copper solids formed in matrix of pH and DIC water conditions.
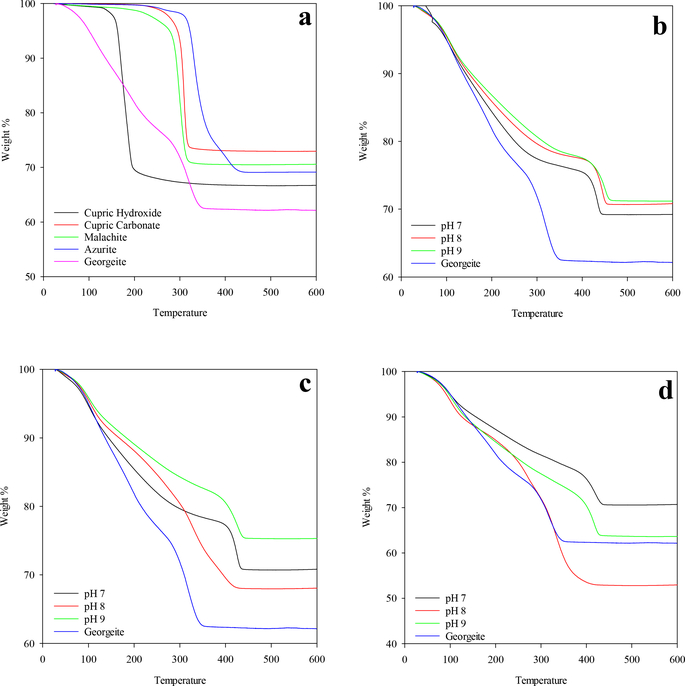
3.2.2. Thermogravimetric analysis (TGA).
A comparison of the decomposition of reference materials by TGA analysis is shown in Figure 3. Malachite (and cupric carbonate) and azurite standards exhibited single weight losses (Figure 3) consistent with work reported by others (Seguin, 1975; Kondrat et al., 2016). Cupric hydroxide decomposition also showed a single weight loss reflecting hydroxide decomposition. Unfortunately, the georgeite mineral standard obtained for this study was not sufficient in quantity to perform TGA, but georgeite (reported formula Cu7(CO3)5(OH)4·5H2O) reference TGA data (Figure 3) were kindly provided by Simon Kondrat (Kondrat et al., 2016). Georgeite exhibited a multistep decomposition with three distinct weight losses as did the solids created under all pH and DIC experimental conditions. Specifically, malachite, and other standards, exhibited a single mass loss associated with the concurrent removal of water and carbon dioxide. Whereas, the decomposition of copper solids generated in the current experiments were consistent with the georgeite and could be separated into three regions identical to that reported by Kondrat et al. (2016): (i) water loss at 80 to 100 °C, (ii) water and carbon dioxide loss at 190 to 210 °C, and (iii) carbon dioxide loss (carbonate decomposition) at 315 to 420 °C.
FIGURE 3.
Thermogravimetric analysis (TGA) of georgeite, synthesized copper solids, and copper samples: (a) TGA of georgeite and copper standards, (b) copper solids formed in 10 mg C/L DIC water, (c) copper solids formed in 50 mg C/L DIC water, and (d) copper solids formed in 100 mg C/L DIC water.
3.2.3. FT-IR Spectroscopy.
IR spectra of relevant copper solid materials cupric hydroxide, Cu(OH)2, cupric carbonate, CuCO3 · CU(OH)2, malachite, Cu2CO3(OH)2, azurite, Cu3(CO3)2(OH)2, and georgeite were collected and compared for reference (Figure 4a). The IR spectrum of georgeite is distinguishable from the other copper minerals and exhibited a broad OH− band at 3251 cm−1 and CO32- bands at 1482, 1384 and 833 cm−1 (Table 3). The georgeite standard IR spectrum (Table 3) is consistent with reports by others (Kondrat et al., 2016; Bridge et al. 1979). Precipitated Cu(II) solids in aqueous suspensions, analyzed immediately after filtration (wet) and following air drying, were analyzed. IR spectra of solids produced overall nine drinking water conditions evaluated in this work (pHs 7, 8, and 9 and 10, 50, and 100 mg C/L DIC) were nearly identical to IR spectra of natural georgeite from the Carr Boyd and Britannia mines (Figures 4a-d, Table 3) and synthesized georgeite (Bridge at al. 1979; Pollard et al. 1989; Pollard et al. 1992; Kondrat et al. 2016) (Table 3).
FIGURE 4.
FT-IR spectra of georgeite, copper standards, and synthesized copper solids: (a) IR spectra of georgeite and copper standards, (b) copper solids formed in 10 mg C/L DIC water, (c) copper solids formed in 50 mg C/L DIC water, and (d) copper solids formed in 100 mg C/L DIC water.
Table 3.
FT-IR frequencies of synthesized copper solids compared against georgeite standard and reference from literature. Values given are for IR band positions in wavenumbers (cm−1).
| Georgeite Standard (Carr Boyd Mine) | Georgeite Reference (Kondrat Paper) | DIC 10 pH 7 | DIC 10 pH 8 | DIC 10 pH 9 | DIC 50 pH 7 | DIC 50 pH 8 | DIC 50 pH 9 | DIC 100 pH 7 | DIC 100 pH 8 | DIC 100 pH 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| 3251 | 3408 | 3247 | 3235 | 3250 | 3243 | 3271 | 3243 | 3221 | 3243 | 3237 |
| 2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
2360 2340 |
|
| 1482 1384 |
1470 1404 |
1456 1367 |
1456 1368 |
1456 1368 |
1455 1369 |
1456 1371 |
1455 1367 |
1455 1366 |
1455 1369 |
1456 1369 |
| 833 | 829 | 833 | 833 | 834 | 832 | 832 | 832 | 830 | 831 | 832 |
IR spectra of precipitated copper solids were repeated multiple times after the samples were stored at room temperature under room atmosphere. Specifically, samples were re-analyzed after one week and three months after initially formed. The analysis showed no change in spectra under all conditions (shown for pH 8 and all DIC conditions in Figure 5).
FIGURE 5.
FT-IR spectra of copper solids formed at pH 8 at all DIC conditions taken over 3 months to show effects of aging on solids left under air: (a) 10 mg C/L DIC, (b) 50 mg C/L DIC, and (c) 100 mg C/L DIC.
3.2.4. XRD.
Malachite, cupric carbonate and azurite are crystalline and belong to the monoclinic crystal system, and cupric hydroxide belongs to the orthorhombic crystal system. XRD scans of the respective solids confirmed crystallinity and were consistent with reference patterns (Fig. 4a). XRD scan of copper solids formed under all pH and DIC conditions, however, only produced a broad bump between a 2 Theta range of 30 to 40ᵒ and did not produce sharp diffraction peaks (Fig. 4b) to d). The large bump distributed in a wide range (2 Theta) and absence of high intensity narrower peaks indicates that the solids were X-ray amorphous or non-crystalline. Georgeite is reported to be an X-ray amorphous copper mineral (Bridge et al., 1979; Pollard et al., 1989). The XRD detection limit is ~2% the sample indicating that the amorphous solid component of experimentally generated copper solids accounted from more than ~98% of the solids.
3.2.5. Isotropic/anisotropic properties.
An optically isotropic mineral has identical optical properties in all directions; the velocity of light is constant in all directions of transmission. As a result, light passes through an isotropic mineral as a single ray. In contrast, the properties of anisotropic minerals vary with different crystallographic directions. Using a polarized light microscope, isotropic minerals will remain dark under crossing polarized light as the stage is rotated. Anisotropic minerals in contrast will allow some light to pass unless in certain orientations. Amorphous materials and isometric crystalline minerals are optically isotropic and are far less common than anisotropic minerals (materials with optical properties that change with orientation). All hexagonal, tetragonal, orthorhombic, monoclinic, and triclinic minerals are anisotropic. Important copper minerals including malachite, cupric hydroxide, and azurite are anisotropic, whereas georgeite is an isotropic mineral. The vast majority of grains examined under the microscope were optically isotropic (a trace fraction of the grains was anisotropic) which is consistent with the optical properties of georgeite.
3.26. HRTEM studies.
Micrographs revealed the existence of a single amorphous phase as the dominant phase with crystallinity as deduced from structure analysis by HRTEM (Fig. 5) which is consistent with the XRD results. Two crystalline structures, a large plant leaf-like structure with average crystal dimensions of 580 nm and 170 nm, and very tiny rice-like grains with particle size ranged from 3.7 nm to 9.0 nm (Fig. 5A) were identified as trace phases. The leaf-like crystals composed from Cu(OH)2 particles aggregates, likely generated via initial interaction between Cu2+ and water molecules. Dehydration of Cu(OH)2 and consequent oxidation produced CuO particles (i.e., rice grains). A similar observation was reported by Nishino et al. (2017) for CuO nano-flowered material synthesized via submerged photosynthesis of Cu crystallites. The presence of Cu(OH)2 and CuO nanoparticles and their distribution on the dominant amorphous were deduced from HRTEM image (Fig. 5) that displayed typical fringes of Cu(OH)2 and CuO crystals. This result was proved via comparison of SAED diffraction rings of amorphous material with the theoretical SAED rings of Cu (OH)2 and CuO materials which are available in the online open-data-base. Indeed, the perfect overlapping between theoretical miller indices (0 2 0), )1 1 0), (1 1 1), (0 0 2 ) and (0 2 2), with amorphous material rings confirmed for SAED patterns, however, the minor of Cu (OH)2 and CuO crystals were identified by comparing the SAED pattern with the online standard crystallographic information of Cu(OH)2 and CuO (Fig. 5C). According to the Fast Fourier transform (FFT) analysis (Fig. 5D), the amorphous material sample was comprised of highly defined patterns corresponding to the Cu. This result was consistent with the FFT micrographs of georgeite and malachite materials synthesized by the sol-gel method combined with supercritical antisolvent precipitation (SAP) using CO2. As could be seen from Fig. 5C broad rings correspond to amorphous georgeite morphology. Although the presence of crystalline materials within the amorphous georgeite was interesting, they represented a trace compositional fraction of the experimentally generated copper solids consistent with XRD results (<2% for each of the two identified crystalline phases) which is consistent with HRTEM scan observations. That is to say that, the solids formed in this work were relatively homogenous amorphous material as opposed to a mixture of solids.
3.2.7. Inorganic Carbon Compositions.
The inorganic carbon content of precipitated copper solids was a critical measurement in correctly identifying precipitated copper minerals (Table 4).
Table 4.
Summary of replicate inorganic carbon content (weight %) of copper solids formed under all experimental conditions.
| Solids Total Inorganic Carbon (%) | |||||
|---|---|---|---|---|---|
| 9/2/2015 | 1/5/2016 | 1/5/2017 | Average | St. Dev | |
| DIC 10, pH 7 | NA* | 3.38 | 3.14 | 3.26 | 0.17 |
| DIC 10, pH 8 | 3.12 | 3.03 | 2.97 | 3.04 | 0.07 |
| DIC 10, pH 9 | 2.72 | 2.95 | 2.88 | 2.85 | 0.12 |
| DIC 50, pH 7 | 4.19 | 3.41 | 3.34 | 3.65 | 0.47 |
| DIC 50, pH 8 | 3.83 | 3.46 | 3.54 | 3.61 | 0.19 |
| DIC 50, pH 9 | 3.74 | 3.41 | 3.48 | 3.54 | 0.17 |
| DIC 100, pH 7 | 4.00 | 4.21 | 3.76 | 3.99 | 0.22 |
| DIC 100, pH 8 | 3.86 | 3.81 | 3.82 | 3.83 | 0.02 |
| DIC 100, pH 9 | 3.73 | 3.69 | 3.69 | 3.70 | 0.02 |
Because XRD, HRTEM and polarizing light microscopy analysis indicated that precipitated solids consisted dominantly (>98%) of a single amorphous phase, carbon analysis results reflect the composition of the amorphous copper solid (i.e., dilution and mixture effects are not significant). The inorganic carbon content of copper solids formed in water containing 10 mg C/L increaded from 2.85 to 3.26% C (average of two or three individual precipitation tests) as the target pH decreased from 9 to 7. The inorganic carbon content of coper solids formed in water containing 50 mg C/L increased from 3.54 to 3.65% C (average of three precipitation tests per condition) as the target pH decreased from 9 to 7. Lastly, the inorganic carbon content of copper solids formed in water containing 100 mg C/L increased from 3.70 to 3.99% C (average of three precipitation tests per condition) as pH decreased from 9 to 7. Cupric hydroxide does not contain carbonate and therefore was not a match. The carbon content of solids precipitated under all conditions was far less than malachite and azurite at 5.43% and 6.97%, respectively. Georgeite is an X-ray amorphous analog of malachite having several reported chemical formulas: Cu2CO3(OH)2·6H2O, Cu2(CO3)(OH)2, Cu7(CO3)5(OH)4·5H2O and Cu5(CO3)3(OH)4·6H2O. Differences in reported chemical formula and carbon composition of georgeite are possibly associated with inconsistencies in the way whereby inorganic carbon content was derived which was often not well addressed if at all. The carbon content of precipitated experimental copper solids was also much lower than georgeite reported with the chemical formula of Cu5(CO3)3(OH)4·6H2O, which is 5.63%. The carbon content, however, is consistent to georgeite with a chemical formula of Cu2(CO3)(OH)2·6H2O, which was 3.65%. Copper solids formed in 10 mg C/L DIC contained less carbon but the carbon content in the water may have been limiting given the relatively large amount of copper precipitated in the tests (32 mg/L).
3.3. Importance of georgeite in water.
The experimentally measured copper concentrations in water associated with freshly precipitated copper were not in good agreement with predicted copper solubility of cupric hydroxide previously believed to be the amorphous Cu(II) phase initially formed in water as described by the cupric hydroxide model. In particular, the experimental data deviated from the theoretical solubility model at the mid to upper pH ranges indicating the influence of carbonate on Cu(II) solubility. The solubility results suggested the likelihood that a malachite-like Cu(II) mineral such as the rare amorphous mineral georgeite was the identity of the precipitated solids. Multiple materials analysis approaches were applied to freshly precipitated copper solids as well as reference materials of interest (malachite, azurite, cupric hydroxide, and georgeite) for comparison, which included physical appearance and crystallographic and compositional characteristics. Although the color of experimental solids was consistent with the color of georgeite, cupric hydroxide, and to a lesser degree azurite, polarized light microscopy, inorganic carbon analysis, FT-IR analysis, and TGA analysis conclusively, XRD analysis and HRTEM analysis results were all consistent with georgeite. Considering these results, the precipitated solids are identified as georgeite. The findings were compared to and consistent with multiple georgeite references. Inorganic carbon analysis of the solids was consistent with georgeite having the specific chemical formula of Cu2(CO3)(OH)2·6H2O.
Due to the rarity of georgeite, very little has been reported on its properties in any environment. The important results of this work represent the first time the georgeite has been formed in drinking water over a wide range of DICs and pH values. The implications may have a large impact on how the aging of corroding copper in drinking water systems and other environments is conceptually understood. Specifically, the long established cupric hydroxide model may be more accurately referred to as the georgeite for at least some conditions. The solubility of georgeite introduced here, and the expanded modelling effort and emphasis of a following manuscript, will be particularly valuable in predicting copper levels associated with corroding copper plumbing in drinking water and dosing of copper to premise plumbing systems for Legionella control. With this information, next steps include searching for georgeite on corroding copper surfaces exposed to water, expanding the solubility modelling efforts initiated in this work, and more precisely incorporating georgeite into the copper aging model.
4. CONCLUSION
Several essential research findings came from this work. Experimentally measured copper concentrations associated with freshly precipitated copper formed in a matrix of three pH and three DIC waters were not in good agreement with the theoretical solubility of cupric hydroxide previously believed to be the amorphous Cu(II) mineral initially formed in water according to the cupric hydroxide model. Instead, experimental data were represented by a hypothetical solid with the same chemical formula as malachite but with a higher solubility. A literature review identified georgeite as possible an amorphous analog of malachite. Multiple solids analysis approaches were used to identify freshly precipitated copper solids. All results were consistent with georgeite based on previous reports and, taken together, confirm the identification: such as: i) The color of the solid was pale blue or sky blue ii) TGA decomposition was consistent with those reported in the literature iii) The IR spectroscopy of samples was consistent with those of naturally occurring georgeite and consistent with spectrums reported by other researchers iv) XRD and HRTEM analyses indicated that samples were a single (> 98%) amorphous phase, consistent with georgeite. v) The majority of sample grains analyzed were optically isotropic. vi) The inorganic carbon content of samples was consistent with Cu2(CO3)(OH)2·6H2O.
The current research represents the first reported occurrence of georgeite over drinking water relevant DIC and pH ranges. The implications may have a large impact on how the aging of corroding copper in drinking water systems and other environments is conceptually understood. Specifically, the long-established cupric hydroxide model may be more accurately referred to as the georgeite for at least some conditions. The solubility of georgeite introduced here, and the expanded modeling effort and emphasis of the following manuscript, will be particularly valuable in predicting copper levels associated with corroding copper plumbing in drinking water and dosing of copper to premise plumbing systems for Legionella control.
Future next research steps include searching for georgeite on corroding copper surfaces exposed to water, expanding the solubility modeling efforts initiated in this work, and more precisely incorporating georgeite into the copper aging model.
5. ACKNOWLEDGEMENTS
The authors thank Maily Pham for water quality analysis, Christy Muhlen from the U.S. Environmental Protection Agency (U.S. EPA) for her assistance in operating the experimental system, and Mitch Wilcox with Pegasus Technical Services for technical review of the manuscript. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the EPA. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the EPA. Any mention of products or trade names does not constitute recommendation for use by the EPA.
Contributor Information
Darren A. Lytle, U.S. Environmental Protection Agency, ORD, NRMRL, WSWRD, TTEB, 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268.
David Wahman, U.S. Environmental Protection Agency, ORD, NRMRL, WSWRD, TTEB, 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268.
Michael R. Schock, U.S. Environmental Protection Agency, ORD, NRMRL, WSWRD, TTEB, 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268
Mallik Nadagouda, U.S. Environmental Protection Agency, ORD, NRMRL, WSWRD, TTEB, 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268.
Stephen Harmon, U.S. Environmental Protection Agency, ORD, NRMRL, WSWRD, TTEB, 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268.
Katherine Webster, Pegasus Technical Services, Inc. 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268.
Jacob Botkins, Pegasus Technical Services, Inc. 26 W. Martin Luther King Drive, Cincinnati, Ohio 45268.
6. REFERENCES
- ASTM (1996) Standard practices for identification of crystalline compounds in water-formed deposits by x-ray diffraction. Vol. 11.02, D 934–80. American Society for Testing and Materials, Conshohocken, PA. [Google Scholar]
- Barbosa VL and Thompson KC (2016). Controlling Legionella in a UK hospital using copper and silver ionisation—A case study, Journal of Environmental Chemical Engineering, 4:3: 3330–3337. [Google Scholar]
- Barthelmy D (accessed 04 April 2017). Webmineral Mineralogy Database[http://webmineral.com/data/Georgeite.shtml#.WOOJDk1U3cs
- Benjamin MM (2002). Water Chemistry. McGraw-Hill, New York, NY. [Google Scholar]
- Bevins RE (1994). A Mineralogy of Wales, Cardiff. [Google Scholar]
- Bridge PJ, Just J and Hey MH (1979). Georgeite, a new amorphous copper carbonate from the Carr Boyd mine, Western Australia. Mineralogical Magazine, 43, 97–98. [Google Scholar]
- Broo AE, Berghult B and Hedberg T (1997). Copper corrosion in drinking water distribution systems — the influence of water quality. Corrosion Science 39(6), 1119–1132. [Google Scholar]
- Edwards M, Powers K, Hidmi L,and Schock MR(2001). The Role of Pipe Aging in Copper Corrosion By-Product Release, Water Science and Technology. 1(3):25–32. [Google Scholar]
- Edwards M, Hidmi L, and Gladwell D (2002). Phosphate inhibition of soluble copper corrosion by-product release. Corr. Sci 44, 1057–1071. [Google Scholar]
- Edwards M, Meyer TE and Schock MR (1996). Alkalinity, pH and Copper Corrosion By-Product Release. Journal of the American Water Works Association 88(3), 81–94. [Google Scholar]
- Edwards M, Jacobs S and Dodrill D (1999). Desktop Guidance in Mitigation of Pb and Cu Corrosion By-Products. Journal of the American Water Works Association 91(5), 66–77. [Google Scholar]
- Edwards M; Schock M; & Meyer TE (1996). Alkalinity, pH, and Copper Corrosion By-Product Release. Jour. AWWA, 88:3. [Google Scholar]
- Register Federal (1991a). Drinking water regulations; maximum contaminant level goals and national primary drinking water regulations for lead and copper. 40 CRF Parts 141 and 142. U. S. EPA 56:32112. [Google Scholar]
- Register Federal (1991b). Maximum contaminant level goals and national primary drinking water regulations for lead and copper. U. S. EPA 56:26460. [Google Scholar]
- Register Federal (1992). Drinking water regulations: Maximum contaminant level goals and national primary drinking water regulations for lead and copper. 40 CFR Parts 141 and 142. U. S. EPA 57:28785. [Google Scholar]
- Ferguson JL, von Franque O & Schock MR (1996). Corrosion of Copper in Potable Water Systems, in Internal Corrosion of Water Distribution Systems; 2nd ed.; AWWA Research Foundation/DVGW-TZW: Denver, CO. [Google Scholar]
- Gulens J, Leeson PM and Séguin (1984). Kinetic Influences on Studies of Copper(II) Hydrolysis by Copper Ion-Selective Electrode. Analytica Chimica Acta 156, 19–31. [Google Scholar]
- Hidmi L and Edwards M (1999). Role of Temperature and pH in Cu(OH)2 Solubility. Environmental Science & Technology 33(15), 2607–2610. [Google Scholar]
- Kondrat SA, Smith PJ, Wells PP, Chater PA, Carter JH, Morgan DJ, Fiordaliso EM, Wagner JB, Davies TE, Lu L, Bartley JK, Taylor SH, Spencer MS, Kiely CJ, Kelly GJ, Park CW, Rosseinsky MJ, Hutchings GJ (2016). Stable amorphous georgeite as a precursor to a high-activity catalyst. Nature. 3;531(7592):83–7. [DOI] [PubMed] [Google Scholar]
- Lehigh University (2016). “Rare mineral georgeite synthesized for first time: Rare mineral is a superior catalyst, researchers say.” ScienceDaily. ScienceDaily, 16 February 2016 <www.sciencedaily.com/releases/2016/02/160216123451.htm>.
- Lyons JJ, Pontes J and Karalekas P (1995). Optimizing Corrosion Control for Lead and Copper Using Phosphoric Acid and Sodium Hydroxide, pp. 2457–2473, New Orleans, LA. [Google Scholar]
- Lytle DA and Schock MR (2008). Pitting Corrosion of Copper in High pH and Low Alkalinity Waters. Jour. AWWA, 100:3:115–128. [Google Scholar]
- Lytle DA and Liggett J (2016). Impact of water quality on chlorine demand of corroding copper. Water Research 92, 11–21. [DOI] [PubMed] [Google Scholar]
- Lytle DA and Schock MR (2000). Impact of Stagnation Time on Metal Dissolution from Plumbing Materials. Aqua in press. [Google Scholar]
- Lagos GE, Cuadrado CA, and Victoria-Letelier M (2001). Aging of copper pipes by drinking water. Jour. AWWA 93(11), 94–103. [Google Scholar]
- Merkel TH (2004). Copper corrosion: understanding and modeling general corrosion. Wat. Sci. Technol 49(2), 63–71. [PubMed] [Google Scholar]
- Merkel TH, Werner WW, Alex T, Klümper TH and Eberle SH (1999). Copper Corrosion Research State of the Art and Recent Projects in Germany, Chicago, IL. [Google Scholar]
- Mindat.org (2015). Georgeite: Georgeite Mineral Information and Data, and the Hudson Institute of Mineralogy 1993-2017 http://www.mindat.org/min-1676.html.mindat.org.
- MWH (2005). Internal Corrosion of Water Conduits, Ch. 21 in Water Treatment Principles and Design; 2nd ed., Crittenden JC, Trussell RR, Hand DW, Howe KJ, and Tchobanoglous G (eds.). John Wiley and Sons: New York, NY. [Google Scholar]
- National Research Council (2001). Committee on Copper in Drinking Water. National Academies Press (US): Washington, DC. [Google Scholar]
- Palit A, Pehkonen SO and Schock M (1998). Copper Corrosion: The Effect of Specific Inorganic Species in Drinking Water and the Evaluation of a Homogeneous Cu2O Film as a Corrosion Inhibitor using Simulated and Real Waters, San Diego, CA. [Google Scholar]
- Paulson AJ and Kester DR (1980). Copper(II) Ion Hydrolysis in Aqueous Solution. J. Solution Chem 9(4), 269–277. [Google Scholar]
- Pollard AM, Thomas RG, Williams PA, Bevins RE and Turgoose S (1989). Carbonatian connellite, a new variety, from the Britannia Mine, North Wales, and from the Botallack Mine. Cornwall. Journal of the Russell Society 2(2), 23–27. [Google Scholar]
- Pollard AM, Spencer RG, Thomas RG, and Williams PA (1992). Georgeite and azurite as precursors in the preparation of co-precipitated copper/zinc oxide catalysts, Applied Catalysis A: General, 85, 1–11. [Google Scholar]
- Pollard AM, Thomas RG, Williams PA, Bevins RE and Turgoose S (1989). Carbonatian connellite, a new variety, from the Britannia Mine, North Wales, and from the Botallack Mine, Cornwall. Journal of the Russell Society. 2 (2), 23–27. [Google Scholar]
- Pollard AM, Thomas RG, Williams PA, Just J and Bridge PJ (1991). The Synthesis and Composition of Georgeite and its Reactions to Form Other Secondary Copper(II) Carbonates . Mineralogical Magazine 55(379), 163–166. [Google Scholar]
- Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjoberg S, and Wanner H (2005). Chemical Speciation of Environmentally Significant Heavy Metals with Inorganic Ligands - Part 1: The Hg2+- Cl−, OH−, CO32-, SO42-, and PO43- Aqueous Systems - (IUPAC technical report). Pure Appl. Chem 77 (4), 739–800. [Google Scholar]
- Powell KJ, Brown PL, Byrne RH, Gajda T, Hefter G, Sjoberg S, and Wanner H (2007). Chemical speciation of environmentally significant metals with inorganic ligands - Part 2: The Cu2+-OH−, Cl−, CO32-, SO42-, and PO43- systems - (IUPAC technical report). Pure Appl. Chem 79 (5), 895–950. [Google Scholar]
- Sandvig A, Kwan P, Kirmeyer G, Maynard B, West D, Trussell R, Trussell S, Cantor A and Prescott A (2008). Contribution of Service Line and Plumbing Fixtures to Lead and Copper Rule Compliance Issues. Denver, Colo.: AWWARF. [Google Scholar]
- Schock MR, Lytle DA, and Clement JA (1995a). Effect of pH, DIC, orthophosphate and sulfate on drinking water cuprosolvency. EPA/600/R-95/085, U.S. EPA, Office of Research and Development, Cincinnati, OH: <http://www.epa.gov/ORD/WebPubs/effect>. [Google Scholar]
- Schock MR, Lytle DA, and Clement JA (1995b). Effects of pH, carbonate, orthophosphate and redox potential on cuprosolvency. NACE Corrosion/95, Orlando, FL. [Google Scholar]
- Schock MR, Lytle DA and Clement JA (1995b) Effects of pH, Carbonate, Orthophosphate and Redox Potential on Cuprosolvency, Orlando, FL. [Google Scholar]
- Schock MR (1999). Water Quality and Treatment: A Handbook of Community Water Supplies. Association, A.W.W (ed), pp. 17.01–17.109, McGraw-Hill, Inc., New York. [Google Scholar]
- Schock MR and Clement JA (1998). Control of Lead and Copper with Non-zinc Orthophosphate. Journal of the New England Water Works Association 112(1), 20–42. [Google Scholar]
- Schock MR, Lytle DA and Clement JA (1995a). Effect of pH, DIC, Orthophosphate and Sulfate on Drinking Water Cuprosolvency, Office of Research and Development, Cincinnati, OH. [Google Scholar]
- Singley JE, Beaudet BA and Markey PH (1984). Corrosion Manual for Internal Corrosion of Water Distribution Systems, Prepared for Office of Drinking Water by Environmental Science and Engineering, Inc., Gainesville, FL. [Google Scholar]
- Triantafyllidou S, Lytle DA, Muhlen C, and Swertfeger J (2016). Copper-silver ionization at a US hospital: Interaction of treated drinking water with plumbing materials, aesthetics and other considerations, Water Research, 102: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek NF, Kasten L, Lytle DA, and Goltz MN Impact of plumbing age on copper levels in tap water. Jour. Water Supply:Res. and Tech.:AQUA, 60:1:1–15 (2011). [Google Scholar]
- Vargas IT, Pavissich JP, Olivares TE, Jeria GA, Cienfuegos RA, Pastén PA and Pizarro GE (2010a) Increase of the concentration of dissolved copper in drinking water systems due to flow-induced nanoparticle release from surface corrosion by-products. Corrosion Science 52(10), 3492–3503. [Google Scholar]
- Vargas IT, Pastén PA and Pizarro GE (2010b) Empirical model for dissolved oxygen depletion during corrosion of drinking water copper pipes. Corrosion Science 52(7), 2250–2257. [Google Scholar]
- Wales N.M.o. (2016) Mineralogy of Wales Database: Georgeite.
- Wales N.M.o. (2017) Mineralogy of Wales Database: Georgeite, https://museum.wales/mineralogy-of-wales/database/?mineral=249.
- USEPA (U.S. Environmental Protection Agency) (1987). Summary Review of the Health Associated with Copper Health Issue Assessment. EPA/600/8–87/001. Environmental Criteria and Assessment Office, U.S. Environmental Protection Agency, Cincinnati, OH. [Google Scholar]
- Kusnetsov Jaana, Iivanainen Eila, Elomaa Nina, Zacheus Outi, and Martikainen Pertti J. (2001). Copper and silver ions more effective against Legionellae than against mycobacteria in a hospital warm water system, Water Research, Volume 35, Issue 17, December 2001, Pages 4217–4225. [DOI] [PubMed] [Google Scholar]
- Schindler P, Althaus H, Hofer F and Minder W (1965). Löslichkeitsprodukte von Zinkoxid, Kupferhydroxid und Kupferoxid in Abhängigkeit von Teilchengrösse und molarer Oberfläche. Ein Beitrag zur Thermodynamik von Grenzflächen fest-flüssig. Helvetica Chim. Acta 48(5), 1204–1215. [Google Scholar]
- Schindler PW (1967). Equilibrium Concepts in Natural Water Systems, American Chemical Society, Washington, DC. [Google Scholar]
- Walraven N, Pool W, and Chapman C (2016). Efficacy of copper-silver ionisation in controlling Legionella in complex water distribution systems and a cooling tower: Over 5 years of practical experience, Journal of Water Process Engineering, 13:196–205. [Google Scholar]